Abstract
S-adenosylmethionine (AdoMet, also known as SAM and SAMe) is the principal biological methyl donor synthesized in all mammalian cells but most abundantly in the liver. Biosynthesis of AdoMet requires the enzyme methionine adenosyltransferase (MAT). In mammals, two genes, MAT1A that is largely expressed by normal liver and MAT2A that is expressed by all extrahepatic tissues, encode MAT. Patients with chronic liver disease have reduced MAT activity and AdoMet levels. Mice lacking Mat1a have reduced hepatic AdoMet levels and develop oxidative stress, steatohepatitis, and hepatocellular carcinoma (HCC). In these mice, several signaling pathways are abnormal that can contribute to HCC formation. However, injury and HCC also occur if hepatic AdoMet level is excessive chronically. This can result from inactive mutation of the enzyme glycine N-methyltransferase (GNMT). Children with GNMT mutation have elevated liver transaminases, and Gnmt knockout mice develop liver injury, fibrosis, and HCC. Thus a normal hepatic AdoMet level is necessary to maintain liver health and prevent injury and HCC. AdoMet is effective in cholestasis of pregnancy, and its role in other human liver diseases remains to be better defined. In experimental models, it is effective as a chemopreventive agent in HCC and perhaps other forms of cancer as well.
I. INTRODUCTION
The discovery of S-adenosyl-l-methionine (AdoMet, also frequently abbreviated as SAM and SAMe) occurred nearly 60 years ago (31). Widely known as the principal biological methyl donor, recent evidence illustrates its essential role in diverse cellular processes including growth and death. Although AdoMet is synthesized in all mammalian cells, liver can be considered as the body's AdoMet factory as this is where ~85% of all transmethylation reactions and 50% of methionine metabolism take place (67, 179). This review is focused mainly on the role of AdoMet in liver health, injury, and cancer. Much of current understanding has been gained through the use of genetic models that exhibit chronic hepatic AdoMet deficiency or excess. AdoMet is also available as a drug in many parts of the world and in the United States as a nutritional supplement. This review will also briefly discuss the current status of AdoMet's therapeutic use in liver diseases and several extrahepatic disorders.
II. STRUCTURE AND BIOSYNTHESIS OF AdoMet
A. Structure of AdoMet
Early in evolution AdoMet emerged as the foremost donor of methyl groups in methylation reactions. Specific amino acid side chains in proteins, nucleotide bases in DNA and RNA, sugars, and many small molecules often carry a methyl group that is derived from AdoMet. Nature's selection of AdoMet for this role is attributable to its unique chemical structure. AdoMet is a typical sulfonium salt, a compound in which the sulfur atom has three single covalent substituents and therefore has a positive charge analogous to an ammonium compound (FIGURE 1). Structurally, sulfonium salts form a pyramidal arrangement of the three substituents, and there is a lone electron pair also associated with the sulfur atom (17). The sulfonium ion of AdoMet is particularly well suited for the transfer of its methyl group to a variety of nucleophilic atoms by a typical sn-2 substitution reaction with the concomitant release of S-adenosyl-l-homocysteine (AdoHcy) (FIGURE 1) (87, 229, 263). In AdoMet-dependent methylation reactions, the nucleophile may be a carbon atom, as in the C-methylation reaction of the C5-cytosine in DNA methylation; a nitrogen atom, as in the N-methylation reaction of the amino group in glycine methylation; an oxygen atom, as in the O-methylation reaction of the hydroxyl moiety in catecholamine methylation; or a sulfur atom, as in the S-methylation reaction of the sulfur atom in thiopurines methylation. AdoMet can also methylate other atoms, such as arsenic, an important step in the metabolism of this toxic metalloid (135).
FIGURE 1.
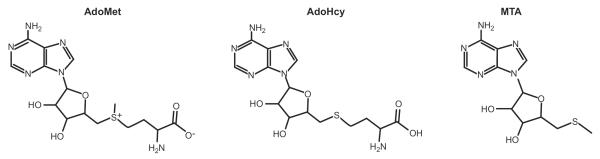
Structure of S-adenosyl-l-methionine (AdoMet), S-adenosyl-l-homocysteine (AdoHcy), and 5′-methylthioadenosine (MTA).
Giulio Cantoni discovered AdoMet in 1953 (31). He elegantly demonstrated that methionine and ATP reacted to form a molecule, and after determination of its chemical structure, he named it AdoMet, capable of transferring a methyl group to nicotinamide or guanidinoacetic acid to form N-methylnicotinamide and creatine, respectively. Since then, investigators have implicated AdoMet in thousands of different methylation reactions in biological systems. In fact, AdoMet competes with ATP as the most widely used enzyme substrate (31, 138).
In addition to the chiral carbon at the α amino position, the sulfur at the sulfonium center of AdoMet is also chiral. Consequently, AdoMet can exist in two diastereomeric forms with respect to its sulfonium ion, (S,S)-AdoMet and (R,S)-AdoMet, where the designations refer to the sulfur and the α carbon, respectively. (S,S)-AdoMet is the only form that is synthesized enzymatically, whereas the AdoMet obtained by chemical methylation of AdoHcy is a racemic mixture of the two isomers (50). The (S,S) diastereomer is also the only form used in almost all methylation reactions (16, 17, 29, 56). Although under physiological conditions, 37°C and pH 7.5, the sulfonium ion of (S,S)-AdoMet can spontaneously racemize to the R form producing (R,S)-AdoMet with a first-order rate constant of 1.8 × 10−6 s−1 (91), the in vivo concentration of this biologically inactive AdoMet form is low to undetectable in the several tissues where it has been determined. Thus the (R,S)-AdoMet form was found to be only 1.5 and 3% of total AdoMet in mouse liver and rat brain, respectively (16, 91). This contrasts with the values predicted from the estimated racemization rate (91), which implies that in vivo there are mechanisms that either dispose of the (R,S)-AdoMet diastereomer or convert it back to (S,S)-AdoMet.
Another two spontaneous AdoMet reactions that take place under physiological conditions are: an intramolecular cleavage reaction that produces homoserine lactone and 5′-methylthioadenosine (MTA) (FIGURE 1) and a hydrolysis reaction that generates adenine and S-pentosylmethionine, which occur with first-order rate constants of 4.6 × 10−6 s−1 and 3 × 10−6 s−1, respectively (91, 265). These two reactions may contribute to balance the racemization of (S,S)-AdoMet in vivo and prevent the accumulation of (R,S)-AdoMet. Two methyltransferases (MT), Mht1 (S-methylmethionine-homocysteine methyltransferase) and Sam4 (AdoMet-homocysteine methyltransferase), which transfer methyl groups from AdoMet to homocysteine to synthesize methionine, have been identified in yeast, worms, plants, and flies (255). Sam4 was found to use both the (S,S) and (R,S) forms of AdoMet, with a higher affinity for the (R,S) diastereomer, while Mht1 was found to use exclusively the (R,S) form. Mht1 and Sam4 deletion in yeast resulted in accumulation of the biologically inactive (R,S) form, indicating that these two enzymes play an important role in controlling the in vivo accumulation of spontaneously generated (R,S)-AdoMet (256). Interestingly, Mht1 and Sam4 activities have not been detected in mammalian cells (257), which indicates that mammals limit (R,S)-AdoMet accumulation using other mechanisms.
It is important to note that spontaneous cleavage, hydrolysis, and racemization of AdoMet also take place in test tubes and tissue culture plates and that this may affect the interpretation of experimental results. For instance, MTA, a product of the spontaneous cleavage of AdoMet, in addition to being metabolized in vivo to form methionine can also inhibit certain MTs (54) (see sect. IIIC). When utilizing AdoMet, it is also important to note that racemization is independent of the pH in a range from 7.5 to 1.5, that the cleavage reaction persists up to a pH of 1.5, and that hydrolysis ceases at pH 6 (91). Finally, the chiral and chemical purity of AdoMet may vary between different commercial sources, which may affect its biological activity when used in the laboratory, as a food supplement, or in clinical settings.
B. Biosynthesis of AdoMet
Methionine adenosyltransferase (MAT) is the enzyme that catalyzes the biosynthesis of AdoMet from ATP and methionine, an essential amino acid (FIGURE 2). All living cells, with the only exception of some parasites and infectious agents that obtain AdoMet from their hosts (170, 236) (see sect. IX), express MAT. MAT is one of the genes that is essential to sustain life (80). Although divergent sequences have been reported for Archea, MAT has been extremely well conserved through evolution, with 59% sequence identity being found between the human and Escherichia coli enzymes (81). In mammals, all cells and tissues that have been studied express MAT, its activity being highest in the liver (67). Consequently, individuals with hepatic MAT activity deficiency are characterized by isolated persistent hypermethioninemia (248). The plasma methionine concentration in these subjects with hepatic MAT activity deficiency can be as high as 1,300 μM, while the upper limit in control individuals is ~35 μM (248).
FIGURE 2.
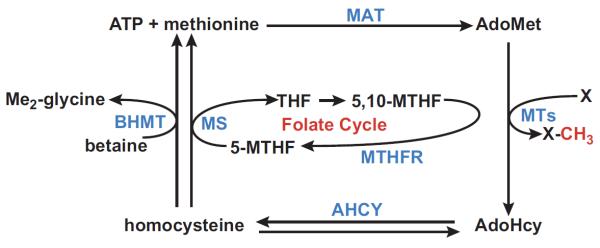
Methionine metabolism: transmethylation pathway. Methionine adenosyltransferase (MAT) catalyzes the conversion of methionine and ATP into AdoMet. In transmethylation reactions, AdoMet donates its methyl group to a large variety of acceptor molecules (X) in reactions catalyzed by methyl transferases (MTs). AdoMet-dependent methylation reactions yield AdoHcy as a byproduct. AdoHcy cellular content is regulated by the enzyme AdoHcy hydrolase (AHCY), which reversibly cleaves this molecule into adenosine and homocysteine. Remethylation of homocysteine to form methionine occurs by two enzymes: methionine synthase (MS), which requires normal levels of folate and vitamin B12, and betaine homocysteine methyltransferase (BHMT), which requires betaine, a metabolite of choline. MS-catalyzed homocysteine remethylation requires 5-methyltetrahydrofolate (5-MTHF), which is derived from 5,10-methylenetetrahydrofolate (5,10-MTHF) in a reaction catalyzed by methylenetetrahydrofolate reductase (MTHFR). 5-MTHF is then converted to tetrahydrofolate (THF) as it donates its methyl group and THF is converted to 5,10-MTHF to complete the folate cycle.
The mechanism of AdoMet biosynthesis has been best studied using MAT purified from Escherichia coli and rat liver (58, 151). MAT activity is the combination of two consecutive reactions, AdoMet synthesis and hydrolysis of tripolyphosphate, with the latter being rate-limiting. The first reaction involves the formation of an enzyme-ATP-methionine complex and the synthesis of AdoMet and tripolyphosphate. In the second reaction, tripolyphosphate is hydrolyzed to orthophosphate and pyrophosphate before the release of the products. Tripolyphosphate is tightly bound to the enzyme so that its hydrolysis is oriented and over 95% of the orthophosphate generated originates from the γ-phosphoryl group of ATP. MAT is also able to hydrolyze exogenously added tripolyphosphate.
Ethionine is a nonproteinogenic amino acid structurally related to methionine with an ethyl group in place of a methyl group attached to the sulfur atom. Ethionine is a substrate of MAT and as such can react with ATP to form S-adenosylethionine, a nonmetabolizable analog of AdoMet (140). In vivo, ethionine causes hepatic AdoMet and ATP depletion, impaired methylation reactions, reduction of glucose and glycogen, lipid accumulation, and drug-induced liver injury (234). Cycloleucine, a nonmetabolizable, nonsubstrate analog of methionine synthesized by cyclization of leucine, is an inhibitor of MAT activity (140) that induces AdoMet depletion and potentiates drug-induced cytochrome P-450 2E1 (CYP2E1)-mediated oxidative stress and toxicity in hepatocytes (279). Nitric oxide (NO) reversibly inhibits liver MAT tripolyphosphatase activity and also induces hepatic AdoMet depletion (58, 203, 222). This inhibitory effect of NO on MAT activity seems to be important for the in vivo regulation of AdoMet concentration during liver regeneration (253; see sect. IVA).
III. METABOLISM OF AdoMet
A. Transmethylation
AdoMet is the principal biological methyl donor made in the cytosol of every mammalian cell, but the liver is where the bulk of AdoMet is generated as it is the site where up to half of the daily intake of methionine is metabolized and up to 85% of all methylation reactions takes place (164, 179). AdoMet is the link to four key metabolic pathways: transmethylation (sect. IIIA), transsulfuration (sect. IIIB), polyamine synthesis (sect. IIIC), and 5′-deoxyadenosyl 5′-radical mediated biochemical transformations (sect. III-D). In transmethylation (FIGURE 2), AdoMet donates its methyl group to a large variety of acceptor molecules in reactions catalyzed by MTs. Over 200 proteins in the human genome have been identified as known or putative AdoMet-dependent MTs (204). These enzymes transfer a methyl group from AdoMet to a variety of nucleophiles, including oxygen, sulfur, nitrogen, and carbon atoms on proteins, nucleic acids, carbohydrates, lipids, and small molecules. The most abundant AdoMet-dependent MT in liver is glycine N-methyltransferase (GNMT), where it accounts for 1% of cytosolic protein (147, 237). Genetic deficiency of GNMT activity in humans has been reported in only three cases (6, 178). Metabolic abnormalities in these patients include elevated plasma methionine and AdoMet, which is consistent with this enzyme being the most abundant MT in liver (6, 178). Patients with GNMT deficiency develop hepatomegaly and liver injury at an early age (6, 178). Similarly, Gnmt knockout (KO) mice spontaneously develop steatohepatitis and HCC (see sect. VIII).
AdoMet-dependent methylation reactions yield AdoHcy as a byproduct. AdoHcy is a potent inhibitor of methylation reactions with an inhibition constant (Ki) that is in the same micromolar range as the Km of most AdoMet-dependent MTs (45, 46). The ratio of AdoMet to AdoHcy is frequently considered as a metabolic gauge controlling in vivo methylation reactions, where a decrease in this ratio predicts reduced methylation capacity. However, it is important to note that since the activity of a given MT depends of its kinetic constants (mainly the Km and Ki for AdoMet and AdoHcy, respectively), the methylation capacity is better determined by measuring the concentration of AdoMet and AdoHcy (69).
AdoHcy cellular content is regulated by the enzyme AdoHcy hydrolase (AHCY, also known as adenosylhomocysteinase), which reversibly cleaves this molecule into adenosine and homocysteine (Hcy) (FIGURE 2) (218). The thermodynamics of AHCY favor AdoHcy biosynthesis rather than hydrolysis (67). In vivo, the reaction proceeds in the direction of hydrolysis only if the products, adenosine and Hcy, are rapidly removed (90, 162), which is essential to prevent accumulation of AdoHcy.
Genetic deficiency of AHCY activity in humans has been reported in only a few cases (11, 12, 21, 82, 258). Metabolic abnormalities in all patients with AHCY deficiency include elevated plasma AdoHcy, AdoMet, and methionine, which confirms the important role of this enzyme in methionine metabolism. Plasma AdoMet and methionine accumulation in AHCY deficiency can be easily explained as the consequence of the inhibition of transmethylation reactions by AdoHcy and, therefore, of AdoMet utilization. Loss of function of the gene encoding AHCY in zebrafish larvae also induces an elevation of the content of AdoHcy and AdoMet with a reduction of the AdoMet-to-AdoHcy ratio (167). AHCY deficiency in zebrafish induced a decrease in global DNA methylation and global protein lysine methylation, which is consistent with a global inhibition of MTs caused by elevated AdoHcy and decreased AdoMet-to-AdoHcy ratio.
Whereas the patients with the mildest clinical symptoms have 20–30% residual AHCY activity and develop myopathy, delayed development, steatosis and abnormal liver function tests, patients with very low or undetectable resid ual AHCY activity have fetal hydrops (probably due to impaired liver function), severe hypotonia/myopathy, feeding problems, respiratory failure, and develop tumors and die soon after birth (11, 12, 21, 82, 258). Since the two published cases of fetal hydrops associated with AHCY deficiency are two siblings (82), it is not possible to exclude that this condition is not due to some other family trait independent of the AHCY deficiency. Hepatic steatosis also develops spontaneously in zebrafish deficient in AHCY activity (167).
AHCY is a reversible enzyme that favors AdoHcy biosynthesis so that impairment in Hcy catabolism will lead to AdoHcy accumulation and inhibition of MTs. In liver, Hcy can undergo remethylation or conversion to cysteine via the transsulfuration pathway. Remethylation of Hcy to form methionine occurs by two enzymes: methionine synthase (MS), which requires normal levels of folate and vitamin B12, and betaine Hcy methyltransferase (BHMT), which requires betaine, a metabolite of choline (FIGURE 2). MS-catalyzed Hcy remethylation requires 5-methyltetrahydrofolate (5-MTHF), which is derived from 5,10-methylenetetrahydrofolate (5,10-MTHF) in a reaction catalyzed by methylenetetrahydrofolate reductase (MTHFR). 5-MTHF is then converted to tetrahydrofolate (THF) as it donates its methyl group and THF is converted to 5,10-MTHF to complete the folate cycle.
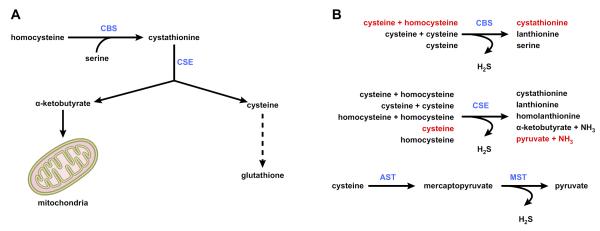
B. Transsulfuration
The transsulfuration pathway (FIGURE 3A) links AdoMet to cysteine biosynthesis. Here Hcy is converted to cysteine (the rate-limiting precursor for GSH synthesis) via a two-step enzymatic process catalyzed by cystathionine β-synthase (CBS) and cystathionase (CSE), both requiring vitamin B6. The transsulfuration pathway is particularly active in the liver, making AdoMet an important precursor of GSH (142). All mammalian tissues express MAT and MS, whereas BHMT is limited to the liver and kidney. In the liver, AdoMet inhibits MTHFR (therefore reduces 5-MTHF, the substrate for MS) and activates CBS (162, 207). This way AdoMet level controls the flux so that when it is depleted, Hcy is channeled to remethylation to regenerate AdoMet, whereas when the level is high, Hcy is channeled to the transsulfuration pathway. Accordingly, whereas mice deficient in BHMT or MTHFR have increased levels of Hcy and AdoHcy as well as reduced AdoMet, CBS deficiency associates with elevated Hcy, AdoHcy, and AdoMet (68).
FIGURE 3.
Methionine metabolism: transsulfuration and hydrogen sulfide synthesis. A: the transsulfuration pathway links AdoMet to cysteine biosynthesis. Here homocysteine is converted to cysteine (the rate-limiting precursor for glutathione) via a two-step enzymatic process catalyzed by cystathionine β-synthase (CBS) and cystathionase (CSE), both requiring vitamin B6. α-Ketobutyrate, the other product of cystathionine cleavage, is further metabolized by the mitochondria through the Kreb's cycle. B: hydrogen sulfide synthesis. Although the classical role of CBS and CSE is to generate cystathionine and cysteine, respectively, these two enzymes catalyze multiple hydrogen sulfide (H2S)-generating reactions using cysteine and homocysteine as substrates. The predominant H2S-generating reactions are shown in red. A third pathway uses aspartate aminotransferase (AST) to yield mercaptopyruvate, which is further converted into H2S in a reaction catalyzed by mercaptopyruvate sulfurtransferase (MST). The function of this pathway is primarily catabolic.
Although the classical role of CBS and CSE is to generate cystathionine and cysteine, respectively, these two enzymes catalyze multiple hydrogen sulfide (H2S)-generating reactions using cysteine and homocysteine as substrates (FIGURE 3B) (104). Whereas H2S production from cysteine is primarily catalyzed by CSE, H2S generation by CBS occurs mainly by condensation of cysteine and homocysteine (104). A third pathway uses aspartate aminotransferase (AST), which also deaminates cysteine to yield mercaptopyruvate that is further converted into H2S and pyruvate in a reaction catalyzed by mercaptopyruvate sulfurtransferase (MST) (FIGURE 3B) (104). Whereas CBS regulates H2S production mainly in the brain, CSE is principally responsible for H2S synthesis in the periphery (75). The high Km of AST for cysteine is consistent with the function of this enzyme, together with MST, in the cysteine catabolic pathway (104).
H2S is a signaling molecule that has been shown to reduce systemic blood pressure causing vasodilation of mesenteric artery, aorta, and portal vein (75). Decreased CSE expression coupled with reduced plasma H2S contributes to the development of portal hypertension (70), the main complication of cirrhotic liver. Cse KO mice develop normally, although they display hypercystationinemia, hyperhomocysteinemia, and reduced levels of serum H2S (100, 269). Hypermethioninemia and hepatic dysfunction/steatosis, a characteristic feature of Cbs KO mice as well as of other mouse models displaying elevated plasma Hcy levels (166), are not observed in Cse-deficient animals (100, 269). These results indicate 1) that peripheral H2S does not play an important role in the initiation of liver injury, although it may be important in the development of portal hypertension in cirrhosis, and 2) that hyperhomocysteinemia alone does not induce hepatic injury in mice.
