Abstract
Background
Weight gain has been described in Parkinson’s disease (PD) patients after subthalamic nucleus (STN) deep brain stimulation (DBS).
Objectives
We examined change in weight following DBS in both PD and dystonia patients to further investigate the role of disease and brain target (STN or GPi) specificity.
Methods
Data was retrospectively collected on 61 PD DBS patients (STN (n=31) or GPi (n=30)) and on 36 dystonia DBS patients (STN (n=9) and GPi (n=27)) before and after surgery. Annual change in body mass index (BMI) was evaluated with non-parametric tests between groups and multiple quantile regression.
Results
PD patients treated with STN DBS had a small increase in median BMI while those with GPi had a small decrease in BMI. Dystonia patients treated with STN DBS had a greater increase in BMI per year compared to those treated with GPi. Multivariable regression analyses for each disease showed little difference between targets in weight gain in those with PD, but STN target was strongly associated with weight gain in dystonia patients (STN vs. GPi, +7.99 kg, p=0.012).
Conclusions
Our results support previous reports of weight gain after DBS in PD. This is the first report to suggest a target-specific increase in weight following STN DBS in dystonia patients.
Keywords: deep brain stimulation, Parkinson’s disease, dystonia, Subthalamic Nucleus, Globus Pallidus, weight change
INTRODUCTION
Deep brain stimulation (DBS) is used to treat a variety of movement disorders, including Parkinson’s disease (PD), dystonia[1–3], and essential tremor[4–6]. PD patients typically have progressive weight loss[7,8] that is thought to be associated with elevated daily energy expenditure (EE). Interestingly, after bilateral[9–12] and even unilateral[13,14] STN DBS, PD patients have been reported to typically gain weight. PD patients treated with STN DBS have body mass indices (weight in kg / height in m2; BMI) that eventually approach those of age-matched healthy controls[15,16].
Recent studies describing weight gain after DBS in PD have focused on STN as the brain target[9–13,16]. Previous studies looking at pallidotomy[17–19]and pallidal DBS[20]have showed less pronounced weight gain in comparison to the STN DBS studies. A few studies have directly compared weight changes in PD patients treated with STN or GPi DBS[20–22]. All of these studied were non-randomized in target selection except one unilateral treatment study[22], which did not find a statistically significant difference in weight between the two target groups. The current study is the first to examine the differential effects of DBS target on weight gain in PD patients randomized to receive either bilateral STN or GPi DBS to further test the hypothesis of weight gain after DBS being due to a correction of an underlying alteration in energy expenditure specific to Parkinson’s disease. Unique to this study, we also evaluated weight gain in a group of dystonia patients receiving STN or GPi DBS, whose disease course is not typically associated with progressive weight loss.
METHODS
Study Population
Parkinson’s disease patients who met standard criteria for DBS surgery were randomized to receive either STN or GPi DBS[23]. Dystonia patients were not randomized to target and included patients enrolled in a recent trial evaluating the use of STN DBS in dystonia[24] and additional dystonia patients who received GPi DBS at our center. IRB approval was obtained both through the UCSF Committee on Human Research and through the San Francisco VA Medical Center (IRB# 10-03456). Patients were included as subjects if they underwent either subthalamic or pallidal DBS at UCSF or the San Francisco Veterans Affairs Medical Center for PD or primary dystonia, had a pre-surgical height and weight measurements, had a post-surgical height and weight measurement at least 12 months after surgery, and were over 18 years of age. Patients were excluded if their stimulators were turned off for a period of more than 3 months at any time during follow-up. Between the two time points used in this study, no patients were counseled or treated with specific dietary interventions outside normal care that may have been provided by their primary care physicians.
Data Collection
Data was collected retrospectively through chart review and stored on a secure server. In addition to the indication for DBS and the brain target, data for the following variables were collected at both the pre-surgical and post-surgical time points: pre-operative weight (kg), pre-operative height (cm), antiparkinsonian medications in PD patients (expressed in levodopa equivalent daily dose – LEDD), age at surgery, sex, percent change in Burke-Fahn-Marsden Dystonia Rating Scale (BFM-DRS) scores in the dystonia patients, and amount of time between pre-operative and post-operative time points (months).Medication usage in our dystonia populationwas not available post-operatively, thus we could not include this as a covariate in our model. Parkinson’s disease rating scales were also not available at the time points when weight was collected.
Statistical Methods
Baseline demographic and clinical characteristics were compared by target for each disease using the Wilcoxon Rank Sum test for continuous variables and Fisher’s exact test for categorical variables. Within group tests of annual change in BMI were conducted using the Wilcoxon signed-rank test, and annual changes in BMI were compared between targets for each disease using the Wilcoxon Rank Sum test. For this part of the analysis, the yearly rate of weight gain was assumed to be constant despite differences in time to follow-up between the dystonia target groups.
