Abstract
Objective
To study the angiogenesis modulation mechanism of Xuefu Zhuyu Decoction(
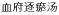 ) on endothelial cell ECV304.
) on endothelial cell ECV304.
Methods
ECV304 cells were treated by 2.5% Xuefu Zhuyu Decoction-containing serum (XFZYD-CS) for 24h, 48h and 72h respectively. MTT, FACS, migration, adhesion and in vitro tube formation assay confirmed an angiogenesis effect of XFZYD at 3 time points. Then an analysis of angiogenesis regulator profiles at 3 times with Real-time PCR Supperarray was performed.
Results
At 48h, XFZYD-CS induced ECV304 significantly improved cell vigor, number in S phase, migration, adhesion and tube formation. At 24h and 72h, only cell migration was elevated. Microarray results showed that 18,14 and 16 genes changed expressions at 24h, 48h and 72h respectively. 6 genes changed consistently and 7 genes varied between 48h and the other two times.
Conclusion
The changes of ECV304 induced by XFZYD-CS at 48h. The regulation mode at 48h had less genetic change but had greater cellular effect.
Keywords: Xuefu Zhuyu Decoction, angiogenesis, Microarray, Chinese herbs
Angiogenesis is important for the treatment and prognosis of ischemic diseases. Xuefu Zhuyu Decoction (Drive Out Stasis from the Mansion of Blood Decoction) was first recorded in 1830 in Wang Qing-ren’s Yi-lin Gaicuo (
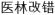 ). It is a classical formula for activating blood and removing stasis, composed of the following ingredients: Angelica sinensis (Oliv.) Diels 9 g, Rehmannia glutinosa Libosch. 9 g, Prunus persica 12 g, Carthamus tinctorius L. 9 g, Citrus aurantium L. 6 g, Paeonia lactiflora Pall. 6 g, Bupleurum chinese DC. 3 g, Glycyrrhiza uralensis Fisch. 6 g, Platycodon grandiflorum 4.5 g, Ligusticum Chuanxiong Hort. 4.5 g, and Cyathula officinalis Kuan 9 g,
). It is a classical formula for activating blood and removing stasis, composed of the following ingredients: Angelica sinensis (Oliv.) Diels 9 g, Rehmannia glutinosa Libosch. 9 g, Prunus persica 12 g, Carthamus tinctorius L. 9 g, Citrus aurantium L. 6 g, Paeonia lactiflora Pall. 6 g, Bupleurum chinese DC. 3 g, Glycyrrhiza uralensis Fisch. 6 g, Platycodon grandiflorum 4.5 g, Ligusticum Chuanxiong Hort. 4.5 g, and Cyathula officinalis Kuan 9 g,
Previous work by our team demonstrated that Xuefu Zhuyu Decoction (XFZYD) has angiogenesis effects that not only increase the vessel number in chicken embryo chorioallantoic membrane (CAM) model(1), but also mobilize marrow endothelial progenitor cell(EPC)(2,3), promoting EPC differentiation(4) and tube formation(5). The mechanism of its pro-angiogenesis is unclear. In this study of angiogenesis modulation function and the regulation mechanism of XFZYD, we first examined relevant endothelial cell migration, proliferation, adhesion and tube formation with endothelial cell line ECV304 to demonstrate the angiogenesis effect of XFZYD. We then used microarray technique analyze gene expression profiles.
MATERIAL AND METHODS
Preparation of XFZYD-containing Serum
XFZYD-containing serum were made according to the protocol adopted in our previous study(3).
Incubation and Grouping of ECV304
Endothelial cell line ECV304 (China Center of Type Culture Collection, Wuhan University, China) was grown in M199 containing 5%FBS(v/v) at 37°C in a 5%CO2 atmosphere. Once confluenced, cells were detached with trypsin-EDTA solution, synchronized by incubation for 24h in serum-free M199, then harvested and plated in 96-well plates (for proliferation assay) or 25cm2 flask at a concentration of 2.5×103 cells/well or 2.5×105 cells/flask in 5%FBS. After 4h, the medium was discarded and the cells were exposed to 2.5%XFZYD-CS or control serum for 24h, 48h and 72h.
Cell vigor assay
The effect of XFZYD-CS in inducing ECV304 proliferation was estimated by methyl thiazolyl tetrazolium(MTT) assay. MTT(5mg/ml) was added to each well and incubated for 4h. After the MTT solution was discarded and replaced by 200μl DMSO, the plates were shaken for 10min. The optical density (OD) was assessed at 570nm (reference wave, 630nm) using a 96-well microplate reader(BioTek Co., USA).
