Abstract
Electrogenic Na+ transport across high resistance epithelial is mediated by the epithelial Na+ channel (ENaC). Our understanding of the mechanisms of ENaC regulation has continued to evolve over the two decades following the cloning of ENaC subunits. This review highlights many of the cellular and extracellular factors that regulate channel trafficking or gating.
Keywords: epithelial Na+ channel, fluid volume, regulation, protein kinases, phosphatidylinositol
The aldosterone-sensitive distal nephron represents the final site within the nephron where filtered Na+ is reabsorbed. ENaCs are expressed in principal cells in the late distal convoluted tubule, connecting tubule and through the collecting duct, and are the major pathway for Na+ entry across the apical plasma membrane. The regulated reabsorption of Na+ via ENaC in the distal nephron has a key role in the control of extracellular fluid volume, blood pressure, and renal K+ secretion. ENaCs are also expressed within the airways [1-5], where they have a key role in the modulating airway fluid volume [6, 7], an important factor facilitating mucociliary clearance [7, 8]. ENaCs are also expressed in the distal colon, sweat ducts, salivary ducts, inner ear, lingual epithelium, keritinocytes, lymphocytes and vascular smooth muscle. ENaC expression has also been reported in endothelium and in various sites within the eye (epithelia within retina, lens, and pigmented ciliary body and iris [9-17]. The functional role(s) of ENaCs within many of these tissues is, at present, unclear.
The role of in ENaC in the control of blood pressure is perhaps best exemplified by two rare inherited disorders. Liddle’s syndrome, an autosomal dominant disorder characterized by extracellular fluid volume expansion, hypertension and hypokalemia, which is associated with gain of function mutations within the channel’s β or γ subunit [18-26]. Pseudohypoaldosteronism type I, an autosomal recessive disorder characterized by volume depletion, hypotension, and hyperkalemia is associated with loss of function ENaC mutations [27]. Some common ENaC polymorphisms are associated with altered channel activity [28-31], and may segregate with blood pressure in selected populations (e.g., βT594M) [32]. Disorders of mineralocorticoid and glucocorticoid metabolism, as well as receptor mutations, are associated with increases in ENaC activity and hypertension [33-35]. In addition, cystic fibrosis is characterized by a decrease in airway fluid volume and increased ENaC activity in airway epithelia. Channel activation reflects, in part, enhanced proteolysis of channel subunits [36-38]. The increase in ENaC activity in cystic fibrosis may contribute to a reduced airway surface liquid volume with associated increases in the viscosity of airway fluids and reduction in mucociliary clearance [39-42].
ENaC subunit structure
ENaC subunits are members of the ENaC/Degenerin gene family [43-45]. ENaCs from mammalian kidney tissues are comprised of three subunits, α, β, and γ that likely form a heterotrimer [46], analogous to the structure of a related gene family member, acid sensing ion channel 1 (ASIC1) [47].
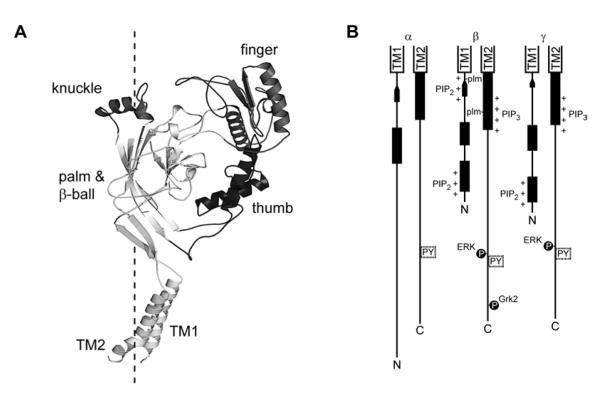
The three subunits share modest (~30% to 40%) sequence identity. Each subunit has two membrane spanning helices (TM1 and TM2) resulting in cytoplasmic amino and carboxyl termini [48, 49]. The cytoplasmic domains have sites that are phosphorylated by specific kinases, have specific motifs that direct protein-protein and protein-lipid interactions that affect channel gating or trafficking, and have sites that may directly influence channel gating or trafficking (Figure 1). Between the TM helices in the linear sequence lies a large extracellular region. Based on homology to ASIC1, this region is organized in distinct domains with well-conserved β-sheet domains at the center of the fold and poorly conserved α-helical domains surrounding the protein core (Figure 1) [50, 51]. This region is responsible for extracellular ligand-dependent gating, proteolytic activation, and mechanosensitivity.
Figure 1.
(A) Model of the extracellular and transmembrane regions of the α subunit of ENaC [50]. Domains within the extracellular region are noted. The dashed line indicates the pseudo three-fold symmetry axis. (B) Linear depiction of the cytoplasmic regions of the three mouse ENaC subunits. Secondary structure prediction was performed using Jpred3 [160]. Predicted α helices (rectangles) and β-strands (arrows) are noted. PY motifs, ERK and Grk2 phosphorylation sites, palmitoylation (plm) sites, and PI(4,5)P2 and PI(3,4,5)P3 binding sites are indicated.
ENaC regulation by cytoplasmic factors
The cytoplasmic region of ENaC is comprised of the amino and carboxyl termini of each subunit. These cytoplasmic domains serve as sites of chemical modification, protein binding, and interactions with components of the plasma membrane, often in response to cell signals that regulate ENaC activity by either changing the number of channels at the cell surface or by changing the open probability of the channel. In addition, the carboxyl termini serve to link the channel to the cytoskeleton by binding α spectrin and possibly actin [52, 53]. Finally, sites within this region have been implicated in the functioning of the channel, having roles in ion conduction, ion selectivity, or gating.
Ubiquitination
Mutations in individuals with Liddle’s syndrome suggested that a proline-rich region (Figure 1B) within the carboxyl-termini of the β or γ subunit have an important role in the regulation of channel trafficking [18-21, 54, 55]. Staub, Rotin and colleagues used this region of the β subunit of ENaC as bait in a yeast two-hybrid screen to identify proteins that interact with ENaC and isolated an E3 ubiquitin ligase, Nedd4 whose WW domains serve to mediate the interaction between ligase and ENaC [22, 56, 57].
Nedd4-2, the isoform that has a key role in modulating channel activity, is expressed in tissues that also express ENaC. The Nedd4-2 WW domains interact in vitro with a proline-rich region that contains a proline-tyrosine (PY) motif in the carboxyl terminus of ENaC subunits [58-60]. Co-expression studies with Nedd4 and ENaC in oocytes demonstrated that Nedd4 decreases ENaC surface expression and that this is dependent both on the E3 ligase domain of Nedd4 and on the presence of lysine residues on the amino-termini of channel subunits that are the sites of ubiquitin modification [56]. Nedd4-2 dependent ubiquitination of channel subunits occurs at the cell surface, targeting channels for internalization [61-63]. Following internalization, channels recycle to the plasma membrane or are degraded. Deubiquitination appears to be an important step that facilitates the recycling of channels to the plasma membrane. Recently, ENaC was identified as a substrate for two deubiquitinating enzymes, UCH-L3 and Usp2-45 [64-66].
