Abstract
Antigen presenting dendritic cells (DC) interpret environmental signals to orchestrate local and systemic immune responses. They govern the balance between tolerance and inflammation at epithelial surfaces, where the immune system must provide robust pathogen responses while maintaining tolerance to commensal flora and food antigens. The Wnt family of secreted proteins, which control epithelial as well as hematopoietic development and homeostasis, is emerging as an important regulator of inflammation. Here we show that canonical and non-canonical Wnts directly stimulate murine DC production of anti-inflammatory cytokines. Wnt3A triggers canonical β-catenin signaling and preferentially induces DC TGF-β and VEGF production, whereas Wnt5A induces IL-10 through alternative pathways. The Wnts also alter DC responses to microbe- or pathogen-associated molecular patterns (PAMPs), inhibiting pro-inflammatory cytokine induction in response to toll like receptor ligands and promoting DC generation of Foxp3+ regulatory T cells. Moreover, although both Wnts suppress pro-inflammatory responses to bacterial endotoxin and to TLR1/2, TLR7 and TLR9 ligands, Wnt5A but not Wnt3A inhibits IL-6 production in response to the viral mimic, polyinosinic:polycytidylic acid. Wnt family members thus directly and differentially regulate DC functions, an ability that may contribute to the balance between tolerance and inflammation at epithelial sites of exposure to microbes and environmental antigens.
Introduction
Dendritic cells process antigens and microenvironmental signals to control innate and adaptive immunity. They detect microbe and pathogen associated molecular patterns (PAMPs) through pattern recognition receptors (PRRs) including the toll like receptors (TLRs). PAMPs trigger increased surface expression of MHC-antigen complexes and co-stimulatory molecules essential for initiation of adaptive immune responses. TLR activation also induces cytokines and other immune stimulatory factors which regulate the recruitment and activation of innate immune cells, and shape the adaptive immune response(1). However, DCs are also critical in maintaining tissue homeostasis by presenting self and food antigens, and commensal microbe-derived products, in a tolerance-promoting context that depends in part on DC secretion of immune suppressive cytokines and on generation of regulatory T cells(2). Dysregulated dendritic cell control of the balance between inflammatory and tolerogenic/homeostatic immune responses contributes to immunopathology in autoimmune disease, and to mechanisms of immune diversion or escape in cancer. In this context, significant attention has focused recently on identification of microenvironmental factors that regulate the balance between pro-inflammatory and tolerogenic functions of DC.
The Wnt family of proteins regulates cell proliferation and differentiation in normal tissues and in cancer. Wnts are expressed in the intestine, lung and skin(3, 4) where they are crucial for regulation of epithelial cell turnover; and in the bone marrow and thymus where they regulate the development of cells of hematopoietic origin including DCs(5). Wnts regulate the development of conventional dendritic cells from monocytes or bone marrow precursors in vitro(6) and have been reported to have both pro-inflammatory and anti-inflammatory effects on monocytes and macrophages(7–10). Studies based on modulation of downstream Wnt signaling molecules suggest a potential role for Wnts in DC function as well: β-catenin, a multifunctional adaptor protein and transcriptional coactivator, is stabilized by canonical Wnt signaling and directs transcriptional activation of many Wnt responsive genes. DC specific deletion of β-catenin increased pro-inflammatory cytokine production and intestinal inflammation in mice(11). However, β-catenin activity is also regulated by cell adhesion and other Wnt-indpendent pathways: indeed, disruption of cell-cell contacts or mechanical agitation of DC induces a tolerogenic DC phenotype dependent on signaling by β-catenin(12, 13). Moreover, Wnts can also activate β-catenin-independent “non-canonical” signaling mechanisms with both pro- and anti-inflammatory effects depending on the cell context and experimental model(7, 9, 10, 14). Interestingly, a non-canonical Wnt controls a primordial mechanism for tolerance and resolution of inflammation in drosophila(15). Here we undertook to assess directly the effects of Wnts on the biology and functional responses of differentiated DCs.
We report that Wnt3A and Wnt5A directly induce immunoregulatory cytokine production by differentiated DC. Moreover, they redirect the DC response to PAMPs, suppressing DC activation and inflammatory cytokines while promoting regulatory T cell generation. Consistent with their signaling in other cell systems, Wnt3A activates canonical β-catenin signaling in DC, while Wnt5A triggers non-canonical signaling cascades. Although both Wnts support a tolerogenic DC phenotype, they induce distinct patterns of tolerogenic cytokine production and differential DC responses to TLRs. The results suggest an important role for the Wnt family in governing dendritic cell function and immune responses.
Materials and Methods
Mice
C57BL/6 and B6.SJL/J mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and bred in the VMU facility of the Veterans Affairs Palo Alto Health Care Systems (VAPAHCS). All animal studies were approved by the institutional animal use and care committee and experimentation was conducted in accordance with AAALAC guidelines.
Cell isolation
DCs were isolated from mesenteric and peripheral lymph nodes of 4–10 week old female or male C57BL/6 or B6.SJL/J mice by Collagenase IV digestion (250U/ml), (Worthington Biochemical Corp., Lakewood, NJ) for 30 min at 37°C, followed by positive selection of CD11c+ cells using CD11c microbeads (Miltenyi, Bergisch Gladbach, Germany), according to the manufacturer’s instructions. In some experiments we took advantage of the ability of Fms-like tyrosine kinase 3 ligand (Flt3L) to expand DC subsets in vivo with minimal alterations in their phenotypic or functional capabilities(16): C57BL/6 or B6.SJL mice were injected s.c. with 5 × 106 Flt3L-secreting B16 melanoma cells (hereafter termed Flt3L-treated mice)(17). After 14 days, mesenteric and peripheral lymph nodes were harvested and DCs were isolated by CD11c microbeads (Miltenyi). For some studies CD11c+ DC were further separated into total conventional DC (cDC) as Lin (CD3, CD19, NK1.1)-CD11c+B220- or CD103+ cDC, Lin-CD11c+B220-CD103+, or plasmacytoid DC (pDC) as Lin-CD11c+B220+CCR9+ by fluorescence activated cell sorting (FACSAria). Spleen CD4+ T cells were isolated by positive selection using CD4 micro beads (Miltenyi).
Cell culture and CFSE labeling
DCs were cultured (1–5 × 106 cells/ml) in complete RPMI 1640 supplemented with 10% FCS either in medium alone or in the presence of R837 (0.5μg/ml), LPS (5μg/ml), Pam3CSK4 (5μg/ml), CpGA-ODN1585 (5μg/ml) or poly(I:C) (5ug/ml) all from InvivoGen, San Diego, CA, recombinant murine Wnt3A (PeproTech or R&D Systems, Minneapolis, MN) or recombinant human/mouse Wnt5A (R&D Systems). Cytokine secretion was determined at 20 hours of culture by ELISA or Luminex analysis of culture supernatants. Expression of MHCII, CD80, CD86 and CCR7 was determined by FACS at 6 hours of culture. For co-culture experiments, DCs were pretreated, (4×106/ml) for 5 hours either with medium alone, CpGA alone or CpGA in the presence of Wnt3A or Wnt5A. Cells were thereafter collected and extensively washed in complete RPMI prior to co culture with T cells. CD4+ T cells were labeled with CFSE (1μM), (R&D Systems) for 5 min at 37°C followed by extensive washing, prior to culture. DCs (5×104/ml) and CD4+ T cells (1.25×105/ml) were cultured in the presence of anti-CD3 (0.5μg/ml) alone or with anti-CD3 in combination with recombinant human TGFβ (2ng/ml), (R&D Systems), 2ml/culture, in 24 well plates. On the last day of culture cells were stimulated for 4 hours with PMA (10ng/ml), (Sigma-Aldrich, St Louis, MO) and Ionomycin (1μg/ml), (Sigma) in the presence of Brefeldin A (eBioscience) to enhance cytokine detection. Cells were thereafter collected and stained with 7-AminoactinomycinD (7AAD), for the exclusion of dead cells and for expression of CD4 followed by intracellular staining of cytokines and Foxp3. IFNγ/TNFα expression and frequency of Foxp3+ cells was determined by FACS, on day 3 and day 4 of culture, respectively. FACS analysis was performed on an LSRII flow cytometer.
