Abstract
Objectives
To develop and validate the Geriatric CompleXity of Care Index (GXI), a comorbidity index of medical, geriatric, and psychosocial conditions that addresses disease severity and intensity of ambulatory care for older adults with chronic conditions.
Design
Development phase: variable selection and rating by clinician panel. Validation phase: medical record review and secondary data analysis.
Setting
Assessing the Care of Vulnerable Elders-2 study.
Participants
Six hundred forty-four older (≥75) individuals receiving ambulatory care.
Measures
Development: 32 conditions categorized according to severity, resulting in 117 GXI variables. A panel of clinicians rated each GXI variable with respect to the added difficulty of providing primary care for an individual with that condition. Validation: Modified versions of previously validated comorbidity measures (simple count, Charlson, Medicare Hierarchical Condition Category), longitudinal clinical outcomes (functional decline, survival), intensity of ambulatory care (primary, specialty care visits, polypharmacy, number of eligible quality indicators (NQI)) over 1 year of care.
Results
The most-morbid individuals (according to quintiles of GXI) had more visits (7.0 vs 3.7 primary care, 6.2 vs 2.4 specialist), polypharmacy (14.3% vs 0% had ≥14 medications), and greater NQI (33 vs 25) than the least-morbid individuals. Of the four comorbidity measures, the GXI was the strongest predictor of primary care visits, polypharmacy, and NQI (p<.001, controlling for age, sex, function-based vulnerability).
Conclusion
Older adults with complex care needs, as measured by the GXI, have healthcare needs above what previously employed comorbidity measures captured. Healthcare systems could use the GXI to identify the most complex elderly adults and appropriately reimburse primary providers caring for older adults with the most complex care needs for providing additional visits and coordination of care.
Keywords: ambulatory care, utilization, comorbidity
The medical home has been hailed as a new system-based strategy for providing high-quality patient-centered primary care,1 but one challenge that medical homes face will be to provide high-quality care to the older adults with the most complex care needs, typically those with multiple chronic conditions.2 Individuals with multimorbidity are at greater risk for mortality and disability than those with no major chronic conditions3, 4 and generate greater healthcare costs.5, 6 Beyond daily medication and self-care routines, individuals with multimorbidity have been described as having frequent pharmacy7 and doctor visits8, 9 and multiple prescribers.7 Recent research has shifted attention to the potential burden of applying individual chronic disease guidelines to individuals with multiple chronic diseases.10, 11 For clinicians, prioritizing patients' multiple conditions is a critical source of complexity.12 As health systems assume responsibility for delivering high-quality care to defined populations of individuals, understanding the intensity of care required to care for the older adults with the most complex needs will become critically important.
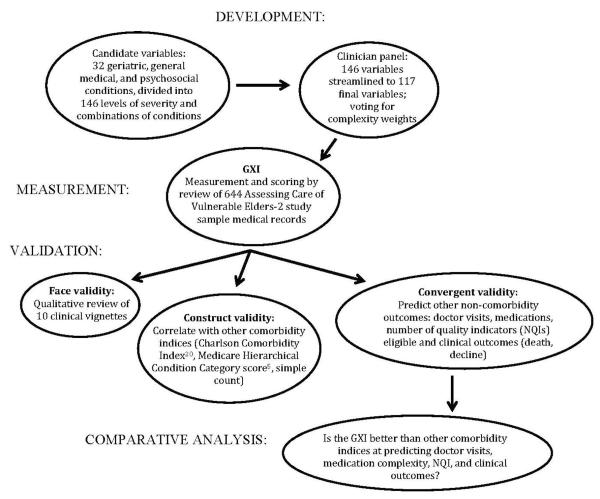
The current approach to measuring multimorbidity and its effect on delivering high-quality care ignores geriatric conditions and the severity of individual diseases.13, 14 Geriatric conditions are prevalent in older adults15 and are associated with functional decline15 and poor quality of care.16 Therefore, as part of the Assessing the Care of Vulnerable Elders-2 (ACOVE-2)17 study, an effort was made to develop a new, more-comprehensive comorbidity index for older adults. The Geriatric CompleXity of Care Index (GXI) includes difficult-to-manage geriatric conditions (e.g., dementia and falls) and severity ratings for geriatric and other chronic diseases. This article describes the development and multistep validation of the GXI index (Figure 1).
Figure 1.
ACOVE-2 was an ideal opportunity to study comorbidity and complexity of care. Participants had geriatric and other conditions and were evaluated for eligibility for a comprehensive set of ambulatory care quality indicators (QIs) as a measure of complexity of care. It was primarily hypothesized that the GXI would be a better predictor of complicated ambulatory care (number of eligible QIs and polypharmacy) than three previously employed comorbidity measures: a simple count of 12 conditions used in prior ACOVE studies,13, 18, 19 the Charlson Comorbidity Index (CCI),20 and Medicare Hierarchical Condition Categories (HCC).5 The secondary hypotheses were that the CCI would be the best at predicting clinical outcomes (function and survival) and that the HCC would be the best at predicting number of ambulatory care visits.
METHODS
Development of the GXI
First, candidate conditions were identified based on a nosology of diseases of aging.21 Candidate conditions were considered if they were chronic, symptomatic, and prevalent and required ambulatory care. Conditions were stratified according to disease severity, including subclinical forms (e.g., pre-diabetes mellitus (DM)), as less-severe versions of the conditions. When available, existing staging methods were used (e.g., New York Heart Association heart failure criteria22). In other cases, commonly documented symptoms were used as indicators of severity (e.g., behavior problems as a severity criterion for dementia). Combinations of common co-prevalent conditions in older adults19 were also considered as sources of additional complexity: hypertension and chronic kidney disease (CKD);23 DM and CKD;23 and depression, insomnia, and anxiety24, 25. In total, 146 candidate variables were considered (32 conditions, stratified according to severity, with potential interactions between conditions and levels of severity).
Expert panel assessment
A panel of six clinician–researchers (including LM, DR, DG, PS, and NW) with experience in clinical care of older adults with complex care needs, quality measurement, and comorbidity was first convened. In two rounds of voting, the panelists first voted on inclusion of candidate variables and then weighted each condition according to their assessment of difficulty involved with caring for an older adults with that condition over 1 year of primary care. Each candidate variable was rated on a scale from 1 to 10, with higher scores representing a more complex form of the condition. Each GXI variable was assigned the median rating of the six raters as the final complexity weight. Two combinations of multiple conditions (DM and CKD; depression, anxiety, and insomnia) were rated as having more complexity than the sum of the individual conditions. The panel's final number of GXI variables (conditions, severity levels, and interactions) was 117 (Appendix A.1).
Setting and data collection
Medical records from ACOVE-217, a practice-based intervention in 2002 that enrolled 644 individuals at two multisite ambulatory care practices in southern California, were reviewed. This cohort comprised older adults (≥75) who had at least one of three geriatric conditions (urinary incontinence (UI), impaired memory, and falls). Within each practice, one site received a multicomponent practice-directed quality improvement intervention26 for dementia, falls, and UI; the other sites (one in the first practice, four at the other) served as controls (only patient screening).
In the original ACOVE-2 study, participants' complete ambulatory medical records (including all primary and specialty care providers for the 13-month study) were collected for evaluation of 98 ACOVE ambulatory care quality indicators (QIs) in 13 areas of medical and geriatric care (dementia, depression, DM, falls and fear of falling, hearing impairment, hypertension, malnutrition, osteoporosis, osteoarthritis, pain, UI, medication use, screening and prevention).27 A detailed medical record review was performed to determine eligibility for 65 QIs. In addition, 578 participants were interviewed upon enrollment, which determined eligibility for an additional 33 interview-based QIs.
For the current study, complete medical records were re-abstracted for the 117 GXI variables during the study year. Two abstractors (LM, AW) were blinded to which QIs were previously measured.16, 17, 27 The mean time needed to abstract a chart for the GXI was 12 minutes. The institutional review boards at RAND Health and the University of California at Los Angeles approved this study.
Primary predictor variable: GXI comorbidity score
Each individual's GXI score was calculated, based on his or her conditions, as the sum of the complexity weights as rated by the expert panel. A higher GXI indicated greater burden. For example, if an individual had uncomplicated atrial fibrillation (AF) (complexity weight= 3), uncomplicated emphysema (complexity weight = 4), and hypercholesterolemia (complexity weight=2), the GXI score was calculated to be 9.
Outcome variables
To validate the GXI, six outcome measures of clinical complexity were studied. These measures were not based on medical conditions and included two clinical outcomes, two provider visit variables, polypharmacy, and number of eligible QIs.
Clinical outcomes
Longitudinal functional decline and mortality outcomes had been previously collected on the ACOVE-2 cohort (2002–07).28 Functional decline was defined using the Short Functional Survey (SES),29, 30 which summarizes independence in five activities of daily living (shopping, light housework, finances, bathing, and walking; 0–5 possible points). Two 5-year outcomes were calculated for each individual: death versus survival and functional decline versus no decline. Full information was available on death outcomes. Information on functional decline outcomes was collected for 295 participants.
Primary care provider and specialty care visits
As a measure of ambulatory care use, available administrative data were used to identify the number of visits to the individual's primary care provider. If more visits were made to an alternate primary care provider, the alternate provider was assigned as the individual's primary care provider. Each individual's specialty visits over the same time period were counted.
Number of eligible quality indicators
Because each ACOVE ambulatory care QI specifies an individual's eligibility for an ambulatory care process,31 it was determined that the number of eligible QIs (NQI) for each individual could serve as a unique marker of the clinical care needed to meet quality standards.
The number of medical-record based ambulatory care QIs for which each individual was eligible (of 65 possible) was calculated for each individual. For participants who provided interview-based eligibility information, an alternative NQI was calculated that combined medical record and interview eligibility methods (98 QIs possible). As in the original ACOVE-2 study, individuals with advanced dementia and limited life expectancy were not eligible for a subset of QIs based on appropriateness criteria.32 Because nearly all QIs require more care (only 2 of 98 were “overuse”-type QIs), a higher NQI indicates an individual needing higher-intensity ambulatory care.
Medication use
As an additional measure of ambulatory care intensity,7 the maximum number of medications documented in the medical record at any visit was counted. Only prescribed medications or over-the-counter medications recommended on a long-term basis for chronic condition management were included. Polypharmacy was categorized as none (≤6 medications), mild (7–9), moderate (10–13), or severe (≥14).