The interaction of Nedd4-2 with its target proteins is modified by kinases that phosphorylate the ubiquitin ligase or ENaC at defined sites. For example, phosphorylation of Nedd4-2 by serum and glucocorticoid-regulated kinase (sgk1) or by protein kinase A (PKA) results in the recruitment of an adaptor 14-3-3 protein that disrupts the binding of Nedd4-2 with the channel and subsequent ubiquitination and channel internalization [67-73]. Sgk1 also increases channel open probability by directly phosphorylating the carboxyl-terminus of the α subunit [74]. PKA also activates ENaC by increasing the delivery of channels from an intracellular pool to the plasma membrane and by increasing channel open probability [75, 76]. The PKA-dependent delivery of channels to the plasma membrane may involve dephosphorylation of sites within the channel that are targeted by ERK [77]. Other kinases, such as IκB kinase-β [78], phosphorylate Nedd4-2 and activate ENaC.
In contrast, there are several kinases that enhance the interaction of Nedd4-2 with the channel and inhibit channel activity. For example, the cellular energy sensor AMP-activated protein kinase (AMPK) [79, 80] inhibits ENaC in a Nedd4-2 dependent manner. While Nedd4-2 is an AMPK target, the mechanism by which AMPK enhances the inhibitory efficacy of Nedd4-2 has not been elucidated. ERK directly phosphorylates βT613 and γT623 in the immediate vicinity of the PY motif essential to Nedd4-2 binding [81]. ERK-dependent phosphorylation of ENaC facilitates interactions between the channel and Nedd4, thereby inhibiting ENaC activity. JNK1 was recently shown to phosphorylate Nedd4-2 and appears to modulate its activity [80].
The glucocorticoid-induced leucine zipper protein GILZ1 is an aldosterone-induced protein that activates ENaC by inhibiting MAP kinase signaling and ERK activation. Soundararajan, Pearce and colleagues have shown that sgk1, GILZ1, Nedd4-2, as well as members of the Raf-MAP kinase signaling pathway are present within a complex that is associated with ENaC, termed the ENaC regulatory complex. GILZ appears to have an important role in recruiting sgk1 to this complex and preventing rapid degradation [62, 82-84].
G protein-coupled receptor kinase, Grk2, has the opposite effect when it phosphorylates S633 in carboxyl-terminus of the β subunit [85]. Phosphorylation at this site renders the channel insensitive to regulation by Nedd4-2, resulting in increased surface expression and channel activity and explains why increased Grk2 activity has been associated with hypertension [86]. Grk2 also activates channel activity in a manner that is dependent on Gαq/11 but independent of kinase activity [87].
Other kinases
Although many kinases regulate ENaC, evidence for direct phosphorylation of ENaC subunits exists for a small subset of these. Protein kinase C (PKC) activation is associated with an increase in phosphorylation of the cytoplasmic carboxyl-termini of the β and γ subunits in both live cells and isolated membranes [88, 89], lowering both channel open probability and the expression of β and γ subunits [89, 90]. PKC activity is required for insulin regulation of ENaC [91]. At present, it is unclear whether PKC isoforms directly phosphorylate channel subunits. The PKC δ isoform seems to differentially regulate the αA/T663 human ENaC polymorphism, although T663 is not a PKC δ phosphorylation site [92].
Casein kinase 2 activates ENaC in association with phosphorylation of intracellular C-terminal sites βS631 and γT599 [93, 94]. Activation likely results from reduced Nedd4-2 binding, increasing the number of channels at the cell surface. Casein kinase 1 also differentially regulates the αA/T663 human ENaC variant, although the effect is indirect [95].
Members of the WNK (with no lysine (K)) kinase family modulate ENaC activity. The N termini of all four WNK kinases activate ENaC. This appears to reflect, in part, activation of sgk1 [96]. Furthermore, sgk1 phosphorylates and appears to modulate WNK4 activity [97]. While one group suggested that full length WNK1 and WNK4 have been reported to activate ENaC [96], another group showed that WNK4 inhibits ENaC [97, 98].
Lipids
The anionic, cellular phosphoinositides phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3) and phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) affect ENaC function. Patch clamp studies have shown that PI(4,5)P2 and PI(3,4,5)P3 can directly activate channels through binding to tracks of cytoplasmic basic residues in the β and γ subunits [99-103]. Increased levels of these phosphoinositides are associated with increased ENaC open probability. PI(4,5)P2 was also associated with increases in ENaC surface expression [104]. In addition to its well defined effects on ENaC gating, the local generation of PI(4,5)P2 via phosphatidylinositol 4-phosphate 5-kinase α enhances ENaC endocytosis [105].
PI(4,5)P2 regulation of ENaC appears to be a convergence point for various regulatory pathways. The activation of luminal purinergic receptors, results in activation of phospholipase C, hydrolysis of PI(4,5)P2 and an inhibition of ENaC activity [106, 107]. Likewise, epidermal growth factor inhibits ENaC by activating receptor tyrosine kinases, which leads to depleted PI(4,5)P2 levels [108].
Methylation reactions have been implicated in the activation of ENaC by aldosterone [109, 110]. Aldosterone stimulates carboxylmethylation of proteins and phospholipids, and inhibition of these reactions blunts the ENaC response to steroid stimulation [111]. Two potential target proteins of methyltransferases are k-ras and the ENaC β subunit. Induction and processing of k-ras appears to be important for regulation of ENaC in A6 cells [112]. Methylation of ENaC in planar lipid bilayers has been shown to lead to an increase in open probability of the channel [113]. Edinger and co-workers have identified a methyltransferase that targets the β subunit of ENaC [114].
In addition, palmitoylation of ENaC β and γ subunits at specific cytoplasmic cysteine residues was recently reported [115]. β subunit palmitoylation increases channel open probability, but does not affect the number of channels at the membrane. Only a small percentage of channels were reported to be palmitoylated, suggesting that ENaC palmitoylation may occur at specific locations within cells. The palmitoyltransferase(s) that regulate the modification of channels by palmitate, as well as the upstream regulators of this process have not been reported.
Sodium
Increases in intracellular Na+ reduce both ENaC surface expression as well as open probability, a process referred to as feedback inhibition. The reduction in surface expression is dependent, in part, on G proteins and on Nedd4-2 and related ubiquitin ligases [116-118]. The reduction in channel open probability may be due to a reduction in proteolytic processing of ENaC subunits (see below) [119, 120].
Trafficking itinerary
The assembly and export of channels from the ER, forward trafficking of assembled channels through the biosynthetic pathway to the plasma membrane, retrieval from the plasma, and the recycling or degradation of internalized channels are events that require specific accessory proteins. Changes in channel trafficking at any of these steps may alter the cellular or surface pool of channels. A growing number of proteins that influence ENaC trafficking have been identified, including ER chaperones [121, 122], members of the rab family of GTPases [123-126], SNARE proteins [127-130], ubiquitin ligases and deubiquitinating enzymes (see above), and members of the ESCRT (endosomal sorting complexes required for transport) complex that targets internalized channels for degradation [131].
ENaC regulation by extracellular factors
The extracellular region of proteins in the ENaC/Degenerin family confers sensitivity to exogenous factors. Based on homology to ASIC1, this region of ENaC is likely organized into several discrete domains (see Figure 1) [47, 51]. At the center of the fold are two β-sheet domains termed the “palm” and “β-ball”. These domains are well conserved across the gene family and form the core of the trimer. Stemming from this foundation are the “finger”, “thumb”, and “knuckle” domains. These are likely formed by helical and coiled regions and are poorly conserved among members of the family. The thumb domain is also characterized by ten conserved cysteines that form five disulfide bonds in the ASIC1 structure, confirming a disulfide bridged helical ladder in the α subunit of ENaC that had been proposed based on functional data [132, 133]. Several extracellular or external factors influence ENaC activity at the cell surface: ions, proteolytic cleavage, and mechanical stress [51].