Antibodies and ELISA reagents
The following anti-mouse antibodies and reagents were used for flow cytometry and cell stimulation. Purified anti-CD16/CD32 (24G2), FITC anti-CD3 (145.2C11), FITC anti-CD19 (1D3), FITC anti-NK1.1 (PK136), Pacific Blue anti-CD11c (N418), PerCp-Cy5.5 anti-B220 (RA3–6B2), APC anti-IFNγ, PE anti-TNFα, Pacific Blue anti-CD4 (RM4–5), AlexaFluor700 anti-MHCII (M5/114.15.2), PE anti-CD80 (16–10A1), PE anti-CD86 (GL1), purified anti-CD3 (145.2C11), PE-Cy7 Streptavidin and 7AAD, all from BD Biosciences; FITC anti-Siglec H (eBio440c), biotin anti-CD103 (2E7), PE anti-CD103 (2E7), APC anti-CCR7 (4B12), PE anti-Foxp3 (FJK-16), all from eBiosciences; APC anti-CCR9 (242503) (R&D Systems, Minneapolis, MN). The following reagents were used for ELISA: mouse IL-6 ELISA set, mouse IL-12p40 ELISA set, mouse TNFα ELISA set, mouse IL-10 ELISA set (BD Biosciences), mouse VEGF ELISA set (R&D Systems) and IFNα ELISA was performed using anti Mu-IFNα (Rmma-1) as capture antibody and Rabbit-Pab against Mu-IFNα for detection (PBL InterferonSource, Piscataway, NJ). Antibodies used for western blot analysis were primary antibodies: rabbit monoclonal anti-mouse β-catenin (6B3), (Cell Signaling Technology, Danvers, MA), mouse monoclonal anti-tubulin alpha Ab-2 (DM1A), (NeoMarkers, Fremont, CA), goat polyclonal anti-lamin B (Santa Cruz Biotech, CA) and secondary antibodies: Alexa Fluor 680 donkey anti-rabbit IgG (Molecular Probes, OR) and IRDye 800CW donkey anti-mouse IgG (LI-COR Biosciences, NE).
Luminex cytokine analysis
Mouse 26-plex kits were purchased from Affymetrix and used according to the manufacturer’s recommendations with modifications as described below. Briefly, samples were mixed with antibody-linked polystyrene beads on 96-well filter-bottom plates and incubated at room temperature for 2 h followed by overnight incubation at 4°C. Plates were vacuum filtered and washed twice with wash buffer, then incubated with biotinylated detection antibody for 2 h at room temperature. Samples were then filtered and washed twice as above and resuspended in streptavidin-PE. After incubation for 40 minutes at room temperature, two additional vacuum washes were performed, and the samples resuspended in Reading Buffer. Plates were read using a Luminex 200 instrument with a lower bound of 100 beads per sample per cytokine.
Western blot analysis
Nuclear and cytoplasmic protein fractions were prepared using the Nuclear Extract Kit (Active Motif) according to manufacturer’s instructions, from Flt3L treated mouse lymph node CD11c+ DC (5×106 cell/mL) cultured in the presence or absence of Wnt3A or Wnt5A (0.3μg/mL and 3μg/ml) for 30 min and 2 hours. Protein concentration was determined with the BCA protein assay kit (Pierce) and 10μg protein was resolved on 4–12% NuPAGE Bis-Tris Mini Gels (Life Technologies) and transferred to Immobilon-FL transfer membrane (Millipore). Membranes were blocked in Odyssey blocking buffer (LI-COR Biosciences), probed with antibodies for β-catenin (1/1000), tubulin (1/2000), and lamin B (1/2000) and proteins were detected using the Odyssey CLx (LI-COR Biosciences) imaging system. Signal intensity was analyzed with Image Studio software.
Kinex protein microarray
Preparation of protein samples was completed according to standard Kinexus recommendations (http://www.kinexus.ca/services). Samples were prepared from lymph node DC of Flt3L-treated mice cultured at 5×106 cell/ml for 30 minutes with 3μg/ml Wnt5A or with control media. Analysis of the data was carried out by C. Laudanna and Simone Zorzan, Università di Verona, Italy in a modification of the standard Kinexus analytics and of applications of Z transformation to microarray studies(18). Z scores (a measure of the amount of change of the signal in relation with its standard deviation) were calculated from the Kinexus logarithmic “log2” read values on the basis of the standard deviation of replicates relative to antibodies with good read (flag = 0 in both the physical replicates). Antibodies with poor reads (flag=1) were excluded. Z ratios are calculated as differences between Z scores on control and Wnt5A treated samples. A percentage error value was obtained as percentage ratio between average and standard deviation on the two good physical replicas of each probe. Proteins that met the following criteria were highlighted as candidate proteins changed by Wnt5A signaling: z-ratios ≥1.7 or ≤−1.7, maximum percentage error between the two physical replicas of the probe as calculated above <20%, and ≥2 fold change in mean value induced by Wnt5A treatment.
Statistics
Data are presented as mean values ± standard error of the mean (SEM) unless otherwise indicated. Statistical significance between sets of data was assessed with the two-tailed unpaired Student’s t test.
Results
Wnts stimulate anti-inflammatory cytokine production in DC
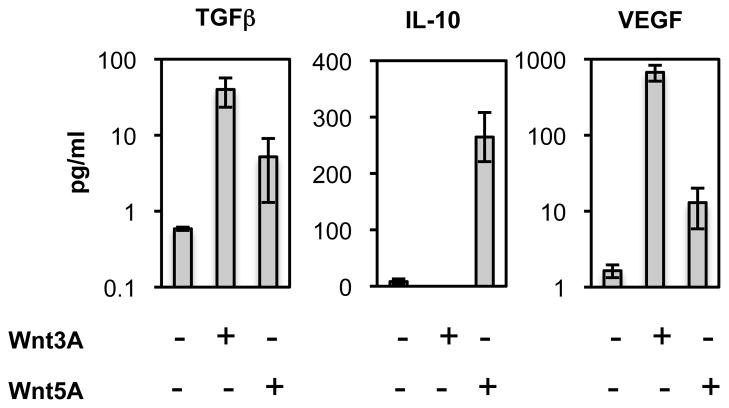
DCs express genes encoding several Wnt receptors and downstream Wnt effector molecules(11, 19) suggesting that DCs have the machinery to respond to Wnts. To determine whether Wnts directly affect differentiated DCs, we assessed the ability of Wnt3A and Wnt5A to induce cytokine production in freshly isolated CD11c+ DCs from mouse lymph nodes. Overnight stimulation of DC with recombinant Wnt3A or Wnt5A stimulated secretion of the immune regulatory cytokine TGFβ. The levels induced by Wnt3A were higher than those induced by Wnt5A (Fig. 1A). Interestingly, Wnt5A (but not Wnt3A) also induced IL-10 while Wnt3A (but not Wnt5A) strongly induced VEGF-A, an angiogenic growth factor that has recently been implicated in immune regulation as well(20–22) (Fig 1A). Not all cytokines were modulated: constitutive low or undetectable expression of MCP-1 (CCL2), MCP-3 (CCL7), IFN-γ-induced protein (IP-10) (CXCL10), G-CSF, GM-CSF, IFNγ, IL-1α, IL-1β, IL-12p70, IL-5 and IL-13 was not affected by the Wnts (data not shown). Thus Wnts directly and selectively promote DC production of immune regulatory cytokines.
FIGURE 1. Wnt3A and Wnt5A differentially stimulate tolerogenic cytokine production in resting DC.
Isolated CD11c+ DC from pooled peripheral and mesenteric lymph nodes were stimulated in vitro with Wnt3A (3μg/ml) or Wnt5A (3μg/ml) for 20 hours. Cytokine secretion was determined by multiplex luminex analysis (VEGF and TGFβ) or ELISA (IL-10) of culture supernatants. Results are presented as mean ± standard error of the mean (SEM), n=3. DC were from normal mice (n=2, TGFβ and VEGF) or Flt3L-treated mice (n=1, TGFβ and VEGF; n=3, IL-10). Wnt5A but not Wnt3A also induced an increase in IL-10 from normal mouse lymph node DC in an independent experiment (not shown). P <0.01 for VEGF, P <0.05 for TGFβ induction (Wnt3A vs control) and P <0.004 for IL-10 (Wnt5A vs control).