Comparison comorbidity measures (simple count, CCI, and HCC)
The ACOVE simple count consisted of 12 conditions: AF, coronary artery disease, heart failure, cerebrovascular disease, DM, hypertension, dementia, falls, hearing impairment, UI, malnutrition, and osteoporosis (starred in Appendix A.1).
All 19 comorbidities from the CCI20 were mapped to GXI variables to calculate a modified CCI score (mCCI) for each individual. Claims data necessary to calculate the HCC directly (based on 80 acute and chronic conditions) were not available. To calculate a modified HCC score (mHCC), 37 ambulatory care conditions were mapped to the HCC, and previously published community-based weights were applied. 5 For mCCI and mHCC, points were omitted for age and sex because these variables are not included in the GXI and simple count.
Covariables
Sex, age, ACOVE-2 intervention site, and the Vulnerable Elders Survey-13 (VES-13) were used in multivariable models.33 The VES-13 is a 13-item screening tool (11 items pertaining to physical abilities and activities of daily living, 1 item regarding self-rated health, and age) that predicts death and further functional decline. It contains no comorbidity variables and predicts outcomes independently of comorbidity.28, 33, 34
Analysis
To validate the GXI (Figure 1), first 10 comorbidity vignettes of ACOVE-2 participants were reviewed for face validity, then the GXI was correlated with previously validated comorbidity measures for construct validity (simple count, mHCC, and mCCI), and finally the GXI was analyzed against external (noncomorbidity) measures for convergent (predictive) validity and its performance was compared with the previously validated comorbidity measures.
Qualitative review of 10 multimorbid individuals receiving ambulatory care
To review for face validity, ACOVE-2 participants with increasing combinations of five conditions (hypertension, coronary artery disease, DM, AF, and dementia) were selected, a method based on prior comorbidity work.19 All individuals with none of the five conditions were identified as the first tier, those with hypertension and none of the remaining conditions as the second tier, and so on, up to individuals with all five conditions for the sixth tier. Two individuals were selected from each tier, one with a low (bottom tertile) GXI score, and one with a high (top tertile) GXI score, for qualitative review as comorbidity vignettes (Appendix A.2).
Quantitative analysis
Correlations were first calculated between the GXI and the previously validated comorbidity measures. Next, to validate the GXI against the noncomorbidity measures, unadjusted effect (logistic and linear regression) was calculated on clinical outcomes, ambulatory care visits, and complexity of care measures for increasing quintiles of GXI scores (Table 2). Multivariable regression was used to introduce control variables (age, sex, VES-13, intervention site) and to calculate the effect of GXI (top minus bottom quintile, last columns of Table 2) on the external validation variables.
Table 2.
Mean Clinical and Intensity of Care Variables Across Quintiles of Geriatric Complexity of Care Index (GXI) in Assessing Care of Vulnerable Elders 2
| Variable | Mean (Unadjusted) Values Across Quintiles of GXI (Range of GXI Points for Individuals in Quintile) n | Adjusted Difference Between Top and Bottom Quintiles | |||||
|---|---|---|---|---|---|---|---|
| 1st (7–20) n=107 | 2nd (21–26) n=138 | 3rd (27–32) n=132 | 4th (33–39) n=134 | Highest (40–72) n=133 | P-Value for Trend Across Quintiles | Effect Sizea (95% Confidence Interval) | |
| Activity of daily living impairments at baseline, n (range 0–5)b | 0.3 | 0.7 | 1.2 | 1.1 | 1.3 | <.001 | 0.8 (0.5–1.1) |
| VES-13 score at baseline (range 0–10, higher = greater risk)c | 3.1 | 3.9 | 5.1 | 5.2 | 5.6 | <.001 | 2.0 (1.6–2.5) |
| Died (5-year), % | 21.5 | 23.2 | 36.4 | 42.5 | 45.1 | <.001 | 4.8 (0–14) |
| Functional decline (5-year), %d | 4.5 | 4.1 | 11.1 | 11.5 | 14.3 | .02 | 2.5 (−2.4–3.1) |
| Primary care visits, n | 3.7 | 4.5 | 5.5 | 6.2 | 7.0 | <.001 | 3.5 (2.7–4.3) |
| Specialty visits, n | 2.4 | 2.6 | 3.8 | 4.3 | 6.2 | <.001 | 3.8 (2.8–5.7) |
| ≥14 chronic medications, %e | 0.0 | 0.7 | 7.6 | 6.7 | 14.3 | <.001 | 13 (6–19) |
| Medical record QIs eligible per individual, n | 9.2 | 10.4 | 12.0 | 13.0 | 15.9 | <.001 | 7.1 (6.1–8.4) |
| All QIs eligible from medical record and interview, n (n=578)f | 23.5 | 26.0 | 27.4 | 28.5 | 31.9 | <.001 | 9.7 (8.0–11.3) |
Predicted outcomes for top versus bottom quintile of GXI, controlling for age, sex, Vulnerable Elders Survey-13 (VES-13), and intervention group. GXI was a five-category variable. Confidence intervals were obtained using bootstrapping. VES-13 was omitted as a control variable to predict VES-13 and ADL disabilities because of colinearity.
Functional status as measured according to the Short Functional Survey (SFS).29
Five-year functional decline was defined as SFS decline of ≥1 abilities.29, 30 Two hundred ninety-five survivors participated in the follow-up SFS. The reduced quintile sample sizes for the 5-year functional decline outcome was (1st through 5th): 66, 74, 54, 52, 59.
Pescribed for chronic conditions; taken routinely; “as needed” if taken on more than half of days; nutritional supplements for management of a chronic condition.
Based on a sample size of 578, the number of participants in the interview. The reduced quintile sample sizes were (1 through 5): 99, 124, 120, 120, 115.
QI=quality improvement.
To compare how well the GXI predicted the external validation measures with the previously validated comorbidity variables, each comorbidity measure was paired with each external outcome using model fit (R2 for linear regression models and area under the receiver operating characteristic curve (AUC) for logistic models) as the comparative metric (Table 3). Last, GXI and each previously validated comorbidity index was entered into fully adjusted models using tests of statistical independence (p<.05) to evaluate whether the more-resource-intensive GXI score would better predict the outcomes than the previously validated comorbidity variables. All analyses were performed using Stata 12 (Stata Corp., College Station, TX).
Table 3.
Comparison Between the Geriatric CompleXity of Care Index (GXI) and Previously Validated Comorbidity Indexes in Predicting Clinical and Healthcare Outcomes
| Simple Count Unadjust % | mCCI | mHCC | GXI | Simple Count Adjustsed | mCCI | mHCC | GXI | |
|---|---|---|---|---|---|---|---|---|
| Continuous outcomes (R2) | ||||||||
| Primary clinician visits | 6.1 | 3.6 | 5.9 | 13.6a | 6.4 | 3.8 | 6.1 | 14.3a |
| Specialty visits | 2.5 | 2.0 | 9.1 | 8.2 | 4.0 | 2.9 | 9.9 | 9.1a |
| NQI (medical record only) | 33.2 | 17.7 | 14.3 | 30.5a | 35.2 | 19.6 | 15.8 | 32.8a |
| NQI (interview and medical record) | 14.2 | 5.3 | 5.6 | 18.1a | 24.8 | 17.5 | 16.6 | 30.6a |
| Dichotomous outcomes (area under the receiver operating characteristic curve) | ||||||||
| 5-year mortality | 64.7 | 67.9 | 69.1 | 61.8 | 77.1 | 77.5 | 78.7 | 76.2 |
| 5-year functional decline | 51.7 | 61.4 | 56.8 | 56.3 | 89.6 | 89.9 | 89.6 | 83.8 |
| Polypharmacy (≥14 medications) | 72.2 | 59.7 | 73.9 | 78.0a | 76.2 | 70.1 | 76.9 | 81.5a |
Adjusted models controlled for age, sex, vulnerability, and intervention versus control site.
GXI R2 or AUC at least 5 percentage points better than any one of the previously validated comorbidity indices.
mCCI= modified Charlson Comorbidity Index,20 risk of death calculated without age; GXI=Geriatric CompleXity of Care Index; mHCC=modified Medicare-Hierarchical Condition Category5, predicted annual cost for community-based sample, calculated without acute diseases, age, and sex; NQI = number of quality indicators.
RESULTS
Clinician panel ratings for 117 GXI comorbidity variables (Appendix A.1)
The range of median ratings by the panel extended from 1 (history of smoking in a nonsmoker) to 8 (severe liver disease, new depression with symptoms of insomnia and anxiety, nonsevere dementia with behavior problems, and severe dementia with behavior problems).
Qualitative review of 10 comorbidity vignettes (Appendix A.2)
Review of the vignettes confirmed that, within similar conditions, individuals selected for high complexity scores were substantively more complex in the nature of their conditions, medication regimens, and NQI and had more doctor visits.
As an example of how GXI distinguished clinical complexity, two individuals were identified as having hypertension but none of the four other conditions (coronary artery disease, DM, AF, and dementia; Tier 2, Appendix A.2). Although one did not have complex care needs (2nd percentile on GXI score) and the other did (80th percentile), both had a simple condition count of 3. The noncomplex individual had well-controlled hypertension (2 points added to GXI), normal weight (0 points), osteoporosis without fracture (2 points), hearing impairment (2 points), and falls (5 points). By contrast, the complex individual's hypertension was moderately severe (3 points), and she had chronic pain (5 points), hypothyroidism (2 points), hearing (2 points) and vision problems (3 points), fatigue (5 points), insomnia (2 points), and stable mild kidney disease (2 points). She developed a new osteoporotic fracture (6 points), weight loss (4 points), new anemia (2 points), and new UI (4 points). Despite the vast difference in complexity, the simple conditio count did not indicate a difference in complexity between these two individuals.
Quantitative results
Sample characteristics are described in Table 1
Table 1.
