Abstract
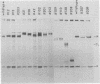
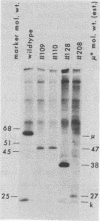
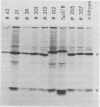
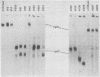
Using a hybridoma cell line which secretes hapten-specific immunoglobulin M (IgM), we have isolated a variety of mutants which produce abnormal immunoglobulin. Immunoglobulin was tested for the size and composition of the component heavy and light chains and for variable and constant region related functional and serological activities. Some mutants secrete IgM which seems to be defective in hapten binding; others make IgM which appears not to activate complement. Many of the mutants secrete monomeric as opposed to pentameric IgM. In some cases, the defect apparently correlates with structural alterations in the mu heavy chain: partial deletion, polypeptide addition, and abnormal glycosylation have been observed. These mutant cell lines provide a means of identifying the structural basis of IgM function and of studying the biochemistry of IgM synthesis and processing.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumal R., Birshtein B. K., Coffino P., Scharff M. D. Mutations in immunoglobulin-producing mouse myeloma cells. Science. 1973 Oct 12;182(4108):164–166. doi: 10.1126/science.182.4108.164. [DOI] [PubMed] [Google Scholar]
- Baumal R., Scharff M. D. Immunoglobulin biosynthesis by the MOPC 173 mouse myeloma tumor and a variant spleen clone. J Immunol. 1976 Jan;116(1):65–74. [PubMed] [Google Scholar]
- Berek C., Schreier M. H., Sidman C. L., Jaton J. C., Kocher H. P., Cosenza H. Phosphorylcholine-binding hybridoma proteins of normal and idiotypically suppressed BALB/c mice I. Characterization and idiotypic analysis. Eur J Immunol. 1980 Apr;10(4):258–263. doi: 10.1002/eji.1830100407. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Koshland M. E. Activation of antibody Fc function by antigen-induced conformational changes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5111–5115. doi: 10.1073/pnas.72.12.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows P., LeJeune M., Kearney J. F. Evidence that murine pre-B cells synthesise mu heavy chains but no light chains. Nature. 1979 Aug 30;280(5725):838–840. doi: 10.1038/280838a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Metzger H. Affinity labeling of a phosphorylcholine binding mouse myeloma protein. Biochemistry. 1972 Feb 29;11(5):766–771. doi: 10.1021/bi00755a014. [DOI] [PubMed] [Google Scholar]
- Claflin J. L., Lieberman R., Davie J. M. Clonal nature of the immune response to phosphorylcholine. I. Specificity, class, and idiotype of phosphorylcholine-binding receptors on lymphoid cells. J Exp Med. 1974 Jan 1;139(1):58–73. doi: 10.1084/jem.139.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Scharff M. D. Antigen-binding mutants of mouse myeloma cells. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5687–5691. doi: 10.1073/pnas.74.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. J., Secher D. S., Milstein C. Intracellular immunoglobulin chain synthesis in non-secreting variants of a mouse myeloma: detection of inactive light-chain messenger RNA. J Mol Biol. 1974 Dec 25;90(4):691–701. doi: 10.1016/0022-2836(74)90533-6. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Della Corte E., Parkhouse R. M. Biosynthesis of immunoglobulin A (IgA) and immunoglobulin M (IgM). Requirement for J chain and a disulphide-exchanging enzyme for polymerization. Biochem J. 1973 Nov;136(3):597–606. doi: 10.1042/bj1360597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Francus T., Dharmgrongartama B., Campbell R., Scharff M. D., Birshtein B. K. IgG2a-producing variants of an IgG2b-producing mouse myeloma cell line. J Exp Med. 1978 Jun 1;147(6):1535–1550. doi: 10.1084/jem.147.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U., Coffino P. Mutagenesis in S49 mouse lymphoma cells: induction of resistance to ouabain, 6-thioguanine, and dibutyryl cyclic AMP. Proc Natl Acad Sci U S A. 1977 Feb;74(2):679–683. doi: 10.1073/pnas.74.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füst G., Csécsi-Nagy M., Medgyesi G. A., Kulics J., Gergely J. Study of the interaction between monoclonal IgM proteins and the complement system. Immunochemistry. 1976 Oct;13(10):793–800. doi: 10.1016/0019-2791(76)90178-6. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Hurst M. M., Volanakis J. E., Stroud R. M., Bennett J. C. C1 fixation and classical complement pathway activation by a fragment of the Cmu4 domain of IgM. J Exp Med. 1975 Nov 1;142(5):1322–1326. doi: 10.1084/jem.142.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. J., Thames K. E. Investigations of the complement-fixing sites of immunoglobulins. J Immunol. 1976 Nov;117(5 Pt 1):1491–1494. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kocher H. P., Berek C., Schreier M. H., Cosenza H., Jaton J. C. Phosphorylcholine-binding hybridoma proteins of normal and idiotypically suppressed BALB/c mice II. Variable region N-terminal amino acid sequences. Eur J Immunol. 1980 Apr;10(4):264–267. doi: 10.1002/eji.1830100408. [DOI] [PubMed] [Google Scholar]