Wnts reprogram DC responses to PAMPs
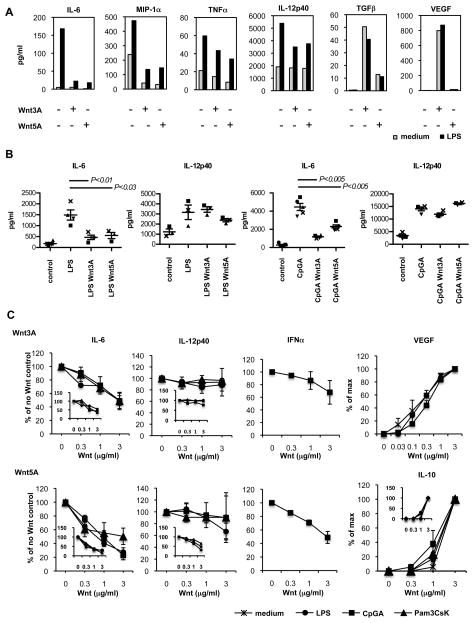
TLR’s activate DCs to produce pro-inflammatory cytokines and chemokines. We assessed the effects of the Wnts on TLR responses of DCs from normal mouse lymph nodes, and from lymph nodes of mice in which DC populations were expanded with Flt3L as previously described(17). DCs were stimulated overnight with TLR ligands in the presence or absence of recombinant Wnt3A or Wnt5A. Both Wnts effectively suppressed LPS induced IL-6 and MIP-1α (CCL3) production by normal lymph node DC, and reduced TNFα (Fig. 2A). IL-12p40 production was variably reduced as well in DC from normal mice (though not in in vivo Flt3L-expanded DC, which otherwise behaved similarly to DC from untreated mice; see below). In contrast Wnt3A induced secretion of VEGF and both Wnts induced secretion of TGFβ in the presence of LPS (Fig. 2A). There was little or no effect on LPS induced secretion of RANTES (CCL5). IFNγ, IL-1β, IL-1α, CCL2, CCL7, CXCL1, GM-CSF, IL-12p70 and IL-13 were not significantly induced by TLR’s or Wnts (data not shown). Moreover, Wnt inhibition of TLR stimulated IL-6 was not limited to LPS responses: both Wnts also suppressed induction of IL-6 by total DC in response to the TLR9 ligand CpGA (Fig. 2B).
FIGURE 2. Wnts suppress TLR induced proinflammatory cytokine production.
a) Effect of Wnts on LPS responses of DC from normal mouse lymph nodes. CD11c+ DC were stimulated in vitro with LPS (5μg/ml) in combination with Wnt3A (3μg/ml) or Wnt5A (3μg/ml) for 20 hours. Cytokine secretion was determined by multiplex luminex analysis of culture supernatants. Data represent cytokine concentrations from one of two experiments performed with similar results. b) Wnts inhibit CpG as well as LPS stimulation of IL-6 in DC. CD11c+ DC isolated from pooled lymph nodes from normal mice were stimulated in vitro with LPS (5μg/ml) or CpGA (5μg/ml) in combination with Wnt3a or Wnt5a (3μg/ml) for 20 hours. Cytokine secretion was determined by ELISA. Data show cytokine concentrations from 3–5 experiments. Mean values ± standard error of the mean (SEM). Symbols indicate individual experiments. c) Dose dependent Wnt effects on TLR responses. Total FACS sorted cDC (CD11c high, B220−) or CD103+ cDC (inserted graphs) isolated from mesenteric lymph nodes (IL-6, IL-12p40 and IL-10 graphs) or total CD11c+ DCs isolated from pooled lymph nodes (VEGF-A and IFN-α graphs) of FLT3L-treated mice were stimulated in vitro with LPS (5μg/ml), CpGA (5μg/ml) or Pam3CSK4 (5μg/ml) in the presence of varying doses of Wnt3A or Wnt5A for 20 hours. Cytokine secretion was determined by ELISA of culture supernatants. For IL-6, IL-12p40 and IFN-α data are presented as % of cytokine concentration in the presence of TLR alone, without Wnt. [In the absence of Wnts values were as follows in pg/ml: IL-6 (for the Wnt3A graph) LPS 820, CpGA 3400, Pam3CSK4 730; IL-6 (for the Wnt5a graph) LPS 670, CpGA 4150, Pam3CSK4 660; IL-12p40 (for the Wnt3A graph) LPS 18310, CpGA 25220, Pam3CSK4 18900; IL-12p40 (for the Wnt5A graph) LPS 16550, CpGA 32140, Pam3CSK4 13530; and IFN-α in units/μl (for both Wnt3A and Wnt5A graphs) 1650.] For IL-10 and VEGF-A induction, data are % of maximum cytokine concentration induced by each TLR ligand or control. [Maximum values in pg/ml were as follows: IL-10 medium 80, LPS 120, CpGA 110, Pam3CSK4 240; VEGF-A medium 22580, LPS 20940, CpGA 17100.] Mean ± SEM are presented, n=3, with the exception of IL-12p40 and IFN-α in response to CpGA, and VEGF-A in response to all stimulations, mean ± StDev n=2. CD103+ DC responses (graph inserts) are from one of two experiments with similar results.
In studies with FACS purified conventional DC (cDC: CD11c high B220−) from Flt3L-treated mice, both Wnts suppressed IL-6 secretion in response to LPS, CpGA, R837 (Imiquimod) and the TLR1/2 ligand Pam3CSK4 as well (Fig. 2C). TLR stimulation of IL-12p40 production was unaffected. Under the same culture conditions Wnt5A induced IL-10 secretion similarly in the presence or absence of TLR stimulation (Fig. 2C). Wnt inhibition of IL-6 and induction of IL-10 were dose-dependent. Both Wnts also inhibited IFN-α secretion induced by CpGA stimulation (Fig. 2C).
We also assessed Wnt effects on the responses of CD103+ cDC (CD11c high, B220−, CD103+ cells), a subset implicated in mucosal immunity and immune regulation(23). The Wnts suppressed TLR induced IL-6 and increased IL-10 production by CD103+ cDC to a similar extent as observed for total cDC (Fig. 2C, inserted graphs). Both Wnts also inhibited secretion of IL-6 and TNFα by sorted CD11c+B220+ CCR9+ plasmacytoid DC stimulated with the pDC-selective TLR7 ligand R837 (data not shown; IL-10 was not analyzed in pDC). Although we cannot exclude other subset- or Wnt-protein specific effects, the results suggest that inhibition of TLR-induced pro-inflammatory cytokines and induction of suppressive cytokines may be a common feature of Wnt-DC interactions.
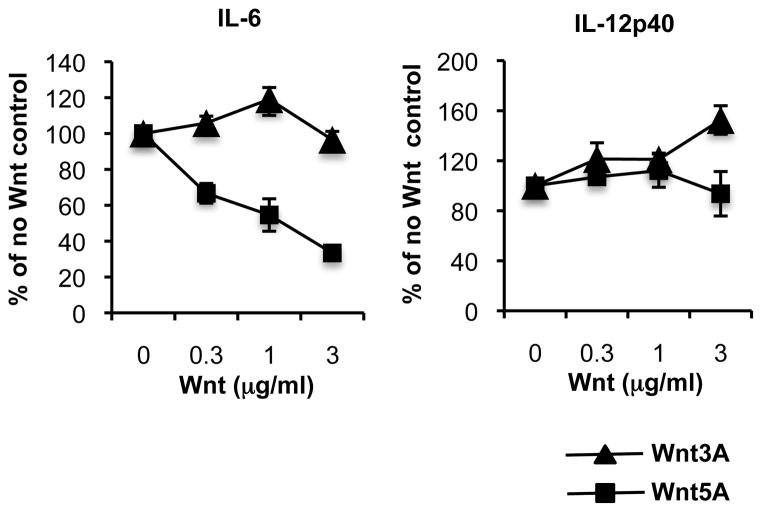
Interestingly, the two Wnts displayed distinct effects on DC responses to the viral RNA mimic poly(I:C): Wnt5A efficiently suppresses poly(I:C)-stimulated IL-6 secretion, while Wnt3A does not (Fig. 3), indicating a unique ability of Wnt3A to differentiate between PAMPs.