We used multivariable quantile regression analyses to estimate the association of disease (PD vs. dystonia) and target (GPi vs. STN) with annual change in BMI (kg/m2/yr), while controlling for potentially confounding factors, such as the amount of time between data collection points. Covariates in the model included age, gender, baseline BMI, time between pre-op and post-op weights,% change in BFM-DRS (dystonia only), and LEDD (in PD only). Tests of the residuals found violations of normality and heteroscedasticity; therefore, we used quantile regression analyses to estimate the differences in weight change between disease and target [25]. All analyses were conducted using Stata version 11 (StataCorp, College Station, TX).
RESULTS
Subjects
The study included 61 patients with PD and 35 with dystonia, whose baseline characteristics are presented in Table 1. Among participants with PD, 30 had GPi and 31 had STN DBS, with a majority of PD participants being male (48M/ 13F). The PD patients’ average age was 61 years, and a follow-up weight obtained at an average of 38 months. Although PD subjects with GPi were heavier than those with STN on average (84 vs. 74 kg, p=0.0078), their baseline BMIs were only slightly higher (27 vs. 25 kg/m2, p=0.21) compared to those treated with STN, due to differences in height (1.77 vs. 1.71, p=0.06).
Table 1.
Baseline characteristics of subjects.
| Parkinson’s Disease | Dystonia | |||||
|---|---|---|---|---|---|---|
| Parameter | GPi | STN | p-value | GPi | STN | p-value |
| # of subjects | 30 | 31 | 27 | 9 | ||
| Gender | ||||||
| Female | 4 (13%) | 9 (29%) | 0.21 | 13 (48%) | 5 (56%) | >0.99 |
| Male | 26 (87%) | 22 (71%) | 14 (52%) | 4 (44%) | ||
| Age (y) | 60.5 ± 7.8 | 61.5 ± 9.5 | 0.69 | 44.6 ± 15.4 | 48.3 ± 13.2 | 0.77 |
| Months at post-op weight | 37.5 ± 15.5 | 38.3 ± 14.7 | 0.83 | 27.1 ± 9.6 | 18.4 ± 9.2 | 0.023 |
| Baseline Weight (kg) | 83.8 ± 14.7 | 74.0 ± 13.2 | 0.0078 | 72.3 ± 20.2 | 83.5 ± 16.1 | 0.065 |
| Baseline BMI (kg/m2) | 26.8 ± 4.7 | 24.8 ± 3.7 | 0.21 | 24.1 ± 5.0 | 27.8 ± 4.8 | 0.065 |
| Change in LEDD | −452 ± 812 | −392 ± 580 | 0.36 | - | - | - |
| Improvement in BFM-DRS Movement | - | - | - | 13.9 ± 12.6 | 8.11 ± 11.6 | 0.18 |
| % Improvement in BFM-DRS Movement | - | - | - | 47.24 ± 34.7 | 45.51 ± 57.7 | 0.67 |
Data are summarized as n (%) or mean ± SD. P-value from Wilcoxon rank-sum test for continuous parameters, and from Fisher's exact test for categorical parameters. (STN = subthalamic nucleus, GPi = globus pallidus internus, BFM-DRS = Burke-Fahn-Marsden Dystonia Rating Scale, LEDD = levodopa equivalent daily dose).
Among dystonia patients, 27 had GPi and 9 had STN DBS implants, with similar proportions of males and females (18M /18F). Those treated with GPi DBS were slightly younger on average (45 vs. 48 years, p=0.68) and had somewhat lower weight (72 vs. 84 kg, p=0.065) and BMI (24 vs. 28 kg/m2, p=0.065) compared to those with STN DBS, though none of these differences were statistically significant. In dystonia patients, the amount of time between the baseline and collection of post-operative data points was longer in the GPi DBS group than in the STN DBS group (27 vs. 18 months, p=0.023).
The distribution of annual change in BMI is presented in Figure 1. The difference between targets in annual change in BMI was much larger for the dystonia patients than for the PD patients (target-by-disease interaction, p=0.012). Therefore, we stratified our analyses by disease and compared the two brain targets in both PD and dystonia separately (Tables 2 and 3).
Figure 1.