Cell proliferation assay
Cell proliferation assay was tested by FACS as protocol described in the instruction book of Cycle TEST™ plus DNA REAGENT KIT. Cell Quest software was used to obtain data and ModiFit Version 3.0 was used for analysis.
Cell migration assay
Cell migration was evaluated by Boyden chamber assay. The upper chamber was covered by a 8μm polycarbonate membrane. All groups ECV304 (2×104 cells) were suspended in 100μl corresponding serum and added on the membrane. The lower chamber was loaded with 100μl corresponding cell culture supernatant. After incubatation at 37°C for 1h, the residual cells on the upper side of the membrane were removed with cotton swabs, and the membrane fixed with 4% neutral formalin for 10 min, and stained with hematoxylin. The stained cells from 6 high power (×400) fields (HPF) were counted. Photographs were taken by an inverted phase contrast microscope (IX70, Olympu Co., Japan).
Cell adhesion assay
96-well plates were coated with 1% gelatin for 1h. XFZYD-CS-treated or control serum-treated ECV304 (1×104 cells) were plated with 5%FBS for 30 min. The culture medium was subsequently removed and adherent cells from 6 random fields (×100) were counted.
In vitro tube formation assay
Matrigel assay was used to evaluate capillary tube formation activity as described in the protocol of In Vitro Angiogenesis Assay Kit (Millipore Co., USA). Briefly, ECMatrix™ solution was thawed on ice overnight, then mixed with diluent and placed in a 96-well plate at 37° for 2h to allow the matrix solution to solidify. Each group ECV304 was added on the polymerized matrigel at 104 cells per well. After incubation at 37° for 10h, capillary tubes were inspected at a magnification of 400× with an inverted phase contrast microscope. Capillary tubes were defined as endothelial cord formations that were connected at both ends. The number of tubes in six random fields per well was determined.
Real-time PCR microarray
Prepared RNA by Trizol, its yield and quality assessed by UV absorbance and denaturing agarose gel electrophoresis as common, synthetized first strand cDNA by Reverse Transcriptase (invitrogen Co., USA), diluted cDNA sample and loaded for the 96-Well Real-time PCR Arrays (SuperArray Co., USA). All assays were performed according to the manufacturer’s protocols.
Data analysis and statistics
Data analysis of PCR array adopted the ΔΔCt Method. We calculate the ΔCt and ΔΔCt as the formula: ΔCt = average Ct − average of HK genes’ Ct, ΔΔCt = ΔCt (XFZYD group) - ΔCt (control group), then calculated the fold-change for each gene as 2-ΔΔCt, Significance was set at fold difference≥2, negative meaned down-regulation. Except that, continuous variables were compared using the Student’s t-test for two independent variables, or one-way ANOVA for comparisons of more than two means. Significance was set at P < 0.05.
RESULTS
Effect of XFZYD-CS on cell vigor
At 24 h, the MTT colorimetric assay revealed that compared to the control group, 2.5% XFZYD-CS treated ECV304 could elevate the mitochondria metabolism capacity to improve the cell vigor (Table1).
Table 1.
Effects of XFZYD on Ecv304 vigor, proliferation, migration, adhesion and tube formation capacity (x̄±s, n=6)
| Time | Group | Vigor (570 nm light absorbance) | Cell cycle (S phase %) | Migration (migratory Cells×400) | Adhesion (adherent cells×100) | Tube formation (tubes ×400) |
|---|---|---|---|---|---|---|
| 24h | XFZYD | 0.193±0.005 | 38.00±1.41 | 36.67±3.50* | 35.00±4.90 | 20.50±3.73 |
| Control | 0.196±0.026 | 38.67±2.16 | 25.50±6.28 | 29.67±2.94 | 23.17±2.99 | |
|
| ||||||
| 48h | XFZYD | 0.253±0.014* | 45.67±2.25# | 39.83±3.71# | 36.50±3.94* | 36.50±4.85# |
| Control | 0.208±0.030 | 33.17±2.23 | 28.67±3.33 | 28.17±3.37 | 21.33±4.41 | |
|
| ||||||
| 72h | XFZYD | 0.387±0.018 | 32.00±1.79 | 35.50±4.93# | 27.33±5.16 | 20.00±3.74 |
| Control | 0.392±0.011 | 29.83±3.66 | 23.50±4.51 | 30.00±5.10 | 20.17±3.92 | |
Compared to control group,
P<0.05;
P<0.01
Effect of XFZYD-CS on cell proliferation
In time course experiments, only at 48h, did results of the Flow Cytometer show, XFZYD-CS treated ECV304 elevated cell proliferation significantly compared to the control group (Table1).