FIGURE 3. Differential effects of Wnt3A and Wnt5A on pIC-induced IL-6 production.
cDC (CD11chigh, B220−) FACS sorted from mesenteric lymph nodes of FLT3-treated mice were stimulated in vitro with pIC (5μg/ml) in the presence of Wnt3A or Wnt5A. Supernatants were collected at 20 hours of culture and analyzed by ELISA to determine concentration of secreted cytokines and is presented as % of cytokine concentration in the presence of TLR alone, without Wnt. [Cytokine concentration in the absence of Wnt: IL-6 (for the Wnt3A graph) 1100pg/ml; IL-6 (for the Wnt5A graph) 1000pg/ml; IL-12p40 (for the Wnt3A graph) 19160pg/ml; IL-12p40 (for the Wnt5A graph) 12800pg/ml.] Results are presented as mean ± SEM, n=3 (IL-6) and mean ± StDev, n=2 (IL-12p40).
Wnts do not prevent DC maturation
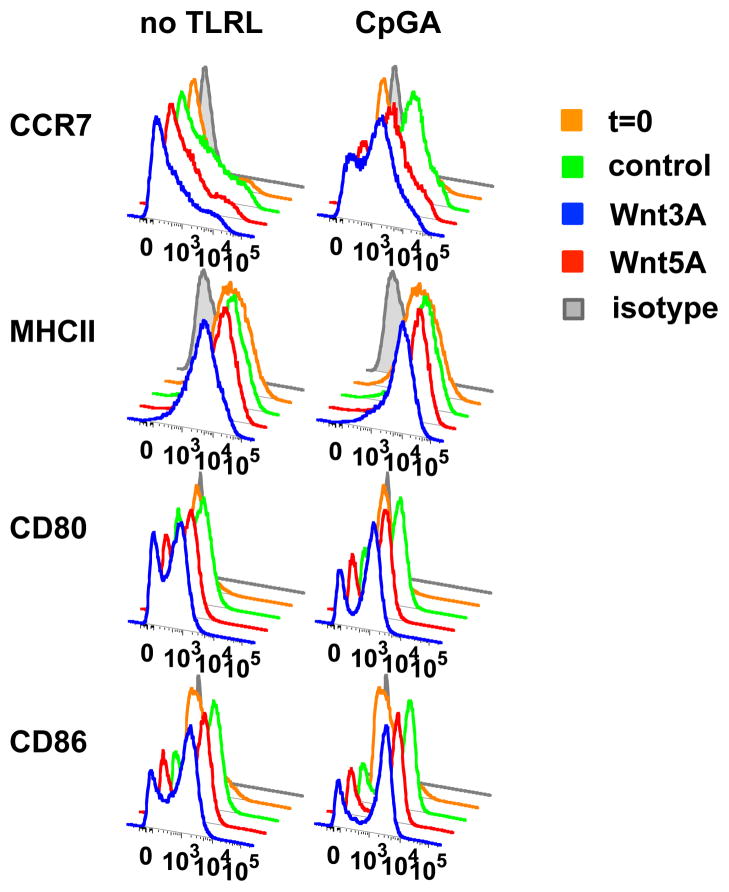
Whether activated by PAMPs or by tolerogenic stimuli such as ‘cluster disruption’ or mechanical manipulation, mature DC are characterized by increased surface display of MHCII-peptide complexes and costimulatory molecules (i.e. CD86, CD80), and expression of the chemokine receptor CCR7 that allows migrating DCs to exit tissue sites of antigen uptake and migrate to draining lymph nodes. Together these features are required for efficient presentation of antigens to T cells in the draining node, whether for tolerance or immune stimulation. In our experiments we isolated DC from lymph nodes of Flt3L treated mice using positive selection with CD11c-conjugated beads, a process that initiates in vitro maturation without inducing inflammatory cytokines(12, 13). Isolated DC were cultured either in medium alone or in the presence of TLR9 ligand CpGA, with or without Wnt3A or Wnt5A as above. Cultured DC rapidly upregulated MHCII, CD80, CD86 and CCR7 in medium alone and further in response to CpGA. Importantly, Wnts had no effect on MHCII and costimulatory molecule induction, and only minimally reduced CCR7 upregulation (Fig. 4). Thus Wnts upregulate immune regulatory cytokines without inhibiting DC maturation, allowing development of a mature tolerogenic DC phenotype.
FIGURE 4. Wnts do not prevent DC maturation.
CD11c+ DC isolated from pooled lymph nodes of FLT3L-treated mice were cultured in medium alone or with CpGA (5μg/ml) in the presence of Wnt3A (2μg/ml) or Wnt5A (2μg/ml). Cells were analyzed by FACS at 6 hours of culture. Expression of CCR7, MHCII, CD80 and CD86 was determined of conventional DC, gated as CD11c+ Siglec H- cells. Data are from one of two experiments performed with similar results.
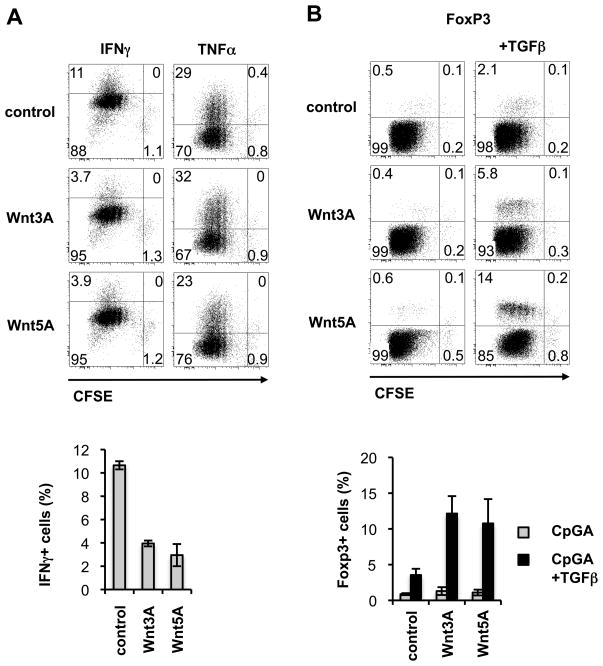
Wnts reprogram DC responses to TLR ligands to promote Foxp3+ Treg generation
To determine whether Wnt preconditioning alters the effects of DC on T cells, we stimulated DCs with TLR ligand either alone or in combination with Wnts as before. After extensive washing the conditioned DCs were used as stimulators in secondary cultures with CD4+ T cells and anti-CD3. Wnt+CpG pre-conditioned DC generated a 50–75% decreased frequency of IFNγ producing T cells, while the generation of TNFα producing cells was only marginally reduced by Wnt5A and overall T cell expansion was unaffected compared to control DC pre-conditioned with TLR ligand alone (Fig. 5A). Pretreatment of DC with Wnt alone, in the absence of TLR ligand, did not alter their ability to promote basal levels of T cell proliferation and showed only slight and variable, (non-significant) inhibitory effects on IFNγ production, as compared to medium control treated DCs (Fig. S1 and data not shown). To examine the effects of Wnts on TLR stimulated DC induction of Tregs we included TGFβ in the secondary cocultures. Wnt+CpG pre-treated DC generated a much higher frequency of Foxp3+ Tregs as compared to control DC pre-conditioned with TLR alone (Fig. 5B). Consistent with the known Treg promoting effect of TGFβ, there was minimal generation of Tregs by CpGA or medium control stimulated DC in the absence of exogenous TGFβ; and this was not significantly enhanced by Wnts (Fig. 5B and Fig. S1). Thus, while the levels of TGFβ induced by Wnts in DC were insufficient to promote expansion of Tregs under the in vitro conditions employed here, there was a clear synergy of Wnt+TLR ligand preconditioned DC with TGFβ resulting in increased Treg generation and reduced expansion of Foxp3− CD4+ T cells (Fig. 5B and Fig. S1). Addition of neutralizing anti-Wnt5A antibody (10μg/ml) to the secondary co-cultures had no effect on Foxp3+ T cell generation by Wnt5A+CpG pre-conditioned DC, ruling out a direct effect of residual Wnt on T cells (not shown).