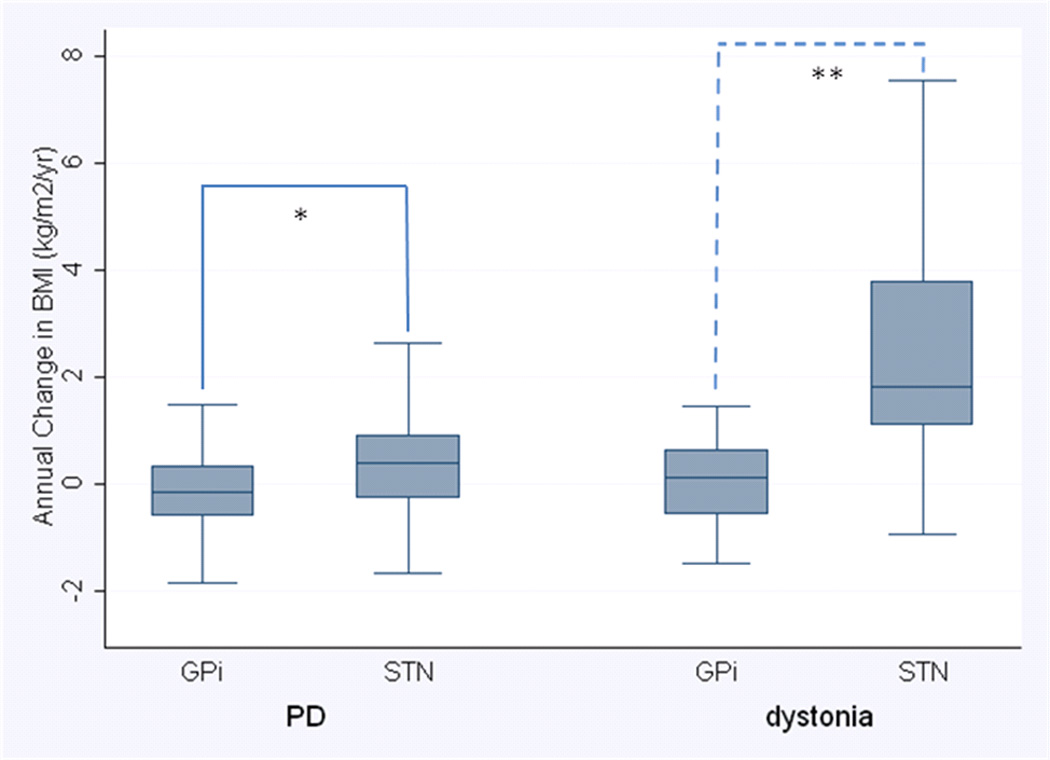
Annual change in BMI after DBS surgery, stratified by disease and surgical target.
Disease-specific differences in median annual BMI change between targets were evaluated with Wilcoxon rank-sum test (*p=0.037, **p=0.020). Median is indicated by black center line, and the inter-quartile range (first and third quartiles) are the edges of the box. Whiskers denote 1.5 × the IQR from the nearest quartile. PD = Parkinson’s disease, GPi = globus pallidus internus, STN = subthalamic nucleus
Table 2.
Median change in weight (kg) per year in DBS patients after surgery.
| Disease | Target | Median change in weight in kg/yr [95% CI] (p†) |
Difference between targets in median annual weight change (p††) |
Total weight gain >10 kg (%) |
|---|---|---|---|---|
| PD | GPi | −0.045 [−1.17, 0.78] (0.59) | 0.93 (0.071) | 1 (3%) |
| STN | 0.89 [−0.47, 1.70] (0.079) | 6 (19%) | ||
| Dystonia | GPi | 0.40 [−1.31, 1.09] (0.68) | 5.70 (0.027) | 2 (7%) |
| STN | 6.10 [−2.02, 11.44] (0.028) | 4 (44%) | ||
P-value from Wilcoxon sign-rank test
P-value from rank-sum test.
Table 3.
Median change in BMI per year in DBS patients after surgery.
| Disease | Target | Median change in BMI in kg/m2/yr [95% CI] (p†) |
Difference between targets in median annual BMI change (p††) |
| PD | GPi | −0.14 [−0.53, 0.21] (0.32) | 0.53 (0.037) |
| STN | 0.39 [−0.18, 0.64] (0.055) | ||
| Dystonia | GPi | 0.14 [−0.38, 0.42] (0.56) | 1.67 (0.020) |
| STN | 1.81 [−0.69, 4.39] (0.021) | ||
P-value from Wilcoxon sign-rank test
P-value from rank-sum test.