Effect of XFZYD-CS on cell migration
At 24h, 48h and 72h, XFZYD-CS treated ECV304 significantly improved the number of the cell migrated to the underlayer of the membrane as compared to the control group (Table1). This demonstrated that XFZYD influenced cell migration ability.
Effect of XFZYD-CS on cell adhesion
At 48h, the cell adhesion assay indicated that XFZYD-CS treatment significantly elevated the number of cells adherent on gelatin compared to the control group. This demonstrated that XFZYD elevated cell adhesion ability. (Table1).
Effect of XFZYD-CS on tube formation
In vitro angiogenesis assay showed that only at 48h did XFZY-CS dramatically increase the tube number compared to controls. (Table1).
The results of Real-time PCR microarray
Microarray analysis illustrated that among the 84 angiogenesis regulator gene chosen, all 27 genes had been effected significantly at 3 times, and 18,14,16 genes changed expression at 24h, 48h and 72h respectively (Table1-3). The 48h was the optimal angiogenesis time demonstrated by the above assay but this time affected the least number of genes. There were 6 identical genes changed at 3 times as TGFβ2 and VEGFC upregulate; CXCL6, CXCL10, CDH5 and EGF downregulate (Table2). 7 genes changed expression differentially between 48h and the other two times (Table3) as EFNA1, CXCL5 and THBS2 downregulated at 48h; EFNB2, TYMP, FGF2 and NRP1 upregulated at 24h or 72h.
Table 3.
Differentially Changed expression genes between 48h and the other two times a
| Symbol | Description | Fold difference
|
||
|---|---|---|---|---|
| 24h | 48h | 72h | ||
| EFNA1 | Ephrin-A1 | -1.15 | -2.03 | -1.12 |
| EFNB2 | Ephrin-B2 | 2.95 | 1.98 | 2.36 |
| CXCL5 | Chemokine (C-X-C motif) ligand 5 | -1.58 | -3.63 | -1.23 |
| THBS2 | Thrombospondin 2 | -1.40 | -7.60 | -1.13 |
| TYMP | Thymidine phosphorylase | -2.27 | -1.28 | -3.55 |
| FGF2 | Fibroblast growth factor 2 (basic) | 3.18 | 1.49 | 2.24 |
| NRP1 | Neuropilin 1 | 2.41 | 1.72 | 2.29 |
Results shown are representative of three experiments.
Table 2.
Same changed angiogenesis regulator genes detected at 3 times a
| Symbol | Description | Fold difference
|
||
|---|---|---|---|---|
| 24h | 48h | 72h | ||
| TGFβ2 | Transforming growth factor, beta 2 | 3.74 | 2.19 | 2.61 |
| VEGFC | Vascular endothelial growth factor C | 2.59 | 3.01 | 2.37 |
| EGF | Epidermal growth factor (beta-urogastrone) | -2.23 | -2.01 | -2.50 |
| CDH5 | Cadherin 5, type 2, VE-cadherin (vascular epithelium) | -2.89 | -6.13 | -2.18 |
| CXCL10 | Chemokine (C-X-C motif) ligand 10 | -5.82 | -5.93 | -4.07 |
| CXCL6 | Chemokine (C-X-C motif) ligand 6 (granulocyte chemotactic protein 2) | -2.11 | -2.39 | -2.11 |
Results shown are representative of three experiments.
DISCUSSION
By comprehensive screening every aspect of endothelial cell in the process of angiogenesis including cell vigor, reproduction, migration, adhesion and tube formation, this experiment demonstrated that, at 48h, XFZYD-CS treated ECV304 had significant effects in all of the above stages. The 48h was the optimal time point for angiogenesis while the 24h and 72h only effected cell migration. There was not only a optimal time but also optimal concentration in angiogenesis induced by XFZYD that made the time and dose-response curve resemble an inverted U(6). This was quite different from a pure angiogenesis factor such as VEGF etc. Previous clinical and laboratory research showed that XFZYD could promote angiogenesis while also reducing atherogenesis (1,7). On the other hand, VEGF also promotes angiogenesis in atherosclerotic plaque by expanding the plaque and accelerate atherogenesis(7). The different angiogenesis effects we observed between XFZYD and VEGF is perplexing and intriguing. Considering the inverted U curve of XFZYD pro-angiogenesis effect, we speculate that XFZYD does not entirely stimulate angiogenesis but rather primarily maintains vessel growth in physiological or repair range to avoid angiogenesis in the atheromatous plaque. This vessel appropriate growth phenomenon could be regulated by different aspect of angiogenesis and our experiment may provide us with a clue to understand the regulation mode. More research is warranted to test our hypothesis and fully develop angiogenesis in therapy of ischemic diseases.