Sodium, chloride and protons
Na+ and Cl− both have physiologic roles in ENaC regulation, while the effect of H+ is species-specific. Extracellular Na+ has long been known to acutely down-regulate ENaC activity, a process referred to as Na+ self-inhibition [134]. This is an allosteric inhibitory effect that results in a reduction in channel open probability [135, 136], and is distinct from the slower “feedback inhibition” due to increases in the intracellular Na+ concentration [116-118]. The extracellular Na+ binding site(s) and the mechanism of transduction remain undefined. Cl− has recently been reported to modulate ENaC activity [137]. The ASIC1 structure revealed 3 Cl− binding sites at each of the intersubunit interfaces [47]. Testing analogous ENaC residues at these sites defined by the interface of the thumb and palm domains of different subunits suggests that two of these are allosteric effector sites for Cl− [138]. Protons activate human, but not rodent ENaCs [139]. In additional, divalent cations such as Zn2+ and Ni2+ modulate ENaC activity [140, 141].
Proteases
Na+ channel subunits undergo assembly in the endoplasmic reticulum, where core, high mannose asparagine-linked glycans are added at specific sites and are later modified to complex-type endoglycosidase H-resistant forms [142, 143]. ENaC α and γ subunits are also cleaved by the pro-protein convertase furin in the biosynthetic pathway [143, 144]. The α subunit is cleaved twice by furin releasing an imbedded inhibitory tract, which partially activates the channel by increasing its open probability [135, 145-147]. The γ subunit is cleaved once by furin. Cleavage of the γ subunit by a second protease at a site distal to the furin cleavage site releases a second, distinct inhibitory tract that further activates the channel [146, 148-150]. Proteases that cleave the γ subunit at a distal site and activate the channel have been found on the plasma membrane and within the epithelial lumen (for reviews, see [149, 151]).
Shear stress
ENaCs are expressed at the apical surface of cells in the distal nephron, where they are subjected to variable mechanical stresses due to variable fluid flow rates. ENaC, like several members of its gene family, is mechanosensitive and is activated by laminar shear stress [152-155]. The structures involved in the mechanical stress response are not yet defined, but altering membrane composition had little effect, making membrane deformation an unlikely mechanism [156]. Conversely, structures that have been implicated in the response of the channel to other ligands appear to modulate the stress response [157-159].
Summary
Mechanisms of ENaC regulation are complex, involving a myriad of both intracellular and extracellular factors. It is notable that the vast majority of these regulatory mechanisms, many of which are in the pathways that we now know are modulated by ENaC regulatory hormones such as aldosterone and vasopressin, have been described over the past two decades. There are clear gaps in our understanding of many of these regulatory mechanisms. For example, how do PKC isoforms modulate ENaC activity? How are the multiple phosphorylation sites within Nedd4-2 coordinated to alter its regulatory functions? How do external and internal factors influence structural transitions that affect channel gating? It is likely that these and other questions will be answered over the next decade.
Acknowledgments
This work was supported by National Institutes of Health grants DK051391, DK065161 DK078734 and DK079307 (the Pittsburgh Center for Kidney Research).
References
- [1].Burch LH, Talbot CR, Knowles MR, Canessa CM, Rossier BC, Boucher RC. Relative expression of the human epithelial Na+ channel subunits in normal and cystic fibrosis airways. Am J Physiol. 1995;269:C511–518. doi: 10.1152/ajpcell.1995.269.2.C511. [DOI] [PubMed] [Google Scholar]
- [2].Talbot CL, Bosworth DG, Briley EL, Fenstermacher DA, Boucher RC, Gabriel SE, Barker PM. Quantitation and localization of ENaC subunit expression in fetal, newborn, and adult mouse lung. Am J Respir Cell Mol Biol. 1999;20:398–406. doi: 10.1165/ajrcmb.20.3.3283. [DOI] [PubMed] [Google Scholar]
- [3].Matsushita K, McCray PB, Jr., Sigmund RD, Welsh MJ, Stokes JB. Localization of epithelial sodium channel subunit mRNAs in adult rat lung by in situ hybridization. Am J Physiol. 1996;271:L332–339. doi: 10.1152/ajplung.1996.271.2.L332. [DOI] [PubMed] [Google Scholar]
- [4].Jain L, Chen XJ, Malik B, Al-Khalili O, Eaton DC. Antisense oligonucleotides against the alpha-subunit of ENaC decrease lung epithelial cation-channel activity. Am J Physiol. 1999;276:L1046–1051. doi: 10.1152/ajplung.1999.276.6.L1046. [DOI] [PubMed] [Google Scholar]
- [5].Borok Z, Liebler JM, Lubman RL, Foster MJ, Zhou B, Li X, Zabski SM, Kim KJ, Crandall ED. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L599–608. doi: 10.1152/ajplung.00130.2000. [DOI] [PubMed] [Google Scholar]
- [6].Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- [7].Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- [8].Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- [9].Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- [10].Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC. Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: localization by in situ hybridization and immunocytochemistry. J Cell Biol. 1994;127:1907–1921. doi: 10.1083/jcb.127.6.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kretz O, Barbry P, Bock R, Lindemann B. Differential expression of RNA and protein of the three pore-forming subunits of the amiloride-sensitive epithelial sodium channel in taste buds of the rat. J Histochem Cytochem. 1999;47:51–64. doi: 10.1177/002215549904700106. [DOI] [PubMed] [Google Scholar]
- [12].Brouard M, Casado M, Djelidi S, Barrandon Y, Farman N. Epithelial sodium channel in human epidermal keratinocytes: expression of its subunits and relation to sodium transport and differentiation. J Cell Sci. 1999;112(Pt 19):3343–3352. doi: 10.1242/jcs.112.19.3343. [DOI] [PubMed] [Google Scholar]
- [13].Grunder S, Muller A, Ruppersberg JP. Developmental and cellular expression pattern of epithelial sodium channel alpha, beta and gamma subunits in the inner ear of the rat. Eur J Neurosci. 2001;13:641–648. doi: 10.1046/j.1460-9568.2001.01426.x. [DOI] [PubMed] [Google Scholar]
- [14].Couloigner V, Fay M, Djelidi S, Farman N, Escoubet B, Runembert I, Sterkers O, Friedlander G, Ferrary E. Location and function of the epithelial Na channel in the cochlea. Am J Physiol Renal Physiol. 2001;280:F214–222. doi: 10.1152/ajprenal.2001.280.2.F214. [DOI] [PubMed] [Google Scholar]
- [15].Drummond HA, Gebremedhin D, Harder DR. Degenerin/epithelial Na+ channel proteins: components of a vascular mechanosensor. Hypertension. 2004;44:643–648. doi: 10.1161/01.HYP.0000144465.56360.ad. [DOI] [PubMed] [Google Scholar]