Among those with PD, there was a non-statistically significant difference in median annual change in weight (0.93 kg/yr, p=0.071) but a statistically significant difference in change in BMI (0.53 kg/m2/yr, p=0.037) by target. Those treated with GPi DBS had a small, non-statistically significant decrease in weight (−0.04 kg/yr, 0%/yr, p=0.59) and BMI (−0.14 kg/m2/year, p=0.32), whilethose treated with STN had a trend towards anincrease in weight (+0.89 kg/yr, 1%/yr, p=0.079) and BMI (+0.39 kg/m2/year, p=0.055). Dystonia patients treated with STN DBS had a greater increase in weight (6.10 kg/yr, 7%/yr, p=0.028) and BMI (1.81 kg/m2/year, p=0.021), while those treated with GPi DBS showed only a small increase in weight (0.40 kg/yr, 1%/year, p=0.68) and BMI (0.14 kg/m2/year, p=0.56) with a statistically significant difference in median weight gain (median 5.70 kg/yr, p=0.027) and BMI increase (1.67 kg/m2/yr, p=0.020) found between the two targets. Of the GPi DBS dystonia patients, 16 had an increase in BMI (range: 0.01 – 3.82 BMI/yr) while 11 had a decrease in BMI (range: −0.04 – −1.47 BMI/yr). In the dystonia patients with STN DBS, 7 had an increase in BMI (range: 1.09 – 7.53 BMI/yr) and only 2 saw a decrease in BMI (range: −0.085 – −0.93). In the dystonia patients who received GPi DBS, 71% of those with generalized dystonia gained weight, while 15% of those with focal or segmental (craniocervical) dystonia gained weight. In dystonia patients with STN DBS, 100% of the generalized dystonia patients gained weight while 71% of the segmental (craniocervical) patients gained weight.
Multivariable median regression analyses, stratified by disease, were performed to control for potentially confounding factors (Table 4). Among those with PD, baseline BMI was negatively associated with annual change in BMI (−0.78 kg per baseline kg/m2/yr, p=.009), demonstrating that those with lower BMI at baseline were more likely to gain weight after surgery. There were no significant associations with other factors in the model (age, time to follow-up, gender, or change in LEDD (p=0.99)) in PD patients. STN target was associated with slightly greater weight gain compared with GPi target, although the difference between targets did not reach statistical significance (+3.09 kg, p=0.26). In the patients with dystonia, those treated with STN DBS gained a median of 7.99 kg more than those treated with GPi DBS (95%CI: 1.87 to 14.11, p=0.012). Baseline BMI in dystonia showed a trend toward a negative association with weight gain (−0.39 kg per pre-op BMI point, p=0.15), but the effect size was small and the association did not reach statistical significance. Dystonia patients also showed a trend toward more weight gain in women (2.03 kg more than men, p=0.38). Percent improvement in BFM-DRS showed a trend toward having a small positive relationship with annual change in BMI (0.034 kg per 1% improvement, 95%, CI: −0.025 to 0.094, p=0.25). Even with a reasonable clinical improvement of 50% in BFM-DRS after DBS, this would only give an effect size of 1.7 kg, which is still overshadowed by the effect size of target.
Table 4.
Results of median regression analyses for each disease with total weight change (kg) as the primary outcome.
| Parameters | PD (n=61) | Dystonia (n=35) | ||
|---|---|---|---|---|
| Estimate (95% CI) | P-value | Estimate (95% CI) | P-value | |
| Male vs. Female | −1.35 (−7.40, 4.69) | 0.65 | −2.03 (−6.72, 2.65) | 0.38 |
| Age (per year) | −0.103 (−0.40, 0.19) | 0.49 | −0.011 (−0.20, 0.17) | 0.9 |
| Target: STN vs. Gpi | 3.09 (−2.25, 8.44) | 0.26 | 7.99 (1.87, 14.11) | 0.012* |
| Pre-DBS BMI | −0.78 (−1.35, −1.98) | 0.009* | −0.39 (−0.95, 0.16) | 0.15 |
| Months between weights | −0.002 (−0.19, 0.18) | 0.98 | 0.031 (−0.21, 0.28) | 0.8 |
| Δ in LEDD (per 100 mg increase) | −0.00003 (−0.0038, 0.0037) | 0.99 | - | - |
| % Δ in BFM-DRS Movement | - | - | 0.034 (−0.025, 0.094) | 0.25 |
p < 0.05
DISCUSSION
This is the first study to look at change in weight following treatment with two different DBS targets (STN and GPi) and in two different patient populations (PD and dystonia). While previous studies have described weight change following DBS for PD, no previous studies have evaluated weight change in dystonia patients with DBS as a separate group.