- Köhler G., Hengartner H., Shulman M. J. Immunoglobulin production by lymphocyte hybridomas. Eur J Immunol. 1978 Feb;8(2):82–88. doi: 10.1002/eji.1830080203. [DOI] [PubMed] [Google Scholar]
- Köhler G., Howe S. C., Milstein C. Fusion between immunoglobulin-secreting and nonsecreting myeloma cell lines. Eur J Immunol. 1976 Apr;6(4):292–295. doi: 10.1002/eji.1830060411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Independent synthesis of light and heavy chains: quantitation of light-chain production by mouse myeloma variants. J Exp Med. 1974 Oct 1;140(4):1112–1116. doi: 10.1084/jem.140.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt D., Cooper M. D. Mouse pre-B cells synthesize and secrete mu heavy chains but not light chains. Cell. 1980 Mar;19(3):617–625. doi: 10.1016/s0092-8674(80)80038-9. [DOI] [PubMed] [Google Scholar]
- Liesegang B., Radbruch A., Rajewsky K. Isolation of myeloma variants with predefined variant surface immunoglobulin by cell sorting. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3901–3905. doi: 10.1073/pnas.75.8.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Adetugbo K., Cowan N. J., Köhler G., Secher D. S., Wilde C. D. Somatic cell genetics of antibody-secreting cells: studies of clonal diversification and analysis by cell fusion. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):793–803. doi: 10.1101/sqb.1977.041.01.090. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Scharff M. D. Heavy chain-producing variants of a mouse myeloma cell line. J Immunol. 1975 Feb;114(2 Pt 1):655–659. [PubMed] [Google Scholar]
- Mosmann T. R., Baumal R., Williamson A. R. Mutations affecting immunoglobulin light chain secretion by myeloma cells. I. Functional analysis by cell fusion. Eur J Immunol. 1979 Jul;9(7):511–516. doi: 10.1002/eji.1830090705. [DOI] [PubMed] [Google Scholar]
- Padlan E. A., Davies D. R., Rudikoff S., Potter M. Structural basis for the specificity of phosphorylcholine-binding immunoglobulins. Immunochemistry. 1976 Nov;13(11):945–949. doi: 10.1016/0019-2791(76)90239-1. [DOI] [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins of mice. Adv Immunol. 1977;25:141–211. [PubMed] [Google Scholar]
- Preud'Homme J. L., Birshtein B. K., Scharff M. D. Variants of a mouse myeloma cell line that synthesize immunoglobulin heavy chains having an altered serotype. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1427–1430. doi: 10.1073/pnas.72.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radbruch A., Liesegang B., Rajewsky K. Isolation of variants of mouse myeloma X63 that express changed immunoglobulin class. Proc Natl Acad Sci U S A. 1980 May;77(5):2909–2913. doi: 10.1073/pnas.77.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. A., Koshland M. E. Identification of a lymphocyte enzyme that catalyzes pentamer immunoglobulin M assembly. J Biol Chem. 1981 May 10;256(9):4633–4639. [PubMed] [Google Scholar]
- Roth R. A., Koshland M. E. Role of disulfide interchange enzyme in immunoglobulin synthesis. Biochemistry. 1981 Nov 10;20(23):6594–6599. doi: 10.1021/bi00526a012. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Siegel R. C., Cathou R. E. Effects of limited denaturation by heat on the dynamic conformation of equine immunoglobulin M antibody and on interaction with antigen and complement. Biochemistry. 1981 Jan 6;20(1):192–198. doi: 10.1021/bi00504a032. [DOI] [PubMed] [Google Scholar]
- Sonenshein G. E., Siekevitz M., Siebert G. R., Gefter M. L. Control of immunoglobulin secretion in the murine plasmacytoma line MOPC 315. J Exp Med. 1978 Jul 1;148(1):301–312. doi: 10.1084/jem.148.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Schlesinger S., Kornfeld S. Processing of high mannose oligosaccharides to form complex type oligosaccharides on the newly synthesized polypeptides of the vesicular stomatitis virus G protein and the IgG heavy chain. J Biol Chem. 1978 Feb 10;253(3):716–722. [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]
- Wims L. A., Morrison S. L. ICR-191 and ethyl methanesulfonate induced mutagenesis at the immunoglobulin locus in the Y5606 cultured myeloma cell line. Mutat Res. 1981 Apr;81(2):215–228. doi: 10.1016/0027-5107(81)90036-1. [DOI] [PubMed] [Google Scholar]
- Winkelhake J. L. Immunoglobulin structure and effector functions. Immunochemistry. 1978 Sep;15(9):695–714. doi: 10.1016/0161-5890(78)90044-5. [DOI] [PubMed] [Google Scholar]