- [16].Mirshahi M, Nicolas C, Mirshahi S, Golestaneh N, d’Hermies F, Agarwal MK. Immunochemical analysis of the sodium channel in rodent and human eye. Exp Eye Res. 1999;69:21–32. doi: 10.1006/exer.1999.0675. [DOI] [PubMed] [Google Scholar]
- [17].Bubien JK, Watson B, Khan MA, Langloh AL, Fuller CM, Berdiev B, Tousson A, Benos DJ. Expression and regulation of normal and polymorphic epithelial sodium channel by human lymphocytes. J Biol Chem. 2001;276:8557–8566. doi: 10.1074/jbc.M008886200. [DOI] [PubMed] [Google Scholar]
- [18].Tamura H, Schild L, Enomoto N, Matsui N, Marumo F, Rossier BC. Liddle disease caused by a missense mutation of beta subunit of the epithelial sodium channel gene. J Clin Invest. 1996;97:1780–1784. doi: 10.1172/JCI118606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hansson JH, Schild L, Lu Y, Wilson TA, Gautschi I, Shimkets R, Nelson-Williams C, Rossier BC, Lifton RP. A de novo missense mutation of the beta subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc Natl Acad Sci U S A. 1995;92:11495–11499. doi: 10.1073/pnas.92.25.11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet. 1995;11:76–82. doi: 10.1038/ng0995-76. [DOI] [PubMed] [Google Scholar]
- [21].Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Jr., Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP. Liddle’s syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79:407–414. doi: 10.1016/0092-8674(94)90250-x. [DOI] [PubMed] [Google Scholar]
- [22].Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. Embo J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- [23].Kamynina E, Tauxe C, Staub O. Distinct characteristics of two human Nedd4 proteins with respect to epithelial Na(+) channel regulation. Am J Physiol Renal Physiol. 2001;281:F469–477. doi: 10.1152/ajprenal.2001.281.3.F469. [DOI] [PubMed] [Google Scholar]
- [24].Goulet CC, Volk KA, Adams CM, Prince LS, Stokes JB, Snyder PM. Inhibition of the epithelial Na+ channel by interaction of Nedd4 with a PY motif deleted in Liddle’s syndrome. J Biol Chem. 1998;273:30012–30017. doi: 10.1074/jbc.273.45.30012. [DOI] [PubMed] [Google Scholar]
- [25].Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC. Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. Embo J. 1996;15:2381–2387. [PMC free article] [PubMed] [Google Scholar]
- [26].Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- [27].Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet. 1996;12:248–253. doi: 10.1038/ng0396-248. [DOI] [PubMed] [Google Scholar]
- [28].Baker EH, Duggal A, Dong Y, Ireson NJ, Wood M, Markandu ND, MacGregor GA. Amiloride, a specific drug for hypertension in black people with T594M variant? Hypertension. 2002;40:13–17. doi: 10.1161/01.hyp.0000022570.02119.75. [DOI] [PubMed] [Google Scholar]
- [29].Samaha FF, Rubenstein RC, Yan W, Ramkumar M, Levy DI, Ahn YJ, Sheng S, Kleyman TR. Functional polymorphism in the carboxyl terminus of the alpha-subunit of the human epithelial sodium channel. J Biol Chem. 2004;279:23900–23907. doi: 10.1074/jbc.M401941200. [DOI] [PubMed] [Google Scholar]
- [30].Cui Y, Su YR, Rutkowski M, Reif M, Menon AG, Pun RY. Loss of protein kinase C inhibition in the beta-T594M variant of the amiloride-sensitive Na+ channel. Proc Natl Acad Sci U S A. 1997;94:9962–9966. doi: 10.1073/pnas.94.18.9962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tong Q, Menon AG, Stockand JD. Functional polymorphisms in the alpha-subunit of the human epithelial Na+ channel increase activity. Am J Physiol Renal Physiol. 2006;290:F821–827. doi: 10.1152/ajprenal.00312.2005. [DOI] [PubMed] [Google Scholar]
- [32].Baker EH, Dong YB, Sagnella GA, Rothwell M, Onipinla AK, Markandu ND, Cappuccio FP, Cook DG, Persu A, Corvol P, Jeunemaitre X, Carter ND, MacGregor GA. Association of hypertension with T594M mutation in beta subunit of epithelial sodium channels in black people resident in London. Lancet. 1998;351:1388–1392. doi: 10.1016/s0140-6736(97)07306-6. [DOI] [PubMed] [Google Scholar]
- [33].Mohaupt MG. The role of adrenal steroidogenesis in arterial hypertension. Endocrine development. 2008;13:133–144. doi: 10.1159/000134830. [DOI] [PubMed] [Google Scholar]
- [34].Thomson SP, Stump CS, Kurukulasuriya LR, Sowers JR. Adrenal steroids and the metabolic syndrome. Current hypertension reports. 2007;9:512–519. doi: 10.1007/s11906-007-0093-4. [DOI] [PubMed] [Google Scholar]
- [35].Geller DS, Farhi A, Pinkerton N, Fradley M, Moritz M, Spitzer A, Meinke G, Tsai FT, Sigler PB, Lifton RP. Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science. 2000;289:119–123. doi: 10.1126/science.289.5476.119. [DOI] [PubMed] [Google Scholar]
- [36].Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- [37].Mall M, Bleich M, Greger R, Schreiber R, Kunzelmann K. The amiloride-inhibitable Na+ conductance is reduced by the cystic fibrosis transmembrane conductance regulator in normal but not in cystic fibrosis airways. J Clin Invest. 1998;102:15–21. doi: 10.1172/JCI2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ling BN, Zuckerman JB, Lin C, Harte BJ, McNulty KA, Smith PR, Gomez LM, Worrell RT, Eaton DC, Kleyman TR. Expression of the cystic fibrosis phenotype in a renal amphibian epithelial cell line. J Biol Chem. 1997;272:594–600. doi: 10.1074/jbc.272.1.594. [DOI] [PubMed] [Google Scholar]
- [39].Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- [40].Gentzsch M, Dang H, Dang Y, Garcia-Caballero A, Suchindran H, Boucher RC, Stutts MJ. The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na+ channel. J Biol Chem. 2010;285:32227–32232. doi: 10.1074/jbc.M110.155259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Myerburg MM, Butterworth MB, McKenna EE, Peters KW, Frizzell RA, Kleyman TR, Pilewski JM. Airway surface liquid volume regulates ENaC by altering the serine protease-protease inhibitor balance: a mechanism for sodium hyperabsorption in cystic fibrosis. J Biol Chem. 2006;281:27942–27949. doi: 10.1074/jbc.M606449200. [DOI] [PubMed] [Google Scholar]
- [42].Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127:591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huang M, Chalfie M. Gene interactions affecting mechanosensory transduction in Caenorhabditis elegans. Nature. 1994;367:467–470. doi: 10.1038/367467a0. [DOI] [PubMed] [Google Scholar]
- [44].Corey DP, Garcia-Anoveros J. Mechanosensation and the DEG/ENaC ion channels. Science. 1996;273:323–324. doi: 10.1126/science.273.5273.323. [DOI] [PubMed] [Google Scholar]
- [45].Goodman MB, Ernstrom GG, Chelur DS, O’Hagan R, Yao CA, Chalfie M. MEC-2 regulates C. elegans DEG/ENaC channels needed for mechanosensation. Nature. 2002;415:1039–1042. doi: 10.1038/4151039a. [DOI] [PubMed] [Google Scholar]