FIGURE 5. Altered T cell responses to dendritic cells stimulated with CpG in the presence of Wnts.
CD11c+ DC isolated from pooled lymph nodes of FLT3L-treated mice were stimulated in vitro for 5 hours with CpGA (5μg/ml) in the presence of Wnt3A (3μg/ml) or Wnt5A (3μg/ml). Cells were extensively washed and co-cultured with spleen derived CFSE labeled CD4+ T cells in the presence of anti-CD3 (0.5μg/ml) alone (in A, and left panels in B) or anti-CD3 and TGFβ (2ng/ml) (right panels in B). On day 3 of culture T cells were analyzed by FACS for proliferation and expression of cytokines (A) and on day 4 for frequency of Foxp3+ cells (B). Intensity of CFSE and expression of IFNγ, TNFα and Foxp3 was determined among gated CD4+ T cells. Dot plots are from one of 3 experiments performed with similar results. Bar graphs show the mean ± standard error of the mean (SEM), n=3 (Foxp3) and mean and range, n=2 (IFN-γ).
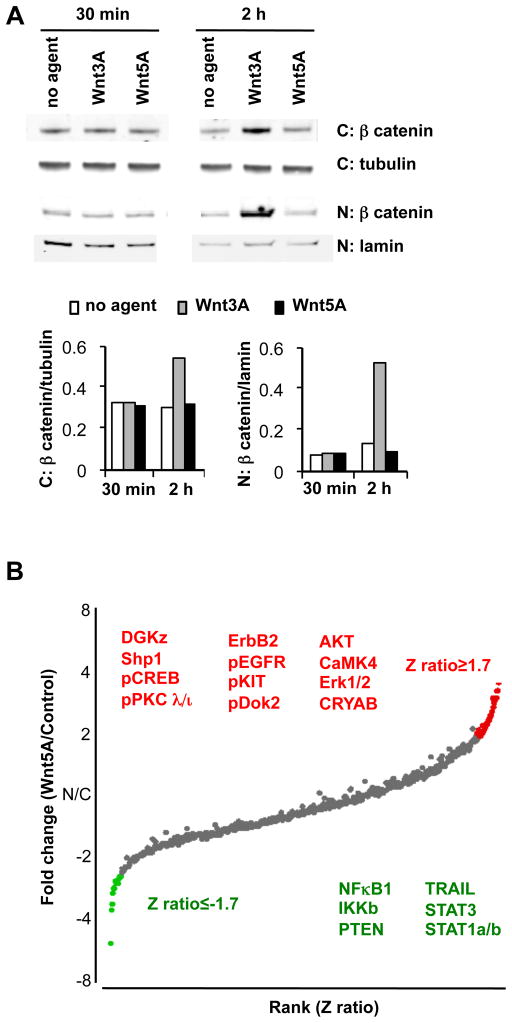
Wnt3A triggers β-catenin activation and Wnt5A non-canonical signaling in DC
Wnt3A treatment of dendritic cells induced accumulation and translocation of β-catenin from the cytoplasm to the nucleus (Fig. 6A), consistent with its well characterized activation of canonical Wnt signaling in other cell systems(24). In contrast Wnt5A failed to alter β–catenin levels or affect its nuclear translocation in isolated DC. To confirm an effect of Wnt5A on DC intracellular signaling, analysis of proteins affected by Wnt5A was carried out using Kinex protein microarrays that interrogate more than 800 proteins and phosphoproteins (Fig. 6B and supplementary Table I). Comparison of control untreated vs Wnt5A treated CD11c+ DC revealed effects on multiple signaling molecules including components of calcium, MAP kinase, growth factor, NFκB and protein kinase C (PKC) and cytokine signal transduction (STAT) pathways (Fig. 6B). Interestingly, several of the modulated proteins have been implicated in IL-10 regulation, with the potential to contribute to Wnt5A induction of IL-10 in DC (see discussion); and others are known moderators of pro-inflammatory NFκB signaling. We conclude that Wnt5A triggers significant alterations in intracellular signaling molecules in DC.
FIGURE 6. Signaling by Wnt3A and Wnt5A.
a) Western blot analysis reveals nuclear translocation of β-catenin in response to Wnt3A but not Wnt5A treatment of DC. CD11c+ DC isolated from lymph nodes of FLT3L treated mice were treated with Wnt3A (0.3μg/ml) or Wnt5A (0.3μg/ml) for 30 min and 2 hours. Data shown include western blots of β-catenin in the cytoplasmic (C) and nuclear fractions (N) and loading controls; tubulin (cytoplasmic) and lamin B (nuclear), and is representative of 1 of 3 independent experiments with similar results. (Treatment with 3μg/ml Wnt3A or Wnt5A gave similar results, data not shown). b) Protein expression analysis using Kinex antibody microarrays comparing control vs Wnt5A treated DC. Positive or negative fold-changes in protein or phosphoprotein expression after 30 minute treatment of DC with 3μg/ml Wnt5A and are plotted (Y axis) vs. rank order of Z scores. Proteins with Z-ratios above 1.7 or below −1.7, and whose intensity was increased or decreased at least 2 fold are colored in the plot and listed in Supplementary Table 1. Significantly affected proteins with potential roles in IL-10 induction, modulation of inflammatory or growth/survival mechanisms, are listed as red (upregulated) or green (downregulated) text on the Figure.
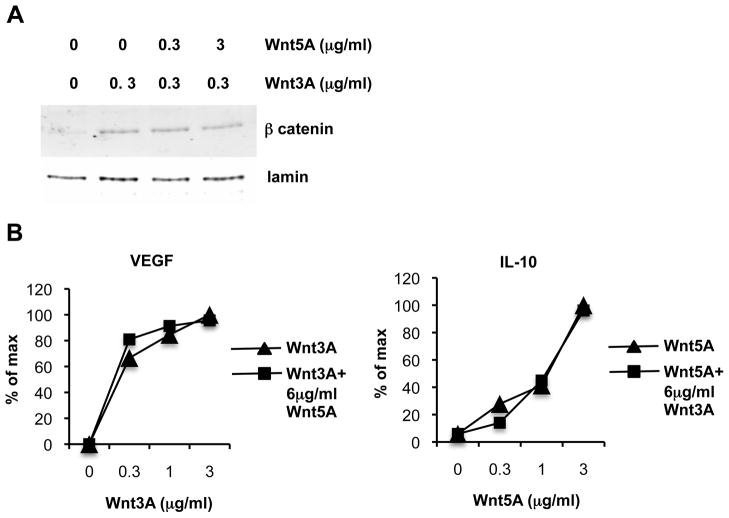
In hematopoietic stem cell differentiation and in colon cancer cells, non-canonical signaling triggered by Wnt5A counteracts and inhibits β-catenin activation by canonical wnts(25, 26). However, in DC Wnt5A failed to inhibit or alter Wnt3A induction of VEGF; nor did it inhibit Wnt3A induced β-catenin translocation (Fig. 7). Moreover, excess Wnt3A had no effect on Wnt5A induced IL-10 secretion (Fig. 7). These findings underscore the specialized nature of dendritic cell responses to Wnt signaling.
FIGURE 7. Wnt5A does not counteract Wnt3A induced β-catenin signaling or VEGF secretion nor does Wnt3A affect Wnt5A induced IL-10 secretion.
CD11c+ DC from pooled lymph nodes of FLT3L treated mice were treated with Wnt3A or Wnt5A or Wnt3A in combination with Wnt5A at the indicated concentrations. a) DC were cultured 2 hours and nuclear extracts were analyzed by western blot for β-catenin and for lamin B as a loading control. Data shown is representative of 3 independent experiments with similar results. b) DC were cultured for 20 hours and supernatants were analyzed by ELISA to determine concentration of secreted cytokines. Results are presented as % of maximum cytokine concentration induced, Maximum values were 120 pg/ml for IL-10, 4200 pg/ml for VEGF-A. Data shown is representative of 2 independent experiments with similar results.