Sample Characteristics
| Characteristic | Value |
|---|---|
| Demographic | |
| Age, mean±SD (range) | 81.2±4.8 (75–100) |
| Male, n (%) | 217 (33.6) |
| Clinical | |
| Vulnerable Elders Survey-13 score, mean± SD (range)a | 4.6±2.9 (1–10) |
| Number of activity of daily living impairments, mean±SD (range)b | 0.9±1.44 (0–5) |
| Simple count of conditions, mean± SD (range)c | 3.6±1.6 (0–9) |
| Modified Charlson Comorbidity Index, mean± SD (range)d20 | 3.7±2.6 (0–11) |
| Modified Hierarchical Condition Count score (predicted annual cost), $, mean± SD (range)e | 7,432±4,665 (2,475–30,125) |
| Geriatric Complexity of Care score, mean± SD (range) | 31.3±11.6 (7–72) |
| Health care | |
| Number of visits with assigned primary care provider, mean± SD (range) | 5.43.3 (0–20) |
| Number of visits with specialists, mean± SD (range) | 3.95.6 (0–62) |
| Polypharmacy (number of chronic medications), n (%) | |
| None (<7) | 316 (49) |
| Moderate (7–9) | 172 (26.8) |
| Severe (10–13) | 117 (18.2) |
| Very severe (≥14) | 39 (6.1) |
| NQI needed (as documented in medical record), mean± SD (range) | 12.2±4.3 (4–27) |
| NQI (as documented in medical record and in interviews), mean± SD (range)f | 27.5±6.8 (8–47) |
The analytical sample is from the Assessing the Care of Vulnerable Elders-2 Study,17 which originally screened 2,671 individuals aged ≥75 for falls, urinary incontinence (UI), and memory impairment; 784 of these (29%) screened positive for one or more of the geriatric conditions, and 649 (83%) agreed to enroll in the study. Adequate medical records were obtained for 644 (99%) to review for quality and complexity of care. The longitudinal outcomes of the overall sample have been previously reported.28: 220 (34%) of the sample died. Two hundred ninety-five of the survivors were contacted for a functional status interview 5 years after enrollment.
A function-based risk score predicting 1-, 2-, and 5-year risk of functional decline or death.28,33,34 A score of ≥3 identifies individuals aged 65 or higher at the 30% highest risk.
Number of self-reported disabilities of 5 possible (shopping, light housework, walking, bathing, finances) reported as difficult and requiring help or not doing because of health according to the Short Functional Survey.29
Unweighted sum of atrial fibrillation, coronary artery disease, congestive heart failure, cerebrovascular disease, diabetes mellitus type 2, hypertension, dementia, falls, hearing impairment, UI, malnutrition, and osteoporosis.
Charlson Comorbidity Index20 was calculated without age and sex points, using comorbidities only, analogous to the simple count and Geriatric Complexity of Care Index score.
Comorbidity using dollar weights from the Medicare Hierarchical Condition Category5 score. The modified HCC did not include acute diseases, age, and sex weights.
The n for these variables was 578 who participated in the quality-of-care interview.
SD=standard deviation; NQI=number of quality indicators.
The GXI was correlated with all of the previously validated comorbidity scores (p<.001): simple count (r=.66), mCCI (r=.62), and mHCC (r=.73).
The GXI defined increasing care intensity, with substantial and statistically significant tests of trends across all of the unadjusted intensity variables (p<.001 for all except functional decline, p<.02) (Table 2). An individual in the top quintile of GXI, on average, visited her primary care provider 7.0 times, versus 3.7 times for an individual in the bottom quintile. No individuals in the healthiest quintile had the most-severe category of polypharmacy, but 14% of those in the highest quintile were in this category. The mean NQI was 16 for the most complex quintile and 9 for the least complex. Although the GXI was associated with functional decline and survival on bivariate analysis, it was not as strong as the VES-13 in the multivariable model (last column, Table 2) and became nonsignificant in adjusted models.
When comparing the GXI with the other comorbidity variables at predicting outcomes (using model fit as the criterion), the GXI was comparable with or worse than previously validated indices for predicting death and decline but better at predicting care intensity: doctor visits, polypharmacy, and NQI (defined as an R2 or AUC of >5%points better than at least one other index, Table 3), with polypharmacy reaching the excellent AUC range (>0.8). The GXI also independently (p<.01) predicted primary care and specialty visits, polypharmacy, and NQI, controlling for age, sex, intervention site, and VES-13 and when any of the three previously validated comorbidity measures (simple count, mCCI, mHCC) was also entered into the model (not shown). The mHCC was the best predictor (p<.001, even with any other three comorbidity variables included) of death. None of the comorbidity variables predicted 5-year functional decline independent of VES-13 (odds ratio=2.0 per point, p<.001 for all models).
DISCUSSION
This article describes the multistage development of a comprehensive measure of geriatric comorbidity, the GXI. The GXI predicted ambulatory care intensity, as measured according to primary and specialty care visits, polypharmacy, and eligibility for complex ambulatory care processes independent of age, functional status, and previously validated measures of comorbidity. The GXI captured complex care as measured according to NQI and complexity of medication management well. The authors believe that they were successful at capturing ambulatory care intensity with the GXI because geriatric conditions and severity of disease were considered.
In contrast with other comorbidity studies,5, 6, 12, 20 the GXI was also tested against functional status, an important outcome for older adults. Functional impairment has also been included as a condition in other comorbidity indices.35, 36 Although comorbidity and function are important health concerns for older adults, by keeping them separate, it was possible to evaluate their relative contributions to the various outcomes tested. As an example, the GXI was independent of the VES-13, the function-based covariable, at predicting NQI, but GXI was less predictive than VES-13 at predicting future decline. This is consistent with prior work, in which it was found that the VES-13 was an effective and parsimonious way to predict clinical outcomes in older community-dwelling adults and that comorbidity added no predictive value.33, 34 Unlike other comorbidity measures that give more weight to acute medical and surgical conditions to predict death20 and cost,5, 6 the current study gave greater weight to the more difficult-to-manage geriatric conditions from the perspective of primary care clinicians.
Therefore, the GXI complements the other indices by focusing on a different, but important, area of care—ambulatory care of older adults with multiple chronic conditions. In this study, the GXI was the best predictor of number of visits a primary care clinician will need to provide in a given year based on the complexity of his or her patients or a way to target valuable medical home resources for individuals with more-complex needs, such as pharmacy review or added care coordination.
The GXI was not as good as the other indices at predicting survival or functional decline, underscoring prior findings that no comorbidity index can predict all outcomes for all individuals.37 The mHCC, originally derived5 to adjust for high-cost individuals, was the best predictor of survival in this sample, even though not all of the acute illness variables necessary to compute a full score were available. It is not surprising that cost and mortality would be closely related, given accelerated costs at the end of life.38, 39 The results suggest that the mHCC would be a more-efficient way to risk-adjust for death outcomes than the GXI. Additionally, the mHCC outperformed the GXI at predicting specialty visits, consistent with the known relationship between mHCC and higher-cost care.
This study highlights the value of NQI, which quantifies the number of care processes that should be provided for an individual as an outcome variable. In prior studies, NQI was used as a proxy variable for comorbidity (as a predictor) rather than as an outcome.40 This analysis validates that it is reasonable to use NQI as a proxy for disease burden.
There are several limitations to this study. First, because this study of comorbidity was planned as a medical record abstraction, the GXI variable definitions were customized to what is typically documented in hand-written or dictated primary care records. Although the GXI was not difficult to collect (12 minutes on average per record), future refinement should occur within evolving health systems that can routinely capture required data. This study provides new insight into which comorbidities should be candidates for systemwide automated data to identify individuals requiring the most complex care. This would also facilitate further validation with other measures of complexity important to healthcare systems such as hospitalizations.
Second, the International Classification of Diseases (ICD) codes used to score the HCC were not available, so mapping of HCC variables to the chart-abstracted information was relied on. A future study is needed to develop an ICD version of the GXI for comparison with the HCC, although ICD codes should not be the criterion standard for measuring geriatric comorbidity because ICD codes do not capture important geriatric conditions such as falls.
A third potential limitation is that the ACOVE-2 study enrolled individuals with at least one geriatric condition, so the results of the current study might not be generalizable to unselected older populations. Inclusion of individuals with geriatric conditions increased the GXI for all participants in the sample, therefore if an unselected population with greater variation in GXI were re-enrolled, it would be expected that association with greater difference in intensity of primary care would be found. Fourth, the data were collected from 2002 to 2003, and practice patterns may have evolved (e.g., with respect to number of visits). Fifth, the sample was recruited from only two communities in southern California, and participants were nearly all white and had at least a high school education. The influence of ethnic and socioeconomic diversity on the predictive ability of this comorbidity index cannot be captured in this study.
Sixth, this study focused on primary care rather than all health care. Unlike the original ACOVE-1 cohort,16 ACOVE-2 did not include the ACOVE hospital and continuity of care QIs that contribute greatly to the overall complexity of medical care managed by a medical home or healthcare system. How the GXI relates to acute care measures of complexity (e.g., transitions and readmissions) requires further study. Last, the number of potential ACOVE QIs doubled in 2007,41 mostly because of inclusion of new conditions; therefore, NQI will increase even more for individuals with complex needs as a result of the newer QI set.
In conclusion, detailed comorbidity and severity-of-disease measures were used to capture the breadth of complex ambulatory care due to geriatric, psychosocial, and general medical conditions. The GXI can identify older adults with complex care needs who require adequate time and resources to provide them the ambulatory care that they need. With refinement for use in an electronic health record, the GXI could prospectively identify individuals who need more attention from future medical homes.
ACKNOWLEDGMENTS
We would like to acknowledge the technical assistance of Patty Smith and Caren Kamberg during the medical record review process. The clinician panel included authors Lillian Min, David Reuben, David Ganz, Paul Shekelle, Neil Wenger, and Joel Belmin from the Geriatric Service, Hốpital Charles-Foix, Assistance Publique Hốpitaux de Paris, Ivry sur Seine, France.
Conflict of Interest: This project was supported by the Agency for Healthcare Research and Quality (Min R21 HS017621). Dr. Min was supported by the Claude Pepper Older Americans Independence Centers at the University of California at Los Angeles (NIA-K12 2006–10) and the University of Michigan (2010–11) and a Hartford-AFAR Geriatric Scholars Research Outcomes Award (2005–6). Dr. Ganz is funded by a Veterans Affairs Greater Los Angeles Health Services Research and Development Center of Excellence Career Development Award (CD2 08–012–1). The original ACOVE-2 study was supported by a contract from Pfizer, Inc. to RAND. These results were presented at the American Geriatrics Society and Society for General Internal Medicine Meetings in 2012.
Sponsor's Role: Neither RAND nor Pfizer participates in the design, analysis, or preparation of this manuscript.
Appendix A.1.