C. Polyamine Biosynthesis
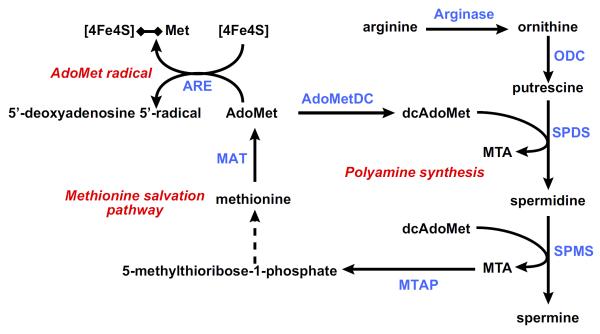
AdoMet is best known as the principal biological methyl donor. However, in addition to this well-recognized function, AdoMet is critically involved in the synthesis of polyamines. Polyamines are low-molecular-weight, positively charged molecules that are ubiquitous in all living cells (201). Polyamines play a crucial role in many biochemical processes including regulation of transcription and translation, cell growth, and apoptosis. To enter this pathway, AdoMet needs first to be decarboxylated, a reaction catalyzed by the enzyme AdoMet decarboxylase, which is the rate-limiting step for polyamine synthesis since decarboxylated AdoMet (dcAdoMet) content is low to not detectable in cells under normal conditions (FIGURE 4). The predominant polyamines in mammalian cells are spermidine (SPD) and spermine (SPM). These polyamines are made by sequential addition of aminopropyl groups from dcAdoMet, yielding MTA as a byproduct. SPD synthase catalyzes the transfer of the first aminopropyl group from dcAdoMet to putrescine to form SPD and MTA, whereas the addition of the second aminopropyl group to the n-10 position of SPD to form SPM and a second molecule of MTA is catalyzed by SPM synthase.
FIGURE 4.
Methionine metabolism: polyamine synthesis, methionine salvation pathway, and AdoMet radical reactions. To synthesize polyamines, AdoMet needs first to be decarboxylated, a reaction catalyzed by the enzyme AdoMet decarboxylase (AdoMetDC), to form decarboxylated AdoMet (dcAdoMet). The predominant polyamines in mammalian cells are spermidine (SPD) and spermine (SPM). These polyamines are made by sequential addition of aminopropyl groups from dcAdoMet, yielding 5′-methylthioadenosine (MTA) as a byproduct. SPD synthase (SPDS) catalyzes the transfer of the first aminopropyl group from dcAdoMet to putrescine to form SPD and MTA, whereas SPM synthase (SPMS) catalyzes the transfer of the second aminopropyl group to SPD to form SPM and a second molecule of MTA. MTA is metabolized through the methionine salvation pathway to regenerate AdoMet. The first reaction in this pathway is the cleavage of MTA by the enzyme MTA phosphorylase (MTAP) yielding adenine and 5-methylthioribose-1-phosphate, which is further metabolized to methionine and adenine to AMP. AdoMet may be also converted to 5′-deoxyadenosyl 5′-radical by a large family of AdoMet radical enzymes (ARE) and initiate a variety of radical chemical reactions. These enzymes share a CX3CX2C motif forming a characteristic [4Fe-4S] cluster. This [4Fe-4S] cluster binds AdoMet catalyzing its reductive cleavage to generate [4Fe-4S]-methionine and a 5′-deoxyadenosyl 5′-radical. ODC, ornithine decarboxylase; MAT, methionine adenosyltransferase.
MTA is a potent inhibitor of SPM synthase with a Ki of ~0.3 μM (89, 192, 193) and a less potent inhibitor of SPD synthase with a Ki of ~2–10 μM (89, 210). This inhibition, however, does not have a great impact on polyamine synthesis in vivo since MTA is rapidly metabolized, through the methionine salvation pathway, to regenerate AdoMet (FIGURE 4) (200). The first reaction in this pathway is the phosphorylation of MTA by the enzyme MTA phosphorylase (MTAP). MTAP cleaves MTA to adenine and 5-methylthioribose-1-phosphate, and the latter compound is further metabolized to methionine. Cells lacking MTAP cannot salvage methionine or adenine from endogenous MTA, which leads to impaired polyamine biosynthesis and to the accumulation of dcAdoMet and MTA. dcAdoMet is an inhibitor of methylation reactions, such as DNA methylation (73, 86), as this compound cannot donate its methyl group. MTA is both an inhibitor of methylation reactions (54, 119, 264) and an inhibitor of AHCY (208). AHCY, as mentioned in section IIIA, is a reversible enzyme that cleaves AdoHcy, a potent inhibitor of methylation reactions, into adenosine and Hcy. Thus MTAP deficiency not only leads to an impaired biosynthesis of polyamines, but also to the inhibition of methylation reactions caused by the cellular accumulation of dcAdoMet, MTA, and AdoHcy.
MTAP has been proposed to be a tumor suppressor gene, and as such, downregulation of MTAP has been associated with tumor progression both in experimental animal models and in humans (18). Loss of Mtap in mice induces embryonic lethality, but heterozygous Mtap KO mice are viable and although indistinguishable from wild-type animals, tend to die prematurely of T-cell lymphoma (103). How MTAP-deficient tumor cells escape from the deleterious effects of an excessive accumulation of MTA is not clear. Changes in AdoMet synthesis (25) and MTA excretion (111) as well as changes in the polyamine biosynthetic and catabolic pathways (108, 109, 199) may be among the mechanisms used by cancer cells to adapt to a deficiency in MTAP activity. Interestingly, MTA has been shown to be pro-apoptotic in Huh-7 and HepG2, two hepatoma cell lines, but anti-apoptotic in normal hepatocytes (4, 9, 275). Similarly, MTA has been shown to be pro-apoptotic in colon cancer cells but not in normal colon epithelial cells (131), and MTA has also been found to reduce the growth of preneoplastic lesions in rat liver during the early stages of hepatocarcinogenesis (197, 198).
Inhibition of residual MTAP with picomolar methylthio-DADMe-immuncillin-A (MTDIA) increases cellular MTA concentration, decreases polyamine synthesis and DNA methylation, and induces apoptosis in FaDu and Cal27, two head and neck squamous cell carcinoma cell lines, but not in normal human cell fibroblasts or in MCF7, a breast cancer cell line where MTAP is deleted (14). Furthermore, inhibition of MTAP with MTDIA produces an increase in the intracellular MTA concentration and blocks A549 (human non-small cell lung carcinoma cells) and H358 (human bronchioalveolar non-small cell lung carcinoma) xenograft tumor growth in immune-deficient nude mice (15).
These findings, along with those made in cells and animal models defective in AdoMet biosynthetic and catabolic pathways (see sects. VII and VIII), indicate that metabolic fluxes through the transmethylation and the polyamine pathways and the consequent changes in levels of various metabolites within the cell (i.e., AdoMet, dcAdoMet, MTA, AdoHcy, and methionine) are critical in tumor development, and that alteration of this onco-metabolic homeostasis, as exemplified by treatment with MTA, can block cell growth and induce apoptosis.
D. AdoMet Radical Reactions
In 1976, Knappe and Schmitt (112) elegantly demonstrated that apart from its well-known function as a methyl donor and precursor of polyamine synthesis, AdoMet also mediates novel radical chemical reactions in organisms grown anaerobically (112). This family of AdoMet radical enzymes currently comprises more than 2,800 members that share a CX3CX2C motif forming a characteristic [4Fe–4S] cluster (72). This [4Fe–4S] cluster binds AdoMet to the only Fe not bound to a cysteine residue catalyzing its reductive cleavage to generate [4Fe–4S]-methionine and a 5′-deoxyadenosyl 5′-radical (FIGURE 4). 5′-Deoxyadenosyl 5′-radical then removes a hydrogen atom from a small molecule, protein, RNA, or DNA substrate to initiate a radical mechanism. AdoMet radical enzymes participate in more than 40 distinct biochemical transformations, and most members have not been biochemically characterized (72). Viperin, an interferon (IFN)-inducible antiviral protein (228), has been recently demonstrated to be an AdoMet radical enzyme, although the substrate for this reaction has not been identified (61). Viperin is one of the IFN-stimulated genes whose expression in liver biopsy specimens of chronic hepatitis C patients is predictive of the response to pegylated-IFN-α/ribavirin treatment (228). Interestingly, AdoMet improves early viral responses and IFN-stimulated gene induction in chronic hepatitis C patients (63, 66), whether AdoMet radical reactions are mediating this effect of AdoMet is unknown.
IV. AdoMet's EFFECT ON GROWTH AND APOPTOSIS
A. AdoMet's Effect on Growth
Accumulating evidence shows that AdoMet regulates cellular proliferation. It is important to point out that the effect is cell-type specific. Thus intracellular AdoMet level increases nearly fivefold during lymphocyte activation, and this increase appears to be essential for activation presumably by providing a source for polyamines (83). This is not the case in hepatocytes, where AdoMet level is related to the differentiation status so that quiescent and proliferating hepatocytes have high and low AdoMet levels, respectively (25, 155). Hepatic AdoMet level is dramatically reduced shortly after two-thirds partial hepatectomy (PH), coinciding with the onset of DNA synthesis and the induction of early response genes (96). Hepatocyte DNA synthesis was inhibited when this fall in AdoMet was prevented by exogenous AdoMet administration (198, 253). This is further illustrated by the Mat1a KO mouse model, which exhibits increased basal hepatocyte proliferation but impaired regeneration following PH (42). In this model, the basal hepatic AdoMet level is reduced but did not change following PH (42). Impaired liver regeneration is also observed in Gnmt KO mice, where hepatic AdoMet levels are markedly elevated (251).
Several molecular mechanisms of AdoMet's growth inhibitory effect in hepatocytes have been elucidated. One important mechanism is the ability of AdoMet to inhibit mitogenic activity of growth factors. Examples include the hepatocyte growth factor (HGF), the most potent hepatocyte mitogen essential for liver regeneration (122), and leptin (214). Both growth factors activate MAT2A transcriptionally and induce hepatocyte DNA synthesis (122, 214). MAT2A induction has been shown to be required for liver cell proliferation (194, 214). AdoMet prevented MAT2A induction and inhibited the mitogenic activity of HGF and leptin (122, 214). Although HGF is a potent mitogen in vitro, it elicits a poor mitogenic response when administered in vivo to normal rats (173). These observations suggest that hepatic parenchymal cells need to be primed to respond to proliferative signals. Two key inflammatory cytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-6, are part of this priming event (52, 268). Both cytokines promote the activation of signaling pathways including NFκB, STAT-3, activator protein 1 (AP-1), and CCAAT/enhancer binding protein β (C/EBP β) that lead to activation of target genes in hepatocytes leading to DNA synthesis and cell proliferation (250). One target gene induced is inducible NO synthase (iNOS), which also occurs before the onset of DNA synthesis (185). Increased NO production is essential for liver regeneration as mice lacking iNOS have impaired liver regeneration (209). NO is known to inactivate MAT I/III (but not MAT II) (see sect. VA), and interestingly, the mitogenic activity of HGF in cultured hepatocytes required iNOS activity and a fall in AdoMet level (77). These observations suggest a scenario where the influx of inflammatory cytokines following PH induces iNOS expression and increases NO production, leading to inactivation of MAT I/III and a fall in AdoMet level. This then releases the inhibitory tone that AdoMet exerts on growth factors. Consistent with this, Mat1a KO mice do not exhibit a fall in AdoMet level following PH, and Mat1a KO hepatocytes are resistant to the mitogenic effect of HGF (42).
Recent work has shed further insight on the HGF-NO link in hepatocyte proliferation. HGF activates the Met receptor tyrosine kinase signaling cascade, leading to the activation of the Ras/ERK/MAPK, phosphatidylinositol 3-kinase (PI3K)/AKT, Rac/Pak (serine/threonine kinase p21-activated kinase), and Crk/Rap1 pathways (20). Recently, a noncanonical signaling pathway was identified for HGF involving serine/threonine kinase 11 (LKB1)/AMP-activated protein kinase (AMPK) that is essential for hepatocyte proliferation (253). LKB1 lies upstream of AMPK, and both are considered commonly as metabolic tumor suppressors (231). However, in hepatocytes, HGF treatment activates LKB1/AMPK signaling, leading to nuclear to cytosolic translocation of the RNA-binding protein HuR, which in turn increases the half-life of target mRNA such as cyclin D1 and A2 to promote hepatocyte proliferation (159, 253). AdoMet can block HGF-mediated activation of LKB1/AMPK signaling by a mechanism that requires protein phosphatase 2A (PP2A) (159), presumably by increasing the level of methylated PP2A (mePP2A), which is known to have higher activity (40, 127). The link between HGF's mitogenic activity and NO is through AMPK, which can phosphorylate and activate endothelial NO synthase (eNOS) (128), and mice lacking eNOS have impaired liver regeneration (253). Taken together, the working scheme is summarized in FIGURE 5 as follows: 1) HGF induces NO production through the activation of eNOS phosphorylation, via LKB1 and AMPK activation, and the eNOS derived NO contributes to the stimulation of NO synthesis via iNOS; 2) this increase in hepatic NO inactivates MATI/III, leading to a fall in AdoMet level and reduction in the mePP2A/demethylatedPP2A ratio; and 3) this reduction in PP2A activity facilitates LKB1/AMPK phosphorylation, cell cycle progression, and growth.
FIGURE 5.
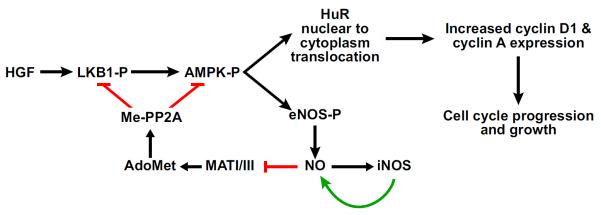
HGF-LKB1/AMPK signaling and AdoMet regulation. Although AMPK and LKB1 are traditionally thought of as metabolic tumor suppressors, they induce hepatocyte proliferation, via HuR cytoplasm translocation and stabilization of cyclin D1 and cyclin A expression, following hepatocyte growth factor (HGF) stimulation, and AdoMet can block this process. Activated AMPK induces the phosphorylation and activation of endothelial nitric oxide synthase (eNOS) leading to the generation of nitric oxide (NO). NO thus generated activates inducible NOS (iNOS) leading to a further increase in NO and the inactivation of MAT I/III. As a result, AdoMet content decreases releasing the inhibitory effect that this molecule exerts on LKB1 and AMPK phosphorylation via methylation and activation of phosphoprotein phosphatases 2A (Me-PP2A).
In the case of leptin, AdoMet was able to block its mitogenic effect in HepG2 cells by preventing leptin-mediated activation of ERK and AKT (214). Interestingly, AdoMet was also shown to block transforming growth factor-β (TGF-β)-mediated ERK activation in hepatic stellate cells (HSCs), and this may be one of its antifibrogenic effects (181). In addition to the liver, AdoMet also blocks the mitogenic effects of leptin, epidermal growth factor (EGF), and insulin-like growth factor (IGF)-I in colon cancer cell lines (41). How AdoMet blocks the effect of these mitogens on ERK, AKT signaling remains largely unknown.
The antiproliferative effect of AdoMet can be recapitulated by MTA (41, 77, 214). As discussed above, MTA is a key metabolite of AdoMet generated in the polyamine pathway as well as a product of AdoMet via nonenzymatic breakdown. In contrast to AdoMet, MTA is not a methyl donor and inhibits polyamine synthesis and MTs by multiple mechanisms (48, 54). While AdoMet is highly unstable with a short half-life (12 h at 37°C in culture medium), MTA is very stable (41). The rate of conversion from AdoMet to MTA is 0.013 mM per hour per mM AdoMet under the same condition (41). These observations also suggest that many of AdoMet's biological effects, when delivered at pharmacological doses, could be mediated in part through its conversion to MTA.
B. AdoMet's Effect on Apoptosis
AdoMet also regulates hepatocyte's death response. AdoMet is known to ameliorate many liver injuries where apoptosis plays a contributory role (see sect. IX). Part of its beneficial effect is due to the fact that in normal hepatocytes, AdoMet is antiapoptotic (4). The protection may be partly related to raising the antioxidant capacity as a GSH precursor and suppressing TNF-α induction (98, 261). However, while AdoMet protected against okadaic acid-induced apoptosis in normal hepatocytes, it induced apoptosis in liver cancer cell lines HepG2 and Huh-7 (4). MTA also recapitulated these actions. These findings are consistent with the chemopreventive action of AdoMet and MTA in an in vivo model of hepatocarcinogenesis in rats, which was accompanied by increased apoptotic bodies in atypical nodules and HCC foci in AdoMet-treated animals (196).
One mechanism of AdoMet and MTA's differential effect on apoptosis in normal hepatocytes and liver cancer cells is due to their ability to selectively induce Bcl-xS in liver cancer cells but not normal hepatocytes (275). Bcl-xS and Bcl-xL are splice variants of Bcl-x; Bcl-xL is anti-apoptotic, while Bcl-xS is pro-apoptotic (19). Alternative splicing requires dephosphorylation of SR proteins (176), which are specific substrates of protein phosphatase 1 (PP1) (36). Both AdoMet and MTA increased PP1 catalytic subunit mRNA and protein levels and dephosphorylation of SR proteins, leading to increased alternative splicing of Bcl-x (275). This also points out that AdoMet and MTA can affect cellular phosphorylation state in liver cancer cells. AdoMet and MTA also have a selective proapoptotic activity against colon cancer cell lines but not normal colonic epithelial cells (131). However, the mechanism is different from in liver cancer cells, as Bcl-xS expression was not affected. Instead, these molecules inhibited the expression of cellular FLICE inhibitory protein (cFLIP), resulting in activation of procaspase-8, Bid cleavage, and release of cytochrome c from the mitochondria (131). This further illustrates that the effects of these molecules are cell-type specific.
V. METHIONINE ADENOSYLTRANSFERASE GENES AND ISOENZYMES
A. MAT Genes, Isoenzymes, Kinetics, and Posttranslational Regulation
Mammals express two genes, MAT1A and MAT2A, which encode for two homologous MAT catalytic subunits, α1 and α2 (118). MAT1A is mostly expressed in normal liver, and the α1 subunit organizes into two MAT isoenzymes, MAT III (dimer) and MAT I (tetramer) (118). MAT2A is widely distributed, and it encodes for a catalytic subunit (α2) found in the MAT isoenzyme MAT II that also exists in polymeric forms that vary from tissue to tissue (92, 117). Mammals also express a third gene, MAT2B, which encodes for a β-regulatory subunit that regulates the activity of MAT II by lowering the Ki for AdoMet and the Km for methionine (83, 125). Recently we reported that MAT2B encodes for two dominant splicing variants, V1 and V2, and V1 is the β-regulatory subunit but V2 differs from V1 by 20 amino acids in the NH2 terminus (272). MAT2B is expressed in most but not all tissues, and the two variants are differentially expressed in normal tissues; prostate, lung, thyroid, adrenal gland, fetal liver, and brain express mostly V1, whereas skeletal muscle and heart express mostly V2. Thymus and kidney express both variants (272). In addition to interacting with MAT II, both MAT2B variants can interact with many other proteins, including HuR (267), and participate in the regulation of growth and apoptosis (155, 272) by mechanisms that appear to be independent of AdoMet.
MAT isoenzymes differ in kinetic and regulatory properties and sensitivities to inhibitors of MAT (146). MAT III has highest (215 μM to 7 mM), MAT I has intermediate (23 μM to 1 mM), and MAT II has the lowest (~4–10 μM) Km for methionine (146). AdoMet strongly inhibits MAT II (IC50 = 60 μM), whereas it minimally inhibits MAT I (IC50 = 400 μM) and stimulates MAT III (up to 8-fold at 500 μM AdoMet) (238). The Km for ATP is also highest for MAT III (1–2 mM), intermediate for MAT I (0.2–0.5 mM), and lowest for MAT II (70 μM) (186, 190). Since the intracellular methionine concentration in hepatocytes is in the range of 50–80 μM (67), MAT II and MAT I would be the predominantly active MAT under physiological conditions, assuming equal amounts of isoenzymes are present. Given the difference in kinetics and regulatory properties, the type of MAT isoenzyme expressed can influence cellular AdoMet levels. Consistent with this, cells that express MAT1A have much higher steady-state AdoMet levels than cells that express MAT2A (25). Cells that express only MAT II have AdoMet levels that should be relatively unaffected by fluctuations in methionine availability because of negative feedback inhibition. However, expression of MAT2B can influence this as the β regulatory subunit renders MAT II more susceptible to feedback inhibition by AdoMet (83). Indeed, during lymphocyte activation, the β-subunit disappears, which may explain the nearly fivefold increase in AdoMet levels compared with resting cells (83). In liver cancer cells, overexpression of MAT2B lowered (155), while knockdown of MAT2B raised AdoMet content (214). In contrast, in cells that express only MAT1A, AdoMet level increases with increasing methionine availability (67), which agrees with the observation that both methionine and AdoMet activate MAT III (24, 58).