Discussion
We have shown that Wnts stimulate DC production of anti-inflammatory cytokines and reprogram DC responses to a wide range of microbial ligands. Wnt3A and Wnt5A, two Wnts with opposing effects in many cellular systems, differentially induce immune regulatory cytokines but share a common ability to inhibit pro-inflammatory DC responses. Brief pretreatment with Wnts during TLR stimulation further altered DC function to promote Foxp3+ Tregs while limiting generation of IFNγ producing effector T cells, suggesting that Wnt reprogramming of DC can be translated into tolerogenic effects on the adaptive immune response.
For our studies we chose to examine Wnt3A as a representative Wnt that characteristically activates the canonical β-catenin pathway; and Wnt5A which in most cell systems mediates non-canonical signaling(24). Both Wnts induced tolerogenic changes in DC, but the responses they produced were distinct. In studies of primary or in vivo Flt3L-expanded lymphoid tissue-derived DC both Wnts stimulated immune regulatory cytokines, but Wnt3A preferentially induced TGFβ while Wnt5A was a potent stimulus for IL-10 production. Wnts also induce TGFβ in fibroblasts(27) and although Wnt induction of IL-10 has not been reported to our knowledge, glycogen synthase kinase3β (Gsk3β) inhibition or β-catenin signaling stimulates IL-10 production in TLR activated human monocytes(28) and T cells(29). TGFβ and IL-10 are critical participants in the maintenance of tolerance and immune homeostasis, acting on innate immune and stromal cells to suppress production of pro-inflammatory cytokines/chemokines and modulate downstream adaptive immune responses(30). They also have important functions in protection at mucosal surfaces: IL-10 acts on epithelial cells to promote epithelial barrier integrity(31), and TGFβ stimulates IgA production(32, 33) and acts on T cells to support the generation and survival of peripheral Foxp3+ Tregs(34, 35); whether the TGFβ is secreted in biologically active form in this setting remains to be determined.
Interestingly, Wnt3A but not Wnt5A strongly stimulated DC production of VEGF-A as well. Wnt3A has previously been reported to upregulate VEGF in osteoblasts(36) and retinal epithelial cells(37) via the canonical β-catenin dependent pathway. In addition to its established role in angiogenesis, VEGF has recently emerged as an immune regulatory cytokine: VEGF produced in the tumor microenvironment inhibited the pro-inflammatory maturation of dendritic cells(20), and generated an increased frequency of Tregs(22); and VEGF can directly suppress T cell activation through VEGFR-2(21). Thus VEGF expression by Wnt-stimulated DC may play an immune regulatory role complementing those of IL-10 and TGFβ.
In addition to these effects on basal DC activity, the Wnts effectively reprogram DC responses to PAMPs. Both Wnt3A and Wnt5A inhibit the induction of the pro-inflammatory cytokines IL-6, MIP-1α (CCL3),TNFα and IFNα in response to TLR ligands. Interestingly however, Wnt5A suppresses IL-6 secretion in response to the viral mimic poly(I:C), just as to other TLR ligands; but Wnt3A does not. Thus Wnt3A can differentiate between PAMPs, allowing DC pro-inflammatory responses to poly(I:C) while suppressing responses to other TLR ligands. Poly(I:C) is a synthetic double stranded RNA which mimics RNA virus infections and initiates cellular responses through TLR3, retinoic acid inducible gene-1 (Rig-1) and melanoma differentiation associated protein-5 (MDA-5). The selective ability of Wnt3A-treated DC to produce pro-inflammatory IL-6 in responses to poly(I:C) may allow a particularly aggressive immune response to RNA viruses in environments containing Wnt3A or related Wnt family members that signal via the canonical pathway (see below). Wnt3, a close structural and functional paralog of Wnt3A, is selectively expressed by Paneth cells of the crypt epithelium in the intestines where epithelial stem cells reside(38, 39). Wnt3 may allow rapid responses of crypt-associated DC to RNA viruses infecting the proliferating epithelial cell compartment, while still inhibiting responses to non-pathogen associated microbial pattern molecules. Wnt5A in contrast is expressed by as yet unidentified mesenchymal cells in the intestinal LP, especially in the apical region of the LP(39), and by fibroblastic reticular cells in lymphoid tissues(19). Together, the results suggest the potential for sophisticated control of DC by Wnts as a function of their microenvironmental localization.
In contrast to their inhibition of pro-inflammatory cytokine production, the Wnts did not inhibit culture-induced or TLR stimulated DC upregulation of costimulatory molecules required for efficient T cell interactions. Moreover, they only had a minor inhibitory effect on upregulation of CCR7, which mediates DC migration from tissues into draining lymph nodes. These hallmarks of DC maturation are required for DC antigen presentation in vivo whether for tolerance or immune activation. Thus the Wnts permit efficient DC maturation while reprogramming their basal and TLR-induced cytokine responses. In accordance with their tolerogenic phenotype, both Wnt3A and Wnt5A pre-conditioned DC supported enhanced generation of Tregs while inhibiting the generation of IFNγ-producing Th1 cells during in vitro T cell coculture. This effect is seen in the context of TLR stimulation and, in our in vitro co-culture setting, required exogenous TGFβ, revealing a synergistic effect of Wnts and TGFβ in DC generation of regulatory T cells. IL-6 is known to suppress TGFβ induced Treg expansion while favoring inflammatory effector responses in the presence of TGFβ (40). Both Wnt3A and Wnt5A potently reduce TLR induced IL-6 production, and it is likely that their stimulation of the Treg response reflects this suppression of DC IL-6 secretion.
Consistent with its well-established role as a canonical Wnt in other cell systems, Wnt3A induced accumulation and nuclear translocation of β-catenin in DC. As outlined above, β-catenin signaling is now well established as a mediator of tolerogenic DC maturation(11–13). While Wnt5A failed to stimulate β-catenin, protein array analysis was consistent with effects on multiple signaling pathways in Wnt5a treated cells. Alterations were observed in calcium (e.g. calcium/calmodulin dependent protein kinase IV (CAMK4), diacylglycerol kinase z (DGKz)), phosphatidylinositol-3-kinase (PI3K) (phosphatase and tensin homolog (PTEN), and AKT), MAPK, and NFκB signaling components. Several of the changes observed in Wnt5A treated DC have been associated in other cell types or settings with anti-inflammatory responses. For example, mediators of inflammatory gene regulation including STAT1a/b, STAT3, PTEN and TNF-related apoptosis inducing ligand (TRAIL) were downregulated, as were proteins associated with activation of NFκB (NFκBp50 and inhibitor of NFκB (IKKβ)), a key mediator of inflammatory responses(41). In contrast, several upregulated proteins have been implicated in anti-inflammatory responses, e.g. heat shock protein-ab-crystallin, protein tyrosine phosphatase Shp1(42), and phospho-Dok2(43). Several Wnt5A upregulated or activated proteins have been implicated in the positive regulation of IL-10 as well, including Shp1(42), AKT(44), Erk1/2(45), the atypical PKCι/λ(46), and phospho-CREB, which can bind the IL-10 promoter and positively regulate its transcription in response to Erk and AKT signaling(45). In addition to immune responsive proteins, changes were observed in several signaling molecules involved in growth/survival; EGFR, erythroblastic leukemia viral oncogene homolog 2 (ErbB2), phospho-KIT, AKT, and Erk1/2 were upregulated while pro-apoptotic TRAIL was downregulated. While these studies were undertaken primarily to confirm an impact of Wnt5A on intracellular signaling, the diverse modifications observed suggest the possibility that Wnt5A induces extensive reprogramming of DC signaling networks. Future studies will be required to define specific pathways involved in the unique DC responses (e.g. IL-10 induction, vs. suppression of TLR-mediated activation) induced by Wnt5A.