Conditions, Severity Levels, Prevalence, and Complexity of Care Weights Used in the Geriatric Complexity of Care (GXI) Score
| Comorbidity | Short Description | Definitions | Prevalence, %b |
Median Complexity Rating Used as Weights in the GXI Score |
|---|---|---|---|---|
| AFa | Old AF, no embolism | Preexisting sustained AF without history of embolism | 9.2 | 4 |
| Old AF, old embolism | Preexisting AF with history of cerebrovascular or systemic embolism |
1.2 | 5 | |
| New AF, no embolism | Sustained AF that developed within the observation period but without new embolism |
1.4 | 6 | |
| New AF, new embolism | New embolic event and new AF | 0 | 7 | |
| CADa | Old CAD, no MI, asymptomatic |
Old CAD without history of MI, asymptomatic | 15.2 | 2 |
| Old CAD, no MI, symptomatic |
Old CAD without history of MI, symptomatic (symptoms: chronic dyspnea, angina pectoris) |
3.9 | 5 | |
| Old CAD or MI asymptomatic |
Old CAD with history of old MI (> 2 years before), asymptomatic |
1.6 | 3 | |
| Old CAD or MI, symptomatic |
Old CAD with history of old MI (< 2 years before), symptomatic (symptoms: chronic dyspnea, angina pectoris) |
1.1 | 5 | |
| Old CAD, new MI, asymptomatic |
Old CAD with new MI (within 2 years), asymptomatic | 9.0 | 5 | |
| Old CAD, new MI, symptomatic |
Old CAD with new MI (within 2 years), symptomatic (symptoms: chronic SOB, angina pectoris) |
2.8 | 6 | |
| New CAD, asymptomatic | New CAD (regardless of MI), asymptomatic | 1.2 | 4 | |
| New CAD, symptomatic | New CAD (regardless of MI), symptomatic (symptoms: chronic SOB, angina pectoris) |
0.8 | 6 | |
| CHFa | Old CHF, not severe | Old CHF (left or right heart failure or pulmonary edema), preserved EF |
13.0 | 3 |
| Old CHF, severe | Old CHF, severe (LV function < 40%, noted as being severe or SOB at rest or NYHA Class IV) |
5.6 | 6.5 | |
| New CHF, not severe | New CHF this year, preserved EF, not noted as severe | 1.9 | 5 | |
| New CHF, severe | New CHF this year, severe (LV function < 40% or noted as being severe, SOB at rest, or NYHA Class IV) |
0.2 | 7.5 | |
| Hypercholest erolemia |
Old hypercholesterolemia | History of hyperlipidemia (low-density lipoprotein cholesterol >130 mg/dL, total cholesterol > 200 mg/dL, or taking lipid-lowering medication) if individual has DM or CAD |
16.1 | 2 |
| New hypercholesterolemia | New hyperlipidemia this year if individual has DM or CAD |
0.1 | 2 | |
| CVDa | Old CVA, not severe | Old CVD (transient ischemic attack, CVA, stroke, carotid artery stenosis, prior carotid endarterectomy, CVD or insufficiency) without documentation of severe stenosis (definition below) |
23.6 | 3.5 |
| Old CVA, severe | Old CVD with documented ≥70%, high-grade, or severe arterial stenosis (any cerebrovascular distribution: carotid or intracranial) according to angiogram (conventional, computed tomography, or magnetic resonance) or Doppler ultrasound |
3.0 | 4.5 | |
| New CVA | New CVD (definition above) during observation window without severe stenosis |
3.0 | 5.5 | |
| New CVA, severe | New cerebrovascular disease (definition above) during observation window with severe or high-grade stenosis (definition above) |
0.5 | 6 | |
| Hypertensiona | Prehypertension | ≥2 consecutive SBP readings between 120 and 139 mmHg during the time period in individuals without diagnosis of hypertension |
10.4 | 2 |
| High BP | ≥2 isolated SBP readings ≥140 mmHg during time period, without diagnosis of hypertension |
3.7 | 3 | |
| Hypertension, controlled | Hypertension diagnosis without ≥2 SBP readings greater than 140 |
39.6 | 2 | |
| Hypertension, high BP | Hypertension diagnosis with ≥2 SBP readings between 140 and 160 mmHg |
22.7 | 3 | |
| Hypertension, poorly controlled |
Hypertension diagnosis with ≥2 SBP readings between 160 and 180 mmHg |
9.2 | 4 | |
| Hypertension, severe | Hypertension diagnosis with ≥2 readings >180 or any documentation of severe or labile hypertension |
1.4 | 4 | |
| DMa | Pre-DM | Subclinical DM: not diagnosed with DM but with any documentation of impaired glucose tolerance, impaired fasting glucose, “borderline” DM, subclinical DM, hyperglycemia, postprandial hyperglycemia, insulin resistance, or metabolic syndrome |
2.0 | |
| HgA1c <6.9% | DM, taking insulin or an antidiabetic agent, or on a diabetic diet, with all HgA1c <6.9% during study period |
7.5 | 4 | |
| HgA1c 7.0–7.9% | DM, taking insulin or an antidiabetic agent, or on a diabetic diet, with highest HgA1c 7–7.9% during study period |
5.0 | ||
| HgA1c 8.0–8.9% | DM, taking insulin or an antidiabetic agent, or on a diabetic diet, with highest HgA1c 8–8.9% during study period |
2.8 | 6 | |
| HgA1c >9.0% | DM, taking insulin or an antidiabetic agent, or on a diabetic diet, with highest HgA1c >9.0% during study period |
1.6 | ||
| Labile DM | DM, taking insulin or an antidiabetic agent, or on a diabetic diet, with any documentation that they are labile, severe, or brittle, supersedes HgA1c classification |
<1 | ||
| Missing HgA1c | DM, taking insulin or an antidiabetic agent, or on a diabetic diet, not in any of the above categories because no HgA1c checked during the time period or documentation as labile DM |
5.3 | ||
| DM and CKD(if both present, additional points assigned) |
DM, low HgA1c, no CKD 4 |
DM as above, with all HgA1c <7.9% and no documentation of CKD (estimated creatinine clearance of ≥60 (MDRD Stage ≥3), CRI, CRF, or proteinuria) |
6.5 | |
| DM, low HgA1c, moderate CKD |
DM with all HgAlc <7.9% and CKD Stage 3 (estimated creatinine clearance 30–60) or diabetic kidney disease (CRI, CRF, or proteinuria) and not in any of the higher CKD categories |
9.2 | +0 | |
| DM, low HgA1c, advanced CKD |
DM with all HgA1c <7.9% but with CKD Stage 4 or 5 (estimated creatinine clearance ≤30), ESRD, or needs hemodialysis |
1.9 | +2 | |
| DM, high HgA1c, no CKD | DM with any HgAlc ≥ 8.0% or labeled as severe or brittle, without CKD |
1.2 | ||
| DM, high HgA1c, CKD | DM with any HgAlc ≥ 8.0% or labeled as severe or brittle, with CKD (Stage 3) |
3.1 | +1 | |
| DM, high HgA1c, severe CKD |
DM with any HgA1c ≥ 8.0% or labeled as severe or brittle, with severe CKD (≥Stage 4, ESRD, or needs hemodialysis) |
0 | +1 | |
| PAD | PAD, not severe | Claudication, evidence of PAD on physical examination (poor pulses), arterial occlusion of >50% seen on radiographic or ultrasound study (not needing bypass or stents), or Doppler ultrasound or ABI 0.5– 0.9. |
4.5 | 3.5 |
| PAD, severe | ABI ≥0.5, stenosis requiring, awaiting, or receiving bypass surgery or amputation associated with ulcerations or inability to ambulate independently |
2.5 | 6 | |
| COPDa | COPD | Symptomatic COPD (GOLD criteria Stage 1 or 2, or FEV1/FVC < 70% with any FEV1), emphysema, or reactive airway disease requiring regular use of any type of inhaler |
16.1 | 4 |
| COPD, severe | Documented as severe, advanced, or Stage III (GOLD criteria, FEV1 < 30% predicted, FEV1 < 1 L in the presence of FEV1/FVC <70%) or COPD resulting in severe shortness of breath, shortness of breath at rest or with minimal exertion (such as during dressing), right-sided heart failure, requiring oxygen supplementation, or resulting in ≥1 exacerbations treated with artificial ventilation |
4.2 | 6 | |
| Anemia | Stable normal hemoglobin | Hemoglobin at baseline ≥14.0 mg/dL; stable (within 1 mg/dL) during study period |
24.2 | Not rated |
| Mild stable anemia | Hemoglobin at baseline 12.0–13.9 mg/dL; stable (within 1 mg/dL) during study period |
40.8 | 2 | |
| Stable anemia | Hemoglobin at baseline 10.0–11.9 mg/dL; stable (within 1 mg/dL) during study period |
10.6 | 2 | |
| Severe but stable anemia | Hemoglobin at baseline was <10.0 mg/dL; stable (within 1 mg/dL) during study period |
1.1 | 3.5 | |
| Normal but with laboratory findings of change |
Hemoglobin at baseline was ≥14 with decrease of 1–2 mg/dL during study period |
2.3 | 2 | |
| Mild anemia, laboratory findings of change |
Hemoglobin at baseline 12.0–13.9 mg/dL with decrease of 1–2 mg/dL during study period |
4.0 | 3 | |
| Anemia, laboratory findings of change |
Hemoglobin at baseline 10.0–11.9 mg/dL with decrease of 1–2 mg/dL during study period |
1.2 | 4 | |
| Severe anemia, laboratory findings of change |
Hemoglobin at baseline < 10 mg/dL with decrease of 1–2 mg/dL during study period |
0.2 | 5 | |
| Normal, with evidence of blood loss |
Hemoglobin at baseline ≥14 with decrease of ≥2 mg/dL during study period |
2.0 | 4 | |
| Mild anemia, with evidence of blood loss |
Hemoglobin at baseline 12.0–13.9 mg/dL with decrease of ≥2 mg/dL during study period |
0.6 | 5 | |
| Anemia, with evidence of blood loss |
Hemoglobin at baseline 10.0–11.9 mg/dL with decrease of ≥2 mg/dL during the study period |
0.8 | 5 | |
| Severe anemia, with evidence of blood loss |
Hemoglobin at baseline <10 mg/dL with decrease of ≥2 mg/dL during study period |
0.2 | 6 | |
| Cancer | Breast cancer, old | History of invasive or malignant breast cancer of any type (carcinoma in situ or higher stage), not awaiting surgery, not receiving any active or hormone chemotherapy |
6.7 | 3 |
| Breast cancer, new | New diagnosis of breast cancer of any type or stage or on active hormone or chemotherapy, surgery, during the study period |
0.5 | 7 | |
| Colon cancer, old | History of colon or rectal cancer (TNM Stage I or higher, Dukes Stage A or higher); completed all medical or surgical therapies |
3.4 | 3 | |
| Colon cancer, new | New colorectal cancer or receiving active chemotherapy or surgery during study period |