MAT isoenzymes differ in responsiveness to sulfhydryl modifying agents. This is because the cysteine at position 121, conserved in rat and human MAT α1 but absent in α2, is a target of covalent modification (10, 203, 222, 226). Based on the model structure of the rat MAT I/III, cysteine 121 is localized at a “flexible loop” over the active site cleft of MAT (203, 226). Although this cysteine is not essential for activity, when cysteine is modified either by oxidation or nitrosylation, the enzyme is inactivated (10, 174, 203, 222, 226). GSH and other thiol-reducing agents can reverse this inactivation. MAT III (dimer) requires 3 mM while MAT I (tetramer) requires 25 mM GSH to reverse the inactivation (226). Since normal hepatic GSH concentration is 5–10 mM, the difference may also contribute to the selective loss of the tetramer in liver disease (23, 51). MAT I/III nitrosylation and inactivation have been demonstrated both in vitro and in vivo in animals treated with LPS (10, 222). MAT I/III have been shown to be phosphorylated by PKC (191), but it did not alter the overall kinetic parameters and is unclear whether phosphorylation plays any physiological role in the regulation of the enzyme activity. Whether MAT II is regulated by phosphorylation is unknown.
In addition to catalyzing the synthesis of AdoMet in cytosol, recent work from Reytor et al. (217) showed that MAT I/III exhibit two partially overlapping areas at its COOH-terminal end that are involved in cytoplasmic retention and nuclear localization. Both tetrameric and monomeric forms of α1 are localized to the nucleus. The authors speculate that the presence of nuclear MAT might be involved in providing a continuous source of nuclear AdoMet. In support of this, nuclear accumulation of the active MAT1A protein correlated with higher levels of histone H3K27 trimethylation, an epigenetic modification associated with gene repression and DNA methylation (217). We reported that MAT2B variants are largely localized to the nucleus and interact with many proteins including HuR, the RNA binding protein known to stabilize many mRNAs (267). Recently, MAT II has also been reported to be mostly nuclear where it serves in conjunction with the β-subunit as a transcriptional corepressor complex of MafK (107). These investigators found that MAT II interacts with many nuclear proteins and may provide a source of AdoMet for methylation of histones or DNA to participate in chromatin-based regulations (107). This accumulating evidence suggests the products of MAT genes are involved in regulation of many pathways, some of which are chromatin-based and some may be independent of AdoMet.
B. MAT Gene Regulation and Dysregulation
Fetal liver expresses MAT2A and MAT2B but not MAT1A (78, 272). MAT1A expression increases a few days after birth and progressively takes over so that the adult liver (mostly hepatocytes) expresses mainly MAT1A, very little MAT2A, or MAT2B (78, 272). Thus MAT1A can be considered a marker of differentiated liver phenotype (164). MAT1A expression is stimulated by glucocorticoid and cAMP (47, 78, 225, 277). Glucocorticoid also induced human MAT1A promoter activity and expression (277). Chronic hypoxia decreased rat MAT1A gene expression (8, 38). Whether the same occurs for the human gene is unknown. In the rat, TNF-α was shown to downregulate MAT1A expression, an action mediated by acidic sphingomyelinase and was achieved via accelerated mRNA degradation (150). Recently, thyroid hormone T3 was shown to positively regulate human MAT1A indirectly via C/EBP α and β at the transcriptional level (266). Several putative C/EBP binding sites are present in the human and murine MAT1A promoter region, and although the human gene is regulated by both C/EBPα and β, only C/EBPβ has been shown to positively regulate the murine gene (99, 266). Lastly, sterol regulatory element-binding protein 1a (SREBP1a), a transcription factor that regulates transcription of many genes involved in fatty acid biosynthesis, was recently identified to positively regulate MAT1A at the mRNA level, although the precise mechanism is unclear (259).
Hepatic MAT1A gene expression is downregulated in HCC (26), during dedifferentiation (146, 254), in most cirrhotic patients (7), and in patients with alcoholic hepatitis (123). The mechanisms of MAT1A downregulation occur both at transcriptional and posttranscriptional levels. The rat and murine MAT1A expression is regulated by epigenetics, namely, promoter methylation and histone acetylation (99, 245). Elevated levels of histone acetylation help to maintain a decondensed and active state of the chromatin, and the underlying pattern of CpG methylation modulates histone acetylation (44, 233). Indeed, the degree of acetylation of histones (H4) associated with the rat MAT1A promoter in the liver is ~15-fold higher than in the kidney (245). The human MAT1A gene is similarly regulated as its expression can be induced by treatment with a demethylating agent or inhibitor of histone deacetylase in HepG2 cells (245). Furthermore, in HepG2 cells and human cirrhotic livers, decreased MAT1A expression is associated with hypermethylation of a HpaII site at −977 position (numbered relative to the translational start site) of the human MAT1A promoter (7, 245). However, more recent examination showed that methylation at +10 and +88, but not −977, correlated better with lack of human MAT1A expression in HCC (242). MAT1A promoter construct methylated at +10 or +88, but not −977, exhibited reduced reporter activity. This was due to reduced transcription. Thus, instead of promoter hypermethylation, it is coding region methylation close to the translational start site of human MAT1A that can interfere with gene transcription (242).
One mechanism of posttranscriptional regulation of human MAT1A involves binding of the AU-rich RNA binding factor 1 (AUF1) to the MAT1A 3′-UTR region (254). AUF1 is known to destabilize target mRNAs (278). HCC specimens express higher AUF1 protein levels, and knockdown of AUF1 increased MAT1A mRNA level (254). In addition, AUF1 expression is high in fetal liver and falls during liver development, which coincides with increased MAT1A expression (254).
While MAT1A is a marker for normal differentiated liver, MAT2A is a marker for rapid liver growth and dedifferentiation. MAT2A is transcriptionally induced in human HCC (26), during rapid liver growth, dedifferentiation, and in response to ethanol feeding in rodents (96, 97, 145). Like MAT1A, MAT2A is also regulated at transcriptional and posttranscriptional levels. We have identified four cis-acting elements and trans-activating factors (Sp1, c-Myb, NFκB, and AP-1) to be involved in MAT2A transcriptional upregulation in HCC (271, 276). TNF-α upregulates MAT2A via NFκB and AP-1 (276). During liver regeneration in rats, MAT2A induction occurs during hepatocyte priming (G0-G1 transition) and at the G1-S interface, and Sp1 plays a critical role for MAT2A induction during the second phase (219). There is an adjacent E2F binding site in both human and rat MAT2A promoter, and cooperation between E2F and Sp1 occurs to induce the MAT2A expression (219). Promoter methylation and histone acetylation status also regulate MAT2A expression. Specifically, the human MAT2A promoter is hypomethylated in HCC but hypermethylated in normal liver (270). Histone acetylation status correlates with MAT2A expression in both human and rat so that a hyperacetylated status correlates with high MAT2A expression (122, 270). Indeed, HGF induces MAT2A expression in rat hepatocytes via stimulation of histone (H4) acetylation associated with the MAT2A promoter (122). Recently, Liu et al. (137) reported that hypoxia-inducible factor 1α (HIF-1α) can induce the human MAT2A promoter activity that was abolished when the putative HIF-1α binding motif was mutated, confirming its functionality (137). This may represent another mechanism of MAT2A induction in HCC as HIF-1α is often overex-pressed (137).
HuR and methylated HuR regulate MAT2A expression at the posttranscriptional level (254). While HuR is a ubiquitously expressed RNA binding protein known to stabilize its target mRNAs, methylated-HuR exerts the opposite effect (254). During hepatocyte dedifferentiation and in HCC, methylated-HuR level falls while HuR level increases, and there is a switch from methylated-HuR to HuR binding to the 3′-UTR of MAT2A, resulting in increased MAT2A mRNA level. The opposite occurs during fetal development, resulting in a fall in MAT2A expression (254).
Similar to MAT2A, MAT2B is also expressed minimally in normal adult liver (272). In human liver, MAT2B expression is increased in cirrhosis and HCC (155, 272). Of the two dominant MAT2B splicing variants, TNF-α induces V1 expression (but not V2) at the transcriptional level by mechanisms that involve AP-1 and NFκB (272). Leptin induces the expression of both MAT2A and MAT2B at the transcriptional level in human hepatoma cells by mechanisms that involve ERK and AKT signaling (214). This induction is essential for leptin to exert its mitogenic effect (214). Since leptin is a profibrogenic factor and MAT2A and MAT2B are essential for growth, we also examined their expression during HSC activation in vitro and in vivo. Both genes are highly induced during HSC activation and are required for HSCs to be activated. During HSC activation, there is a fall in cellular AdoMet level and global DNA hypomethylation. MAT2A induction appears to be essential to provide a source of AdoMet for the growing cells, while MAT2B (not MAT2A) expression is required for ERK and PI3K signaling to occur so that blocking the expression of either MAT2A or MAT2B inhibited HSC activation (213). MAT2B expression is also required for leptin-mediated activation of ERK, PI3K, and STAT3 in hepatoma cells (214). The molecular mechanism(s) of how MAT2B regulates these signaling pathways remains to be elucidated. Whether MAT2B is regulated posttranscriptionally is unknown.
While MAT expression can influence the steady-state AdoMet level (25), AdoMet level can influence MAT expression in return. MAT1A expression falls while MAT2A is induced in primary cultures of hepatocytes due to dedifferentiation (76, 241). This change is prevented by the addition of AdoMet. The opposite occurs with MAT2A so that MAT2A gene expression is rapidly induced when AdoMet level falls (by restricting l-methionine in medium) and downregulated when AdoMet is added (156, 270). AdoMet treatment results in higher methylated-HuR level, which destabilizes the MAT2A mRNA and may explain its inhibitory effect on MAT2A expression (254). AdoMet can also inhibit MAT2B expression at baseline and prevent its induction by leptin in hepatoma cells (214). TABLE 1 summarizes properties and regulation of mammalian MATs.
Table 1.
Properties and regulation of mammalian MAT genes
| MAT Gene | Protein Product | Isoenzymes | Km for Methionine | Inhibition by | Expression Pattern in Healthy Liver | HCC | Reference Nos. |
|---|---|---|---|---|---|---|---|
| MAT1A | α1 | MATI-(α1)4 | 23 μM-1 mM | ROS, NO (activity) | High | Low | 26, 78, 117, 164 |
| MATIII-(α1)2 | 215 μM-7 mM | High | Low | ||||
| MAT2A | α2 | MATII-Structure varies in tissue-specific manner | 4–10 μM | AdoMet (activity and mRNA level) | Low | High | 26, 78, 117, 164, 214 |
| MAT2B | β (V1) | Interacts with MATII | Lowers Km of MATII | AdoMet (mRNA level) | Low | High | 117, 155, 214, 272 |
| V2 | Interacts with MATII | ? | ? | Low | High |
See text for details.
VI. GLYCINE N-METHYLTRANSFERASE
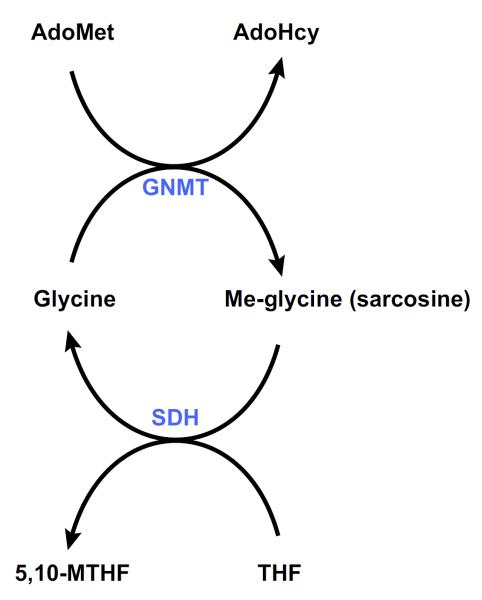
After a protein-rich meal, serum methionine concentration increases transiently as it gets rapidly metabolized in the liver through the concerted action of MAT III and GNMT, which are allosterically activated by methionine and AdoMet, respectively (161). The products of the reaction catalyzed by GNMT are AdoHcy and sarcosine (FIGURE 6). Sarcosine has no known essential metabolic function and is demethylated by the mitochondrial flavoprotein sarcosine dehydrogenase (SDH) to regenerate glycine. During this reaction, THF bound to SDH is converted to 5,10-MTHF as a byproduct (FIGURE 6) (206). 5,10-MTHF is then used to regenerate methionine, via its conversion to 5-MTHF, an inhibitor of GNMT (FIGURE 2). Through this intricate set of reactions, the methyl group of AdoMet used by GNMT is not lost but is saved from being used in undesired methylation reactions of nucleic acids, proteins, etc., to be recovered, via the folate cycle, to regenerate methionine (see sect. IIIA). Together, these results indicate that the function of GNMT is to act as a cellular buffer that maintains constant the concentration of AdoMet.
FIGURE 6.
AdoMet catabolism. Excess AdoMet generated in the liver after a protein-rich meal is rapidly eliminated by the enzyme glycine N-methyltransferase (GNMT), which catalyzes the synthesis of methyl-glycine (sarcosine) from glycine. Sarcosine dehydrogenase (SDH) is a flavoprotein that catalyzes the oxidative demethylation of sarcosine to glycine. In this reaction, tetrahydrofolate (THF) is converted to 5,10-methyllenetrahydrofolate (5,10-MTHF).
Consistent with this concerted action of MAT III/GNMT to maintain AdoMet concentration within a tight range and to prevent aberrant methylation reactions, the tissue expression profile of GNMT parallels that of MAT1A. Thus GNMT is present in large amounts in the liver (1–3% cytosolic protein) and in pancreas and prostate (0.4% of cytosolic protein) and in smaller amounts in other tissues such as kidney tubules, submaxillary glands, intestinal mucosa, cortical neurons, and Purkinje and Schwann cells (149). Similar to MAT1A, the expression of GNMT is minimal in livers of embryos, increases rapidly after birth, and is low or undetectable in liver and prostate tumors; both MAT1A and GNMT expression are induced by glucocorticoids (79, 149). As it occurs with MAT1A, GNMT is also expressed in the nuclei of liver cells, as well as other cells, although in small amounts (149). The function of nuclear GNMT is still a matter of speculation, but a simple hypothesis is that the content of AdoMet in this organelle needs to be particularly well regulated to prevent aberrant epigenetic modifications.
In Gnmt KO mice, hepatic AdoMet concentration is increased ~40-fold, methionine is increased ~7-fold, AdoHcy content is reduced ~3-fold, and the AdoMet-to-AdoHcy ratio, which is an index of the liver methylating capacity, is increased ~100-fold (148, 158). Consistently there is global DNA hypermethylation and increased histone methylation (148, 158). These results confirm the hypothesis that the main biological function of GNMT is to regulate AdoMet content and thus control the AdoMet-to-AdoHcy ratio. In patients with GNMT deficiency and in Gnmt KO mice, serum methionine is also markedly increased (6, 158, 178). Because MAT1A expression is not affected in Gnmt KO mice (158) and MAT III has a large capacity to catabolize methionine and is activated by both methionine and AdoMet (see sect. VA), it is puzzling that Gnmt KO mice have increased serum and liver methionine levels. One possible explanation is that GNMT deficiency causes an increased flux of AdoMet through the methylation-independent pathways (polyamine biosynthesis and the AdoMet radical superfamily) leading to a rapid regeneration of methionine (see FIGURE 4 and sect. III, C and D). Since methionine is rapidly and reversibly transported through the hepatocytes (116), this may provide a mechanism to explain why serum and liver methionine levels are increased in Gnmt KO mice. Increased serum methionine may also result from the inhibition of MAT II in extrahepatic tissues caused by AdoMet effluxing from the liver. However, none of these hypotheses has been experimentally explored.
VII. CONSEQUENCES OF CHRONIC HEPATIC AdoMet DEFICIENCY
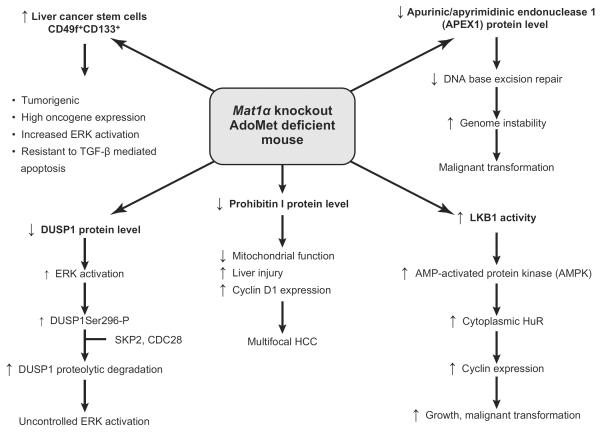
The Mat1a KO mouse model has provided much insight into the consequences of chronic hepatic AdoMet deficiency and altered signaling pathways that may lead to malignant degeneration. This model is highly relevant to human liver disease as MAT1A expression is markedly diminished in the majority of patients with cirrhosis (7). This is the result of reduced or absent MAT1A mRNA and inactivation of MAT I/III as a result of nitrosative and oxidative stress (164). Reduced hepatic MAT activity also explains why many cirrhotic patients have hypermethioninemia and delayed plasma clearance of methionine after intravenous injection (7, 94, 110, 223). Recently, methionine metabolism (whole body flux of methionine, its rate of transmethylation and transsulfuration) has been studied in patients with nonalcoholic steatohepatitis (NASH) after intravenous injection of trace amounts of l-[1-13C]methionine and l-[C2H3]methionine. A lower rate of methionine transmethylation in NASH compared with control subjects was observed, suggesting that hepatic MAT activity is impaired in these subjects (105). Hypermethioninemia also occurs in Mat1a KO mice, confirming the importance of MAT1A in methionine clearance (144). Mat1a KO mice appear normal at a young age but are more susceptible to steatosis induced by a choline-deficient diet and liver injury induced by CCl4, and they develop NASH and HCC spontaneously on a normal diet by 8 and 18 mo, respectively (144, 154). The following summarizes the current understanding of pathways that are abnormal in this model that may contribute to the phenotype observed (FIGURE 7).
FIGURE 7.
Signaling pathways leading to HCC in AdoMet-deficient Mat1a KO mice. Several abnormal pathways have been identified in Mat1a KO mice that can contribute to hepatocellular carcinoma (HCC) formation: 1) a fall in apurinic/apyrimidinic endonuclease 1 (APEX1) activity, which leads to a reduction in DNA base excision repair, genome instability, and malignant transformation; 2) an increase in LKB1 activity, which induces the activation of AMPK and an increased translocation of HuR from the nucleus to the cytoplasm leading to the stabilization of several cyclin mRNAs and enhanced growth; 3) a reduction in prohibitin 1 (PHB1) content, which leads to impaired mitochondrial function and liver injury, increased cyclin D1 expression, and multifocal HCC; 4) a decrease in dual-specificity phosphatase 1 (DUSP1), which leads to uncontrolled ERK activation, a hallmark of HCC; and 5) expansion of tumorigenic oval stem cells positive for CD133, a cancer stem cell marker. See text for details.
A. Increased Oxidative Stress
Mat1a KO mice have increased hepatic oxidative stress (154). There are several contributing factors. The first is decreased hepatic GSH level as AdoMet is an important precursor for GSH through the trans-sulfuration pathway (142). Mat1a KO mice have 75 and 40% lower hepatic AdoMet and GSH levels, respectively (144). In addition, there is impaired mitochondrial function and enhanced CYP2E1 activity (154, 227). Thus Mat1a KO mice are more susceptible to liver injury induced by CCl4 (154), a hepatotoxin that requires CYP2E1 to mediate its toxicity (262). Interestingly, AdoMet has been shown to be a noncompetitive inhibitor of CYP2E1 catalytic activity, and depletion of AdoMet sensitizes hepatocytes to CYP2E1 toxicity in vitro while exogenous AdoMet protects against CYP2E1-dependent hepatotoxicity in vivo (35). Regarding impaired mitochondrial function, one of the mechanisms is reduced protein level of prohibitin 1 (PHB1), a well-known mitochondrial chaperone protein (175, 183). PHB1 is one of the proteins found to be downregulated from the time of birth to development of NASH at 8 mo (227). PHB1 is essential for mitochondrial function, and liver-specific Phb1 KO mice have significant liver injury at a young age with markedly abnormal mitochondria and increased oxidative stress (113). Mice lacking Phb1 develop progressive fibrosis and multi-focal HCC by ~8–10 mo of age (113).
Reduced PHB1 protein level is also found in obese patients and ob/ob mice (227). A possible role of PHB1 in HCC development is discussed further below in section VIIG.
B. Abnormal Lipid Homeostasis
Mat1a KO mice have abnormal hepatic lipid homeostasis as evident by increased susceptibility to steatosis induced by a choline-deficient diet and spontaneous development of NASH (144, 154). Recently, the role of MAT1A in very-low-density lipoprotein (VLDL) assembly was investigated. Loss of MAT1A increased the number of VLDL (apoB) particles secreted by 3-mo-old KO mice but decreased the mobilization of hepatic triglycerides (TG) into these particles resulting in lipoproteins that contained less TG and were smaller in diameter (30). Plasma lipid homeostasis was also altered with an increase in lipid transport in low-density lipoprotein (LDL) subclasses and decrease in high-density lipoprotein (HDL) subclasses. Importantly, these abnormalities were corrected after AdoMet treatment for 7 days (30), which normalized hepatic AdoMet levels in these mice (241). Of interest, rats fed a choline-deficient diet, which would deplete hepatic AdoMet content, were noted over 40 years ago to have impaired release of hepatic TGs (139). In addition, recently Walker et al. (259) reported acute knockdown of MAT1A resulted in activation of SREBP-1a, which would enhance lipogenesis (see also sect. IXE). Taken together, normal hepatic AdoMet level is essential to maintain normal lipid homeostasis.