It was perhaps surprising that Wnt3A and Wnt5A both condition DC for tolerogenesis. In most cellular systems as in DC Wnt3A acts through the canonical pathway, inhibiting Gsk3β and inducing downstream β-catenin accumulation, whereas Wnt5A activates non-canonical signaling and can even antagonize the canonical pathway such that the two Wnts frequently have opposing functions(24). Indeed, while Wnt3A acting through β-catenin is anti-inflammatory for macrophages(8), Wnt5A can be either pro-inflammatory(9) or anti-inflammatory(10). Similarly, Wnt5A inhibits while Wnt3A promotes cDC development from mouse and human bone marrow(6); and Wnt3A and Wnt5A often display opposing roles in developing T and B cells(5). While the two Wnts trigger distinct responses in differentiated DC as well, they nonetheless both induce immune regulatory phenotypic features and suppress the pro-inflammatory DC response to the majority of TLRs. These shared immune suppressive effects on DC suggests a unique importance of tolerogenic immune regulation through Wnt-DC interactions. This concept is further supported by the fact that Wnt5A, which in other cell systems counteracts Wnt3A/canonical wnt signaling, had no effect on Wnt3A induced β-catenin translocation, nor on Wnt3A stimulation of VEGF in DC.
Consistent with our findings, Hack et al recently reported that Wnt5A suppresses human blood pDC activation by CpG(47). Together with this recent report, our findings demonstrate a direct effect of Wnt proteins on differentiated tissue DC, confirming the hypothesized role for canonical Wnts in inducing DC tolerogenesis, but also contrasting DC responses to canonical vs non-canonical Wnts. Previous studies of β-catenin-dependent mechanisms in immune tolerance, and of Wnt effects on in vitro development of dendritic cells from monocytes, provided the rationale for assessing the role of Wnts in dendritic cell function: Two studies showed that disruption of E-cadherin dependent cell clustering of immature in vitro bone marrow (BM)-derived dendritic cells resulted in a mature (costimulatory molecule high, MHCII high and CCR7+) tolerogenic phenotype, induction of which involved β-catenin(12, 13). Cluster disruption-induced DC failed to secrete inflammatory cytokines (IL-6, TNFα, IL-12p40 and IL-1α) as compared to bacteria stimulated DC, much like the Wnt stimulated DC described here; and they generated a tolerogenic rather than immunogenic T cell response(12). However, unlike Wnt5A, cluster disruption of DC did not induce IL-10 secretion by DC, and DC matured by prior cluster disruption displayed decreased levels of IL-10 in response to secondary LPS stimulation(12). Thus β-catenin activation through disruption of cadherin-mediated adhesion, at least in DC generated in vitro, results in a DC phenotype distinct from that induced by Wnt treatment of DC as shown here. While non-canonical Wnts have not been studied in the context of differentiated cDC functions, Valencia et al(48) showed that Wnt5A altered the process of dendritic cell differentiation from human monocytes: inclusion of Wnts in monocyte cultures with GM-CSF and IL-4 resulted in dendritic cells with an immature tolerogenic phenotype, displaying reduced levels of pro-inflammatory cytokines but (in contrast to our results) also failing to upregulate costimulatory molecules, MHCII and CCR7 in response to TLR ligands. It is noteworthy that the tolerogenic effect on DC differentiation from monocytes was mostly limited to Wnt5A and not shared by Wnt3A. Moreover, the fact that inclusion of Wnts during the first 24 hours of the 6 day differentiation culture was sufficient to generate the tolerogenic end stage DC phenotype, suggests that Wnt signaling at the early monocyte stage rather than at a later DC stage was responsible for Wnt mediated modulation in that study. The difference in the induced DC phenotypes and Wnt3A/Wnt5A responses reported here, vs those reported by Valencia et al, could therefore reflect differential and cell-type specific Wnt signaling/Wnt effects in monocytes vs DC. Indeed, Wnt5A displayed pro-inflammatory effects in the differentiating monocyte system: supernatants of Wnt5A treated monocytes contained high levels of IL-6 as compared to control cultures(48), which is in line with previous reports of Wnt5A effects on macrophages(9) but in clear contrast to our observation of its anti-inflammatory effects on differentiated tissue DC.
Finally, elegant studies of Manicassamy et al(11) showed that mice with a selective dendritic cell deficiency in β-catenin display enhanced sensitivity to chemically induced colitis (dextran sulfate sodium), associated with increased production of inflammatory cytokines and a deficiency in DC IL-10 and TGFβ production. In combination with our finding that Wnt3A triggers β-catenin activation and TGFβ secretion in DC, the demonstrated importance of DC-intrinsic β-catenin signaling to immune tolerance in vivo provides strong support for the proposed role of Wnts in regulating dendritic cell responses and maintaining tolerance under homeostatic conditions.
Interestingly, Wnt expression is increased during infection and inflammation: Wnt5A is upregulated in sepsis(9), in granulomas of mycobacterium tuberculosis patients(7, 8) and in bacterial periodontitis(49) and Wnts are increased or abnormally expressed in arthritis synovial tissue(50), atherosclerotic plaques(51), psoriatic or wounded skin(3, 52), and in inflammatory bowel disease(53). Our results suggest that induced Wnt proteins may modulate dendritic cell responses to limit inflammation after trauma or infection. In this context, it is interesting that WntD, a drosophila Wnt protein that signals through non-canonical pathways, plays a critical role in resolving inflammatory responses in the fly(15), raising the possibility that Wnt regulation of immune response is a primordial mechanism for achieving immune homeostasis. However, Wnt5A is also upregulated in granulomas(7, 8), and Wnt-DC interactions could play a negative role in the persistence of chronic infections (e.g. tuberculosis). Finally, while tolerance to innocuous antigens and commensal organisms is advantageous, suppression of the immune response is undesirable in the context of tumor immunity. Wnts are frequently overexpressed in cancers(4), and their immune suppressive and tolerogenic effects on DC could contribute to tumor immune evasion and cancer progression.
Supplementary Material
Acknowledgments
We thank L. Rott for assistance with flow cytometry, Professor Carlo Laudanna and Dr. Simone Zorzan for help with analysis of Kinexus data, the Immunologic Genome Consortium (www.Immgen.org), G. Dranoff for the Flt3L-expressing B16 melanoma line, the Stanford Immune Monitoring Core for Luminex assays, and Robert Coffman, Katharina Lahl, Brian Zabel, and Ruizhu Zeng for critical review of the manuscript.
Source of support
This work was supported by grants R37-AI047822, RO1-AI072618, RO1-DK084647 and RC1-AI087257 from the National Institutes of Health and a Merit Award from the Department of Veterans Affairs (ECB), and by the Stanford Digestive Disease Center FACS Core facility under DK56339. CO was supported by Fellowships from the Wenner-Gren Foundation and the Crohn’s & Colitis Foundation. ML was a recipient of fellowship support under NIH training grants 5 T32 AI07290 and T32CA09151.
Abbreviations
- cDC
conventional dendritic cell
- DC
dendritic cell
- FLt3L
FLt3 ligand
- MLN
mesenteric lymph node
- PAMP
pathogen associated molecular pattern
- pDC
plasmacytoid dendritic cell
- PLN
peripheral lymph node
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
Footnotes
Author Contributions
CO and ML performed the experiments. CO, ML, and ECB designed experiments, analyzed data and wrote the paper.
Disclosure of Interests
The authors declare no competing financial interests.