0.6 | 7 | |
| Prostate cancer, old | History of prostate cancer (definitively diagnosed by biopsy, abnormal examination, or imaging) or (suspected prostate cancer and diagnostic testing recommended based on abnormal examination, imaging, symptoms or high prostate-specific antigen test (> 10 mg/dL)). |
4.2 | 2 | |
| Prostate cancer, new | Newly diagnosed or new highly suspected prostate cancer or under active chemotherapy, hormone, or surgical therapy during study period |
0.8 | 6 | |
| Hypothyroidi sm |
Old | Old hypothyroidism or undergoing thyroid replacement therapy |
25.6 | 2 |
| New | New hypothyroidism or starting on thyroid therapy this year |
0 | 3 | |
| Under and overweighta |
Underweight, weight loss | BMI <18.5 kg/m2, ≥10% weight loss documented during study period |
3.0 | 5 |
| Underweight | BMI <18.5 kg/m2, no new weight loss (<10% during time period) documented |
0.5 | 4 | |
| Normal weight, weight loss |
BMI 18.5–24.9 kg/m2, ≥10% weight loss documented during study period |
33.4 | 4 | |
| Normal weight, no weight loss |
BMI 18.5–24.9 kg/m2, weight stable | 0.8 | Not rated | |
| Overweight | BMI 25.0–29.9 kg/m2, no weight loss documented | 0.6 | 2 | |
| Overweight, weight loss | BMI 25.0–29.9 kg/m2, ≥10% weight loss documented during study period |
31.2 | 2 | |
| Obesity | BMI 30.0–39.9 kg/m2 | 1.9 | 3 | |
| Morbid obesity | BMI ≥40 kg/m2 or ≥35.0 kg/m2 with comorbidity (AF, CAD, recent MI, CHF, new stroke, DM, PAD, Stage 4 or 5 CKD) |
14.3 | 6 | |
| Liver disease | Liver disease | Chronic liver disease, cirrhosis, hepatic or liver insufficiency, hepatic or liver fibrosis, chronic hepatitis B or C, chronic liver failure, or abnormal liver function tests or liver enzymes > 3 months not due to cancer or sepsis |
1.9 | 4 |
| Severe liver disease | Chronic liver disease characterized as severe, end- stage, Stage IV by liver biopsy, cirrhosis, or resulting in hepatic encephalopathy, ascites, hepatorenal syndrome, or resulting in any hospitalization during the study period, institutionalization, or dependence in ≥2 activities of daily living |
1.1 | 8 | |
| Fallsa | Old | History of falls or gait impairment at the beginning of the study period, without history of fall-related fracture |
2.0 | 3 |
| New | New fall or fear of falling documented in chart,aa including falls, gait impairment, fear of falling; excludes any with fall-related fracture |
66.0 | 5 | |
| Severe | History of low-velocity ground-level fall resulting in a fracture (any bone) regardless of OP status |
6.2 | 5 | |
| Severe and new | New fall with fracture during time period. | 5.4 | 6 | |
| Pain | Chronic | Chronic pain, defined as pain reported for ≥12 weeks or reason to see a physician for ≥ 3 visits over ≥ 2 months during the time period, not severe as defined below |
40.5 | 5 |
| Chronic and severe | Chronic pain resulting in inability to perform ≥2 activities of daily living or being wheelchair or bedbound |
1.2 | 7 | |
| OPa | OP | Known history of OP at beginning of time period, not including those with history of severe OP |
16.1 | 2 |
| OP, new | New OP during time period | 2.5 | 3 | |
| OP, severe | History of OP, history of fracture (any bone) from a low-impact fall at the time of or after the diagnosis of OP |
9.0 | 4 | |
| New OP fracture | Osteoporotic fracture during time period | 3.4 | 6 | |
| Parkinson's disease | Parkinson's disease | Parkinson's disease or parkinsonism (drug, vascular, Parkinson syndrome) |
3.6 | 7 |
| Psychiatric | Insomnia | Insomnia or taking hypnotic medication at bedtime only and no diagnosis of anxiety or depression. |
10.4 | 2 |
| Anxiety | Diagnosed with anxiety, taking anxiolytic medication, or documented as having anxiety symptoms for ≥2 visits but not with diagnosis of depression |
2.5 | 6 | |
| Depression, old | Old depression (any type) or currently taking antidepressant for depressive symptoms but not with diagnosis of anxiety or insomnia |
11.0 | 3 | |
| Depression, new | New depression (any type) or new prescription of any antidepressant for depressive symptoms but not with diagnosis of anxiety or insomnia |
2.8 | 3 | |
| Insomnia, anxiety | Anxiety and insomnia | 1.4 | 6 | |
| Anxiety, old depression | Anxiety and old depression | 0.2 | 7 | |
| Anxiety, new depression | Anxiety and new depression this year | 5.4 | 7 | |
| Insomnia, old depression | Insomnia and old depression | 0.6 | 5 | |
| Insomnia, new depression | Insomnia and new depression this year | 4.5 | 7 | |
| Insomnia, anxiety, old depression |
Insomnia and anxiety and old depression this year | .8 | 7 | |
| Insomnia, anxiety, new depression |
Insomnia, anxiety, and new depression | 1.6 | 8 | |
| Fatigue | Fatigue | Fatigue, lack of energy, or being tired and symptoms persistent or ongoing in nature |
17.4 | 5 |
| Dementiaa | Memory problems, new | New-onset or worsening memory loss (failed a memory screen or presented with symptoms of memory loss, forgetfulness, or confusion and no previous diagnosis of dementia or Alzheimer's disease) this year and not taking a cholinesterase inhibitor |
7.5 | 5 |
| Dementia, old | Old diagnosis of dementia or chronic cognitive impairment (of any type) at beginning of time period, diagnosis of Alzheimer's disease, or treatment with a cholinesterase inhibitor; does not include severe dementia or dementia with behavior problems, hallucinations, or need for antipsychotic medication |
9.8 | 4 | |
| Dementia, new | New dementia or new prescription with cholinesterase inhibitor during time period; does not include any new cases classified as severe or with psychiatric or behavior problems |
3.4 | 5 | |
| Dementia, severe | Severe dementia (Mini-Mental State Examination score <10, in late- or end-stage or advanced, or no longer has intelligent communication), but no psychiatric problems or antipsychotic medication |
2.3 | 5 | |
| Dementia with psychiatric symptoms |
Dementia with positive psychiatric symptoms, defined as hallucinations, psychosis, agitation, or any disruptive behavior (yelling, wandering, hitting, danger to others) or receiving antipsychotic medication to control their behavior (as needed or routine), but does not include those classified as having severe or advanced dementia |
0.8 | 8 | |
| Severe dementia with psychiatric symptoms |
Documented as having severe dementia and behavioral or psychiatric problems |
1.1 | 8 | |
| Other psychosocial conditions |
Alcohol use, problematic | Alcohol abuse, dependence, “problem” with alcohol, risky or heavy drinking behavior, consumption of (on average) ≥2 drinks/day, >7 drinks/week, or >3 drinks on heavier drinking occasions, drinking attributed to any drinking-related dysfunction (social, legal, or medical), or positive alcohol screen |
5.7 | 6 |
| Nonadherence | Medically nonadherent or nonadherent behavior interfered with recommended medical care or advice on >1 occasion, counseling was repeatedly performed but not followed, or labeled as difficult |
2.8 | 7 | |
| Smoking, past | Smoker in the past but not now | 44.7 | 1 | |
| Smoking, now | Current smoker | 4.2 | 3 | |
| CKD | No, but with worsening of creatinine |
No CKD at baseline (defined by MDRD equation, serum creatinine, weight, sex), with worsening in CKD stage during time period, with “change in CKD stage” defined as worsening by ≥1 stages according to MDRD staging system and at increase in creatinine by ≥0.2 mg/dL during time period |
0.8 | 3 |
| Stage 2, stable | MDRD Stage 2 at baseline (mild CKD, eGFR 60.1– 90.0 mL/min per 1.73 m2) without decline in CKD stage during time period |
43.8 | 2 | |
| Stage 2, decline | MDRD Stage 2 at baseline (mild CKD), with decline in CKD stage during time period (to Stage 3 or worse and creatinine worsened by ≥0.2 mg/dL) |
3.0 | 4 | |
| Stage 3 | MDRD Stage 3 at baseline (moderate CKD, eGFR 30.1–60.0 mL/min per 1.73 m2), without decline in CKD stage during time period |
34.5 | 3 | |
| Stage 3, decline | MDRD Stage 3 at baseline (moderate CKD), with decline in CKD stage during time period (to Stage 4 or worse and creatinine worsened by >0.2 mg/dL) |
2.2 | 6 | |
| Stage 4 | MDRD Stage 4 or 5 (severe CKD, eGFR ≤30.0 mL/min per 1.73 m2), without decline in CKD during time period |
3.7 | 5 | |
| Stage 4, decline | MDRD Stage 4 at baseline, with decline in CKD during time period (to Stage 5 and creatinine worsened by ≥0.2 mg/dL) |
0.0 | 7 | |
| BPH | Symptomatic BPH, new or known |
Urgency, frequency, hesitancy, difficulty stopping stream, incontinence |
14.4 | 3 |
| UIa | UI, old | Of any type present at baseline | 13.0 | 3 |
| UI, new | Of any type that developed during time period | 31.2 | 4 | |
| Hearing | Hearing problem, old | Hearing problem at baseline, defined as positive audiometry results, use of hearing aid, or self-reported hearing problems |
24.2 | 2 |
| Hearing problem, new | New or worsening hearing problem or failed a formal hearing screening or test during study period |
5.9 | 4 | |
| Severe hearing problem | Severe hearing impairment, defined as being deaf, severe, or interfering with communication |
<1 | 6 | |
| Vision | Old vision problem | Vision impairment, poor vision, or blindness in one or both eyes |
21.4 | 3 |
| New vision problem | New vision impairment this year | 3.3 | 5 |
Conditions used in the simple condition count. Malnutrition was counted in the simple comorbidity count if weight loss or underweight was documented, whereas in the GXI, overweight was also considered.