C. Increased Genomic Instability
Several abnormal pathways have been identified in Mat1a KO mice that can contribute to HCC formation. One is increased genomic instability (GI) (241), which is the result of chromosomal rearrangements, duplications, genomic mutations, deletion, and replication errors (49). Acquisition of GI is thought to occur in the early process of carcinogenesis (49). Recently Calvisi et al. (28) showed that early changes in methionine/AdoMet metabolism (fall in AdoMet) and global DNA hypomethylation have a prognostic value for hepatocarcinogenesis in most patients possibly acting through a modulation of GI. Multiple cellular pathways defend against GI; one of the most critical is the apurinic/apyrimidinic endonuclease 1 (APEX1), which is a multifunctional protein possessing both DNA repair and redox regulatory activities (241). Oxidative stress induces APEX1 expression, which is an adaptive response to defend against GI (211). Since Mat1a KO mice have increased oxidative stress, one would expect higher APEX1 expression. However, APEX1 expression was actually downregulated in Mat1a KO mice livers despite increased GI (241). Both mRNA and protein levels of APEX1 were lower in Mat1a KO livers, with the protein level more reduced than the mRNA level, suggesting posttranslational regulation. A useful in vitro model to examine the consequences of MAT1A downregulation and AdoMet depletion is cultured primary hepatocytes, as they rapidly dedifferentiate and lose MAT1A expression (241). Supplementing culture medium with AdoMet can prevent AdoMet depletion, blunt the fall in MAT1A expression, and completely prevent the fall in APEX1 protein level (241). Thus an important consequence of AdoMet depletion is decreased APEX1 protein stability and increased GI, possibly facilitating malignant degeneration.
D. Dysregulated ERK Signaling
AdoMet's ability to stabilize APEX1 is not unique. In subsequent work, Tomasi et al. (243) found that AdoMet also stabilizes dual-specificity phosphatase 1 (DUSP1) (243). DUSP1 belongs to a family of dual-specificity MAPK phosphatases that can dephosphorylate both serine/threonine and tyrosine residues and control MAPK signaling activity (244). In highly malignant HCC, ERK is overexpressed while DUSP1 expression is decreased (243). DUSP1 and ERK regulate each other in a reciprocal manner. Prolonged ERK activation promotes phosphorylation at the Ser296 residue of DUSP1, which renders the DUSP1 protein susceptible to proteasomal degradation (136). In contrast, transient ERK activation activates DUSP1, which in turn shuts off ERK by dephosphorylation (136). Mat1a KO mice livers have increased basal ERK activity (42, 243). On the other hand, hepatic DUSP1 expression is low in Mat1a KO mice both at the mRNA and protein level, with protein level lower than mRNA (243). AdoMet treatment corrected the AdoMet deficiency in Mat1a KO mice and normalized DUSP1 expression (243). AdoMet normalized DUSP1 mRNA level likely by enhancing p53 binding to its consensus element in the DUSP1 promoter, which is known to activate DUSP1 transcriptionally (243). AdoMet also stabilized DUSP1 protein by inhibiting the proteasomal activity, which was increased in Mat1a KO livers (243). Importantly, with normalization of DUSP1 expression, ERK activity returned to that of control level (243). Thus AdoMet deficiency can lead to uncontrolled ERK activity due at least in part to decreased DUSP1 expression.
It should be noted that there are conflicting data on the effect of AdoMet on proteasomal activity. Osna et al. (188) reported that ethanol's inhibitory effect on proteasomal activity in cultured cells may be related to lower AdoMet/AdoHcy ratio, and AdoMet treatment prevented this. French et al. (71) also reported that in an animal model of diet-induced formation of Mallory-Denk bodies (MDBs), there is a shift from 26S proteasome to immunoproteasome formation, which was prevented by AdoMet administration. In our study, incubation of purified 26S proteasomes with AdoMet led to rapid degradation of some of the 26S proteasomal subunits, which was blocked by the proteasome inhibitor MG132 (243). This suggests either AdoMet activates the intrinsic protease activity of the proteasome, leading to degradation of some of its subunits, or it modifies the proteasome posttranslationally, which makes the protein more susceptible to protease degradation. D'Anselmi et al. (53) have also reported a similar inhibition by AdoMet on proteasomal activity. The explanation for the conflicting report is unclear at present.
E. Dysregulated LKB1/AMPK Signaling
In addition to enhanced ERK signaling, Mat1a KO mice livers also exhibit enhanced LKB1 and AMPK activity (250, 253). Although AMPK and LKB1 are traditionally thought of as metabolic tumor suppressors, they induce proliferation in hepatocytes following HGF stimulation, and AdoMet can block this (253) (see sect. IVA). AMPK activation in hepatocytes results in nuclear to cytoplasmic HuR translocation, which leads to stabilization of several cyclin mRNAs to enhance growth (FIGURE 5). AMPK can also activate eNOS, leading to NO formation, which can inactivate MAT I/III and result in lowering of AdoMet level and releasing the inhibitory tone exerted on mitogens (253). Recently, the increase in LKB1 activity in HCC cells derived from Mat1a KO livers was found to be required for cell survival, and increased LKB1 activity was also found in human HCC samples (160). Whether increased LKB1 contributes to tumor growth survival in other models of hepatocarcinogenesis or is specific of the Mat1a KO is not known.
F. Cancer Stem Cell Expansion
In recent years the role of cancer stem cells (or tumor initiating stem cells) has received increasing attention in the pathogenesis of many cancers, including HCC (121, 230). Hepatic cancer stem cells can be derived from hepatic stem/progenitor cells (also known as oval cells) and dedifferentiation of matured hepatocytes, although there is no evidence for the latter (121). Oval cells reside near the terminal bile ducts, at the hepatocyte-cholangiocyte interface. Oval cells are quiescent and few in number in adult normal liver and proliferate only during severe, prolonged liver injury and in models of experimental carcinogenesis (221). Mat1a KO mice have expansion of oval cells as they age (221). A sub-population of these oval cells stains positive for CD133, a cancer stem cell marker, and they are tumorigenic when injected into immune-deficient mice (221). This was the first demonstration of adult liver stem cells possessing tumorigenic potential in a model without using carcinogenic treatment or manipulating the expression of tumor-suppressors or oncogenes. The cancer stem cells from Mat1a KO mice possess highly enhanced MAPK signaling with increased ERK activity (59), similar to Mat1a KO livers (42, 243). These cancer stem cells use their constitutive ERK activation to evade the apoptotic effect of TGF-β, a well-known growth inhibitor in hepatocytes (59, 184). How AdoMet deficiency allows expansion of oval cells remains unknown.
G. Decreased Prohibitin 1 Expression
Using proteomics, prohibitin 1 (PHB1) was identified as one of several mitochondrial proteins downregulated in Mat1a KO livers from birth until development of NASH (227). Similar to APEX1 and DUSP1, AdoMet also stabilizes PHB1 protein during culture of hepatocytes (227). PHB1 belongs to an evolutionary conserved and ubiquitously expressed family of proteins with a variety of suggested activities in different cellular compartments (175, 183). Two homologous PHB proteins, PHB1 and PHB2, form a large multimeric complex that is found largely in the inner mitochondrial membrane where it exerts a chaperone-like function to stabilize newly synthesized mitochondrial proteins (182) and maintains the organization and stability of mitochondrial nucleoids (106). They are essential for mitochondrial function and biogenesis in yeast (235) and mammals (172). PHB1 is also found in the nucleus, where it has been shown to interact with Rb and p53 among other proteins to bring about a change in transcriptional activities of E2F (259) and p53 (74). These nuclear events have been associated with inhibition of cell cycle progression (260) and induction of apoptosis (74). PHB1 was originally cloned from regenerating livers (its expression was found to be nearly absent shortly after 2/3 partial hepatectomy), identified as having antiproliferative activity and thought to be a tumor suppressor (hence its name) (168). However, the tumor suppressor activity of PHB1 is highly controversial as PHB1 expression is actually higher in many transformed cells and tumors (183). Deletion of PHB1 leads to embryonic lethality so that its in vivo function was unexplored until the development of liver-specific Phb1 KO mice (113). There is significant fetal wastage and biochemical and histological liver injury at 3 wk of age. The KO livers have markedly abnormal mitochondria and exhibit increased oxidative stress, progressive fibrosis, and hepatocyte dysplasia. From 20 wk on, KO mice have multiple liver nodules, and from 35 to 46 wk, 38% have multifocal HCC. Since HCC may have resulted from chronic injury and inflammation, the effect of acute PHB1 knockdown was also examined in murine nontransformed AML12 cells. Knockdown by 30% at the protein level raised cyclin D1, H19, and IGF2 expression as well as increased E2F binding to the cyclin D1 promoter and proliferation. The opposite occurred with PHB1 overexpression (113). These results support PHB1 as a tumor suppressor at least in hepatocytes.
VIII. CONSEQUENCES OF CHRONIC HEPATIC AdoMet EXCESS
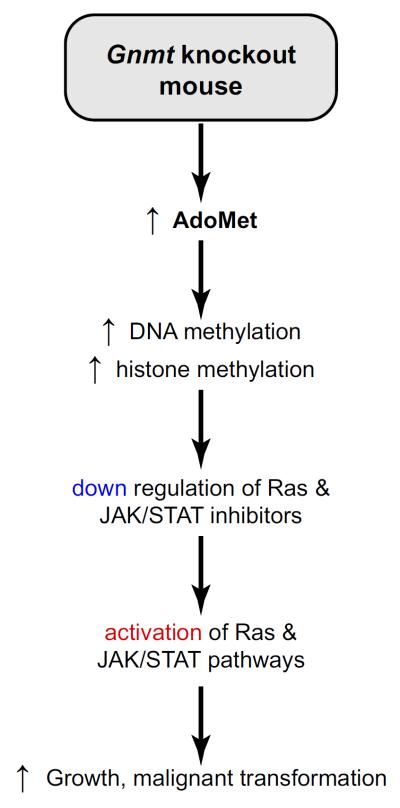
GNMT expression is reduced or absent in HCC (43) and downregulated in the livers of patients at risk of developing HCC, such as in those with hepatitis C virus- and alcohol-induced cirrhosis (7). Moreover, a loss of heterozygosity of GNMT has been reported in ~40% of HCC patients, and GNMT has been proposed to be a tumor-susceptibility gene for liver cancer (43, 246). Identification of several children with mutations of GNMT as having mild to moderate liver disease with elevated serum aminotransferases (6, 178) further suggests that a defect in this enzyme leads to liver disease. Two Gnmt KO mouse models have been developed (133, 148, 158). These mouse models have provided much insight into the consequences of chronic hepatic AdoMet excess and altered signaling pathways that may lead to malignant degeneration. Both mouse models appear normal at birth but develop HCC spontaneously, although at different ages (133, 158). The Gnmt KO mouse model generated by Luka et al. (158) develops NASH and HCC at 3 and 8 mo of age, respectively, whereas the Gnmt KO model generated by Liao et al. (133) develops HCC at 17 mo of age. The reason for these differences is not clear. The following summarizes the current understanding of pathways that are abnormal in Gnmt KO mice that may contribute to the phenotype observed (FIGURE 8).
FIGURE 8.
Signaling pathways leading to HCC in Gnmt KO mice. Loss of GNMT activity induces an abnormal accumulation of hepatic AdoMet leading to a major epigenetic alteration in gene expression regulation. These epigenetic changes include the hypermethylation of Rassf1 and Socs2 promoters, as well as the trimethylation of histone 3 lysine 27 associated with these genes. As a consequence, the expression of Rassf1 and Socs2, as well as of other inhibitors of Ras and JAK/STAT signaling pathways, is downregulated. This leads to the activation of Ras and JAK/STAT signaling pathways, to abnormal growth, and to malignant transformation.
A. Impaired Lipid Metabolism
In Gnmt KO mice, the expression of peroxisome proliferator-activated receptor alpha (PPARα), a lipid-activated transcription factor primarily expressed in the liver that activates fatty acid β-oxidation (126), is downregulated, and the activity of AMPK, a key regulator of cellular energy that once activated switches on the oxidation of fatty acids while switching off the synthesis of lipids (84), is inhibited (252). Additionally, the expression of CD36, a fatty acid translocase whose elevated expression is associated with increased hepatic fatty acid uptake (115); ADFP, a gene related to hepatic lipid droplet formation and whose absence relieves steatosis (37); PPARγ, a transcription factor whose overexpression in the liver induces steatosis (249); and of the Cyp4a omega-oxidation system that metabolizes long-chain and very long-chain fatty acids generating H2O2 and toxic dicarboxylic acids in the process (216) are up-regulated in Gnmt KO mice (252). Serum bile acids, which may contribute to the higher hepatic fatty acid oxidation in NASH (55), are also elevated in Gnmt KO mice (13). Thus both a deficiency and an excess of AdoMet can lead to uncontrolled hepatic lipid metabolism, steatosis, and oxidative stress, albeit with distinct mechanisms (see sect. VIIB). How AdoMet excess influences the expression of these genes involved in lipid metabolism is unclear.
B. DNA and Histone Hypermethylation
As in human HCC, the Ras and JAK/STAT pathways are activated in Gnmt KO mice liver, and also as found in human HCC, activation of these signaling pathways coincided with the downregulation of the expression of the Ras and JAK/STAT inhibitors Rassf1, Rassf4, Socs1, Socs2, Socs3, and Cis (158). These inhibitors of the Ras and JAK/ STAT signaling pathways, which are frequently inactivated by promoter methylation, have been involved in human carcinogenesis (27). Hypermethylation of Rassf1 and Socs2 promoters, and trimethylation of histone 3 lysine 27 (H3K27) bound to Rassf1 and Socs2 promoters, has been identified in livers from Gnmt KO mice. Moreover, and consistent with its function as a cellular AdoMet buffer that prevents the occurrence of aberrant methylation reactions, Gnmt KO mice show global liver DNA hypermethylation (158). Thus absence of GNMT results in increased hepatic AdoMet and aberrant DNA and histone hypermethylation, which can lead to major epigenetic alteration in gene expression regulation.
Consistent with these findings, treatment of Gnmt KO mice with nicotinamide, a substrate of nicotinamide N-methyltransferase (NNMT, an enzyme mainly expressed in the liver), leads to the reduction of hepatic AdoMet content, normalization of lipid metabolism, and prevention of global DNA methylation and of the activation of the Ras and JAK/STAT pathways, fatty liver, and fibrosis formation (252).
C. Expansion of Hepatic Progenitor Cells
Liver regeneration is inhibited in Gnmt KO mice as a result of a sustained increase in AdoMet content (251). The concept of intrahepatic stem/progenitor cells activation to replicate and differentiate to replenish the liver parenchyma when regeneration is suppressed is well established (153). With time, these intrahepatic stem/progenitor cells, which are resistant to liver injury, may selectively proliferate and form preneoplastic nodules that ultimately form liver tumors. Consistent with this concept, stem/progenitor cells staining positive for antibodies to oval cells were visualized in livers of Gnmt KO mice at 8 mo of age (157). Thus both a deficiency and an excess of AdoMet can lead to expansion of liver stem/progenitor cells (see sect. VIIF). Whether there are also cancer stem cells among the progenitor cells in the Gnmt KO livers remains to be examined. How abnormal hepatic AdoMet content sets the stage for normally dormant stem/progenitor cells to replicate and differentiate is not known.
IX. THERAPEUTIC USE OF AdoMet IN LIVER INJURY
AdoMet treatment is well established to ameliorate liver injury in multiple animal models, including galactosamine, acetaminophen, alcohol, thioacetamide, endotoxemia, CCl4, bile duct ligation (BDL), NASH, and ischemia-reper-fusion (34, 35, 114, 162, 165, 273, 274). AdoMet not only protected against acute injury, it also reduced fibrosis in multiple experimental models (35, 165, 274). The underlying mechanisms include AdoMet's ability to raise GSH level (particularly in the mitochondria), inactivate CYP2E1, suppress TNF-α expression, maintain mitochondrial function at least in part via membrane dynamics and changes in mitochondrial proteome, protect hepatocytes against apoptosis, and suppress collagen synthesis by HSCs (3, 22, 35, 164) (see sect. IVB). In hepatocytes, AdoMet not only serves as a precursor for GSH synthesis, it is also able to positively regulate the expression of glutamate-cysteine ligase catalytic (GCL), the rate-limiting enzyme in GSH synthesis (114, 274). Thus, through its multiple actions, AdoMet has been a well-known hepatoprotective agent in experimental liver injury.
Evidence for AdoMet's efficacy in human liver disease is much more limited. In humans, AdoMet is available in oral and parenteral formulations. Bioavailability is much better with parenteral administration. AdoMet was found to be 80–90% bioavailable after an intramuscular injection, with a half-life of 81 min after 100 mg injected dose and 101 min after a 500 mg dose (85). In the United States, AdoMet is only available as an oral dietary supplement, while it is a prescribed medication in Russia, India, China, Italy, Germany, Vietnam, and Mexico (information provided by María Roche, International Pharmaceuticals Abbott). Bio-availabilty of the orally administered AdoMet is poor due to a significant first-pass effect and rapid hepatic metabolism. Peak plasma concentrations of 0.5–1 mg/l are achieved 3–5 h after single AdoMet doses ranging from 400 to 1,000 mg and decline to baseline within 24 h (85). One study showed a gender difference in bioavailability, with women showing three- to sixfold greater peak plasma values than men (85). Plasma-protein binding of AdoMet is no more than 5%, and it crosses the blood-brain barrier, with slow accumulation in the cerebrospinal fluid. Unmetabolized AdoMet is excreted in urine and feces. Thus far, no significant adverse events have been reported in clinical trials using either parenteral or enteral AdoMet (85). However, there is a theoretical concern of administering AdoMet to human immune deficiency (HIV)-infected and other immunocompromised individuals at risk for Pneumocystis carinii infection. This is because P. carinii is an AdoMet auxotroph so that AdoMet administration enhanced its growth and exacerbated the infection in animal models (171). P. carinii actively transports AdoMet, and targeting AdoMet transporters is being proposed as a potential therapy (202). Some species of Rickettsia and Leishmania also do not synthesize AdoMet (202), and it is possible that AdoMet administration could exacerbate these infections as well. The only eukaryotic plasma membrane AdoMet transporters characterized to date are the Saccharomyces cerevisiae SAM3 protein, belonging to the amino acid permease superfamily (220), and the Leishmania AdoMet transporter (AdoMetT1) that belongs to the folate biopterin transporter family (60). In mammalian cells, transport of AdoMet across the plasma membrane to the blood occurs when intracellular AdoMet abnormally accumulates as in Gnmt KO mice and in patients with GNMT or AHCY deficiency (6, 11, 12, 21, 82, 158, 178, 258). This transport mechanism probably operates in the opposite direction after either parenteral or enteral AdoMet administration, or during cell incubation with high AdoMet. The proteins mediating AdoMet transport across the plasma membrane in mammalian cells are not known. Mitochondrial transporters of AdoMet (SAMC) in yeast and mammalian cells have been also functionally characterized. They belong to the mitochondrial carrier protein family (MCF), a class of proteins mediating the transport of various substrates (1, 152). As AdoMet is not synthesized in the mitochondria, the main function of SAMC is probably to facilitate the uptake of AdoMet into the mitochondria in exchange for internal AdoHcy generated by MTs, which is hydrolyzed exclusively in the cytoplasm.
Below are several nonmalignant human liver diseases where AdoMet treatment may be of benefit.
A. Cholestasis of Pregnancy
The Agency for Healthcare Research and Quality (AHRQ) reviewed 102 individual clinical trials of AdoMet in 2002 (85). Forty-one studies were in liver diseases and six were included in a meta-analysis of the efficacy of AdoMet to relieve pruritus and decrease elevated serum bilirubin levels associated with cholestasis of pregnancy (85). AdoMet treatment (mostly 800 mg iv) was associated with a significant decrease in pruritus and serum bilirubin levels compared with placebo (85). AdoMet did not appear to be superior to ursodeoxycholic acid (UDCA), the conventional therapy, and combining AdoMet with UDCA in some studies appeared to be better than either alone (85).
B. Alcoholic Liver Disease
Abnormal methionine metabolism and reduced hepatic AdoMet levels are well established in animal models as well as patients with alcoholic liver disease (ALD) (123, 247). In 2001, the Cochrane Hepato-Biliary Group analyzed 8 clinical trials with a total of 330 ALD patients treated with AdoMet (215). However, most of the studies were small with variable quality, and the meta-analysis did not find a significant benefit of AdoMet treatment. Only 1 trial with 123 patients with alcoholic cirrhosis used adequate methodology and reported clearly on mortality and liver transplantation (163). In this study patients were randomized to receive AdoMet or placebo at 1,200 mg/day in three divided doses for 2 years. Mortality decreased from 30% in the placebo group to 16% in the AdoMet group (P = 0.077), and if patients with more advanced cirrhosis (Child's class C) were excluded (8 patients), the mortality was significantly less in the AdoMet group (12%) compared with the placebo group (25%, P = 0.025). However, this was a subgroup analysis that was data driven so that confirmation with another larger placebo-controlled trial is needed. A smaller trial using the same dosage of AdoMet in less advanced ALD patients showed no benefit of AdoMet on biochemical parameters or histology after 24 wk (169). However, the study included only 24 subjects, and a post-liver biopsy was done in 14 of them due to the high drop-out rate. This along with the short duration of the trial precluded any conclusion to be made.