- [46].Stewart AP, Haerteis S, Diakov A, Korbmacher C, Edwardson JM. Atomic force microscopy reveals the architecture of the epithelial sodium channel (ENaC) The Journal of biological chemistry. 2011;286:31944–31952. doi: 10.1074/jbc.M111.275289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- [48].Renard S, Lingueglia E, Voilley N, Lazdunski M, Barbry P. Biochemical analysis of the membrane topology of the amiloride-sensitive Na+ channel. J Biol Chem. 1994;269:12981–12986. [PubMed] [Google Scholar]
- [49].Canessa CM, Merillat AM, Rossier BC. Membrane topology of the epithelial sodium channel in intact cells. Am J Physiol. 1994;267:C1682–1690. doi: 10.1152/ajpcell.1994.267.6.C1682. [DOI] [PubMed] [Google Scholar]
- [50].Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M. Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel α subunit reveals a mechanism of channel activation by proteases. J Biol Chem. 2011;286:649–660. doi: 10.1074/jbc.M110.167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kashlan OB, Kleyman TR. ENaC structure and function in the wake of a resolved structure of a family member, American journal of physiology. Renal physiology. 2011;301:F684–696. doi: 10.1152/ajprenal.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rotin D, Bar-Sagi D, O’Brodovich H, Merilainen J, Lehto VP, Canessa CM, Rossier BC, Downey GP. An SH3 binding region in the epithelial Na+ channel (alpha rENaC) mediates its localization at the apical membrane. Embo J. 1994;13:4440–4450. doi: 10.1002/j.1460-2075.1994.tb06766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Copeland SJ, Berdiev BK, Ji HL, Lockhart J, Parker S, Fuller CM, Benos DJ. Regions in the carboxy terminus of alpha-bENaC involved in gating and functional effects of actin. Am J Physiol Cell Physiol. 2001;281:C231–240. doi: 10.1152/ajpcell.2001.281.1.C231. [DOI] [PubMed] [Google Scholar]
- [54].Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- [55].Warnock DG. Liddle syndrome: genetics and mechanisms of Na+ channel defects. Am J Med Sci. 2001;322:302–307. doi: 10.1097/00000441-200112000-00002. [DOI] [PubMed] [Google Scholar]
- [56].Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. Embo J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kidney Int. 2000;57:809–815. doi: 10.1046/j.1523-1755.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- [58].Fotia AB, Dinudom A, Shearwin KE, Koch JP, Korbmacher C, Cook DI, Kumar S. The role of individual Nedd4-2 (KIAA0439) WW domains in binding and regulating epithelial sodium channels. Faseb J. 2003;17:70–72. doi: 10.1096/fj.02-0497fje. [DOI] [PubMed] [Google Scholar]
- [59].Lott JS, Coddington-Lawson SJ, Teesdale-Spittle PH, McDonald FJ. A single WW domain is the predominant mediator of the interaction between the human ubiquitin-protein ligase Nedd4 and the human epithelial sodium channel. Biochem J. 2002;361:481–488. doi: 10.1042/0264-6021:3610481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kamynina E, Staub O. Concerted action of ENaC, Nedd4-2, and Sgk1 in transepithelial Na(+) transport. Am J Physiol Renal Physiol. 2002;283:F377–387. doi: 10.1152/ajprenal.00143.2002. [DOI] [PubMed] [Google Scholar]
- [61].Kabra R, Knight KK, Zhou R, Snyder PM. Nedd4-2 induces endocytosis and degradation of proteolytically cleaved epithelial Na+ channels. The Journal of biological chemistry. 2008;283:6033–6039. doi: 10.1074/jbc.M708555200. [DOI] [PubMed] [Google Scholar]
- [62].Soundararajan R, Melters D, Shih IC, Wang J, Pearce D. Epithelial sodium channel regulated by differential composition of a signaling complex. Proc Natl Acad Sci U S A. 2009;106:7804–7809. doi: 10.1073/pnas.0809892106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zhou R, Patel SV, Snyder PM. Nedd4-2 catalyzes ubiquitination and degradation of cell surface ENaC. The Journal of biological chemistry. 2007;282:20207–20212. doi: 10.1074/jbc.M611329200. [DOI] [PubMed] [Google Scholar]
- [64].Butterworth MB, Edinger RS, Ovaa H, Burg D, Johnson JP, Frizzell RA. The deubiquitinating enzyme UCH-L3 regulates the apical membrane recycling of the epithelial sodium channel. The Journal of biological chemistry. 2007;282:37885–37893. doi: 10.1074/jbc.M707989200. [DOI] [PubMed] [Google Scholar]
- [65].Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. Journal of the American Society of Nephrology : JASN. 2007;18:1084–1092. doi: 10.1681/ASN.2006080902. [DOI] [PubMed] [Google Scholar]
- [66].Oberfeld B, Ruffieux-Daidie D, Vitagliano JJ, Pos KM, Verrey F, Staub O. Ubiquitin-specific protease 2-45 (Usp2-45) binds to epithelial Na+ channel (ENaC)-ubiquitylating enzyme Nedd4-2. American journal of physiology. Renal physiology. 2011;301:F189–196. doi: 10.1152/ajprenal.00487.2010. [DOI] [PubMed] [Google Scholar]
- [67].Chen SY, Bhargava A, Mastroberardino L, Meijer OC, Wang J, Buse P, Firestone GL, Verrey F, Pearce D. Epithelial sodium channel regulated by aldosterone-induced protein sgk. Proc Natl Acad Sci U S A. 1999;96:2514–2519. doi: 10.1073/pnas.96.5.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- [69].Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na(+) channel cell surface expression. Embo J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bhalla V, Daidie D, Li H, Pao AC, Lagrange LP, Wang J, Vandewalle A, Stockand JD, Staub O, Pearce D. SGK1 regulates ubiquitin ligase Nedd4-2 by inducing interaction with 14-3-3. Mol Endocrinol. 2005 doi: 10.1210/me.2005-0193. [DOI] [PubMed] [Google Scholar]
- [71].Ichimura T, Yamamura H, Sasamoto K, Tominaga Y, Taoka M, Kakiuchi K, Shinkawa T, Takahashi N, Shimada S, Isobe T. 14-3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J Biol Chem. 2005;280:13187–13194. doi: 10.1074/jbc.M412884200. [DOI] [PubMed] [Google Scholar]
- [72].Liang X, Butterworth MB, Peters KW, Walker WH, Frizzell RA. An obligatory heterodimer of 14-3-3beta and 14-3-3epsilon is required for aldosterone regulation of the epithelial sodium channel. J Biol Chem. 2008;283:27418–27425. doi: 10.1074/jbc.M803687200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Snyder PM, Olson DR, Kabra R, Zhou R, Steines JC. cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2. J Biol Chem. 2004;279:45753–45758. doi: 10.1074/jbc.M407858200. [DOI] [PubMed] [Google Scholar]
- [74].Diakov A, Korbmacher C. A novel pathway of epithelial sodium channel activation involves a serum- and glucocorticoid-inducible kinase consensus motif in the C terminus of the channel’s alpha-subunit. J Biol Chem. 2004;279:38134–38142. doi: 10.1074/jbc.M403260200. [DOI] [PubMed] [Google Scholar]
- [75].Butterworth MB, Edinger RS, Johnson JP, Frizzell RA. Acute ENaC Stimulation by cAMP in a Kidney Cell Line is Mediated by Exocytic Insertion from a Recycling Channel Pool. J Gen Physiol. 2005;125:81–101. doi: 10.1085/jgp.200409124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bugaj V, Pochynyuk O, Stockand JD. Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol. 2009;297:F1411–1418. doi: 10.1152/ajprenal.00371.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yang LM, Rinke R, Korbmacher C. Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel’s beta- and gamma-subunit. J Biol Chem. 2006;281:9859–9868. doi: 10.1074/jbc.M512046200. [DOI] [PubMed] [Google Scholar]