Weight Gain in PD
In this study, as has been shown previously, PD patients receiving bilateral DBS (regardless of target) showed a significant increase in BMI per year after surgery. The relative change in BMI was not as great as reported in other studies[9,10,13,16,21] with the exception of Strowd et al. which showed a similar modest weight gain[26]. When weight change was compared between STN and GPi patients in another prospective study, there was weight gain seen in both groups (mean of 5.7 kg in 6 months in the STN group and a mean of 1.7 kg in 6 months in the GPi group[21]), which was almost 10-fold of that seen in our study when these values are converted to annual weight change. However, this group reported weight gain >10 kg in 4 of 32 (12.5%) STN patients and 1 of 14 (7%) GPi patients, which was similar to the 5 of 31 (19%) and 1 of 30 (3.3%) in our STN and GPi patients, respectively. In another study which reported weight gain in both targets one year after surgery, there was a weight gain of >10 kg in 6 out of 16 (38%) STN patients and 3 of 11 (27%) GPi patients[20]. In comparison to the studies with more weight gain, our sample of PD patients appeared to have a greater proportion of males and have a slightly higher baseline weight.
Weight Gain in PD (STN vs. GPi)
Interestingly, even though PD patients receiving STN DBS had a higher median weight gain compared to those treated with GPi DBS, the effect of target did not meet statistical significance using a multivariable regression analysis when baseline BMI was considered as a predictor of weight gain. The weak effect of brain target on the degree of weight gain in PD patients was also found in a similar study when PD patients were randomized to receive unilateral DBS in either target (GPi or STN) and showed no difference in weight gain between the two brain targets[22]. This finding conflicts with the study by Sauleau et al., where PD patients were not randomized to DBS target but were preferentially treated with GPi DBS rather than STN DBS if “cognitive impairment” was present. They found a statistically significant weight gain (mean of 5.7 kg in 6 months, p<0.0001) in the bilateral STN group, while there was a non-statistically significant trend toward mild weight gain (mean of 1.7 kg in 6 months, p=0.384) in the bilateral GPi group[21]. As mentioned previously, the Volkmann et al. study reported a difference in the number of patients in each target group who gained >10 kg that was similar to that seen in our study as well as the study by Sauleau et al. Considering the number of patients with a large amount of weight gain in each group leads us to conclude that despite the lower median amount of weight gain seen in our study population, the target-specific weight gain after DBS for PD is similar to that which has been reported by other authors.
Multivariable quantile regression analysis in PD patients revealed trends toward a moderate effect of target and an even smaller effect of sex on the degree of weight gain, with the STN target and female sex promoting more weight gain. Baseline (pre-DBS) BMI was the only variable to show a statistically significant (but small) effect on weight gain in this model. When baseline BMI is removed from the regression analysis, the effect of target becomes stronger (4.18 kg greater in STN, 95% CI: 0.75to 7.62, p=0.018), suggesting that this difference might be explained by the effect of preoperative BMI. Since our STN and GPi DBS PD patients had a similar preoperative BMI’s, it may be that underweight patients receiving STN DBS are more likely to gain weight than underweight patients receiving GPi DBS. Alternatively, the difference between targets in median baseline BMI (by 2 kg/m2, p=0.21) may have been statistically significant in a larger sample, in which case the contribution of the “baseline BMI effect” to the “target effect” could be explained by a difference between the two target groups in baseline BMI.
Weight Change in Dystonia
Dystonia patients treated with STN DBS also experienced a statistically significant weight gain after DBS which was not found in the dystonia patients treated with GPi DBS. This target-dependent difference in annual increase in BMI was much larger in the dystonia patients than in the PD patients, and remained large and statistically significant even after accounting for variability in patient age, gender, percent improvement in BFM-DRS, length to follow-up, and baseline BMI between the two groups (Table 4). Only one other study included dystonia patients treated with GPi DBS in their report of weight gain after surgery, and while their DBS population as a whole gained weight, the number of dystonia patients was not large enough to analyze as a separate group[26]. To our knowledge, this study is the first to evaluate weight change following both GPi DBS and STN DBS in dystonia.
Mechanism for Weight Gain after DBS
The mechanism for weight gain seen after DBS is still debated[13,15,22]. Most studies have focused on the PD population with proposed explanations for this effect after STN DBS including: decreased dosage of Parkinson’s disease medications, an increase in dietary energy intake, a decrease in muscle activity as tremor or muscle rigidity, a decrease in dyskinesias[9], a central mechanism of alteration in metabolism mitigated by the direct effects of stimulation on nearby brain structures[15,16,27], and/or a decrease in energy expenditure related to improvement in motor fluctuations[15]. The latter of these hypotheses has been promoted by Montaurier et al., where PD patients underwent highly controlled calorimetry to measure energy expenditure (EE) before and after STN DBS surgery. Daily EE was significantly lower after STN DBS; however, a high pre-operative UPDRS III score was associated with less change in daily EE. The drop in EE following DBS was not found to be predictive of post-operative weight gain.There was no correlation between weight gain and reduction of Parkinson’s disease medications or with improvement in UPDRS IV score (measures severity of dyskinesias and motor fluctuations). While it has been proposed that an improvement in motor symptoms corrects EE[28] allows for weight gain[15], this has not necessarily been shown in the Montaurier study or other studies assessing a correlation between motoric improvement and weight change[9,10]. This led the authors to conclude that post-DBS weight gain may be due to improvement in spontaneous motor fluctuations, but that they could not rule out the possibility of an effect on central regulation of energy expenditure, possibly through stimulation of the nearby thalamus. This study only used STN DBS patients, so it is unclear whether or not this decrease in EE would also be seen in GPi DBS.