C. Chronic Hepatitis C Treatment
Recent data suggest AdoMet improves interferon signaling and early viral response to HCV treatment (63, 66). Potential mechanism(s) include increasing STAT1 methylation to enhance STAT1-DNA binding and greater IFN-stimulated gene responses (63). Interestingly, the expression of viperin, one of the IFN-stimulated genes, predicts response to pegylated-IFN-α/ribavirin treatment (228). Since AdoMet is also being used to treat depression (85) and depression is a well-known side effect of IFN, AdoMet may be particularly attractive as adjunct to IFN-based therapy in HCV.
D. Potential Use in Chronic Cholestatic Disorders
While the evidence supporting the use of AdoMet in cholestasis of pregnancy is strong (85), there are no randomized controlled trials that examined the efficacy of AdoMet in chronic cholestatic liver diseases. In the United States, cholestatic liver injury is a major cause of chronic liver disease. Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease in which the only Federal Drug Administration-approved medication is UDCA (120). However, UDCA has no proven efficacy against other chronic cholestatic liver diseases such as primary sclerosing cholangitis and even in PBC, only 25–30% of the patients have a complete response (232). Moreover, high-dose UDCA was actually found to be associated with higher risk of developing colon cancer in patients with primary sclerosing cholangitis and ulcerative colitis (62). Therefore, more effective treatment against chronic cholestatic liver diseases is sorely needed.
We recently reported that in mice subjected to BDL, adding AdoMet to UDCA was better than either treatment alone in protecting the liver against BDL-induced chronic liver injury (274). The molecular mechanism may be related to the ability of AdoMet and UDCA to enhance the nuclear binding activity of Nrf2, a key transcription factor that activates many genes involved in defense against oxidative stress via binding to the antioxidant response element (ARE) present in the promoter region of these genes (142, 273, 274). AdoMet and UDCA seem to act by different mechanisms in this regard as they exerted additive effects when combined (273). Some of these ARE-dependent genes are involved in the biosynthesis of GSH, a key antioxidant that is also known to induce choleresis (65). Combined AdoMet and UDCA treatment protected against the fall in GSH biosynthesis during chronic cholestasis (273, 274). Thus an interesting area to examine is whether AdoMet has a role in the treatment of PBC, where UDCA is ineffective in 40% of patients (232), either alone or in combination with UDCA. If AdoMet is effective, then it can be studied in other chronic cholestatic liver diseases as well.
E. Potential Use in NASH
A diet deficient in methyl groups (methionine and choline, also known as the MCD diet) is known to cause hepatic steatosis for almost 100 years (166), and the steatotic effect of ethionine, an inhibitor of AdoMet biosynthesis, is also known for many years (102). Phenotype of the Mat1a KO mouse links a reduction in the transmethylation capacity of the liver with hepatic steatosis and liver injury (144, 154). Interestingly, downregulation of Mat-1 (also known as sams-1) in Caenorhabditis elegans leads to the accumulation of TG in lipid droplets in the intestine, which was rescued by choline feeding (132), suggesting that the link between AdoMet and fat metabolism is widely extended in nature. Recent elegant work from Walker et al. (259) has provided further insight into the link between AdoMet, transmethylation capacity, and lipogenesis. Their work using C. elegans, mammalian cells, and rodent liver suggests reducing transmethylation capacity by various mechanisms (including knocking down sams-1) can result in decreased phosphatidylcholine biosynthesis, and this in turn affects Golgi membrane dynamics leading to activation of SREBP-1 and lipogenesis (259). Consistent with these findings, Caballero et al. (22) examined the specific contribution of methionine and choline in the development of NASH using the MCD diet in mice. They found that choline deficiency is responsible for steatosis (TG and free fatty acids accumulation) while methionine deficiency is responsible for weight loss, liver injury, oxidative stress, inflammation, and fibrosis (22). Both methionine-deficient diet and MCD diet resulted in significant depletion of mitochondrial AdoMet and GSH pools, and correcting this depletion with GSH prodrugs as well as AdoMet has been reported to be of benefit (22, 34). Taken together, these findings strongly support the use of AdoMet in NASH, and McClain and colleagues (34) have initiated a National Institutes of Health-funded clinical trial examining the efficacy of AdoMet in NASH patients, and results are pending.
X. POTENTIAL USE OF AdoMet IN LIVER CANCER
A. Chemoprevention
The idea of using AdoMet in chemoprevention of HCC is not new as several studies from Feo and co-workers from the mid 1980s on demonstrated in several rodent models of hepatocarcinogenesis that AdoMet is effective in preventing HCC (64, 196–198). In these models, hepatic AdoMet level fell, and several protooncogenes became hypomethylated and their expression induced. The mechanism of chemoprevention was thought to be related to preventing the hypomethylation that occurred. Recently AdoMet's chemopreventive effect was examined in a different HCC model where liver cancer cells were directly injected into the liver parenchyma without changing the hepatic AdoMet level. In this model, AdoMet was able to inhibit the establishment of HCC (147). In addition to AdoMet's selective pro-apoptotic effect in liver cancer cells, AdoMet also exhibited anti-angiogenic properties (147). There are more than 500 million people in the world at risk for HCC development (143). Most of these individuals have chronic hepatitis B or C viral infection. The incidence of HCC is expected to rise in the west. Chemoprevention is an important area that has received very little to no attention (143). Given AdoMet's excellent safety profile, abundance of basic science data showing hepatic AdoMet deficiency predisposes to HCC, and well-established efficacy of AdoMet in preventing HCC, it is important to examine AdoMet in both primary and secondary chemoprevention of HCC in humans. Currently there is one phase II clinical trial that is evaluating AdoMet as a potential chemopreventive agent in patients with hepatitis C cirrhosis (177).
B. Treatment
In an orthotopic xenograft HCC model, AdoMet was ineffective in treating already existing HCC as the normal liver was able to upregulate compensatory mechanisms to dispose excess AdoMet (147). Thus chronic delivery of AdoMet at large doses induced the expression of GNMT in the normal liver so that AdoMet could not accumulate (147). This is important as a certain level of AdoMet may be required to exert its pro-apoptotic and anti-angiogenic effect. The caveat is that in human HCC, GNMT is frequently silenced, so it remains to be examined what the outcome of AdoMet treatment is when GNMT is not expressed. While exogenous AdoMet cannot maintain a high hepatic AdoMet level, forced expression of MAT1A in a liver cancer cell can (129). This strategy inhibited HCC growth in vitro and in vivo, by inhibiting angiogenesis and promoting apoptosis (129). Two key growth pathways that are upregulated in HCC, ERK and AKT, were inhibited when intracellular AdoMet level was doubled by forcing the expression of MAT1A (129). Thus understanding why MAT1A is silenced in HCC is important, as increasing MAT1A expression in HCC can inhibit HCC growth.
XI. ROLE OF AdoMet OUTSIDE OF THE LIVER
A. Lymphocyte Activation
AdoMet level increases during T-lymphocyte activation due to increased MAT2A gene expression and rapid downregulation of the β subunit (83, 240). This was thought to be necessary to provide a source of polyamine biosynthesis, and if AdoMet accumulation is prevented, lymphocyte activation was inhibited (57). More recently, Barve and colleagues (95) found that ethanol inhibited MAT2A transcription to result in lower MAT II activity and AdoMet level in T lymphocytes. This sensitized the CD4+ T lymphocytes to caspase-3-dependent activation-induced cell death and may be one of the explanations for alcohol-induced immune suppression (95). The same group of investigators also found that proliferating and transformed T leukemic cells express higher MAT II activity, and inhibiting MAT II expression in these cells resulted in higher caspase-8-dependent apoptosis (101). These studies support targeting changes in MAT II/AdoMet level as a treatment strategy to either increase, as in the case of ethanol, or inhibit, as in the case of T-cell leukemia. These in vitro studies await confirmation using relevant in vivo models.
B. Depression
The AHRQ also analyzed the efficacy of AdoMet in the treatment of depression (85). They included 28 of 39 studies involving AdoMet treatment in depression in a meta-analysis. When compared with placebo, AdoMet treatment was associated with an improvement of ~6 points in the score of the Hamilton Rating Scale for Depression measured at 3 wk (95% CI 2.2 to 9.0), which was significant statistically and clinically (85). However, AdoMet treatment was not significantly different when compared with conventional antidepressants. The AHRQ concluded, “good dose-escalation studies have not been performed using the oral formulation of AdoMet for depression” and that “additional smaller clinical trials of an exploratory nature should be conducted to investigate uses of AdoMet to decrease the latency of effectiveness of conventional antidepressants and to treat postpartum depression” (85). In light of these studies, the efficacy of AdoMet as an adjunctive treatment for patients who are antidepressant nonresponders has been recently evaluated. In a preliminary open clinical study, AdoMet treatment (400–800 mg/twice daily) was associated with an improvement among 30 outpatients with serotonin reuptake inhibitor (SRI)-resistant major depressive disorder (MDR) (2). More recently, this group carried out a single-center, double-blind, placebo-controlled trial where 73 SRI nonresponders with MDR were treated for 6 wk with AdoMet (800 mg/twice daily) (195). The results of this study suggest that AdoMet can be an effective adjunctive treatment strategy for SRI nonresponders with MDR (180, 195).
C. Arthritis
AHRQ included 10 of 13 studies in osteoarthritis in a meta-analysis of the efficacy of AdoMet to decrease pain of osteoarthritis (85). One large randomized trial showed a decrease in the pain of osteoarthritis with AdoMet treatment compared with placebo and fewer adverse effects compared with treatment with nonsteroidal anti-inflammatory medications without a significant difference in pain reduction. In 2009, the Cochrane Osteoarthritis Group analyzed four clinical trials including 656 patients that compared AdoMet with placebo (224). The Cochrane Group concluded, “the effects of AdoMet on both pain and function may be potentially clinically relevant and, although effects are expected to be small, deserve further clinical evaluation in adequately sized randomized, parallel-group trials in patients with knee or hip osteoarthritis. Meanwhile, routine use of AdoMet should not be advised” (224). Thus, although improving mental and joint health is widely publicized in support of AdoMet use, the overall consensus remains that additional studies are needed.
D. Colon Cancer
Other than HCC, MAT2A expression is also induced in human colon cancers and polyps from Min mice (41). MAT2A induction was required for mitogens like leptin, EGF, and IGF-I to exert their proliferative effect (41). AdoMet and MTA treatment lowered MAT2A expression and inhibited the proliferative effect of these mitogens (41). In addition, AdoMet and MTA exerted a selective proapoptotic effect in colon cancer cells in part through down-regulation of cFLIP (both isoforms) and sensitized colon cancer cells to TNF-α-related apoptosis-inducing ligand (TRAIL)-induced apoptosis (131). Recently, TNF-α was shown to play a key role in the development of inflammation-induced colon cancer model in mice as the TNF-α blocker entanercept reduced the number of colon tumors in this model (205). Since both AdoMet and MTA can inhibit LPS-induced TNF-α expression in macrophages and whole liver (5, 39, 88), we examined whether these agents might be effective in the same model using axozymethane and dextran sulfate sodium to induce colon cancer (130). We found that AdoMet and MTA treatment reduced tumor load by ~40%, which is comparable to several known chemopreventive agents (130). Both treatments inhibited IL-6 expression and IL-6 downstream signaling (STAT3 activation and IL-10) and NFκB and AKT activation, all key pathways that participate in colon carcinogenesis. MTA treatment also reduced the expression of several pro-survival genes such as cFLIP, MAT2A, MAT2B, cyclooxygenase-2, and IL-1β. Interestingly, neither agent exerted any influence on TNF-α or iNOS mRNA levels, even though both were shown to inhibit their expression induced by LPS in macrophages or liver (5, 39). In addition, AdoMet pre-treatment raised hepatic IL-10 level following LPS (114), but in the colon cancer model, it lowered IL-10 level. Thus the effect of these agents on various cytokines and proinflammatory mediators is very much tissue specific. In vivo, both treatments induced apoptosis but inhibited proliferation and β-catenin activation on immunohistochemistry (130). Taken together, AdoMet and MTA appear to target multiple signaling pathways known to be important in colon carcinogenesis. They may have a potential role as chemopreventive agents in patients at risk for colon cancer (such as those with inflammatory bowel disease). AdoMet has a high safety profile, making it particularly attractive. MTA is not yet available for human use but is being developed into a drug by a pharmaceutical company in Spain.
XII. SUMMARY AND FUTURE DIRECTIONS
AdoMet, derived from methionine catabolism in all mammalian cells, is not only the principal methyl donor, but also a precursor of 1) GSH, the major endogenous antioxidant; 2) polyamines, a critical group of molecules involved in the regulation of transcription, translation, cell growth, and apoptosis; 3) MTA, a byproduct of spontaneous AdoMet degradation and polyamine synthesis that mimics many of AdoMet's actions when given in pharmaceutical doses; and 4) 5′-deoxyadenosyl 5′-radical, which can initiate a radical reaction by removing a hydrogen atom from a variety of substrates including proteins and nucleic acids. From NASH to cirrhosis and HCC, methionine metabolism is increasingly impaired. Accordingly, the expression of MAT1A and GNMT, the main two genes involved in hepatic AdoMet synthesis and catabolism, respectively, are markedly diminished in patients with liver cirrhosis and minimal to absent in HCC. Mat1a- and Gnmt-KO mice spontaneously develop NASH and HCC, indicating that the loss in the expression of these two genes in liver cancer is the cause of the carcinogenic process and not its consequence. Downregulation of MAT1A and GNMT in HCC is accompanied by an increased expression of MAT2A and MAT2B. The accumulating evidence indicates that this switch in the expression of MAT and GNMT genes affects multiple pathways, some of which may be independent of AdoMet. Future research should focus on identifying molecular mechanisms responsible for how dysregulation of MAT/GNMT gene expression leads to oxidative stress, impaired lipid metabolism, and malignant transformation. Finally, AdoMet treatment has been shown to ameliorate liver injury in multiple animal models. Although there is evidence for its efficacy in humans, new clinical trials are necessary to unambiguously establish its benefit in several nonmalignant liver diseases such as NASH, chronic cholestatic disorders, alcoholic liver cirrhosis, and hepatitis C, as well as in the chemoprevention of HCC. The therapeutic potential of MTA in both HCC and colon cancer should be also investigated.
ACKNOWLEDGMENTS
GRANTS This work was supported by National Institutes of Health Grants R01DK51719 (to S. C. Lu), R01DK092407 (to S. C. Lu), P30DK48522 (to S. C. Lu), R01AT1576 (to S. C. Lu and J. M. Mato), and R01AT004896 (to S. C. Lu and J. M. Mato) as well as Plan Nacional of I+D SAF 2011–29851 and Departamento de Educación Gobierno Vasco 2011 (to J. M. Mato).
Footnotes
DISCLOSURES No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Agrimi G, Di Noia MA, Marobbio CM, Fiermonte G, Lasorsa FM, Palmieri F. Identification of the human mitochondrial S-adenosylmethionine transporter: bacterial expression, reconstitution, functional characterization and tissue distribution. Biochem J. 2004;379:183–190. doi: 10.1042/BJ20031664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpert JE, Papakostas G, Mischoulon D, Worthington JJ, 3rd, Petersen T, Mahal Y, Burns A, Bottiglieri T, Nierenberg AA, Fava M. S-adenosyl-l-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol. 2004;24:661–664. doi: 10.1097/01.jcp.0000145339.45794.cd. [DOI] [PubMed] [Google Scholar]
- 3.Andringa KK, King AL, Eccleston HB, Mantena SK, Lander A, Jhala NC, Dickinson DA, Squadrito GL, Bailey SM. Analysis of the liver mitochondrial proteome in response to ethanol and S-adenosylmethionine treatments: novel molecular targets of disease and hepatoprotection. Am J Physiol Gastrointest Liver Physiol. 2010;298:G732–G745. doi: 10.1152/ajpgi.00332.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansorena E, García-Trevijano ER, Martínez-Chantar ML, Huang ZZ, Chen L, Mato JM, Iraburu M, Lu SC, Avila MA. S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatology. 2002;35:274–280. doi: 10.1053/jhep.2002.30419. [DOI] [PubMed] [Google Scholar]
- 5.Ara AI, Xia M, Ramani K, Mato JM, Lu SC. S-adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–1666. doi: 10.1002/hep.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augoustides-Savvopoulou P, Luka Z, Karyda S, Stabler SP, Allen RH, Patsiaoura K, Wagner C, Mudd SH. Glycine N-methyltransferase deficiency: a new patient with a novel mutation. J Inherit Metab Dis. 2003;26:745–759. doi: 10.1023/B:BOLI.0000009978.17777.33. [DOI] [PubMed] [Google Scholar]
- 7.Avila MA, Berasain C, Torres L, Martin-Duce A, Corrales FJ, Yang HP, Prieto J, Lu SC, Caballeria J, Rodes J, Mato JM. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–914. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 8.Avila MA, Carretero MV, Rodriguez EN, Mato JM. Regulation by hypoxia of methionine adenosyltransferase activity and gene expression in rat hepatocytes. Gastroenterology. 1998;114:364–371. doi: 10.1016/s0016-5085(98)70489-5. [DOI] [PubMed] [Google Scholar]
- 9.Avila MA, García-Trevijano ER, Lu SC, Corrales FJ, Mato JM. Methylthioadenosine. Int J Biochem Cell Biol. 2004;36:2125–2130. doi: 10.1016/j.biocel.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Avila MA, Mingorance J, Martínez-Chantar ML, Casado M, Martín-Sanz P, Bascá L, Mato JM. Regulation of rat liver S-adenosylmethionine synthetase during septic shock: role of nitric oxide. Hepatology. 1997;25:391–396. doi: 10.1002/hep.510250222. [DOI] [PubMed] [Google Scholar]