- [78].Edinger RS, Lebowitz J, Li H, Alzamora R, Wang H, Johnson JP, Hallows KR. Functional regulation of the epithelial Na+ channel by IkappaB kinase-beta occurs via phosphorylation of the ubiquitin ligase Nedd4-2. J Biol Chem. 2009;284:150–157. doi: 10.1074/jbc.M807358200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2. J Biol Chem. 2006;281:26159–26169. doi: 10.1074/jbc.M606045200. [DOI] [PubMed] [Google Scholar]
- [80].Hallows KR, Bhalla V, Oyster NM, Wijngaarden MA, Lee JK, Li H, Chandran S, Xia X, Huang Z, Chalkley RJ, Burlingame AL, Pearce D. Phosphopeptide screen uncovers novel phosphorylation sites of Nedd4-2 that potentiate its inhibition of the epithelial Na+ channel. J Biol Chem. 2010;285:21671–21678. doi: 10.1074/jbc.M109.084731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem. 2002;277:13539–13547. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- [82].Soundararajan R, Wang J, Melters D, Pearce D. Differential activities of glucocorticoid-induced leucine zipper protein isoforms. J Biol Chem. 2007;282:36303–36313. doi: 10.1074/jbc.M707287200. [DOI] [PubMed] [Google Scholar]
- [83].Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein in epithelial sodium channel-mediated sodium transport. J Biol Chem. 2005;280:39970–39981. doi: 10.1074/jbc.M508658200. [DOI] [PubMed] [Google Scholar]
- [84].Soundararajan R, Wang J, Melters D, Pearce D. Glucocorticoid-induced Leucine zipper 1 stimulates the epithelial sodium channel by regulating serum- and glucocorticoid-induced kinase 1 stability and subcellular localization. J Biol Chem. 2010;285:39905–39913. doi: 10.1074/jbc.M110.161133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dinudom A, Fotia AB, Lefkowitz RJ, Young JA, Kumar S, Cook DI. The kinase Grk2 regulates Nedd4/Nedd4-2-dependent control of epithelial Na+ channels. Proc Natl Acad Sci U S A. 2004;101:11886–11890. doi: 10.1073/pnas.0402178101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Feldman RD. Deactivation of vasodilator responses by GRK2 overexpression: a mechanism or the mechanism for hypertension? Mol Pharmacol. 2002;61:707–709. doi: 10.1124/mol.61.4.707. [DOI] [PubMed] [Google Scholar]
- [87].Lee IH, Song SH, Campbell CR, Kumar S, Cook DI, Dinudom A. Regulation of the Epithelial Na+ Channel by the RH Domain of G Protein-coupled Receptor Kinase, GRK2, and G{alpha}q/11. J Biol Chem. 2011;286:19259–19269. doi: 10.1074/jbc.M111.239772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shimkets RA, Lifton R, Canessa CM. In vivo phosphorylation of the epithelial sodium channel. Proc Natl Acad Sci U S A. 1998;95:3301–3305. doi: 10.1073/pnas.95.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Awayda MS, Ismailov II, Berdiev BK, Fuller CM, Benos DJ. Protein kinase regulation of a cloned epithelial Na+ channel. J Gen Physiol. 1996;108:49–65. doi: 10.1085/jgp.108.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Stockand JD, Bao HF, Schenck J, Malik B, Middleton P, Schlanger LE, Eaton DC. Differential effects of protein kinase C on the levels of epithelial Na+ channel subunit proteins. J Biol Chem. 2000;275:25760–25765. doi: 10.1074/jbc.M003615200. [DOI] [PubMed] [Google Scholar]
- [91].Zhang YH, de la Rosa D. Alvarez, Canessa CM, Hayslett JP. Insulin-induced phosphorylation of ENaC correlates with increased sodium channel function in A6 cells. Am J Physiol Cell Physiol. 2005;288:C141–147. doi: 10.1152/ajpcell.00343.2004. [DOI] [PubMed] [Google Scholar]
- [92].Yan W, Suaud L, Kleyman TR, Rubenstein RC. Differential Modulation of a Polymorphism in the Carboxyl Terminus of the Alpha Subunit of the Human Epithelial Sodium Channel by Protein Kinase C {delta} Am J Physiol Renal Physiol. 2005 doi: 10.1152/ajprenal.00277.2005. [DOI] [PubMed] [Google Scholar]
- [93].Shi H, Asher C, Yung Y, Kligman L, Reuveny E, Seger R, Garty H. Casein kinase 2 specifically binds to and phosphorylates the carboxy termini of ENaC subunits. Eur J Biochem. 2002;269:4551–4558. doi: 10.1046/j.1432-1033.2002.03154.x. [DOI] [PubMed] [Google Scholar]
- [94].Bachhuber T, Almaca J, Aldehni F, Mehta A, Amaral MD, Schreiber R, Kunzelmann K. Regulation of the epithelial Na+ channel by the protein kinase CK2. J Biol Chem. 2008;283:13225–13232. doi: 10.1074/jbc.M704532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Yan W, Spruce L, Rosenblatt MM, Kleyman TR, Rubenstein RC. Intracellular trafficking of a polymorphism in the COOH terminus of the alpha-subunit of the human epithelial sodium channel is modulated by casein kinase 1. Am J Physiol Renal Physiol. 2007;293:F868–876. doi: 10.1152/ajprenal.00194.2007. [DOI] [PubMed] [Google Scholar]
- [96].Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH. Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem. 2010;285:25161–25167. doi: 10.1074/jbc.M110.103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci U S A. 2007;104:4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc Natl Acad Sci U S A. 2007;104:4020–4024. doi: 10.1073/pnas.0611727104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yue G, Malik B, Yue G, Eaton DC. Phosphatidylinositol 4,5-bisphosphate (PIP2) stimulates epithelial sodium channel activity in A6 cells. J Biol Chem. 2002;277:11965–11969. doi: 10.1074/jbc.M108951200. [DOI] [PubMed] [Google Scholar]
- [100].Ma HP, Saxena S, Warnock DG. Anionic phospholipids regulate native and expressed epithelial sodium channel (ENaC) J Biol Chem. 2002;277:7641–7644. doi: 10.1074/jbc.C100737200. [DOI] [PubMed] [Google Scholar]
- [101].Pochynyuk O, Staruschenko A, Tong Q, Medina J, Stockand JD. Identification of a functional phosphatidylinositol 3,4,5-trisphosphate binding site in the epithelial Na+ channel. J Biol Chem. 2005 doi: 10.1074/jbc.M509071200. [DOI] [PubMed] [Google Scholar]
- [102].Kunzelmann K, Bachhuber T, Regeer R, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ transport via hydrolysis of PIP2. Faseb J. 2005;19:142–143. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- [103].Pochynyuk O, Tong Q, Medina J, Vandewalle A, Staruschenko A, Bugaj V, Stockand JD. Molecular determinants of PI(4,5)P2 and PI(3,4,5)P3 regulation of the epithelial Na+ channel. J Gen Physiol. 2007;130:399–413. doi: 10.1085/jgp.200709800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Staruschenko A, Nichols A, Medina JL, Camacho P, Zheleznova NN, Stockand JD. Rho small GTPases activate the epithelial Na(+) channel. J Biol Chem. 2004;279:49989–49994. doi: 10.1074/jbc.M409812200. [DOI] [PubMed] [Google Scholar]