The observation from our study that weight gain seems relatively site-specific in dystonia supports a hypothesis of a centrally-mediated change in energy metabolism as a result of DBS, especially given the similar motor outcomes in both targets (47.2% vs. 45.5% improvement in BFM-DRS in GPi and STN, respectively). Because dystonia patients are not generally known to experience chronic wasting, the effect of subthalamic stimulation on weight occurs even without the presence chronic weight loss that is typical of PD[7,8,29]. This suggests that the effect of STN DBS may be a stimulation-induced side effect rather than a “regression toward the mean” as it is considered in PD. This idea is also supported by a recent study showing a significant change in hypothalamic hormonal function following STN DBS [27]. It is also possible the weight gain seen in dystonia patients may be due to site-specific changes in impulse control[30,31], and have an effect oneating habits, although multiple studies have not shown an association between increase in energy (dietary) intake and post-DBS weight gain in PD[15,21].
Limitations of the Study
Weaknesses of this study include the retrospective study design, the lack of randomization of brain target in the dystonia population, the relatively small sample size of dystonia patients treated with STN DBS, and the lack of prospectively collected weights at multiple pre-defined time points. While we do not have other time points to evaluate this possibility given the retrospective nature, other studies have reported sustained weight gain throughout the 1–2 years of prospective follow-up [9,13,26], highlighting that changes in weight after DBS are typically stable. Also, the mean total change in weight (irrespective of time to follow-up) in the GPi dystonia group was 0.56 kg while it was 8.62 kg in the STN dystonia group, indicated that unless the GPi dystonia patients lost weight during their increased length of follow-up, the yearly rate likely represents a consistent trend describing weight fluctuations in both sets of patients. To further address the difference in follow-up time between the GPi and STN dystonia groups, the time between pre-op and post-op weight recordings was evaluated as an independent variable in a multivariable quantile regression analysis and did not have a significant effect on the amount of weight gain in either brain target group (table 4). Our dystonia patients were also younger and more often female than our PD patients; however, results were similar in unadjusted and adjusted analyses, and there was no association of gender or age with weight gain in multivariable analysis. Another weakness is the lack of information of daily energy intake, which could be altered in either PD or dystonia. Several studies have shown no increase in daily energy intake after STN DBS for PD [9,10,12,15,19], but there is no data confirming that this is also the case in dystonia.
Conclusion
While the retrospective design and small sample size of this study should be taken into consideration when interpreting the study’s findings, the potential for target-specific effects on weight introduces another important variable (along with many other factors) to consider when making a decision about the most appropriate DBS brain target. PD and dystonia patients should be counseled that STN DBS may result in some weight gain after surgery, though the impact of this weight gain on overall health is unknown in these patients. Future prospective DBS studies using alternative brain targets for dystonia should include weight as a variable to better understand the short and long-term impact it may have on health.
Acknowledgement
The authors would like to acknowledge Larisa Collins for her help with data collection and Leslie Markun for her help with review of the manuscript.
Financial Disclosures: Kelly Mills is funded by the University of California San Francisco Clinical and Translational Science Institute Resident Research Funding Award, he is also Co-PI on a grant from the Clinical and Translational Science Institute Strategic Opportunities Support (CTSI-SOS) Novel Clinical/Translational Methods Catalyst Awards, NIH/NCRR UCSF-CTSI grant number UL1RR024131. Philip Starr is a consultant for Medtronic Inc. and Boston Scientific Inc. and holds a research grant from MRI Interventions Inc. (formerly called Surgivision). Jill Ostrem is a consultant for IpsenInc, MerzInc, Boston Scientific Inc. and is on an advisory board for Ipsen Inc., and Merz Inc. She has received honoraria from Medtronic Inc. and Allergan Inc and currently receives research grant funding from St. Jude Medical and MRI Interventions Inc. (formally called SurgiVision). She is also mentor for a fellowship grant from Medtronic Inc.