- 11.Barić I, Cuk M, Fumić K, Vugrek O, Allen RH, Glenn B, Maradin M, Pazanin L, Pogribny I, Rados M, Sarnavka V, Schulze A, Stabler S, Wagner C, Zeisel SH, Mudd SH. S-Adenosylhomocysteine hydrolase deficiency: a second patient, the younger brother of the index patient, outcomes during therapy. J Inherit Metab Dis. 2005;28:885–902. doi: 10.1007/s10545-005-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barić I, Fumic K, Glenn B, Cuk M, Schulze A, Finkelstein JD, James SJ, Mejaski-Bosnjak V, Pazanin L, Pogribny IP, Rados M, Sarnavka V, Scukanec-Spoljar M, Allen RH, Stabler S, Uzelac L, Vugrek O, Wagner C, Zeisel S, Mudd SH. S-adenosylhomocysteine hydrolase deficiency in a human: a genetic disorder of methionine metabolism. Proc Natl Acad Sci USA. 2004;101:4234–4239. doi: 10.1073/pnas.0400658101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barr J, Vázquez-Chantada M, Alonso C, Pérez-Cormenzana M, Mayo R, Galán A, Caballería J, Martín-Duce A, Tran A, Wagner C, Luka Z, Lu SC, Castro A, Le Marchand-Brustel Y, Martínez-Chantar ML, Veyrie N, Clément K, Tordjman J, Gual P, Mato JM. Liquid chromatography-mass spectrometry-based parallel metabolic profiling of human and mouse model serum reveals putative biomarkers associated with the progression of nonalcoholic fatty liver disease. J Proteome Res. 2010;9:4501–4512. doi: 10.1021/pr1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu I, Cordovano G, Das I, Belbin TJ, Guha C, Schramm VL. A transition state analogue of 5′-methylthioadenosine phosphorylase induces apoptosis in head and neck cancers. J Biol Chem. 2007;282:21477–21486. doi: 10.1074/jbc.M702287200. [DOI] [PubMed] [Google Scholar]
- 15.Basu I, Locker J, Cassera MB, Belbin TJ, Merino EF, Dong X, Hemeon I, Evans GB, Guha C, Schramm VL. Growth and metastases of human lung cancer are inhibited in mouse xenografts by a transition state analogue of 5′-methylthioadenosine phosphorylase. J Biol Chem. 2011;286:4902–4911. doi: 10.1074/jbc.M110.198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beaudouin C, Haurat G, Laffitte JA, Renaud B. The presence of (+)-S-adenosyl-l-methionine in the rat brain and its lack of effect on phenylethanolamine N-methyltransferase activity. J Neurochem. 1993;61:928–935. doi: 10.1111/j.1471-4159.1993.tb03604.x. [DOI] [PubMed] [Google Scholar]
- 17.Bentley R. Role of sulfur chirality in the chemical processes of biology. Chem Soc Rev. 2005;34:609–624. doi: 10.1039/b418284g. [DOI] [PubMed] [Google Scholar]
- 18.Bertino JR, Waudd WR, Parker WB, Lubin M. Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity. Cancer Biol Ther. 2011;11:627–631. doi: 10.4161/cbt.11.7.14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nuñez G, Thompson CB. Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 20.Borowiak M, Garratt AN, Wüstefeld T, Strehle M, Trautwein C, Birchmeier C. Met provides essential signals for liver regeneration. Proc Natl Acad Sci USA. 2004;101:10608–10613. doi: 10.1073/pnas.0403412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buist NR, Glenn B, Vugrek O, Wagner C, Stabler S, Allen RH, Pogribny I, Schulze A, Zeisel SH, Barić I, Mudd SH. S-adenosylhomocysteine hydrolase deficiency in a 26-year-old man. J Inherit Metab Dis. 2006;29:538–545. doi: 10.1007/s10545-006-0240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caballero F, Fernández A, Matías N, Martínez L, Fucho R, Elena M, Caballeria J, Morales A, Fernández-Checa JC, García-Ruiz C. Specific contribution of methionine and choline in nutritional nonalcoholic steatohepatitis. Impact on mitochondrial S-adenosyl-l-methionine and glutathione. J Biol Chem. 2010;285:18528–18536. doi: 10.1074/jbc.M109.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabrero C, Duce AM, Ortiz P, Alemany A, Mato JM. Specific loss of the high-molecular weight form of S-adenosyl-l-methionine synthetase in human liver cirrhosis. Hepatology. 1988;8:1530–1534. doi: 10.1002/hep.1840080610. [DOI] [PubMed] [Google Scholar]
- 24.Cabrero C, Puerta J, Alemany S. Purification and comparison of two forms of S-adenosyl-l-methionine synthetase from rat liver. Eur J Biochem. 1987;170:299–304. doi: 10.1111/j.1432-1033.1987.tb13699.x. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, Mao Z, Hwang JJ, Lu SC. Differential expression of methionine adenosyltransferase genes influences the rate of growth of human hepatocellular carcinoma cells. Cancer Res. 1998;58:1444–1450. [PubMed] [Google Scholar]
- 26.Cai J, Sun W, Hwang JJ, Stain S, Lu SC. Changes in S-adenosylmethionine synthetase in human liver cancer: molecular characterization and significance. Hepatology. 1996;24:1090–1097. doi: 10.1002/hep.510240519. [DOI] [PubMed] [Google Scholar]
- 27.Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Calvisi DF, Simile MM, Ladu S, Pellegrino R, De Murtas V, Pinna F, Tomasi ML, Frau M, Virdis P, De Miglio MR, Muroni MR, Pascale RM, Feo F. Altered methionine metabolism and global DNA methylation in liver cancer: relationship with genomic instability and prognosis. Int J Cancer. 2007;121:2410–2420. doi: 10.1002/ijc.22940. [DOI] [PubMed] [Google Scholar]
- 29.Cannon LM, Butler FN, Wan W, Zhou ZS. A stereospecific colororimetric assay for (S,S)-adenosyl methionine quantification bases on thiopurine methyltransferase-catalyzed thiol methylation. Anal Biochem. 2002;308:358–363. doi: 10.1016/s0003-2697(02)00267-1. [DOI] [PubMed] [Google Scholar]
- 30.Cano A, Buqué X, Martínez-Uña M, Aurrekoetxea I, Menor A, García-Rodriguez JL, Lu SC, Martínez-Chantar ML, Mato JM, Ochoa B, Aspichueta P. Methionine adenosyltransferase 1A gene deletion disrupts hepatic VLDL assembly in mice. Hepatology. 2011;54:1975–1986. doi: 10.1002/hep.24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cantoni GL. The nature of the active methyl donor formed enzymatically from l-methionine and adenosinetriphosphate. J Am Chem Soc. 1952;74:2942–2943. [Google Scholar]
- 32.Cantoni GL. Biochemical methylations: selected aspects. Annu Rev Biochem. 1975;45:285–306. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- 33.Castro A, Le Marchand-Brustel Y, Martínez-Chantar ML, Veyrie N, Clément K, Tordjman J, Gual P, Mato JM. Liquid chromatography-mass spectrometry-based parallel metabolic profiling of human and mouse model serum reveals putative biomarkers associated with the progression of nonalcoholic fatty liver disease. J Proteome Res. 2010;9:4501–4512. doi: 10.1021/pr1002593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007;18:184–195. doi: 10.1016/j.jnutbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 35.Cederbaum AI. Hepatoprotective effects of S-adenosyl-l-methionine against alcohol- and cytochrome P450 2E1-induced liver injury. World J Gastroenterol. 2010;16:1366–1376. doi: 10.3748/wjg.v16.i11.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, Hannun YA. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 37.Chang BH, Li L, Paul A, Taniguchi S, Nannegari V, Heird WC, Chan L. Protection against fatty liver but normal adipogenesis in mice lacking adipose differentiation-related protein. Mol Cell Biol. 2006;26:1063–1076. doi: 10.1128/MCB.26.3.1063-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chawla RK, Jones DP. Abnormal metabolism of S-adenosyl-l-methionine in hypoxia rat liver. Similarities to its abnormal metabolism in alcoholic cirrhosis. Biochim Biophys Acta. 1994;1199:45–51. doi: 10.1016/0304-4165(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 39.Chawla RK, Watson WH, Eastin CE, Lee EY, Schmidt J, McClain CJ. S-adenosylmethionine deficiency and TNFα in lipopolysaccharide-induced hepatic injury. Am J Physiol Gastrointest Liver Physiol. 1998;38:G125–G129. doi: 10.1152/ajpgi.1998.275.1.G125. [DOI] [PubMed] [Google Scholar]
- 40.Chen CL, Lin CF, Chiang CW, Jan MS, Lin YS. Lithium inhibits ceramide- and etopo-side-induced protein phosphatase 2A methylation, Bcl-2 dephosphorylation, caspase-2 activation, and apoptosis. Mol Pharmacol. 2006;70:510–517. doi: 10.1124/mol.106.024059. [DOI] [PubMed] [Google Scholar]
- 41.Chen H, Xia M, Lin M, Yang H, Kuhlenkamp J, Li T, Sodir NM, Chen YH, Josef-Lenz H, Laird PW, Clarke S, Mato JM, Lu SC. Role of methionine adenosyltransferase 2A and S-adenosylmethionine in mitogen-induced growth of human colon cancer cells. Gastroenterology. 2007;133:207–218. doi: 10.1053/j.gastro.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Zeng Y, Yang HP, Lee TD, French SW, Corrales FJ, García-Trevijano ER, Avila MA, Mato JM, Lu SC. Impaired liver regeneration in mice lacking methionine adenosyltransferase 1A. FASEB J. 2004 doi: 10.1096/fj.03-1204fje. 10.1096/fj.03-1204fje. [DOI] [PubMed] [Google Scholar]
- 43.Chen YM, Shiu JY, Tzeng SJ, Shih LS, Chen YJ, Lui WY, Chen PH. Characterization of glycine-N-methyltransferase-gene expression in human hepatocellular carcinoma. Int J Cancer. 1998;75:787–93. doi: 10.1002/(sici)1097-0215(19980302)75:5<787::aid-ijc20>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 45.Chiang PK. Biological effects of inhibitors of S-adenosylhomocysteine hydrolase. Pharmacol Ther. 1998;77:115–134. doi: 10.1016/s0163-7258(97)00089-2. [DOI] [PubMed] [Google Scholar]
- 46.Chiang PK, Gordon RK, Tal J, Zeng GC, Doctor BP, Pardhasaradhi K, McCann PP. S-Adenosylmethionine and methylation. FASEB J. 1996;10:471–480. [PubMed] [Google Scholar]
- 47.Chou JY, Ruppert S, Shelly LL, Pan C. Isolation and characterization of mouse hepatocyte lines carrying a lethal albino deletion. J Biol Chem. 1991;266:5716–5722. [PubMed] [Google Scholar]
- 48.Clarke SG. Inhibition of mammalian protein methyltransferases by 5′-methylthioadenosine (MTA): a mechanism of action of dietary SAMe? The Enzymes. 2006;24:467–493. doi: 10.1016/S1874-6047(06)80018-1. [DOI] [PubMed] [Google Scholar]
- 49.Coleman WB, Tsongalis GJ. Multiple mechanisms account for genomic instability and molecular mutation in neoplastic transformation. Clin Chem. 1995;41:644–657. [PubMed] [Google Scholar]
- 50.Cornforth JW, Reichard SA, Talalay P, Carrell HL, Glusker JP. Determination of the absolute configuration at the sulfonium center of S-adenosylmethionine. Correlation with the absolute configuration of the diastereomeric S-carboxymethyl-(S)-methionine salt. J Am Chem Soc. 1977;99:7292–7300. doi: 10.1021/ja00464a032. [DOI] [PubMed] [Google Scholar]
- 51.Corrales F, Cabrero C, Pajares MA, Ortiz P, Martin-Duce A, Mato JM. Inactivation and dissociation of S-adenosylmethionine synthetase by modification of sulfhydryl groups and its possible occurrence in cirrhosis. Hepatology. 1990;11:216–222. doi: 10.1002/hep.1840110210. [DOI] [PubMed] [Google Scholar]
- 52.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1369–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 53.D'Anselmi F, Cucina A, Cavallaro G, Bizzarri M, Cavallaro RA, Fuso A, Cavallaro A, Scarpa S. S-adenosylmethionine inhibits ubiquitin-proteasome system in vitro and on rat vascular smooth muscle cells. Protein Pept Lett. 2008;15:58–62. doi: 10.2174/092986608783330396. [DOI] [PubMed] [Google Scholar]
- 54.Dante R, Anaud M, Niveleau A. Effects of 5′-deoxy-5′-methylthioadenosine on the metabolism of S-adenosyl methionine. Biochem Biophys Res Commun. 1983;114:214–221. doi: 10.1016/0006-291x(83)91615-7. [DOI] [PubMed] [Google Scholar]
- 55.Dasarathy S, Yang Y, McCullough AJ, Marczewski S, Bennett C, Kalhan SC. Elevated hepatic fatty acid oxidation, high plasma fibroblast growth factor 21, and fasting bile acids in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2011;23:382–388. doi: 10.1097/MEG.0b013e328345c8c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De La Haba G, Jamieson GA, Mudd SH, Richards HH. S-adenosylmethionine: the relation of configuration at the sulfonium center to enzymatic activity. J Am Chem Soc. 1959;81:3975–3980. [Google Scholar]
- 57.De La Rosa J, LeGros HL, Jr, Geller AM, Kotb M. Changes in the relative amount of subunits of methionine adenosyltransferase in human lymphocytes upon stimulation with a polyclonal T cell mitogen. J Biol Chem. 1992;267:10699–10704. [PubMed] [Google Scholar]
- 58.Del Pino MM, Corrales FJ, Mato JM. Hysteretic behavior of methionine adenosyltransferase. III. Methionine switches between two conformations of the enzyme with different specific activity. J Biol Chem. 2000;275:23476–23482. doi: 10.1074/jbc.M002730200. [DOI] [PubMed] [Google Scholar]
- 59.Ding W, Mouzaki M, You H, Laird JC, Mato J, Lu SC, Rountree CB. CD133+ liver cancer stem cells from methionine adenosyltransferase 1A-deficient mice demonstrate resistance to transforming growth factor (TGF)-beta-induced apoptosis. Hepatology. 2009;49:1277–1286. doi: 10.1002/hep.22743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dridi L, Ahmed Ouameur A, Ouellette M. High affinity S-adenosylmethionine plasma membrane transporter of Leishmania is a member of the folate biopterin transporter (FBT) family. J Biol Chem. 2010;285:19767–19775. doi: 10.1074/jbc.M110.114520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duschene KS, Broderick JB. The antiviral protein viperin is a radical SAM enzyme. FEBS Lett. 2010;584:1263–1267. doi: 10.1016/j.febslet.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eaton JE, Silveira MG, Pardi DS, Sinakos E, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Lindor KD. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106:1638–1645. doi: 10.1038/ajg.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Feld JJ, Modi AA, El-Diwany R, Rotman Y, Thomas E, Ahlenstiel G, Titerence R, Koh C, Cherepanov V, Heller T, Ghany MG, Park Y, Hoofnagle JH, Liang TJ. S-adenosyl methionine improves early viral responses and interferon-stimulated gene induction in hepatitis C nonresponders. Gastroenterology. 2011;140:830–839. doi: 10.1053/j.gastro.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feo F, Garcea R, Daino L, Pascale R, Pirisi L, Frassetto S, Ruggiu ME. Early stimulation of polyamine biosynthesis during promotion by Phenobarbital of diethylnitrosamine-induced rat liver carcinogenesis. The effects of variations of the S-adenosyl-l-methionine cellular pool. Carcinogenesis. 1985;6:1713–1720. doi: 10.1093/carcin/6.12.1713. [DOI] [PubMed] [Google Scholar]
- 65.Fernandez-Checa J, Lu SC, Ookhtens M, DeLeve L, Runnegar M, Yoshida H, Saiki H, Kannan R, Garcia-Ruiz C, Kuhlenkamp JF, Kaplowitz N. The regulation of hepatic glutathione. In: Tavoloni N, Berk PD, editors. Hepatic Anion Transport and Bile Secretion: Physiology and Pathophysiology. 2nd ed. Raven; New York: 1993. pp. 363–395. [Google Scholar]
- 66.Filipowicz M, Bernsmeier C, Terracciano L, Duong FH, Heim MH. S-adenosyl-methionine and betaine improve early virological response in chronic hepatitis C patients with previous nonresponse. PLoS One. 2010;5:e15492. doi: 10.1371/journal.pone.0015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Finkelstein JD. Methionine metabolism in mammals. J Nutr Biochem. 1990;1:228–237. doi: 10.1016/0955-2863(90)90070-2. [DOI] [PubMed] [Google Scholar]
- 68.Finkelstein JD. Inborn errors of sulfur-containing amino acid metabolism. J Nutr. 2006;136:1750S–1754S. doi: 10.1093/jn/136.6.1750S. [DOI] [PubMed] [Google Scholar]
- 69.Finkelstein JD. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med. 2007;45:1694–1699. doi: 10.1515/CCLM.2007.341. [DOI] [PubMed] [Google Scholar]
- 70.Fiorucci S, Antonelli E, Mencarelli A, Orlandi S, Renga B, Rizzo G, Distrutti E, Shah V, Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–48. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 71.French SW, Bardag-Gorce F, Li J, French BA, Oliva J. Mallory-Denk body pathogenesis revisited. World J Hepatol. 2010;2:295–301. doi: 10.4254/wjh.v2.i8.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frey PA, Hegeman AD, Ruzicka FJ. The radical SAM superfamily. Crit Rev Biochem Mol Biol. 2008;43:63–88. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 73.Frostesjö L, Holm I, Grahn B, Page AW, Bestor TH, Heby O. Interference with DNA methyltransferase activity and genome methylation during F9 teratocarcinoma stem cell differentiation induced by polyamine depletion. J Biol Chem. 1997;272:4359–4366. doi: 10.1074/jbc.272.7.4359. [DOI] [PubMed] [Google Scholar]
- 74.Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem. 2003;278:47853–47861. doi: 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- 75.Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem. 2010;113:14–26. doi: 10.1111/j.1471-4159.2010.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garcia-Trevijano ER, Latasa MU, Carretero MV, Berasain C, Mato JM, Avila MA. S-adenosylmethionine regulates MAT1A and MAT2A gene expression in cultured rat hepatocytes: a new role for S-adenosylmethionine in the maintenance of the differentiated status of the liver. FASEB J. 2000;14:2511–2518. doi: 10.1096/fj.00-0121com. [DOI] [PubMed] [Google Scholar]
- 77.García-Trevijano ER, Martínez-Chantar ML, Latasa MU, Mato JM, Avila MA. NO sensitizes rat hepatocytes to proliferation by modifying S-adenosylmethionine levels. Gastroenterology. 2002;122:1355–1363. doi: 10.1053/gast.2002.33020. [DOI] [PubMed] [Google Scholar]
- 78.Gil B, Casado M, Pajares M, Boscá L, Mato JM, Martín-Sanz P, Alvarez L. Differential expression pattern of methionine adenosyltransferase isoenzymes during rat liver development. Hepatology. 1996;24:876–881. doi: 10.1002/hep.510240420. [DOI] [PubMed] [Google Scholar]
- 79.Gil B, Pajares MA, Mato JM, Alvarez L. Glucocorticoid regulation of hepatic S-adenosylmethionine synthetase gene expression. Endocrinology. 1997;138:1251–1258. doi: 10.1210/endo.138.3.4967. [DOI] [PubMed] [Google Scholar]
- 80.Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Hutchinson CA 3rd, Smith HO, Venter JC. Essential genes of a minimal bacterium. Proc Natl Acad Sci USA. 2006;103:425–430. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Graham DE, Bock CL, Schalk-Hihi C, Lu ZJ, Markham GD. Identification of a highly diverged class of S-adenosylmethionine synthetases in the Archea. J Biol Chem. 2000;275:4005–4059. doi: 10.1074/jbc.275.6.4055. [DOI] [PubMed] [Google Scholar]
- 82.Grubbs R, Vugrek O, Deisch J, Wagner C, Stabler S, Allen R, Barić I, Rados M, Mudd SH. S-adenosylhomocysteine hydrolase deficiency: two siblings with fetal hydrops and fatal outcomes. J Inherit Metab Dis. 2010;33:705–713. doi: 10.1007/s10545-010-9171-x. [DOI] [PubMed] [Google Scholar]
- 83.Halim AB, LeGros L, Geller A, Kotb M. Expression and functional interaction of the catalytic and regulatory subunits of human methionine adenosyltransferase in mammalian cells. J Biol Chem. 1999;274:29720–29725. doi: 10.1074/jbc.274.42.29720. [DOI] [PubMed] [Google Scholar]
- 84.Hardie DG. Sensing of energy and nutrients by AMP-activated protein kinase. Am J Clin Nutr. 2011;93:891S–896S. doi: 10.3945/ajcn.110.001925. [DOI] [PubMed] [Google Scholar]
- 85.Hardy M, Coulter I, Morton SC, Favreau J, Venuturupalli S, Chiappelli F, Rossi F, Orshansky G, Jungvig LK, Roth EA, Suttorp MJ, Shekelle P. S-Adenosyl-l-Methionine for Treatment of Depression, Osteoarthritis, and Liver Disease. Agency for Healthcare Research and Quality; Rockville, MD: Oct, 2002. Evidence Reports/Technology Assessments, No. 64. [PMC free article] [PubMed] [Google Scholar]
- 86.Heby O. DNA methylation and polyamines in embryonic development and cancer. Int J Dev Biol. 1995;39:737–757. [PubMed] [Google Scholar]
- 87.Hegazi MF, Borchardt RT, Schowen RL. Letter: SN2-like transition state for methyl transfer catalyzed by catechol-O-methyl-transferase. J Am Chem Soc. 1976;98:3048–3049. doi: 10.1021/ja00426a079. [DOI] [PubMed] [Google Scholar]
- 88.Hevia H, Varela-Rey M, Corrales FJ, Berasain C, Martinez-Chantar ML, Latasa MU, Lu SC, Mato JM, García-Trevijano ER, Avila MA. 5′-Methylthioadenosine modulates the inflammatory response to endotoxin in mice and in rat hepatocytes. Hepatology. 2004;39:1088–1098. doi: 10.1002/hep.20154. [DOI] [PubMed] [Google Scholar]
- 89.Hibasami H, Borchardt RT, Chen SY, Coward JK, Pegg AE. Studies of inhibition of rat spermidine synthase and spermine synthase. Biochem J. 1980;187:419–428. doi: 10.1042/bj1870419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoffman DR, Marion DW, Cornatzer WE, Duerre JA. S-Adenosylmethionine and S-adenosylhomocysteine metabolism in isolated rat liver. Effects of l-methionine, l-homocysteine, and adenosine. J Biol Chem. 1980;255:10822–10827. [PubMed] [Google Scholar]
- 91.Hoffman JL. Chromatographic analysis of the chiral and covalent instability of S-adenosyl-l-methionine. Biochemistry. 1986;25:4444–4449. doi: 10.1021/bi00363a041. [DOI] [PubMed] [Google Scholar]
- 92.Horikawa S, Ozasa H, Ota K, Tsukada K. Immunohistochemical analysis of rat methionine adenosyltransferase isozymes in developmental liver. FEBS Lett. 1993;330:307–311. doi: 10.1016/0014-5793(93)80894-z. [DOI] [PubMed] [Google Scholar]