- [105].Weixel KM, Edinger RS, Kester L, Guerriero CJ, Wang H, Fang L, Kleyman TR, Welling PA, Weisz OA, Johnson JP. Phosphatidylinositol 4-phosphate 5-kinase reduces cell surface expression of the epithelial sodium channel (ENaC) in cultured collecting duct cells. J Biol Chem. 2007;282:36534–36542. doi: 10.1074/jbc.M703970200. [DOI] [PubMed] [Google Scholar]
- [106].Pochynyuk O, Bugaj V, Vandewalle A, Stockand JD. Purinergic control of apical plasma membrane PI(4,5)P2 levels sets ENaC activity in principal cells. Am J Physiol Renal Physiol. 2008;294:F38–46. doi: 10.1152/ajprenal.00403.2007. [DOI] [PubMed] [Google Scholar]
- [107].Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, Stockand JD. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008 doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tong Q, Stockand JD. Receptor tyrosine kinases mediate epithelial Na+ channel inhibition by epidermal growth factor. Am J Physiol Renal Physiol. 2005;288:F150–161. doi: 10.1152/ajprenal.00261.2004. [DOI] [PubMed] [Google Scholar]
- [109].Sariban-Sohraby S, Burg M, Wiesmann WP, Chiang PK, Johnson JP. Methylation increases sodium transport into A6 apical membrane vesicles: possible mode of aldosterone action. Science. 1984;225:745–746. doi: 10.1126/science.6463652. [DOI] [PubMed] [Google Scholar]
- [110].Stockand JD, Edinger RS, Eaton DC, Johnson JP. Toward Understanding the Role of Methylation in Aldosterone-Sensitive Na(+) Transport. News Physiol Sci. 2000;15:161–165. doi: 10.1152/physiologyonline.2000.15.4.161. [DOI] [PubMed] [Google Scholar]
- [111].Wiesmann WP, Johnson JP, Miura GA, Chaing PK. Aldosterone-stimulated transmethylations are linked to sodium transport. Am J Physiol. 1985;248:F43–47. doi: 10.1152/ajprenal.1985.248.1.F43. [DOI] [PubMed] [Google Scholar]
- [112].Stockand JD, Spier BJ, Worrell RT, Yue G, Al-Baldawi N, Eaton DC. Regulation of Na(+) reabsorption by the aldosterone-induced small G protein K-Ras2A. J Biol Chem. 1999;274:35449–35454. doi: 10.1074/jbc.274.50.35449. [DOI] [PubMed] [Google Scholar]
- [113].Rokaw MD, Wang JM, Edinger RS, Weisz OA, Hui D, Middleton P, Shlyonsky V, Berdiev BK, Ismailov I, Eaton DC, Benos DJ, Johnson JP. Carboxylmethylation of the beta subunit of xENaC regulates channel activity. J Biol Chem. 1998;273:28746–28751. doi: 10.1074/jbc.273.44.28746. [DOI] [PubMed] [Google Scholar]
- [114].Edinger RS, Yospin J, Perry C, Kleyman TR, Johnson JP. Regulation of epithelial Na+ channels (ENaC) by methylation: a novel methyltransferase stimulates ENaC activity. J Biol Chem. 2006;281:9110–9117. doi: 10.1074/jbc.M509232200. [DOI] [PubMed] [Google Scholar]
- [115].Mueller GM, Maarouf AB, Kinlough CL, Sheng N, Kashlan OB, Okumura S, Luthy S, Kleyman TR, Hughey RP. Cys palmitoylation of the beta subunit modulates gating of the epithelial sodium channel. J Biol Chem. 2010;285:30453–30462. doi: 10.1074/jbc.M110.151845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Komwatana P, Dinudom A, Young JA, Cook DI. Cytosolic Na+ controls and epithelial Na+ channel via the Go guanine nucleotide-binding regulatory protein. Proc Natl Acad Sci U S A. 1996;93:8107–8111. doi: 10.1073/pnas.93.15.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Cook DI, Dinudom A, Komwatana P, Kumar S, Young JA. Patch-clamp studies on epithelial sodium channels in salivary duct cells. Cell Biochem Biophys. 2002;36:105–113. doi: 10.1385/cbb:36:2-3:105. [DOI] [PubMed] [Google Scholar]
- [118].Harvey KF, Dinudom A, Cook DI, Kumar S. The Nedd4-like protein KIAA0439 is a potential regulator of the epithelial sodium channel. J Biol Chem. 2001;276:8597–8601. doi: 10.1074/jbc.C000906200. [DOI] [PubMed] [Google Scholar]
- [119].Anantharam A, Tian Y, Palmer LG. Open probability of the epithelial sodium channel is regulated by intracellular sodium. J Physiol. 2006;574:333–347. doi: 10.1113/jphysiol.2006.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Knight KK, Wentzlaff DM, Snyder PM. Intracellular sodium regulates proteolytic activation of the epithelial sodium channel. J Biol Chem. 2008;283:27477–27482. doi: 10.1074/jbc.M804176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Kashlan OB, Mueller GM, Qamar MZ, Poland PA, Ahner A, Rubenstein RC, Hughey RP, Brodsky JL, Kleyman TR. Small heat shock protein alphaA-crystallin regulates epithelial sodium channel expression. J Biol Chem. 2007;282:28149–28156. doi: 10.1074/jbc.M703409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Buck TM, Kolb AR, Boyd CR, Kleyman TR, Brodsky JL. The endoplasmic reticulum-associated degradation of the epithelial sodium channel requires a unique complement of molecular chaperones. Mol Biol Cell. 2010;21:1047–1058. doi: 10.1091/mbc.E09-11-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Butterworth MB, Edinger RS, Silvis MR, Gallo LI, Liang X, Apodaca G, Frizzell RA, Johnson JP. RAB11b REGULATES THE TRAFFICKING AND RECYCLING OF THE EPITHELIAL SODIUM CHANNEL (ENaC) American journal of physiology. Renal physiology. 2011 doi: 10.1152/ajprenal.00304.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Karpushev AV, Levchenko V, Pavlov TS, Lam VY, Vinnakota KC, Vandewalle A, Wakatsuki T, Staruschenko A. Regulation of ENaC expression at the cell surface by Rab11. Biochem Biophys Res Commun. 2008;377:521–525. doi: 10.1016/j.bbrc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Saxena SK, Horiuchi H, Fukuda M. Rab27a regulates epithelial sodium channel (ENaC) activity through synaptotagmin-like protein (SLP-5) and Munc13-4 effector mechanism. Biochem Biophys Res Commun. 2006;344:651–657. doi: 10.1016/j.bbrc.2006.03.160. [DOI] [PubMed] [Google Scholar]
- [126].Saxena SK, Singh M, Shibata H, Kaur S, George C. Rab4 GTP/GDP modulates amiloride-sensitive sodium channel (ENaC) function in colonic epithelia. Biochem Biophys Res Commun. 2006;340:726–733. doi: 10.1016/j.bbrc.2005.12.036. [DOI] [PubMed] [Google Scholar]
- [127].Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Saxena S, Quick MW, Tousson A, Oh Y, Warnock DG. Interaction of syntaxins with the amiloride-sensitive epithelial sodium channel. J Biol Chem. 1999;274:20812–20817. doi: 10.1074/jbc.274.30.20812. [DOI] [PubMed] [Google Scholar]
- [129].Condliffe SB, Carattino MD, Frizzell RA, Zhang H. Syntaxin 1A regulates ENaC via domain-specific interactions. J Biol Chem. 2003;278:12796–12804. doi: 10.1074/jbc.M210772200. [DOI] [PubMed] [Google Scholar]
- [130].Condliffe SB, Zhang H, Frizzell RA. Syntaxin 1A regulates ENaC channel activity. J Biol Chem. 2004;279:10085–10092. doi: 10.1074/jbc.M313592200. [DOI] [PubMed] [Google Scholar]
- [131].Zhou R, Kabra R, Olson DR, Piper RC, Snyder PM. Hrs controls sorting of the epithelial Na+ channel between endosomal degradation and recycling pathways. J Biol Chem. 2010;285:30523–30530. doi: 10.1074/jbc.M110.150755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Firsov D, Robert-Nicoud M, Gruender S, Schild L, Rossier BC. Mutational analysis of cysteine-rich domains of the epithelium sodium channel (ENaC). Identification of cysteines essential for channel expression at the cell surface. J Biol Chem. 1999;274:2743–2749. doi: 10.1074/jbc.274.5.2743. [DOI] [PubMed] [Google Scholar]