Screening questions pertaining to falls and fear of falling, memory impairment, and bothersome urinary incontinence (UI) symptoms were used to enroll individuals into the Assessing Care of Vulnerable Elders (ACOVE)-2 study; results were placed into all participant's medical records, resulting in higher prevalence than expected compared with a general geriatric population. Two physicians (LM, AW) abstracted the medical records of a 10% subsample of ACOVE-2 participants (n=65) using the prespecified definitions above and compared them for interrater reliability. These conditions had interrater reliability (kappa > 0.6), prevalence (> 0.5%), and face validity, and this final list was included after two rounds of expert panel voting to accept or reject as appropriate measures of complexity of care.
AF=atrial fibrillation; CAD=coronary artery disease; MI=myocardial infarction; SOB=shortness of breath; CHF=congestive heart failure; EF=ejection fraction; LV=left ventricular; NYHA=New York Heart Association; DM=diabetes mellitus; CVA=cerebrovascular accident; CVD=cerebrovascular disease; SBP=systolic blood pressure; HbA1c=glycosylated hemoglobin; CKD= chronic kidney disease; MDRD=Modified Diet in Renal Disease; CRI=chronic renal insufficiency; CRF=chronic renal failure; ESRD=end-stage renal disease; PAD=peripheral artery disease; ABI= ankle-brachial index; COPD=chronic obstructive pulmonary disease; GOLD=Global Initiative for Obstructive Lung Disease; FEV1=forced expiratory volume in 1 second; FVC=forced vital capacity; BMI=body mass index; BPH=benign prostatic hyperplasia.
Appendix A.2.
A Case Series of Comorbidity and Complexity in Assessing Care of Vulnerable Elders 2 The following comorbid condition combinations were used to identify subsamples of individuals with increasing complexity.
| R o w |
Hypertension | CAD | DM | AF | Dementia | N | Mean Simple Count |
Mean GXI Score |
Number of QIs Eligible, Mean±SD |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Absent | Absent | Absent | Absent | Absent | 88 | 1.6 | 24 | 9.4±3.2 |
| 2 | Present | Absent | Absent | Absent | Absent | 182 | 2.8 | 25 | 10.6±3.5 |
| 3 | Present | Present | Absent | Absent | Absent | 86 | 4.3 | 35 | 12.5±3.7 |
| 4 | Present | Present | Present | Absent | Absent | 38 | 5.2 | 41 | 16.0±4.0 |
| 5 | Present | Present | Present | Present | Absent | 8 | 6.3 | 48 | 17.8±4.8 |
| 6 | Present | Present | Present | Present | Present | 3 | 7.7 | 51 | 22.0±4.6 |
One to two individuals were selected from each row for qualitative review based on having a low (where possible) versus high complexity score.
Row 1 Individual without complex care needs (Screened by ACOVE for urinary incontinence (UI) and falls): A 78-year-old man without a diagnosis of hypertension, coronary artery disease (CAD), diabetes mellitus (DM), atrial fibrillation (AF), or dementia. The patient had history of prehypertension (2), mild chronic kidney disease (CKD) (2), fall with fracture (5), mild stable anemia (2), obesity (3).
Complexity score: 14 (~15th percentile)
Simple comorbidity count = 1
No polypharmacy (≤6 medications)
Primary care provider visits = 5
No specialty care
NQI (5 QIs to be documented in the medical record, and an additional 11 to be collected by interview):
Gait and balance evaluation
Weighed at each visit
Education for new medication (I)
Drug regimen review annually (I)
Counsel calcium and vitamin D intake (I)
Counsel weight bearing exercise (I)
Discuss risk for OP risks and prevention (I)
Recommend calcium and vitamin D for OP (I)
Screen pain every 2 years (I)
Advance directive or surrogate in OP charts
Pneumococcal vaccine
Annual influenza vaccine
Screen and take history alcohol use (I)
Assessment or counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
Row 1 Individual with Complex Care Needs (Screened by ACOVE for falls): A 77-year-old man also without hypertension, CAD, DM, AF, or dementia.
The individual had prehypertension (2), anemia with mild decline (4), chronic nonsevere pain (5), old hypothyroidism (2), history of fall with fracture (5), history of osteoporosis (OP) with fracture (4), comorbid insomnia and anxiety with new depression (8), smoked in the past but not presently (1), stable CKD (Stage 4 or 5) (5), tobacco history (1), fatigue (5), problematic alcohol use (6), new bothersome UI symptoms (4).
Complexity score: 51 (~95% percentile)
Simple comorbidity count = 3
Moderate polypharmacy (7–9 medications)
Primary care provider visits = 13
Specialty care: 6 specialist visits (neurology, other medical specialty, psychiatry)
NQI (21 QIs to be documented in the medical record, and an 17 additional QIs to be collected by interview:
Depression symptoms, screen within 2 weeks
Document 3 of 9 DSM-IV symptoms
Document suicidality and psychosis
Treat depression within 2 weeks
If depressed, do not prescribe tricyclics, monoamine oxidase inhibitors, benzodiazepines, or stimulants (except methylphenidate) as first- or second-line therapy
Take a falls history for fall
Perform a fall examination
Perform a gait and balance examination for balance problem (I)
Audiologist teach how to use hearing aid (I)
Weigh at each visit
Follow-up response to new medications
Drug regimen review annually (I)
Check electrolytes within 1 month of starting diuretics
Assess function and pain annually (I)
Recommend physical therapy for OA pain within 3 months (I)
Education for self-management of OA > symptomatic 6 months (I)
Acetaminophen 1st line med for OA (I)
Counsel calcium and vitamin D intake (I)
Counsel weight-bearing exercise (I)
Discuss risk for OP risks and prevention (I)
Recommend calcium and vitamin D for OP (I)
Screen pain every 2 years (I)
History for pain within 1 month
Examination for pain within 1 month
Offer bowel regimen for chronic opioids
Offer treatment for new pain (I)
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Screen and take history alcohol use (I)
Assessment or counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
New UI: take history within 3 months
New UI: perform examination within 3 months
New or persist UI: test urinalysis
New UI: perform post void residual before pharmacological therapy
New UI: discuss treatment options within 3 months
Row 2 individual without complex care needs (Screened by ACOVE for falls): An 85-year-old woman with hypertension only and none of the other conditions.
The individual had well-controlled hypertension (2), normal weight without recent changes (0), new fear of falling or fall (5), OP without fracture (2), stable history of hearing impairment (2).
Complexity score: 11 (~2nd percentile)
Simple comorbidity count = 3
No polypharmacy
Primary care provider visits = 5
Specialty care: 1 visit
NQI (6 QIs to be documented in the medical record, and an additional 12 to be collected by interview):
Perform falls history (I)
Perform a falls examination
Gait and balance evaluation (I)
Weigh at each visit
Drug regimen review annually (I)
Annual electrolytes for diuretic use
Counsel calcium and vitamin D intake (I)
Counsel weight-bearing exercise (I)
Discuss risk for OP risks and prevention (I)
Recommend calcium and vitamin D for OP (I)
Screen pain every 2 years (I)
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Screen and take history alcohol use (I)
Assessment and counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
Row 2 Individual with Complex Care Needs (Screened by ACOVE for worsening UI): An 80-year-old woman with hypertension and none of the other conditions.
The individual had hypertension with high blood pressure (BP) (≥2 high BP readings (140–160 mmHg)) (3). She also had normal hemoglobin at the beginning of the study but with a 0- to 2-mg/dL change during the study period (2), chronic nonsevere pain (5), old hypothyroidism (2), normal weight but with >10% weight loss during the study (4), stable, mild CKD (Stage 2) (2), old hearing problem (2), old vision problem (3), new osteoporotic fracture (6), fatigue (5), insomnia (2), new or bothersome urinary symptoms (4).
Complexity score: 40 (~80th percentile)
Simple comorbidity count = 3
Moderate polypharmacy (7–9 medications)
Primary care provider visits = 12 (saw another provider during time frame, died before assigned primary care provider visit).
Specialty care: 9 visits to a medical specialty
NQI (17 QIs to be documented in the medical record, and an additional 16 to be collected by interview):
Gait and balance evaluation (I)
New depression symptoms, screen within 2 weeks
Counsel nonpharmacological treatment of hypertension (I)
Perform an intervention (any including diet, exercise, counseling) for high BP
Education for new medication (I)
Drug regimen review annually (I)
Weigh at each visit
Document weight loss
Evaluate reversible causes of malnutrition
Evaluate for comorbidities and medications that can cause loss of appetite
Calcium and vitamin D if on steroids for >1 month
Assess function and pain annually (I)
Recommend physical therapy for OA pain within 3 months (I)
Refer to surgery for severe hip and knee OA (I)
Counsel weight-bearing exercise (I)
Discuss risk for OP risks and prevention (I)
Recommend calcium and vitamin D for OP (I)
Screen pain every 2 years (I)
History for pain within 1 month
Examination for pain within 1 month
Offer treatment for new pain
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Screen and take history alcohol use (I)
Assessment and counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
New UI: take history within 3 months
New UI: examine within 3 months
New or persistent UI: order urinalysis
New UI: perform postvoid residual before pharmacological intervention (I)
New UI: Discuss treatment options within 3 months
Row 3 Individual with Moderately Complex Care Needs (Screened by ACOVE for falls): A 79-year-old woman with hypertension and CAD.
The patient had well-controlled hypertension (2), the CAD was old or asymptomatic (2), and there was no history of MI. She had history of fall or fear of falling (5), new OP (3), chronic pain (5), mild but stable anemia (2), normal stable weight (0), history of depression (3), mild stable CKD (estimated glomerular filtration rate 60–90 mL/min per 1.73 m2) (2).