- 93.Horikawa S, Tsukada K. Molecular cloning and developmental expression of a human kidney methionine adenosyltransferase. FEBS Lett. 1992;312:37–41. doi: 10.1016/0014-5793(92)81405-b. [DOI] [PubMed] [Google Scholar]
- 94.Horowitz JH, Rypins EB, Henderson JM, Heymsfield SB, Moffitt SD, Bain RP, Chawla RK, Bleier JC, Rudman D. Evidence for impairment of transsulfuration pathway in cirrhosis. Gastroenterology. 1981;81:668–675. [PubMed] [Google Scholar]
- 95.Hote PT, Sahoo R, Jani TS, Ghare SS, Chen T, Joshi-Barve S, McClain CJ, Barve SS. Ethanol inhibits methionine adenosyltransferase II activity and S-adenosylmethionine biosynthesis and enhances caspase-3-dependent cell death in T lymphocytes: relevance to alcohol-induced immunosuppression. J Nutr Biochem. 2008;19:384–391. doi: 10.1016/j.jnutbio.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang ZZ, Mao Z, Cai J, Lu SC. Changes in methionine adenosyltransferase during liver regeneration in the rat. Am J Physiol Gastrointest Liver Physiol. 1998;294:G14–G21. doi: 10.1152/ajpgi.1998.275.1.G14. [DOI] [PubMed] [Google Scholar]
- 97.Huang ZZ, Mato JM, Kanel G, Lu SC. Differential effect of thioacetamide on hepatic methionine adenosyltransferase expression. Hepatology. 1999;29:1471–1468. doi: 10.1002/hep.510290525. [DOI] [PubMed] [Google Scholar]
- 98.Iglesias Ara A, Xia M, Ramani K, Mato JM, Lu SC. S-Adenosylmethionine inhibits lipopolysaccharide-induced gene expression via modulation of histone methylation. Hepatology. 2008;47:1655–1666. doi: 10.1002/hep.22231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ikeda R, Nishida T, Watanabe F, Shimizu-Saito K, Asahina K, Horikawa S, Teraoka H. Involvement of CCAAT/enhancer binding protein-b (C/EBP-b) in epigenetic regulation of mouse methionine adenosyltransferase 1A gene expression. Int J Biochem Cell Biol. 2008;40:1956–1969. doi: 10.1016/j.biocel.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 100.Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, Suematsu M. Cystathionine gamma-lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem. 2010;285:26358–26368. doi: 10.1074/jbc.M110.147439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jani TS, Gobejishvili L, Hote PT, Barve AS, Joshi-Barve S, Kharebava G, Suttles J, Chen T, McClain CJ, Barve S. Inhibition of methionine adenosyltransferase II induces FasL expression, Fas-DISC formation and caspase-8-dependent apoptotic death in T leukemic cells. Cell Res. 2009;19:358–369. doi: 10.1038/cr.2008.314. [DOI] [PubMed] [Google Scholar]
- 102.Jensen D, Chaikoff IL, Tarver H. The ethionine-induced fatty liver: dosage, prevention, and structural specificity. J Biol Chem. 1951;192:395–403. [PubMed] [Google Scholar]
- 103.Kadariya Y, Yin B, Tang B, Shinton SA, Quinlivan EP, Hua X, Klein-Szanto A, Al-Saleem TI, Bassing CH, Hardy RR, Kruger WD. Mice heterozygous for germ-line mutations in methylthioadenosine phosphorylase (MTAP) die prematurely of T-cell lymphoma. Cancer Res. 2009;69:5961–5969. doi: 10.1158/0008-5472.CAN-09-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kabil O, Banerjee R. Redox biochemistry of hydrogen sulfide. J Biol Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalhan SC, Edmison J, Marczewski S, Dasarathy S, Gruca LL, Bennett C, Duenas C, Lopez R. Methionine and protein metabolism in non-alcoholic steatohepatitis: evidence for lower rate of transmethylation of methionine. Clin Sci. 2011;121:179–189. doi: 10.1042/CS20110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kasashima K, Sumitani M, Satoh M, Endo H. Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp Cell Res. 2008;314:988–996. doi: 10.1016/j.yexcr.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 107.Katoh Y, Ikura T, Hoshikawa Y, Tashiro S, Ito T, Ohta M, Kera Y, Noda T, Igarashi K. Methionine adenosyltransferase II serves as a transcriptional corepressor of Maf oncoproteins. Mol Cell. 2011;41:554–566. doi: 10.1016/j.molcel.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 108.Kee K, Foster BA, Merali S, Kramer DL, Hensen ML, Diegelman P, Kisiel N, Vujcic S, Mazurchuk RV, Porter CW. Activated polyamine catabolism depletes acetyl-CoA pools and suppresses prostate tumor growth in TRAMP mice. J Biol Chem. 2004;279:40076–40083. doi: 10.1074/jbc.M406002200. [DOI] [PubMed] [Google Scholar]
- 109.Kee K, Vujcic S, Merali S, Diegelman P, Kisiel N, Powell CT, Kramer DL, Porter CW. Metabolic and antiproliferative consequences of activated polyamine catabolism in LNCaP prostate carcinoma cells. J Biol Chem. 2004;279:27050–27058. doi: 10.1074/jbc.M403323200. [DOI] [PubMed] [Google Scholar]
- 110.Kinsell LW, Harper HA, Barton HC, Michaels GD, Weiss HA. Rate of disappearance from plasma of intravenously administered methionine in patients with liver damage. Science. 1947;106:589–594. doi: 10.1126/science.106.2763.589. [DOI] [PubMed] [Google Scholar]
- 111.Kirovski G, Stevens AP, Czech B, Dettmer K, Weiss TS, Wild P, Hartmann A, Bosser-hoff AK, Oefner PJ, Hellerbrand C. Down-regulation of methylthioadenosine phosphorylase (MTAP) induces progression of hepatocellular carcinoma via accumulation of 5′-deoxy-5′-methylthioadenosine (MTA) Am J Pathol. 2011;178:1145–1152. doi: 10.1016/j.ajpath.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Knappe J, Schmitt T. A novel reaction of S-adenosyl-l-methionine correlated with the activation of pyruvate formate-lyase. Biochem Biophys Res Commun. 1976;71:1110–1117. doi: 10.1016/0006-291x(76)90768-3. [DOI] [PubMed] [Google Scholar]
- 113.Ko K, Iglesias-Ara A, French BA, French SW, Ramani K, Tomasi ML, Lozano JJ, Oh P, He L, Stiles BL, Li TWH, Yang HP, Martínez-Chantar ML, Mato JM, Lu SC. Liver-specific deletion of prohibitin 1 results in spontaneous liver injury, fibrosis and hepatocellular carcinoma in mice. Hepatology. 2010;52:2096–2108. doi: 10.1002/hep.23919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ko K, Yang HP, Noureddin M, Iglesia-Ara A, Xia M, Wagner C, Luka Z, Mato JM, Lu SC. Changes in S-adenosylmethionine and glutathione homeostasis during endotoxemia in mice. Lab Invest. 2008;88:1121–1129. doi: 10.1038/labinvest.2008.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koonen DP, Jacobs RL, Febbraio M, Young ME, Soltys CL, Ong H, Vance DE, Dyck JR. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 2007;56:2863–2871. doi: 10.2337/db07-0907. [DOI] [PubMed] [Google Scholar]
- 116.Korendyaseva TK, Martinov MV, Dudchenko AM, Vitvitsky VM. Distribution of methionine between cells and incubation medium in suspension of rat hepatocytes. Amino Acids. 2010;39:1281–1289. doi: 10.1007/s00726-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 117.Kotb M, Kredich NM. Methionine adenosyltransferase from human lymphocytes. Purification and characterization. J Biol Chem. 1985;260:3923–3930. [PubMed] [Google Scholar]
- 118.Kotb M, Mudd SH, Mato JM, Geller AM, Kredich NM, Chou JY, Cantoni GL. Consensus nomenclature for the mammalian methionine adenosyltransferase genes and gene products. Trends Genet. 1997;13:51–52. doi: 10.1016/s0168-9525(97)01013-5. [DOI] [PubMed] [Google Scholar]
- 119.Kujubu DA, Stimmel JB, Law RE, Herschman HR, Clarke S. Early responses of PC-12 cells to NGF and EGF: effect of K252a and 5′-methylthioadenosine on gene expression and membrane protein methylation. J Neurosci Res. 1993;36:58–65. doi: 10.1002/jnr.490360107. [DOI] [PubMed] [Google Scholar]
- 120.Kumar D, Tandon RK. Use of ursodeoxycholic acid in liver disease. J Gastro Hepatol. 2001;16:3–14. doi: 10.1046/j.1440-1746.2001.02376.x. [DOI] [PubMed] [Google Scholar]
- 121.Kumar M, Zhao X, Wang XW. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: one step closer to personalized medicine? Cell Biosci. 2011;1:5. doi: 10.1186/2045-3701-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Latasa MU, Boukaba A, García-Trevijano ER, Torres L, Rodríguez JL, Caballería J, Lu SC, López-Rodas G, Franco L, Mato JM, Avila MA. Hepatocyte growth factor induces MAT2A expression and histone acetylation in rat hepatocytes. Role in liver regeneration. FASEB J. 2001;10:1096. doi: 10.1096/fj.00-0556fjev1. [DOI] [PubMed] [Google Scholar]
- 123.Lee TD, Sadda ME, Mendler MH, Bottiglieri T, Kanel G, Mato JM, Lu SC. Abnormal hepatic methionine and GSH metabolism in patients with alcoholic hepatitis. Alcoholism Clin Exp Res. 2004;28:173–181. doi: 10.1097/01.ALC.0000108654.77178.03. [DOI] [PubMed] [Google Scholar]
- 124.LeGros LH, Jr, Halim AB, Chamberlin ME, Geller A, Kotb M. Regulation of the human MAT2b gene encoding the regulatory β subunit of methionine adenosyltransferase, MAT II. J Biol Chem. 2001;276:24918–24924. doi: 10.1074/jbc.M102816200. [DOI] [PubMed] [Google Scholar]
- 125.LeGros LH, Jr, Halim AB, Geller A, Kotb M. Cloning, expression, functional characterization of the b regulatory subunit of human methionine adenosyltransferase (MAT II) J Biol Chem. 2000;275:2359–2366. doi: 10.1074/jbc.275.4.2359. [DOI] [PubMed] [Google Scholar]
- 126.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA. 1999;196:7473–7478. doi: 10.1073/pnas.96.13.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leulliot N, Quevillon-Cheruel S, Sorel I, de La Sierra-Gallay IL, Collinet B, Graille M, Blondeau K, Bettache N, Poupon A, Janin J, van Tilbeurgh H. Structure of protein phosphatase methyltransferase 1 (PPM1), a leucine carboxyl methyltransferase involved in the regulation of protein phosphatase 2A activity. J Biol Chem. 2004;279:8351–8358. doi: 10.1074/jbc.M311484200. [DOI] [PubMed] [Google Scholar]
- 128.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase in endothelial cells: evidence for a AMP→Rac→Akt→eNOS pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 129.Li J, Ramani K, Sun Z, Zee C, Grant EG, Yang HP, Xia M, Oh P, Ko K, Mato JM, Lu SC. Forced expression of methionine adenosyltransferase 1A in human hepatoma cells suppresses in vivo tumorigenesis in mice. Am J Pathol. 2010;176:2456–2466. doi: 10.2353/ajpath.2010.090810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li TWH, Yang HP, Peng H, Xia M, Mato JM, Lu SC. Effects of S-adenosylmethionine and methylthioadenosine on inflammation-induced colon cancer in mice. Carcinogenesis. doi: 10.1093/carcin/bgr295. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li TWH, Zhang Q, Oh P, Xia M, Chen H, Bemanian S, Lastra N, Circ M, Moyer MP, Mato JM, Aw TY, Lu SC. S-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cells. Mol Pharmacol. 2009;76:192–200. doi: 10.1124/mol.108.054411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y, Na K, Lee HJ, Lee EY, Paik YK. Contribution of sams-1 and pmt-1 to lipid homoeostasis in adult Caenorhabditis elegans. J Biochem. 2011;149:529–538. doi: 10.1093/jb/mvr025. [DOI] [PubMed] [Google Scholar]
- 133.Liao YJ, Liu SP, Lee CM, Yen CH, Chuang PC, Chen CY, Tsai TF, Huang SF, Lee YH, Chen YM. Characterization of a glycine N-methyltransferase gene knockout mouse model for hepatocellular carcinoma: implications of the gender disparity in liver cancer susceptibility. Int J Cancer. 2009;124:816–826. doi: 10.1002/ijc.23979. [DOI] [PubMed] [Google Scholar]
- 134.Liau MC, Chang CF, Belanger L, Grenier A. Correlation of isozyme patterns of methionine adenosyltransferase with fetal stages and pathological states of the liver. Cancer Res. 1979;39:162–169. [PubMed] [Google Scholar]
- 135.Lin S, Shi Q, Nix FB, Styblo M, Beck MA, Herbin-Davis KM, Hall LL, Simeonsson JB, Thomas DJ. A novel S-adenosyl-l-methionine:arsenic(III) methyltransferase form rat liver cytosol. J Biol Chem. 2002;277:10795–10803. doi: 10.1074/jbc.M110246200. [DOI] [PubMed] [Google Scholar]
- 136.Lin YW, Chuang SM, Yang JL. ERK1/2 achieves sustained activation by stimulating MAPK phosphatase-1 degradation via the ubiquitin-proteasome pathway. J Biol Chem. 2003;278:21534–21541. doi: 10.1074/jbc.M301854200. [DOI] [PubMed] [Google Scholar]
- 137.Liu Q, Liu L, Zhao Y, Zhang J, Wang D, Chen J, He Y, Wu J, Zhang Z, Liu Z. Hypoxia induces genomic DNA demethylation through the activation of HIF-1a and transcriptional upregulation of MAT2A in hepatoma cells. Mol Cancer Ther. 2011;10:1113–1123. doi: 10.1158/1535-7163.MCT-10-1010. [DOI] [PubMed] [Google Scholar]
- 138.Loenen WA. S-adenosylmethionine: jack of all trades and master of everything? Biochem Soc Trans. 2006;34:330–333. doi: 10.1042/BST20060330. [DOI] [PubMed] [Google Scholar]
- 139.Lombardi B, Pani P, Schlunk FF. Choline-deficiency fatty liver: impaired release of hepatic triglycerides. J Lipid Res. 1968;9:437–446. [PubMed] [Google Scholar]
- 140.Lombardini JB, Chou TC, Talalay P. Regulatory properties of adenosine triphosphate-l-methionine S-adenosyltransferase of rat liver. Biochem J. 1973;135:43–57. doi: 10.1042/bj1350043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lombardini JB, Sufrin JR. Chemotherapeutic potential of methionine analogue inhibitors of tumor-derived methionine adenosyltransferases. Biochem Pharmacol. 1983;32:489–495. doi: 10.1016/0006-2952(83)90528-2. [DOI] [PubMed] [Google Scholar]
- 142.Lu SC. Regulation of glutathione synthesis. Mol Asp Med. 2009;30:42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu SC. Where are we in the chemoprevention of hepatocellular carcinoma? Hepatology. 2010;51:734–736. doi: 10.1002/hep.23497. [DOI] [PubMed] [Google Scholar]
- 144.Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci USA. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lu SC, Huang ZZ, Yang HP, Mato JM, Avila MA, Tsukamoto H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in the alcoholic rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G178–G185. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- 146.Lu SC, Mato JM. S-adenosylmethionine in cell growth, apoptosis and liver cancer. J Gastroenterol Hepatol. 2008;23:S73–S77. doi: 10.1111/j.1440-1746.2007.05289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lu SC, Ramani K, Ou XP, Lin M, Yu V, Ko K, Park R, Bottiglieri T, Tsukamoto H, Kanel G, French S, Mato JM, Moat R, Grant E. S-adenosylmethionine in the chemoprevention and treatment of hepatocellular carcinoma in a rat model. Hepatology. 2009;50:462–471. doi: 10.1002/hep.22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Luka Z, Capdevila A, Mato JM, Wagner C. A glycine N-methyltransferase knockout mouse model for human with deficiency of this enzyme. Transgenic Res. 2006;15:393–397. doi: 10.1007/s11248-006-0008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luka Z, Mudd SH, Wagner C. Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J Biol Chem. 2009;284:22507–22511. doi: 10.1074/jbc.R109.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Marí M, Colell A, Morales A, Pañeda C, Varela-Nieto I, Garía-Ruiz C, Fernández-Checa JC. Acidic sphingomyelinase downregulates the liver-specific methionine adenosyltransferase 1A, contributing to tumor necrosis factor-induced lethal hepatitis. J Clin Invest. 2004;113:895–904. doi: 10.1172/JCI19852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Markham GD, Pajares MA. Structure-function relationships in methionine adenosyltransferases. Cell Mol Life Sci. 2009;66:636–648. doi: 10.1007/s00018-008-8516-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marobbio CM, Agrimi G, Lasorsa FM, Palmieri F. Identification and functional reconstitution of yeast mitochondrial carrier for S-adenosylmethionine. EMBO J. 2003;22:5975–82. doi: 10.1093/emboj/cdg574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Marongiu F, Doratiotto S, Montisci S, Pani P, Laconi E. Liver repopulation and carcinogenesis: two sides of the same coin? Am J Pathol. 2008;172:857–864. doi: 10.2353/ajpath.2008.070910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Martínez-Chantar ML, Corrales FJ, Martínez-Cruz A, García-Trevijano ER, Huang ZZ, Chen LX, Kanel G, Avila MA, Mato JM, Lu SC. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;10:1096. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 155.Martínez-Chantar ML, Garcia-Trevijano ER, Latasa MU, Martin-Duce A, Fortes P, Caballeria J, Avila MA, Mato JM. Methionine adenosyltransferase II β subunit gene expression provides a proliferative advantage in human hepatoma. Gastroenterology. 2003;124:940–948. doi: 10.1053/gast.2003.50151. [DOI] [PubMed] [Google Scholar]
- 156.Martínez-Chantar ML, Latasa MU, Varela-Rey M, Lu SC, García-Trevijano E, Mato JM, Avila MA. l-Methionine availability regulates the expression of methionine adenosyltransferase 2A gene in human hepatocarcinoma cells. J Biol Chem. 2003;278:19885–19890. doi: 10.1074/jbc.M211554200. [DOI] [PubMed] [Google Scholar]
- 157.Martinez-Chantar ML, Lu SC, Mato JM, Luka Z, Wagner C, French BA, French SW. The role of stem cells/progenitor cells in liver carcinogenesis in glycine N-methyltransferase deficient mice. Exp Mol Pathol. 2010;88:234–237. doi: 10.1016/j.yexmp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Martínez-Chantar ML, Vázquez-Chantada M, Ariz U, Martínez N, Varela M, Luka Z, Capdevila A, Rodríguez J, Aransay AM, Matthiesen R, Yang H, Calvisi DF, Esteller M, Fraga M, Lu SC, Wagner C, Mato JM. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martínez-Chantar ML, Vázquez-Chantada M, Garnacho M, Latasa MU, Varela-Rey M, Dotor J, Santamaria M, Martínez-Cruz LA, Parada LA, Lu SC, Mato JM. S-adenosylmethionine regulates cytoplasmic HuR via AMP-activated kinase. Gastroenterology. 2006;131:223–232. doi: 10.1053/j.gastro.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 160.Martínez-López N, Varela-Rey M, Fernández-Ramos D, Woodhoo A, Vázquez-Chantada M, Embade N, Espinosa-Hevia L, Bustamante FJ, Parada LA, Rodriguez MS, Lu SC, Mato JM, Martínez-Chantar ML. Activation of LKB1-Akt pathway independent of phosphoinositide 3-kinase plays a critical role in the proliferation of hepatocellular carcinoma from nonalcoholic steatohepatitis. Hepatology. 2010;52:1621–1631. doi: 10.1002/hep.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martinov MV, Vitvitsky VM, Banerjee R, Ataullakhanov FI. The logic of the hepatic methionine metabolic cycle. Biochim Biophys Acta. 2010;1804:89–96. doi: 10.1016/j.bbapap.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mato JM, Alvarez L, Ortiz P, Pajares MA. S-adenosylmethionine synthesis: molecular mechanisms and clinical implications. Pharmacol Ther. 1997;73:265–280. doi: 10.1016/s0163-7258(96)00197-0. [DOI] [PubMed] [Google Scholar]
- 163.Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, García-Buey L, Beltrán J, Benita V, Caballería J, Solà R, Moreno-Otero R, Barrao F, Martín-Duce A, Correa JA, Parés A, Barrao E, García-Magaz I, Puerta JL, Moreno J, Boissard G, Ortiz P, Rodés J. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized placebo-controlled, double-blind, multicentre trial. J Hepatol. 1999;30:1081–1089. doi: 10.1016/s0168-8278(99)80263-3. [DOI] [PubMed] [Google Scholar]
- 164.Mato JM, Lu SC. Role of S-adenosyl-l-methionine in liver health and injury. Hepatology. 2007;45:1306–1312. doi: 10.1002/hep.21650. [DOI] [PubMed] [Google Scholar]
- 165.Mato JM, Lu SC. S-adenosylmethionine. In: Coates PM, Blackman MR, Levine M, Moss J, White JD, editors. Encyclopedia of Dietary Supplements. 2nd ed. Dekker; New York: 2010. pp. 1–6. [Google Scholar]
- 166.Mato JM, Martínez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annu Rev Nutr. 2008;28:273–93. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- 167.Matthews RP, Lorent K, Mañoral-Mobias R, Huang Y, Gong W, Murray IV, Blair IA, Pack M. TNFalpha-dependent hepatic steatosis and liver degeneration caused by mutation of zebrafish S-adenosylhomocysteine hydrolase. Development. 2009;136:865–875. doi: 10.1242/dev.027565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McClung JK, Danner DB, Stewart DA, Smith JR, Schneider EL, Lumpkin CK, Dell'Orco RT, Nuell MJ. Isolation of a cDNA that hybrid selects antiproliferative mRNA from rat liver. Biochem Biophys Res Commun. 1989;164:1316–1322. doi: 10.1016/0006-291x(89)91813-5. [DOI] [PubMed] [Google Scholar]