- [133].Sheng S, Maarouf AB, Bruns JB, Hughey RP, Kleyman TR. Functional role of extracellular loop cysteine residues of the epithelial Na+ channel in Na+ self-inhibition. J Biol Chem. 2007;282:20180–20190. doi: 10.1074/jbc.M611761200. [DOI] [PubMed] [Google Scholar]
- [134].Fuchs W, Larsen EH, Lindemann B. Current-voltage curve of sodium channels and concentration dependence of sodium permeability in frog skin. J Physiol. 1977;267:137–166. doi: 10.1113/jphysiol.1977.sp011805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol. 2006;290:F1488–1496. doi: 10.1152/ajprenal.00439.2005. [DOI] [PubMed] [Google Scholar]
- [136].Maarouf AB, Sheng N, Chen J, Winarski KL, Okumura S, Carattino MD, Boyd CR, Kleyman TR, Sheng S. Novel determinants of epithelial sodium channel gating within extracellular thumb domains. J Biol Chem. 2009;284:7756–7765. doi: 10.1074/jbc.M807060200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Collier DM, Snyder PM. Extracellular chloride regulates the epithelial sodium channel. J Biol Chem. 2009;284:29320–29325. doi: 10.1074/jbc.M109.046771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Collier DM, Snyder PM. Identification of epithelial Na+ channel (ENaC) intersubunit Cl− inhibitory residues suggests a trimeric αγβ channel architecture. J Biol Chem. 2010;286:6027–6032. doi: 10.1074/jbc.M110.198127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Collier DM, Snyder PM. Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem. 2009;284:792–798. doi: 10.1074/jbc.M806954200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Sheng S, Perry CJ, Kleyman TR. Extracellular Zn2+ activates epithelial Na+ channels by eliminating Na+ self-inhibition. J Biol Chem. 2004;279:31687–31696. doi: 10.1074/jbc.M405224200. [DOI] [PubMed] [Google Scholar]
- [141].Sheng S, Perry CJ, Kleyman TR. External nickel inhibits epithelial sodium channel by binding to histidine residues within the extracellular domains of alpha and gamma subunits and reducing channel open probability. J Biol Chem. 2002;277:50098–50111. doi: 10.1074/jbc.M209975200. [DOI] [PubMed] [Google Scholar]
- [142].De La Rosa DA, Li H, Canessa CM. Effects of aldosterone on biosynthesis, traffic, and functional expression of epithelial sodium channels in a6 cells. J Gen Physiol. 2002;119:427–442. doi: 10.1085/jgp.20028559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Hughey RP, Mueller GM, Bruns JB, Kinlough CL, Poland PA, Harkleroad KL, Carattino MD, Kleyman TR. Maturation of the epithelial Na+ channel involves proteolytic processing of the α- and γ-subunits. J Biol Chem. 2003;278:37073–37082. doi: 10.1074/jbc.M307003200. [DOI] [PubMed] [Google Scholar]
- [144].Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem. 2004;279:48491–48494. doi: 10.1074/jbc.C400460200. [DOI] [PubMed] [Google Scholar]
- [145].Carattino MD, Passero CJ, Steren CA, Maarouf AB, Pilewski JM, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the α-subunit of the epithelial sodium channel. Am J Physiol Renal Physiol. 2008;294:F47–52. doi: 10.1152/ajprenal.00399.2007. [DOI] [PubMed] [Google Scholar]
- [146].Carattino MD, Hughey RP, Kleyman TR. Proteolytic processing of the epithelial sodium channel γ subunit has a dominant role in channel activation. J Biol Chem. 2008;283:25290–25295. doi: 10.1074/jbc.M803931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Carattino MD, Sheng S, Bruns JB, Pilewski JM, Hughey RP, Kleyman TR. The epithelial Na+ channel is inhibited by a peptide derived from proteolytic processing of its α subunit. J Biol Chem. 2006;281:18901–18907. doi: 10.1074/jbc.M604109200. [DOI] [PubMed] [Google Scholar]
- [148].Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR. Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the γ-subunit. J Biol Chem. 2007;282:6153–6160. doi: 10.1074/jbc.M610636200. [DOI] [PubMed] [Google Scholar]
- [149].Kleyman TR, Carattino MD, Hughey RP. ENaC at the cutting edge: regulation of epithelial sodium channels by proteases. J Biol Chem. 2009;284:20447–20451. doi: 10.1074/jbc.R800083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Passero CJ, Carattino MD, Kashlan OB, Myerburg MM, Hughey RP, Kleyman TR. Defining an inhibitory domain in the gamma subunit of the epithelial sodium channel. Am J Physiol Renal Physiol. 2010;299:F854–F861. doi: 10.1152/ajprenal.00316.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Passero CJ, Hughey RP, Kleyman TR. New role for plasmin in sodium homeostasis. Curr Opin Nephrol Hypertens. 2010;19:13–19. doi: 10.1097/MNH.0b013e3283330fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na+ channels are regulated by flow. Am J Physiol Renal Physiol. 2001;280:F1010–1018. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- [153].Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol. 2006;291:F663–669. doi: 10.1152/ajprenal.00514.2005. [DOI] [PubMed] [Google Scholar]
- [154].Satlin LM, Carattino MD, Liu W, Kleyman TR. Regulation of cation transport in the distal nephron by mechanical forces. Am J Physiol Renal Physiol. 2006;291:F923–931. doi: 10.1152/ajprenal.00192.2006. [DOI] [PubMed] [Google Scholar]
- [155].Althaus M, Bogdan R, Clauss WG, Fronius M. Mechano-sensitivity of epithelial sodium channels (ENaCs): laminar shear stress increases ion channel open probability. FASEB J. 2007;21:2389–2399. doi: 10.1096/fj.06-7694com. [DOI] [PubMed] [Google Scholar]
- [156].Carattino MD, Liu W, Hill WG, Satlin LM, Kleyman TR. Lack of a role of membrane-protein interactions in flow-dependent activation of ENaC. Am J Physiol Renal Physiol. 2007;293:F316–324. doi: 10.1152/ajprenal.00455.2006. [DOI] [PubMed] [Google Scholar]
- [157].Carattino MD, Sheng S, Kleyman TR. Mutations in the pore region modify epithelial sodium channel gating by shear stress. J Biol Chem. 2004 doi: 10.1074/jbc.M413123200. [DOI] [PubMed] [Google Scholar]
- [158].Abi-Antoun T, Shi S, Tolino LA, Kleyman TR, Carattino MD. Second transmembrane domain modulates epithelial sodium channel gating in response to shear stress. Am J Physiol Renal Physiol. 2011;300:F1089–1095. doi: 10.1152/ajprenal.00610.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Shi S, Ghosh DD, Okumura S, Carattino MD, Kashlan OB, Sheng S, Kleyman TR. Base of the thumb domain modulates epithelial sodium channel gating. J Biol Chem. 2011;286:14753–14761. doi: 10.1074/jbc.M110.191734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Cole C, Barber JD, Barton GJ. The Jpred 3 secondary structure prediction server. Nucleic Acids Res. 2008;36:W197–201. doi: 10.1093/nar/gkn238. [DOI] [PMC free article] [PubMed] [Google Scholar]