Funding Sources: None
Footnotes
Author Roles:
Kelly Mills was involved in conception, organization, and execution of the research project, as well as design and execution of the statistical analysis and writing of the first draft of the manuscript. Rebecca Scherzer was involved in the design and execution of the statistical analysis and review and critique of the manuscript. Philip Starr contributed review and critique of the manuscript. Jill Ostrem was involved in the conception and organization of the research project as well as review and critique of the statistical analysis and the manuscript.
Bibliography
- 1.Cif L, El Fertit H, Vayssiere N, Hemm S, Hardouin E, Gannau A, Tuffery S, Coubes P. Treatment of dystonic syndromes by chronic electrical stimulation of the internal globus pallidus. J Neurosurg Sci. 2003;47:52–55. [PubMed] [Google Scholar]
- 2.Kupsch A, Benecke R, Muller J, Trottenberg T, Schneider GH, Poewe W, Eisner W, Wolters A, Muller JU, Deuschl G, Pinsker MO, Skogseid IM, Roeste GK, Vollmer-Haase J, Brentrup A, Krause M, Tronnier V, Schnitzler A, Voges J, Nikkhah G, Vesper J, Naumann M, Volkmann J. Deep-Brain Stimulation for Dystonia Study G: Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- 3.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tezenas du Montcel S, Dormont D, Grand S, Blond S, Detante O, Pillon B, Ardouin C, Agid Y, Destee A, Pollak P. French Stimulation du Pallidum Interne dans la Dystonie Study G: Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 4.Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord. 2001;16:464–468. doi: 10.1002/mds.1089. [DOI] [PubMed] [Google Scholar]
- 5.Obwegeser AA, Uitti RJ, Turk MF, Strongosky AJ, Wharen RE. Thalamic stimulation for the treatment of midline tremors in essential tremor patients. Neurology. 2000;54:2342–2344. doi: 10.1212/wnl.54.12.2342. [DOI] [PubMed] [Google Scholar]
- 6.Pahwa R, Lyons KL, Wilkinson SB, Carpenter MA, Troster AI, Searl JP, Overman J, Pickering S, Koller WC. Bilateral thalamic stimulation for the treatment of essential tremor. Neurology. 1999;53:1447–1450. doi: 10.1212/wnl.53.7.1447. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Weight loss in parkinson's disease. Ann Neurol. 2003;53:676–679. doi: 10.1002/ana.10577. [DOI] [PubMed] [Google Scholar]
- 8.Beyer P. Weight change and body composition in patients with parkinson's disease. J Am Diet Assoc. 1995;95:979–983. doi: 10.1016/S0002-8223(95)00269-3. [DOI] [PubMed] [Google Scholar]
- 9.Barichella M, Marczewska AM, Mariani C, Landi A, Vairo A, Pezzoli G. Body weight gain rate in patients with parkinson's disease and deep brain stimulation. Mov Disord. 2003;18:1337–1340. doi: 10.1002/mds.10543. [DOI] [PubMed] [Google Scholar]
- 10.Macia F, Perlemoine C, Coman I, Guehl D, Burbaud P, Cuny E, Gin H, Rigalleau V, Tison F. Parkinson's disease patients with bilateral subthalamic deep brain stimulation gain weight. Mov Disord. 2004;19:206–212. doi: 10.1002/mds.10630. [DOI] [PubMed] [Google Scholar]
- 11.Novakova L, Ruzicka E, Jech R, Serranova T, Dusek P, Urgosik D. Increase in body weight is a non-motor side effect of deep brain stimulation of the subthalamic nucleus in parkinson's disease. Neuro Endocrinol Lett. 2007;28:21–25. [PubMed] [Google Scholar]
- 12.Tuite PJ, Maxwell RE, Ikramuddin S, Kotz CM, Billington CJ, Laseski MA, Thielen SD. Weight and body mass index in parkinson's disease patients after deep brain stimulation surgery. Parkinsonism Relat Disord. 2005;11:247–252. doi: 10.1016/j.parkreldis.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Walker HC, Lyerly M, Cutter G, Hagood J, Stover NP, Guthrie SL, Guthrie BL, Watts RL. Weight changes associated with unilateral stn dbs and advanced pd. Parkinsonism Relat Disord. 2009;15:709–711. doi: 10.1016/j.parkreldis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Lee EM, Kurundkar A, Cutter GR, Huang H, Guthrie BL, Watts RL, Walker HC. Comparison of weight changes following unilateral and staged bilateral stn dbs for advanced pd. Brain and Behavior. 2011;1:12–18. doi: 10.1002/brb3.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montaurier C, Morio B, Bannier S, Derost P, Arnaud P, Brandolini-Bunlon M, Giraudet C, Boirie Y, Durif F. Mechanisms of body weight gain in patients with parkinson's disease after subthalamic stimulation. Brain. 2007;130:1808–1818. doi: 10.1093/brain/awm113. [DOI] [PubMed] [Google Scholar]