Literature Cited
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 3.Fathke C, Wilson L, Shah K, Kim B, Hocking A, Moon R, Isik F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. BMC Cell Biol. 2006;7:4. doi: 10.1186/1471-2121-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 5.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Cheng P, Youn JI, Cotter MJ, Gabrilovich DI. Notch and wingless signaling cooperate in regulation of dendritic cell differentiation. Immunity. 2009;30:845–859. doi: 10.1016/j.immuni.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumenthal A, Ehlers S, Lauber J, Buer J, Lange C, Goldmann T, Heine H, Brandt E, Reiling N. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 8.Neumann J, Schaale K, Farhat K, Endermann T, Ulmer AJ, Ehlers S, Reiling N. Frizzled1 is a marker of inflammatory macrophages, and its ligand Wnt3a is involved in reprogramming Mycobacterium tuberculosis-infected macrophages. FASEB J. 2010;24:4599–4612. doi: 10.1096/fj.10-160994. [DOI] [PubMed] [Google Scholar]
- 9.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 10.Bergenfelz C, Medrek C, Ekstrom E, Jirstrom K, Janols H, Wullt M, Bredberg A, Leandersson K. Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J Immunol. 2012;188:5448–5458. doi: 10.4049/jimmunol.1103378. [DOI] [PubMed] [Google Scholar]
- 11.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vander Lugt B, Beck ZT, Fuhlbrigge RC, Hacohen N, Campbell JJ, Boes M. TGF-beta suppresses beta-catenin-dependent tolerogenic activation program in dendritic cells. PLoS One. 2011;6:e20099. doi: 10.1371/journal.pone.0020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halleskog C, Dijksterhuis JP, Kilander MB, Becerril-Ortega J, Villaescusa CJ, Lindgren E, Arenas E, Schulte G. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J Neuroinflammation. 2012;9:111. doi: 10.1186/1742-2094-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon MD, Dionne MS, Schneider DS, Nusse R. WntD is a feedback inhibitor of Dorsal/NF-kappaB in Drosophila development and immunity. Nature. 2005;437:746–749. doi: 10.1038/nature04073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 17.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O’Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheadle C, Vawter MP, Freed WJ, Becker KG. Analysis of microarray data using Z score transformation. J Mol Diagn. 2003;5:73–81. doi: 10.1016/S1525-1578(10)60455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Immunologic Genome Consortium 2011 [Google Scholar]
- 20.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 21.Ziogas AC, Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Terpos E, Rodolakis A, Vlahos G, Thomakos N, Haidopoulos D, Antsaklis A, Dimopoulos MA, Bamias A. VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer. 2012;130:857–864. doi: 10.1002/ijc.26094. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, Prell R, VanRoey MJ, Simmons AD, Jooss K. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res. 2006;12:6808–6816. doi: 10.1158/1078-0432.CCR-06-1558. [DOI] [PubMed] [Google Scholar]
- 23.Annacker O, Coombes JL, Malmstrom V, Uhlig HH, Bourne T, Johansson-Lindbom B, Agace WW, Parker CM, Powrie F. Essential role for CD103 in the T cell-mediated regulation of experimental colitis. J Exp Med. 2005;202:1051–1061. doi: 10.1084/jem.20040662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Amerongen R, Nusse R. Towards an integrated view of Wnt signaling in development. Development. 2009;136:3205–3214. doi: 10.1242/dev.033910. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth MJ, Topol L, Anderson SM, Yang Y, Bodine DM. Wnt5a inhibits canonical Wnt signaling in hematopoietic stem cells and enhances repopulation. Proc Natl Acad Sci U S A. 2007;104:15436–15441. doi: 10.1073/pnas.0704747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLeod RJ, Hayes M, Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G403–411. doi: 10.1152/ajpgi.00119.2007. [DOI] [PubMed] [Google Scholar]
- 27.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-beta signaling through SMAD2 in a beta-catenin-dependent manner. PLoS One. 2011;6:e19809. doi: 10.1371/journal.pone.0019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Notani D, Gottimukkala KP, Jayani RS, Limaye AS, Damle MV, Mehta S, Purbey PK, Joseph J, Galande S. Global regulator SATB1 recruits beta-catenin and regulates T(H)2 differentiation in Wnt-dependent manner. PLoS Biol. 2010;8:e1000296. doi: 10.1371/journal.pbio.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madsen KL, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology. 1997;113:151–159. doi: 10.1016/s0016-5085(97)70090-8. [DOI] [PubMed] [Google Scholar]
- 32.Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989;170:1039–1044. doi: 10.1084/jem.170.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S, Tominaga A, Yamaguchi N, Takatsu K. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989;170:1415–1420. doi: 10.1084/jem.170.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokuda H, Adachi S, Matsushima-Nishiwaki R, Kato K, Natsume H, Otsuka T, Kozawa O. Enhancement of basic fibroblast growth factor-stimulated VEGF synthesis by Wnt3a in osteoblasts. Int J Mol Med. 2011;27:859–864. doi: 10.3892/ijmm.2011.644. [DOI] [PubMed] [Google Scholar]
- 37.Zhou T, Hu Y, Chen Y, Zhou KK, Zhang B, Gao G, Ma JX. The pathogenic role of the canonical Wnt pathway in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2010;51:4371–4379. doi: 10.1167/iovs.09-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, I, Verma M. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 42.Kaneko T, Saito Y, Kotani T, Okazawa H, Iwamura H, Sato-Hashimoto M, Kanazawa Y, Takahashi S, Hiromura K, Kusakari S, Kaneko Y, Murata Y, Ohnishi H, Nojima Y, Takagishi K, Matozaki T. Dendritic cell-specific ablation of the protein tyrosine phosphatase shp1 promotes Th1 cell differentiation and induces autoimmunity. J Immunol. 2012;188:5397–5407. doi: 10.4049/jimmunol.1103210. [DOI] [PubMed] [Google Scholar]
- 43.Shinohara H, Inoue A, Toyama-Sorimachi N, Nagai Y, Yasuda T, Suzuki H, Horai R, Iwakura Y, Yamamoto T, Karasuyama H, Miyake K, Yamanashi Y. Dok-1 and Dok-2 are negative regulators of lipopolysaccharide-induced signaling. J Exp Med. 2005;201:333–339. doi: 10.1084/jem.20041817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin M, Schifferle RE, Cuesta N, Vogel SN, Katz J, Michalek SM. Role of the phosphatidylinositol 3 kinase-Akt pathway in the regulation of IL-10 and IL-12 by Porphyromonas gingivalis lipopolysaccharide. J Immunol. 2003;171:717–725. doi: 10.4049/jimmunol.171.2.717. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez Y, Municio C, Alonso S, Sanchez Crespo M, Fernandez N. The induction of IL-10 by zymosan in dendritic cells depends on CREB activation by the coactivators CREB-binding protein and TORC2 and autocrine PGE2. J Immunol. 2009;183:1471–1479. doi: 10.4049/jimmunol.0900312. [DOI] [PubMed] [Google Scholar]
- 46.Sudan R, Srivastava N, Pandey SP, Majumdar S, Saha B. Reciprocal regulation of protein kinase C isoforms results in differential cellular responsiveness. J Immunol. 2012;188:2328–2337. doi: 10.4049/jimmunol.1101678. [DOI] [PubMed] [Google Scholar]
- 47.Hack K, Reilly L, Proby C, Fleming C, Leigh I, Foerster J. Wnt5a inhibits the CpG oligodeoxynucleotide-triggered activation of human plasmacytoid dendritic cells. Clin Exp Dermatol. 37:557–561. doi: 10.1111/j.1365-2230.2012.04362.x. [DOI] [PubMed] [Google Scholar]
- 48.Valencia J, Hernandez-Lopez C, Martinez VG, Hidalgo L, Zapata AG, Vicente A, Varas A, Sacedon R. Wnt5a skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J Immunol. 2011;187:4129–4139. doi: 10.4049/jimmunol.1101243. [DOI] [PubMed] [Google Scholar]
- 49.Nanbara H, Wara-aswapati N, Nagasawa T, Yoshida Y, Yashiro R, Bando Y, Kobayashi H, Khongcharoensuk J, Hormdee D, Pitiphat W, Boch JA, Izumi Y. Modulation of Wnt5a expression by periodontopathic bacteria. PLoS One. 2012;7:e34434. doi: 10.1371/journal.pone.0034434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sen M, Lauterbach K, El-Gabalawy H, Firestein GS, Corr M, Carson DA. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Christman MA, 2nd, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, Silver MJ, Kohn LD, Malgor R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008;294:H2864–2870. doi: 10.1152/ajpheart.00982.2007. [DOI] [PubMed] [Google Scholar]
- 52.Gudjonsson JE, Johnston A, Stoll SW, Riblett MB, Xing X, Kochkodan JJ, Ding J, Nair RP, Aphale A, Voorhees JJ, Elder JT. Evidence for altered Wnt signaling in psoriatic skin. J Invest Dermatol. 2010;130:1849–1859. doi: 10.1038/jid.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.You J, Nguyen AV, Albers CG, Lin F, Holcombe RF. Wnt pathway-related gene expression in inflammatory bowel disease. Dig Dis Sci. 2008;53:1013–1019. doi: 10.1007/s10620-007-9973-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.