Complexity score: 24 (~23th percentile)
Simple comorbidity count = 4
Severe polypharmacy (10–13 medications)
Primary care provider visits = 6
Specialty care: 2 visits (medical subspecialist)
NQI (12 QIs to document in the medical record and an additional 13 to be collected in interviews):
Take fall history
Perform fall examination
Gait and balance evaluation (I)
Aspirin for patient with CAD
Weigh at each visit
Annual medication review (I)
Advise risk for NSAIDs (I)
If started on chronic NSAID offer gastric protection
Treatment for new OP
Counsel calcium and vitamin D intake (I)
Counsel weight bearing exercise (I)
Discuss risk for OP and prevention (I)
Recommend calcium and vitamin D for OP (I)
Screen pain every 2 years (I)
History for pain with 1 month
Examination for pain with 1 month
Offer treatment for new pain
Assess response to pain treatment (I)
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Screen and take history alcohol use (I)
Assessment and counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
Row 3 Individual with Complex Care Needs (Screened by ACOVE for falls): A 77-year-old woman with hypertension and CAD.
She had well-controlled hypertension (2). Her diagnosis of CAD was old. and she is currently asymptomatic, but she had a new MI within past 2 years (5) and a history of nonsevere congestive heart failure (3). She also had chronic nonsevere pain (5), mild but stable anemia (2), stable CKD Stage 3 (3), breast cancer that was old or not active (3), old hypothyroidism (2), weight was stable and normal (0), new falls problem with new fracture (6), old OP diagnosis (2), comorbid insomnia and anxiety and old depression (7), known hearing problem (2), chronic pain (5) old bothersome UI (3), medical nonadherence (7).
Complexity score: 57 (~97th percentile)
Simple comorbidity count = 5
Very severe polypharmacy (≥14 medications)
Primary care visits = 4
Specialty care: 3 visits total (neurology and psychiatry)
NQI (10 QIs to be documented and another 10 to be collected by interview):
Take fall history
Perform fall examination
Aspirin for patient with CAD
Weigh at each visit
Document weight loss
Evaluate reversible causes of malnutrition
Evaluate for comorbidities and medications that can cause loss of appetite
Assess function and pain annually (I)
Education for self-management of OA symptomatic for >6 months (I)
Offer acetaminophen as first-line therapy for OA (I)
Refer to surgery for severe hip and knee OA (I)
Recommend calcium and vitamin D for OP (I)
History for pain within 1 month (I)
Examination for pain within 1 month (I)
Screen pain every 2 years (I)
Offer treatment for new pain (I)
Assess response to new pain treatment (I)
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Row 4 Moderately Complex Patient (Screened by ACOVE for falls): A 82 year-old man with hypertension and CAD and DM.
The patient had hypertension with mildly high BP (3), the CAD was old/asymptomatic and without history of MI (2), diabetes was well controlled (hgbA1c all ≤8, without significant CKD) (4). He also had mild but stable anemia (hgb 12–14) (2), history of prostate cancer (2), overweight but with weight loss (2), new fall/fear of falling (5), and past smoking (1).
Complexity score: 21 (~20th percentile)
Simple comorbidity count = 4
Moderate polypharmacy (7–9 medications)
Primary care provider visits = 6
Specialty care: 5 total visits (urology and other surgical specialty)
NQI (12 QIs to document and another 11 to collect in interviews):
Annual HbA1c
Check BP at each visit if diabetic
Annual proteinuria test if diabetic
Offer intervention for hypercholesterolemia and DM (I)
Daily aspirin therapy for diabetes
ACE-I or ARB for cardiac risk and DM
Annual foot examination (I)
Gait and balance examination
Aspirin for individual with CAD
Offer nonpharmacological intervention for hypertension (I)
Once or twice daily dosing for hypertension treatment (I)
Weigh at each visit
Annual medication regimen review (I)
Annual electrolytes for diuretic
Recommend calcium and vitamin D for OP (I)
Screen for pain every 2 years (I)
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Screen and take history alcohol use (I)
Assessment and counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
Row 4 Individual with Complex Care Needs (Screened by ACOVE for falls): An 87-year-old woman with hypertension and CAD and DM.
The individual had hypertension (moderately well controlled, with mildly high BP (3), CAD that was old and asymptomatic but with new recent MI (5), controlled DM without CKD (4). She also had history of well-controlled hypercholesterolemia (2), history of nonsevere cerebrovascular disease (CVD) (3.5), morbidly obese (6), history of falls with old fracture (5), anxiety (6), smoked in the past (1), old hearing (2) and vision problem (3).
Complexity score: 40.5 (~80th percentile)
Simple comorbidity count = 4
Moderate polypharmacy (7–9 medications)
Primary care provider visits = 4
Specialty care: none
NQI (10 QIs to document in the medical record, and another 19 to be collected by interview):
Offer intervention for hypercholesterolemia and DM (I)
Annual HbA1c
Annual proteinuria test
Check BP at each visit if diabetic
Daily aspirin therapy for DM
Annual foot examination (I)
Aspirin for CAD
Stroke prophylaxis for CVA
Take fall history
Perform fall examination
Gait and balance examination for balance problem (I)
Audiologist counseling if qualifies for hearing aids (I)
Offer nonpharmacological intervention for hypertension (I)
Once or twice daily dosing for hypertension treatment (I)
Annual medication review (I)
Recommend calcium and vitamin D for OP (I)
Assess pain and function annually in OA (I)
Education for self-management of OA symptomatic for >6 months (I)
Screen for pain every 2 years (I)
Counseling weight-bearing exercise (I)
Weighed at each visit
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Discuss risk for OP and prevention (I)
Screen and take history alcohol use (I)
Assessment and counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
Row 5 Individual with Moderately Complex Care Needs (Screened by ACOVE for falls): A 79-year-old man with hypertension, CAD, DM, and AF.
The individual had controlled hypertension, the CAD was old or asymptomatic and without history of MI, well-controlled DM, AF that was old and stable (without embolic history). He also had Stage 3 CKD, a new diagnosis of CVD with severe stenosis, history of well-controlled hypercholesterolemia, past history of smoking, old hypothyroidism, normal stable weight, old hearing problem, new falls problem but without fracture.
Complexity score: 30 (~50th percentile)
Simple comorbidity count = 6
Severe polypharmacy (10–13 medications)
Primary care provider visits = 8
Specialty care: 14 visits (neurology, urology, other medical subspecialty, other surgical specialty)
NQI (15 QIs to document in the medical record, and another 23 to collect by interview) :
Annual HbA1c
Annual proteinuria test (I)
Check BP at each visit if diabetic
Daily aspirin therapy for DM
Offer intervention for hypercholesterolemia and DM (I)
Annual foot examination (I)
ACE-I or ARB for cardiac risk and DM
Take fall history
Perform fall examination
Gait and balance examination for balance problem (I)
Offer nonpharmacological treatment for hypertension (I)
Once or twice daily dosing for hypertension treatment (I)
Aspirin for patient with CAD
Anticoagulant for high-risk AF
CVA stroke prophylaxis for recurrent stroke
Weigh at each visit
Education for new medication (I)
Annual medication review (I)
Warfarin monitoring every 6 weeks
Check electrolytes within 1 month of starting diuretics
Assess function and pain annually (I)
Recommend physical therapy for OA pain within 3 months (I)
Education for self-management of OA symptomatic for >6 months (I)
Acetaminophen first-line medication for OA (I)
Counsel calcium and vitamin D intake (I)
Counsel weight-bearing exercise (I)
Discuss risk for OP risks and prevention (I)
Recommend calcium and vitamin D for OP (I)
Screen pain every 2 years (I)
History for pain within 1 month
Examination for pain within 1 month
Pneumococcal vaccine
Annual influenza vaccine
Discuss risk for OP and prevention (I)
Screen and take history alcohol use (I)
Assessment and counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
Row 5 Individual with Complex Care Needs (Screened by ACOVE due falls): A 75-year-old man with hypertension, CAD, DM, and AF.
The individual had well-controlled hypertension (2), CAD that was old and asymptomatic and an MI within 2 years (5), well-controlled DM (4) but with stable severe CKD (5 points for CKD plus additional 2 points for CKD+DM combination), and the AF was old and stable without embolic history (4). In addition, he had CHF that was well controlled and nonsevere with preserved ejection fraction (3), new hypercholesterolemia (2), severe peripheral artery disease (6) , stable anemia (2), new falls problem without fracture (5), smoking history not current (1), new-onset or worsening memory but without diagnosis of dementia (5), problematic alcohol use (6), vision impairment (3), benign prostatic hyperplasia (3), newly diagnosed depression (7), chronic pain (5).
Complexity score: 70 (~99th percentile)
Simple comorbidity count = 6
Moderate polypharmacy (7–9 medications)
Primary care provider visits = 7
Specialty care: none
NQI (21 QIs to be documented in the medical record and an additional 11 to be collected in interviews):
Failed memory screen, assess memory
New depression symptoms, screen within 2 weeks
New depression: document 3 of 9 DSM-IV symptoms
New depression: document suicidality and psychosis
Treat depression within 2 weeks
Annual HbA1c
Annual proteinuria test
Check BP at each visit if diabetic
Daily aspirin therapy for DM
Offer intervention for hypercholesterolemia and DM (I)
Take fall history
Perform fall examination
Offer nonpharmacological treatment for hypertension (I)
Once or twice daily dosing for hypertension treatment (I)
ACE-I if HTN and renal insufficiency
ASA for CAD
Anticoagulant for high-risk AF
Stroke prophylaxis for CVA (I)
Weighed at each visit
Follow-up response to new med
Warfarin monitoring 6 weeks
Annual electrolytes for diuretic
Annual medication review (I)
Screen for pain every 2 years (I)
Endocarditis prophylaxis for risk procedures (I)
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Screen and take history alcohol use (I)
Assessment and counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
Row 6 Individual with Very Complex Care Needs (Screened by ACOVE for all three conditions: falls, dementia and UI): A 76-year-old woman with hypertension, CAD, DM, AF, and dementia.
The individual had ≥2 moderately high SBP readings (3), CAD that was old but with ongoing symptoms and history of MI (5), controlled DM (4) with stable Stage 3 CKD (3, with no additional points for the combination of DM and CKD at these levels of severity), AF that was old and without embolism (4), and her dementia was an existing diagnosis without psychiatric symptoms (4). She also had severe chronic obstructive pulmonary disease (COPD) (6), history of well-controlled hypercholesterolemia (2), history of nonsevere CVD (4.5), nonsevere chronic congestive heart failure (6.5), chronic severe pain (7), severe anemia with decrease in Hgb >2 mg/dL (5), old hypothyroidism (2), new falls problem without fracture (5), anxiety with comorbid depression (7), normal weight without weight loss (0), new or bothersome UI (4).