- 16.Perlemoine C, Macia F, Tison F, Coman I, Guehl D, Burbaud P, Cuny E, Baillet L, Gin H, Rigalleau V. Effects of subthalamic nucleus deep brain stimulation and levodopa on energy production rate and substrate oxidation in parkinson's disease. Br J Nutr. 2005;93:191–198. doi: 10.1079/bjn20041297. [DOI] [PubMed] [Google Scholar]
- 17.Lang AE, Lozano A, Tasker R, Duff J, Saint-Cyr J, Trepanier L. Neuropsychological and behavioral changes and weight gain after medial pallidotomy. Ann Neurol. 1997;41:834–836. doi: 10.1002/ana.410410624. [DOI] [PubMed] [Google Scholar]
- 18.Gironell A, Pascual-Sedano B, Otermin P, Kulisevsky J, et al. Weight gain after functional surgery for parkinsons disease. Neurologia. 2002;17:310–316. [PubMed] [Google Scholar]
- 19.Ondo WG, Ben-Aire L, Jankovic J, Lai E, Contant C, Grossman R. Weight gain following unilateral pallidotomy in parkinson's disease. Acta Neurol Scand. 2000;101:79–84. doi: 10.1034/j.1600-0404.2000.101002079.x. [DOI] [PubMed] [Google Scholar]
- 20.Volkmann J, Allert N, Voges J, Weiss PH, Freund HJ, Sturm V. Safety and efficacy of pallidal or subthalamic nucleus stimulation in advanced pd. Neurology. 2001;56:548–551. doi: 10.1212/wnl.56.4.548. [DOI] [PubMed] [Google Scholar]
- 21.Sauleau P, Leray E, Rouaud T, Drapier S, Drapier D, Blanchard S, Drillet G, Peron J, Verin M, et al. Comparison of weight gain and energy intake after subthalamic versus pallidal stimulation in parkinson's disease. Mov Disord. 2009;24:2149–2155. doi: 10.1002/mds.22765. [DOI] [PubMed] [Google Scholar]
- 22.Locke MC, Wu SS, Foote KD, Sassi M, Jacobson CE, Rodriguez RL, Fernandez HH, Okun MS. Weight changes in stn versus gpi dbs: Results from the compare parkinson's disease dbs cohort. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e31820b52c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Follett KA, Weaver FM, Stern M, Hur K, Harris CL, Luo P, Marks WJ, Jr, Rothlind J, Sagher O, Moy C, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein JM, Stoner G, Starr PA, Simpson R, Baltuch G, De Salles A, Huang GD, Reda DJ, Group CSPS. Pallidal versus subthalamic deep-brain stimulation for parkinson's disease. N Engl J Med. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 24.Ostrem JL, Racine CA, Glass GA, Grace JK, Volz MM, Heath SL, Starr PA. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76:870–878. doi: 10.1212/WNL.0b013e31820f2e4f. [DOI] [PubMed] [Google Scholar]
- 25.Koenker RHK. Quantile regression. J Econ Perspect. 2001;15:143–156. [Google Scholar]
- 26.Strowd RE, Cartwright MS, Passmore LV, Ellis TL, Tatter SB, Siddiqui MS. Weight change following deep brain stimulation for movement disorders. J Neurol. 2010;257:1293–1297. doi: 10.1007/s00415-010-5509-4. [DOI] [PubMed] [Google Scholar]
- 27.Markaki E, Ellul J, Kefalopoulou Z, Trachani E, Theodoropoulou A, Kyriazopoulou V, Constantoyannis C. The role of ghrelin, neuropeptide y and leptin peptides in weight gain after deep brain stimulation for parkinson's disease. Stereotact Funct Neurosurg. 2012;90:104–112. doi: 10.1159/000335045. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen HU, Werdelin L, Lokkegaard A, Westerterp KR, Simonsen L. Free-living energy expenditure reduced after deep brain stimulation surgery for parkinson's disease. Clinical Physiology and Functional Imaging. 2012;32:214–220. doi: 10.1111/j.1475-097X.2011.01079.x. [DOI] [PubMed] [Google Scholar]
- 29.Palhagen S, Lorefalt B, Carlsson M, Ganowiak W, Toss G, Unosson M, Granerus AK. Does l-dopa treatment contribute to reduction in body weight in elderly patients with parkinson's disease? Acta Neurol Scand. 2005;111:12–20. doi: 10.1111/j.1600-0404.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 30.Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (mri) and functional mri. J Neurosci. 2007;27:3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: Impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]