Among the changed genes at the 3 time points in the 84 angiogenesis regulators, the changes of Eph super family interested us most. EphA1 and EphB2 were both pro-angiogenesis, but their expression changed in differentially at 48h and the other two times as EphA1 down-regulation in 48h and EphB2 up-regulation in 24h or 72h. In recent years it has become known that Eph receptor and ephrin interactions also regulate critical steps of angiogenesis, blood vessel formation and remodeling during vascular development(8-10). EphrinA1 was the first to be isolated from cultured human umbilical vein endothelial cells in the whole family(10-12). It has a major impact on endothelial cell behavior and can modulate capillary sprout formation in the rat cornea assay and promote capillary-tube formation in vitro angiogenesis assays(10). Besides EphrinA1 function in angiogenesis, EphrinB2 is also a key factor in both vascular development(13,14) and postnatal angiogenesis(15,16), In our experiment, that EphA1 and EphB2 changed bidirectionally at different time points and caused different angiogenesis effects may reflect the key work of Eph family in angiogenesis induced by XFZYD. Again, amore definitive understanding will require further research.
From our perspective, our most intriguing finding was that 48h was the time point having the optimal angiogenesis effect but it impacted the least number of gene: 14 genes of 84 selected regulation genes. As we know, angiogenesis is a highly complex process which is regulated by numerous positive and negative factors. Common positive factors include vascular endothelial growth factor, transforming growth factor, epithelial growth factor and fibroblast growth factor(17) and common negative factors include angiostatin, endostatin, thrombospondin etc(18,19). Balance between angiogenesis promoting factors and inhibitors allows healthy angiogenesis to occur only in embryonic development, wound healing and the female reproductive cycle. Any imbalance will lead to proangiogenesis or antiangiogenesis. Proangiogenesis is caused by the inadequate production of angiogenesis inhibitors and/or excessive amounts of angiogenesis growth factors. Our experimental results may reflect not only the imbalance but also the complicated modulation of proangiogenesis. XFZYD did not elevate all positive factors, for example it upregulated VEGFC and TGFβ2 but downregulated EGF, the same effect as the inhibitors, and the general trend in the end is proangiogenesis.
In conclusion, our study showed that the angiogenesis mechanism of this classical Chinese herbal formula was multilevel, multi-targeted and had multiplepathways from endothelial progenitor cell to endothelial cell, from vascular development to adult angiogenesis. These findings provide a scientific rationale for the basis of adopting XFZYD in the treatment of ischemic disease. Further basic science and clinical research is necessary to gain a precise and full understanding of appropriate angiogenesis modulation mode.
Table 4.
The other Changed expression significantly genes at 48h a
| Symbol | Description | Gene Name | Fold difference
|
||
|---|---|---|---|---|---|
| 24h | 48h | 72h | |||
| ID3 | Inhibitor of DNA binding 3, dominant negative helix-loop-helix protein | HEIR-1 | 2.54 | 2.89 | 1.81 |
| IFNA1 | Interferon, alpha 1 | IFL/IFN | 1.52 | 2.93 | 2.10 |
| CCL11 | Chemokine (C-C motif) ligand 11 | SCYA11 | -2.03 | -2.09 | -1.58 |
| COL4A3 | Collagen, type IV, alpha 3 (Goodpasture antigen) | TUMSTATIN | 1.01 | -2.35 | -5.18 |
| ITGB3 | Integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61) | CD61/GP3A | 1.04 | -3.52 | -2.22 |
Results shown are representative of three experiments.
Footnotes
Supported by the National Natural Science Foundation of China (NO. 81072933); Fujian Natural Science Foundation (No. 2007J0101); Fujian Academy of Integrative medicine Foundation (No. CKJ2008001), NIH-NCCAM, US (No. K24 AT004095).