Complexity score: 72 (the most complex person in the sample)
Simple comorbidity count = 9
Very severe polypharmacy (≥14 medications)
Primary care provider visits = 14
Specialty care (2 total visits): psychiatry
NQI (26 QIs to be documented in the medical record and an additional 15 to be collected in interviews):
Counseling about cholinesterase inhibitors in dementia (I)
Dementia: provide caregiver education about safety, conflicts and resources
Annual HbA1c
Annual proteinuria test
Check BP at each visit if diabetic
Daily aspirin therapy for DM
Cholesterol intervention for DM
Annual foot examination (I)
ACE-I or ARB for cardiac risk factors and DM
Offer non-pharmacologic therapy for hypertension (I)
Fall history (I)
Fall examination (I)
Gait and balance examination
ACE-I or ARB for heart failure and low ejection fraction
Intervene to decrease low-density lipoprotein cholesterol if >130 mg/dL and CHD
Aspirin for CAD
Beta-blocker after MI in the past 2 years (I)
Anticoagulant for high risk AF
CVA stroke prophylaxis against recurrent stroke
Weigh at each visit
Warfarin monitoring 6 weeks
Electrolytes within 1 month for diuretic
Annual medication review (I)
History for pain within 1 month
Examination for pain within 1 month
Offer treatment for new pain
Counsel weight-bearing exercise (I)
Discuss risk for OP risks and prevention (I)
Screen pain every 2 years (I)
Advanced directives or surrogate in outpatient charts
Pneumococcal vaccine
Annual influenza vaccine
Screen and take history alcohol use (I)
Assessment and counseling to increase physical activity (I)
Colon cancer screening (I)
Eye examination every 2 years (I)
New UI: take history within 3 months
New UI: examine within 3 months
New or persistent UI: order urinalysis
New UI prescription medication: postvoid residual before pharmacological therapy (I)
New UI: Discuss treatment options within 3 months
I = QI strictly collected in interviews (cannot be collected from the medical record). All other QIs are obtained strictly from medical record or can be collected from the medical record or interview.
NQI= number of quality indicators (QIs); HbA1c= glycosylated hemoglobin; ACE-I= angiotensin-converting enzyme inhibitor; ARB=angiotensin receptor blocker; DSM-IV= Diagnostic and Statistical Manual of Mental Diseases, Fourth Edition.
Footnotes
Author Contributions: Lillian Min and Anne Walling: study concept and design, acquisition of data, analysis and interpretation of data, and preparation of manuscript. Neil Wenger: study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript. Caroline Blaum: study concept and design, interpretation of data and preparation of manuscript. Chris Cigolle: interpretation of data and preparation of manuscript. David Ganz: study concept and design, acquisition of data, interpretation of data, and preparation of manuscript. David Reuben and Paul Shekelle: study concept and design, acquisition of subjects and data, interpretation of data, and preparation of manuscript. Carol Roth: acquisition of subjects and data, interpretation of data, and preparation of manuscript. Eve Kerr: study concept and design, analysis and interpretation of data, and preparation of manuscript.
REFERENCES
- 1.Stange KC, Nutting PA, Miller WL, et al. Defining and measuring the patient-centered medical home. J Gen Intern Med. 2010;25:601–612. doi: 10.1007/s11606-010-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rich ELD, Libersky J, Parchman M. White Paper (Prepared by Mathematica Policy Research under Contract No HHSA290200900019I/HHSA29032005T) AHRQ Publication No 12–0010-EF. Agency for Healthcare Research and Quality; Rockville, MD: 2012. Coordinating Care for Adults With Complex Care Needs in the Patient-Centered Medical Home: Challenges and Solutions. [Google Scholar]
- 3.Gijsen R, Hoeymans N, Schellevis FG, et al. Causes and consequences of comorbidity: A review. J Clin Epidemiol. 2001;54:661–674. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 4.de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity. A critical review of available methods. J Clin Epidemiol. 2003;56:221–229. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 5.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 6.Weiner JP, Starfield BH, Lieberman RN, Johns Hopkins Ambulatory Care Groups (ACGs) A case-mix system for UR, QA and capitation adjustment. HMO Pract. 1992;6:13–19. [PubMed] [Google Scholar]
- 7.Choudhry NK, Fischer MA, Avorn J, et al. The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med. 2011;171:814–822. doi: 10.1001/archinternmed.2010.495. [DOI] [PubMed] [Google Scholar]
- 8.Starfield B, Lemke KW, Herbert R, et al. Comorbidity and the use of primary care and specialist care in the elderly. Ann Fam Med. 2005;3:215–222. doi: 10.1370/afm.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starfield B, Lemke KW, Bernhardt T, et al. Comorbidity: Implications for the importance of primary care in `case' management. Ann Fam Med. 2003;1:8–14. doi: 10.1370/afm.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd CM, Darer J, Boult C, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: Implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 11.Tinetti ME, Bogardus ST, Jr., Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]
- 12.Grant RW, Ashburner JM, Hong CC, et al. Defining patient complexity from the primary care physician's perspective: A cohort study. Ann Intern Med. 2011;155:797–804. doi: 10.7326/0003-4819-155-12-201112200-00001. [DOI] [PubMed] [Google Scholar]
- 13.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356:2496–2504. doi: 10.1056/NEJMsa066253. [DOI] [PubMed] [Google Scholar]
- 14.Fung CH, Setodji CM, Kung FY, et al. The relationship between multimorbidity and patients' ratings of communication. J Gen Intern Med. 2008;23:788–793. doi: 10.1007/s11606-008-0602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cigolle CT, Langa KM, Kabeto MU, et al. Geriatric conditions and disability: The Health and Retirement Study. Ann Intern Med. 2007;147:156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 16.Wenger NS, Solomon DH, Roth CP, et al. The quality of medical care provided to vulnerable community-dwelling older patients. Ann Intern Med. 2003;139:740–747. doi: 10.7326/0003-4819-139-9-200311040-00008. [DOI] [PubMed] [Google Scholar]
- 17.Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc. 2009;57:547–555. doi: 10.1111/j.1532-5415.2008.02128.x. [DOI] [PubMed] [Google Scholar]
- 18.Min LC, Reuben DB, MacLean CH, et al. Predictors of overall quality of care provided to vulnerable older people. J Am Geriatr Soc. 2005;53:1705–1711. doi: 10.1111/j.1532-5415.2005.53520.x. [DOI] [PubMed] [Google Scholar]
- 19.Min LC, Wenger NS, Fung C, et al. Multimorbidity is associated with better quality of care among vulnerable elders. Med Care. 2007;45:480–488. doi: 10.1097/MLR.0b013e318030fff9. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Karlamangla A, Tinetti M, Guralnik J, et al. Comorbidity in older adults: Nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci. 2007;62A:296–300. doi: 10.1093/gerona/62.3.296. [DOI] [PubMed] [Google Scholar]
- 22.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 23.Kalaitzidis R, Li S, Wang C, et al. Hypertension in early-stage kidney disease: An update from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2009;53:S22–31. doi: 10.1053/j.ajkd.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Taylor DJ, Lichstein KL, Durrence HH, et al. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28:1457–1464. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 25.Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–880. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuben DB, Roth C, Kamberg C, et al. Restructuring primary care practices to manage geriatric syndromes: The ACOVE-2 intervention. J Am Geriatr Soc. 2003;51:1787–1793. doi: 10.1046/j.1532-5415.2003.51565.x. [DOI] [PubMed] [Google Scholar]
- 27.Ganz DA, Wenger NS, Roth CP, et al. The effect of a quality improvement initiative on the quality of other aspects of health care: The law of unintended consequences? Med Care. 2007;45:8–18. doi: 10.1097/01.mlr.0000241115.31531.15. [DOI] [PubMed] [Google Scholar]
- 28.Min L, Yoon W, Mariano J, et al. The Vulnerable Elders-13 Survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57:2070–2076. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saliba D, Orlando M, Wenger NS, et al. Identifying a short functional disability screen for older persons. J Gerontol A Biol Sci Med Sci. 2000;55A:M750–M756. doi: 10.1093/gerona/55.12.m750. [DOI] [PubMed] [Google Scholar]
- 30.Min LC, Wenger NS, Reuben DB, et al. A short functional survey is responsive to changes in functional status in vulnerable older people. J Am Geriatr Soc. 2008;56:1932–1936. doi: 10.1111/j.1532-5415.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shekelle PG, MacLean CH, Morton SC, et al. Assessing care of vulnerable elders: Methods for developing quality indicators. Ann Intern Med. 2001;135:647–652. doi: 10.7326/0003-4819-135-8_part_2-200110161-00003. [DOI] [PubMed] [Google Scholar]
- 32.Solomon DH, Wenger NS, Saliba D, et al. Appropriateness of quality indicators for older patients with advanced dementia and poor prognosis. J Am Geriatr Soc. 2003;51:902–907. doi: 10.1046/j.1365-2389.2003.513331.x. [DOI] [PubMed] [Google Scholar]
- 33.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: A tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 34.Min LC, Elliott MN, Wenger NS, et al. Higher Vulnerable Elders Survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc. 2006;54:507–511. doi: 10.1111/j.1532-5415.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song X, Mitnitski A, MacKnight C, et al. Assessment of individual risk of death using self-report data: An artificial neural network compared with a frailty index. J Am Geriatr Soc. 2004;52:1180–1184. doi: 10.1111/j.1532-5415.2004.52319.x. [DOI] [PubMed] [Google Scholar]
- 37.Byles JE, D'Este C, Parkinson L, et al. Single index of multimorbidity did not predict multiple outcomes. J Clin Epidemiol. 2005;58:997–1005. doi: 10.1016/j.jclinepi.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 38.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45:565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoover DR, Crystal S, Kumar R, et al. Medical expenditures during the last year of life: Findings from the 1992–1996 Medicare current beneficiary survey. Health Serv Res. 2002;37:1625–1642. doi: 10.1111/1475-6773.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min LC, Reuben DB, Keeler E, et al. Is patient-perceived severity of a geriatric condition related to better quality of care? Med Care. 2011;49:101–107. doi: 10.1097/MLR.0b013e3181f53523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wenger NS, Roth CP, Shekelle P. Introduction to the Assessing Care of Vulnerable Elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(Suppl 2):S247–S252. doi: 10.1111/j.1532-5415.2007.01328.x. [DOI] [PubMed] [Google Scholar]