References
- 1.Gao D, Song J, Hu J, Lin JM, Zheng LP, Cai J, et al. Angiogenesis Promoting Effects of Chinese Herbal Medicine for Activating Blood Circulation to Remove Stasis on Chick Embryo Chorio-allantoic Membrane. Chinese Journal of Integrated Traditional and Western Medicine. 2005;25(10):912–915. [PubMed] [Google Scholar]
- 2.Gao D, Lin W, Zhen LP, Lin JM, Chen XZ, Song J, et al. Effect of Xuefu zhuyu Decoction on Endothelial Progenitor Cells Migrating from Rat Marrow. Chinese Journal of Integrated Medicine on Cardio-/Cerebrovascular Disease. 2007;5(9):829–831. [Google Scholar]
- 3.Gao Dong, Wu LY, Jiao YH, Chen WY, Zheng LP, Song J, et al. The Experimental analysis of Xuefu Zhuyu Decoction on EPC mobilization factor. Journal of Traditional Chinese Medicine. 2010;51(5):457–459. [Google Scholar]
- 4.Gao Dong, Wu LY, Jiao YH, Chen WY, Song J, Chen KJ. The Experimental Study of effect on EPCs differentiation function by Xuefu Zhuyu Decoction. Chinese Journal of Basic Medicine in Traditional Chinese Medicine. 2009;15(12):917–919. [Google Scholar]
- 5.Gao D, Wu LY, Jiao YH, Chen WY, Chen Y, Kaptchuk TJ, et al. The Effect of Xuefu Zhuyu Decoction on in vitro Endothelial Progenitor Cell Tube Formation. Chin J Integr Med. 2010;16(1):50–53. doi: 10.1007/s11655-010-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao D, Chen WY, Wu LY, Zheng LP, Lin W, Song J. The study on angiogenesis regulation gene profile of ECV304 by Xuefu Zhuyu Decoction. Chinese Journal of Integrated Traditional and Western Medicine. 2010;30(2):153–156. [PubMed] [Google Scholar]
- 7.Wang PL, Lei Y, Chen KJ. Angiogenesis——A Novel Therapeutic Strategy for Cardiovascular Diseases. Chinese Journal of Integrated Traditional and Western Medicine. 2006;26(2):173–176. [PubMed] [Google Scholar]
- 8.Brantley Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: From development to disease. Angiogenesis. 2004;7:17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Hughes SE. Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol. 2006;208:453–461. doi: 10.1002/path.1937. [DOI] [PubMed] [Google Scholar]
- 10.Pandey A, Shao H, Marks RM, Polverini PJ, Dixit VM. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha induced angiogenesis. Science. 1995;268:567–569. doi: 10.1126/science.7536959. [DOI] [PubMed] [Google Scholar]
- 11.Daniel TO, Stein E, Cerretti DP, St John PL, Robert B, Abrahamson DR. ELK and LERK-2 in developing kidney and microvascular endothelial assembly. Kidney Int Suppl. 1996;57:S73–S81. [PubMed] [Google Scholar]
- 12.Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, et al. Eph receptors discriminate specific ligand oligomers to determine alternative signalling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor EphB4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 14.Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002;129(6):1397–1410. doi: 10.1242/dev.129.6.1397. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi S, Asahara T, Masuda H, Isner JM, Losordo DW. Functional ephrin-B2 expression for promotive interaction between arterial and venous vessels in postnatal neovascularization. Circulation. 2005;111:2210–2218. doi: 10.1161/01.CIR.0000163566.07427.73. [DOI] [PubMed] [Google Scholar]
- 16.Zamora DO, Davies MH, Planck SR, Rosenbaum JT, Powers MR. Soluble forms of ephrinB2 and EphB4 reduce retinal neovascularization in model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2005;46:2175–2182. doi: 10.1167/iovs.04-0983. [DOI] [PubMed] [Google Scholar]
- 17.Sakamaki K. Regulation of endothelial cell death and its role in angiogenesis and vascular regression. Curr Neurovasc Res. 2004;1(4):305–315. doi: 10.2174/1567202043362072. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y. Update on endogenous inhibitors of angiogenesis. Endothelium. 2006;13(2):147–155. doi: 10.1080/1062332060069110. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Jiang L, Wang Y, Xiao Y, Huang Y, Yao Q, et al. Inhibition of angiogenesis by a novel small peptide consisting of the active fragments of platelet factor-4 and vasostatin. Cancer Lett. 2007;256(1):29–32. doi: 10.1016/j.canlet.2007.05.002. [DOI] [PubMed] [Google Scholar]



 ) on Endothelial Cell
) on Endothelial Cell