- 169.Medici V, Virata MC, Peerson JM, Stabler SP, French SW, Gregory JF, 3rd, Albanese A, Bowlus CL, Devaraj S, Panacek EA, Richards JR, Halsted CH. S-adenosyl-l-methionine treatment for alcoholic liver disease: a double-blinded, randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2011;35:1960–1965. doi: 10.1111/j.1530-0277.2011.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Merali S, Clarkson AB., Jr. S-adenosylmethionine and Pneumocystis. FEMS Microbiol Lett. 2004;237:179–186. doi: 10.1016/j.femsle.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 171.Merali S, Vargas D, Frankli M, Clarkson AB., Jr. S-adenosylmethionine and Pneumocystis carinii. J Biol Chem. 2000;275:14958–14963. doi: 10.1074/jbc.275.20.14958. [DOI] [PubMed] [Google Scholar]
- 172.Merkwirth C, Langer T. Prohibitin function within the mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta. 2009;1793:27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 173.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 174.Mingorance J, Alvarez L, Sánchez-Góngora E, Mato JM, Pajares MA. Site-directed mutagenesis of rat liver S-adenosylmethionine synthetase. Biochem J. 1996;315:761–766. doi: 10.1042/bj3150761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mishra S, Murphy LC, Murphy LJ. The Prohibitins: emerging roles in diverse functions. J Cell Mol Med. 2006;10:353–363. doi: 10.1111/j.1582-4934.2006.tb00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Misteli T. RNA splicing: what has phosphorylation got to do with it? Curr Biol. 1999;9:R198–200. doi: 10.1016/s0960-9822(99)80128-6. [DOI] [PubMed] [Google Scholar]
- 177.Morgan TR. Chemoprevention of hepatocellular carcinoma in chronic hepatitis C. Recent Results Cancer Res. 2011;188:85–99. doi: 10.1007/978-3-642-10858-7_7. [DOI] [PubMed] [Google Scholar]
- 178.Mudd SH, Cerone R, Schiaffino MC, Fantasia AR, Minniti G, Caruso U, Lorini R, Watkins D, Matiaszuk N, Rosenblatt DS, Schwahn B, Rozen R, LeGros L, Kotb M, Capdevila A, Luka Z, Finkelstein JD, Tangerman A, Stabler SP, Allen RH, Wagner C. Glycine N-methyltransferase deficiency: a novel inborn error causing persistent isolated hypermethioninaemia. J Inherit Metab Dis. 2001;24:448–464. doi: 10.1023/a:1010577512912. [DOI] [PubMed] [Google Scholar]
- 179.Mudd SH, Poole JR. Labile methyl balances for normal humans on various dietary regimens. Metabolism. 1975;24:721–735. doi: 10.1016/0026-0495(75)90040-2. [DOI] [PubMed] [Google Scholar]
- 180.Nelson JC. S-adenosyl methionine (SAMe) augmentation in major depressive disorder. Am J Psychiatry. 2010;167:889–891. doi: 10.1176/appi.ajp.2010.10040627. [DOI] [PubMed] [Google Scholar]
- 181.Nieto N, Cederbaum AI. S-adenosylmethionine blocks collagen I production by preventing transforming growth factor-β induction of the COL1A2 promoter. J Biol Chem. 2005;280:30963–30974. doi: 10.1074/jbc.M503569200. [DOI] [PubMed] [Google Scholar]
- 182.Nijtmans LG, de Jong L, Artal Sanz M, Coates PJ, Berden JA, Back JW, Muijsers AO, van der Spek H, Grivell LA. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000;19:2444–2451. doi: 10.1093/emboj/19.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nijtmans LGJ, Sanz MA, Grivell LA, Coates PJ. The mitochondrial PHB complex: roles in mitochondrial respiratory complex assembly, ageing and degenerative disease. Cell Mol Life Sci. 2002;59:143–155. doi: 10.1007/s00018-002-8411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nguyen LN, Furuya MH, Wolfraim LA, Nguyen AP, Holdren MS, Campbell JS, Knight B, Yeoh GC, Fausto N, Parks WT. Transforming growth factor-beta differentially regulates oval cell and hepatocyte proliferation. Hepatology. 2007;45:31–41. doi: 10.1002/hep.21466. [DOI] [PubMed] [Google Scholar]
- 185.Obolenskaya M, Schulze-Specking A, Plaumann B, Frenzer K, Freudenberg N, Decker K. Nitric oxide production by cells isolated from regenerating rat liver. Biochem Biophys Res Commun. 1994;204:1305–1311. doi: 10.1006/bbrc.1994.2605. [DOI] [PubMed] [Google Scholar]
- 186.Okada G, Teraoka H, Tsukada K. Multiple species of mammalian methionine adenosyltransferase. Partial purification and characterization. Biochemistry. 1981;20:934–940. doi: 10.1021/bi00507a045. [DOI] [PubMed] [Google Scholar]
- 187.Okada G, Watanabe Y, Tsukada K. Changes in patterns of methionine adenosyltransferase in fetal and postnatal rat liver. Cancer Res. 1980;40:2895–2897. [PubMed] [Google Scholar]
- 188.Osna NA, White RL, Donohue TM, Jr, Beard MR, Tuma DJ, Kharbanda KK. Impaired methylation as a novel mechanism for proteasome suppression in liver cells. Biochem Biophys Res Commun. 2010;391:1291–1296. doi: 10.1016/j.bbrc.2009.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pajares MA, Corrales F, Ochoa P, Mato JM. The role of cysteine-150 in the structure and activity of rat liver S-adenosyl-l-methionine synthetase. Biochem J. 1991;274:225–229. doi: 10.1042/bj2740225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pajares MA, Duran C, Corrales F, Pliego M, Mato JM. Modulation of rat liver S-adenosylmethionine synthetase activity by glutathione. J Biol Chem. 1992;267:17598–17605. [PubMed] [Google Scholar]
- 191.Pajares MA, Duran C, Corrales F, Mato JM. Protein kinase C phosphorylation of rat liver methionine adenosyltransferase: dissociation and product of an active monomer. Biochem J. 1994;303:949–955. doi: 10.1042/bj3030949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pajula RL, Raina A. Methylthioadenosine, a potent inhibitor of spermine synthase from bovine brain. FEBS Lett. 1979;99:343–345. doi: 10.1016/0014-5793(79)80988-6. [DOI] [PubMed] [Google Scholar]
- 193.Pajula RL, Raina A, Eloranta T. Polyamine synthesis in mammalian tissues. Isolation and characterization of spermine synthase from bovine brain. Eur J Biochem. 1979;101:619–626. doi: 10.1111/j.1432-1033.1979.tb19756.x. [DOI] [PubMed] [Google Scholar]
- 194.Pañeda C, Gorospe I, Herrera B, Nakamura T, Fabregat I, Varela-Nieto I. Liver cell proliferation requires methionine adenosyltransferase 2A mRNA up-regulation. Hepatology. 2002;35:1381–1391. doi: 10.1053/jhep.2002.32538. [DOI] [PubMed] [Google Scholar]
- 195.Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry. 2010;167:942–948. doi: 10.1176/appi.ajp.2009.09081198. [DOI] [PubMed] [Google Scholar]
- 196.Pascale RM, Simile MM, De Miglio MR, Nufris A, Daino L, Seddaiu MA, Rao PM, Rajalakshmi S, Sarma DS, Feo F. Chemoprevention by S-adenosyl-l-methionine of rat liver carcinogenesis initiated by 1,2-dimethylhydrazine and promoted by orotic acid. Carcinogenesis. 1995;16:427–430. doi: 10.1093/carcin/16.2.427. [DOI] [PubMed] [Google Scholar]
- 197.Pascale R, Simile MM, Ruggiu ME, Seddaiu MA, Satta G, Sequenza MJ, Daino L, Vannini MG, Lai P, Feo F. Reversal by 5-azacytidine of the S-adenosyl-l-methionine-induced inhibition of the development of putative preneoplastic foci in rat liver carcinogenesis. Cancer Lett. 1991;56:259–265. doi: 10.1016/0304-3835(91)90011-6. [DOI] [PubMed] [Google Scholar]
- 198.Pascale RM, Simile MM, Satta G, Seddaiu MA, Daino L, Pinna G, Vinci MA, Gaspa L, Feo F. Comparative effects of L-methionine, S-adenosyl-l-methionine and 5′-methylthioadenosine on the growth of preneoplastic lesions and DNA methylation in rat liver during the early stages of hepatocarcinogenesis. Anticancer Res. 1991;11:1617–1624. [PubMed] [Google Scholar]
- 199.Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294:E995–E1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- 200.Pegg AE, Wiliams-Ashman HG. Phosphate-stimulated breakdown of 5′-methylthioadenosine by rat ventral prostate. Biochem J. 1969;115:241–247. doi: 10.1042/bj1150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Perez-Leal O, Merali S. Regulation of polyamine metabolism by translational control. Amino Acids. 2012;42:611–617. doi: 10.1007/s00726-011-1036-6. [DOI] [PubMed] [Google Scholar]
- 202.Perez-Leal O, Moncada C, Clarkson AB, Merali S. Pneumocystis S-adenosylmethionine transport: a potential drug target. Am J Respir Cell Mol Biol. 2011;45:1142–1146. doi: 10.1165/rcmb.2011-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Perez-Mato I, Castro C, Ruiz FA, Corrales FJ, Mato JM. Methionine adenosyltransferase S-nitrosylation is regulated by the basic and acidic amino acids surrounding the target thiol. J Biol Chem. 1999;274:17075–17079. doi: 10.1074/jbc.274.24.17075. [DOI] [PubMed] [Google Scholar]
- 204.Petrossian TC, Clarke SG. Uncovering the human methyltransferasome. Mol Cell Proteomics. 2011 doi: 10.1074/mcp.M110.000976. Epub 2010 Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fuji C. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–570. doi: 10.1172/JCI32453. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Porter DH, Cook RJ, Wagner C. Enzymatic properties of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver. Arch Biochem Biophys. 1985;243:396–407. doi: 10.1016/0003-9861(85)90516-8. [DOI] [PubMed] [Google Scholar]
- 207.Prudova A, Bauman Z, Braun A, Vitvitsky V, Lu SC, Banerjee R. AdoMet regulation of cystathionine β-synthase: relevance to oxidative stress in liver disease. Proc Natl Acad Sci USA. 2006;103:6489–6494. doi: 10.1073/pnas.0509531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ragione FD, Pegg AE. Effect of analogous of 5′-methylthioadenosine on cellular metabolism. Biochem J. 1983;210:429–435. doi: 10.1042/bj2100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rai RM, Lee FYJ, Rosen A, Yang SQ, Lin HZ, Koteish A, Liew FY, Zaragoza C, Lowenstein C, Diehl AM. Impaired liver regeneration in inducible nitric oxide synthase-deficient mice. Proc Natl Acad Sci USA. 1998;95:13829–13834. doi: 10.1073/pnas.95.23.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Raina A, Hyvonen T, Eloranta T, Voutilainen M, Samejima K, Yamanoha B. Inhibition of the synthesis of polyamines and macromolecules by 5′-methylthioadenosine and 5′-alkylthiotubercidins in BHK21 cells. Biochem J. 1984;219:991–1000. doi: 10.1042/bj2190991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ramana CV, Boldogh I, Izumi T, Mitra S. Activation of apurinic/apyrimidinic endonuclease in human cells by reactive oxygen species and its correlation with their adaptive response to genotoxicity of free radicals. Proc Natl Acad Sci USA. 1998;95:5061–5066. doi: 10.1073/pnas.95.9.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ramani K, Mato JM, Lu SC. Role of methionine adenosyltransferase genes in hepatocarcinogenesis. Cancers. 2011;3:1480–1497. doi: 10.3390/cancers3021480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ramani K, Yang HP, Kuhlenkamp J, Tomasi ML, Tsukamoto H, Mato JM, Lu SC. Changes in methionine adenosyltransferase and S-adenosylmethionine during hepatic stellate cell activation. Hepatology. 2010;51:986–995. doi: 10.1002/hep.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ramani K, Yang HP, Xia M, Iglesias Ara A, Mato JM, Lu SC. Leptin's mitogenic effect in human liver cancer cells requires induction of both methionine adenosyltransferase 2A and 2β. Hepatology. 2008;47:521–531. doi: 10.1002/hep.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rambaldi A, Gluud C. S-adenosyl-l-methionine for alcoholic liver disease. Cochrane Database Syst Rev. 2001;4:CD002235. doi: 10.1002/14651858.CD002235. [DOI] [PubMed] [Google Scholar]
- 216.Rao MS, Reddy JK. Peroxisomal beta-oxidation and steatohepatitis. Semin Liver Dis. 2001;21:43–55. doi: 10.1055/s-2001-12928. [DOI] [PubMed] [Google Scholar]
- 217.Reytor E, Perez-Miguelsanz J, Alvarez L, Perez-Sala D, Pajares MA. Conformational signals in the C-terminal domain of methionine adenosyltransferase I/III determine its nucleocytoplasmic distribution. FASEB J. 2009;23:3347–3360. doi: 10.1096/fj.09-130187. [DOI] [PubMed] [Google Scholar]
- 218.Richards HH, Chiang PK, Cantoni Adenosylhomocysteine hydrolase GL. Crystallization of the purified enzyme and its properties. J Biol Chem. 1978;253:4476–4480. [PubMed] [Google Scholar]
- 219.Rodríguez JL, Boukaba A, Sandoval J, Georgieva EI, Latasa MU, García-Trevijano ER, Serviddio G, Nakamura T, Avila MA, Sastre J, Torres L, Mato JM, López-Rodas G. Transcription of the MAT2A gene, coding for methionine adenosyltransferase, is up-regulated by E2F and Sp1 at a chromatin level during proliferation of liver cells. Int J Biochem Cell Biol. 2007;39:842–850. doi: 10.1016/j.biocel.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 220.Rouillon A, Surdin-Kerjan Y, Thomas D. Transport of sulfonium compounds. Characterization of the s-adenosylmethionine and s-methylmethionine permeases from the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:28096–28105. doi: 10.1074/jbc.274.40.28096. [DOI] [PubMed] [Google Scholar]
- 221.Rountree CB, Senadheera S, Mato JM, Crooks GM, Lu SC. Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A deficient mice. Hepatology. 2008;47:1288–1297. doi: 10.1002/hep.22141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ruiz F, Corrales FJ, Miqueo C, Mato JM. Nitric oxide inactivates rat hepatic methionine adenosyltransferase in vivo by S-nitrosylation. Hepatology. 1998;128:1051–1057. doi: 10.1002/hep.510280420. [DOI] [PubMed] [Google Scholar]
- 223.Russmann S, Junker E, Lauterburg BH. Remethylation and transsulfuration of methionine in cirrhosis: studies with l-[H3-methyl-1-C]methionine. Hepatology. 2002;36:1190–1196. doi: 10.1053/jhep.2002.36499. [DOI] [PubMed] [Google Scholar]
- 224.Rutjes AW, Nüesch E, Reichenbach S, Jüni P. S-adenosylmethionine for osteoarthritis of the knee or hip. Cochrane Database Syst Rev. 2009;4:CD007321. doi: 10.1002/14651858.CD007321.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sakata SF, Shelly LL, Ruppert S, Schutz G, Chou JY. Cloning and expression of murine methionine adenosyltransferase. J Biol Chem. 1993;268:13978–13986. [PubMed] [Google Scholar]
- 226.Sánchez-Góngora E, Ruiz F, Mingorance J, An W, Corrales FJ, Mato JM. Interaction of liver methionine adenosyltransferase with hydroxyl radical. FASEB J. 1997;11:1013–1019. doi: 10.1096/fasebj.11.12.9337154. [DOI] [PubMed] [Google Scholar]
- 227.Santamaria E, Avila MA, Latasa MU, Rubio A, Martin-Duce A, Lu SC, Mato JM, Corrales FJ. Functional proteomics of nonalcoholic steatohepatitis: mitochondrial proteins as targets of S-adenosylmethionine. Proc Natl Acad Sci USA. 2003;100:3065–3070. doi: 10.1073/pnas.0536625100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sarasin-Filipowicz M, Oakeley EJ, Duong FH, Christen V, Terracciano L, Filipowicz W, Heim MH. Interferon signaling and treatment outcome in chronic hepatitis C. Proc Natl Acad Sci USA. 2008;105:7034–7039. doi: 10.1073/pnas.0707882105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scavetta RD, Thomas CB, Walsh MA, Szegedi S, Jooachimiak A, Gumport RI, Churchill MEA. Structure of RsrI methyltransferase, a member of the N6-adenine β class of DNA methyltransferases. Nucleic Acids Res. 2000;28:3950–3961. doi: 10.1093/nar/28.20.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sell S. Cellular origin of hepatocellular carcinomas. Semin Cell Dev Biol. 2002;13:419–424. doi: 10.1016/s1084952102001295. [DOI] [PubMed] [Google Scholar]
- 231.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, DePinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Silveira MG, Lindor KD. Treatment of primary biliary cirrhosis: therapy with choleretic and immunosuppressive agents. Clin Liver Dis. 2008;12:425–443. doi: 10.1016/j.cld.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 233.Singal R, Ginder GD. DNA methylation. Blood. 1999;93:4059–4070. [PubMed] [Google Scholar]
- 234.Skordi E, Claus SP, Martin FP, Cloarec O, Lindberg J, Schuppe-Kolstinen I, Holmes E, Nicholson JK. Analysis of time-related metabolic fluctuations induced by ethionine in the rat. J Proteome Res. 2007;6:4572–4581. doi: 10.1021/pr070268q. [DOI] [PubMed] [Google Scholar]
- 235.Steglich G, Neupert W, Langer T. Prohibitins regulate membrane protein degradation by the m-AAA protease in mitochondria. Mol Cell Biol. 1999;19:3435–3442. doi: 10.1128/mcb.19.5.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, Aravind L, Mitchell W, Olinger L, Tatusov RL, Zhao Q, Koonin EV, Davis RW. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 237.Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr. 2004;24:539–577. doi: 10.1146/annurev.nutr.24.012003.132418. [DOI] [PubMed] [Google Scholar]
- 238.Sullivan DM, Hoffman J. Fractionation and kinetic properties of rat liver and kidney methionine adenosyltransferase isozymes. Biochemistry. 1983;22:1636–1641. doi: 10.1021/bi00276a017. [DOI] [PubMed] [Google Scholar]
- 239.Teng YW, Mehedint MG, Garrow TA, Zeisel SH. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J Biol Chem. 2011;286:36258–36267. doi: 10.1074/jbc.M111.265348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tobeña R, Horikawa S, Calvo V, Alemany S. Interleukin-2 induces γ-S-adenosyl-l-methionine synthetase gene expression during T-lymphocyte activation. Biochem J. 1996;319:929–933. doi: 10.1042/bj3190929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Tomasi ML, Iglesias Ara A, Yang H, Ramani K, Feo F, Pascale MR, Martínez-Chantar ML, Mato JM, Lu SC. S-adenosylmethionine regulates apurinic/apyrimidinic endonuclease 1 stability: implication in hepatocarcinogenesis. Gastroenterology. 2009;136:1025–1036. doi: 10.1053/j.gastro.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Tomasi ML, Li TWH, Li M, Mato JM, Lu SC. Inhibition of methionine adenosyltransferase 1A transcription by coding region methylation. J Cell Physiol. 2012;227:1583–1591. doi: 10.1002/jcp.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Tomasi ML, Ramani K, Lopitz-Otsoa F, Rodríguez MS, Li TWH, Ko K, Yang HP, Bardag-Gorce F, Iglesias-Ara A, Feo F, Pascale MR, Mato JM, Lu SC. S-adenosylmethionine regulates dual-specificity MAPK phosphatase expression in mouse and human hepatocytes. Hepatology. 2010;51:2152–2161. doi: 10.1002/hep.23530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tonks NK, Neel BG. From form to function: signaling by protein tyrosine phosphatases. Cell. 1996;87:365–368. doi: 10.1016/s0092-8674(00)81357-4. [DOI] [PubMed] [Google Scholar]
- 245.Torres L, Avila MA, Carretero MV, Latasa MU, Caballeria J, López-Rodas G, Boukaba A, Lu SC, Franco L, Mato JM. Liver-specific methionine adenosyltransferase MAT1A gene expression is associated to a specific pattern of promoter methylation and histone acetylation. Implications for MAT1A silencing during transformation. FASEB J. 2000;14:95–102. doi: 10.1096/fasebj.14.1.95. [DOI] [PubMed] [Google Scholar]
- 246.Tseng TL, Shih YP, Huang YC, Wang CK, Chen PH, Chang JG, Yeh KT, Chen YM, Buetow KH. Genotypic and phenotypic characterization of a putative tumor susceptibility gene, GNMT, in liver cancer. Cancer Res. 2003;63:647–654. [PubMed] [Google Scholar]
- 247.Tsukamoto HC, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 248.Ubagai T, Lei KJ, Huang S, Mudd SH, Levy HL, Chou Y. Molecular mechanisms of an inborn error of methionine pathway. J Clin Invest. 1995;96:1943–1947. doi: 10.1172/JCI118240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Uno K, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Imai J, Hasegawa Y, Gao J, Kaneko K, Iwasaki H, Ishihara H, Sasano H, Inukai K, Mizuguchi H, Asano T, Shiota M, Nakazato M, Oka Y. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- 250.Varela-Rey M, Beraza N, Lu SC, Mato JM, Martínez-Chantar ML. Role of AMPK in the control of hepatocyte priming and proliferation during liver regeneration. Exp Biol Med. 2011;236:402–408. doi: 10.1258/ebm.2011.010352. [DOI] [PubMed] [Google Scholar]
- 251.Varela-Rey M, Fernández-Ramos D, Martínez-López N, Embade N, Gómez-Santos L, Beraza N, Vázquez-Chantada M, Rodríguez J, Luka Z, Wagner C, Lu SC, Martínez-Chantar ML, Mato JM. Impaired liver regeneration in mice lacking glycine N-methyltransferase. Hepatology. 2009;50:443–452. doi: 10.1002/hep.23033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Varela-Rey M, Martínez-López N, Fernández-Ramos D, Embade N, Calvisi DF, Woodhoo A, Rodríguez J, Fraga MF, Julve J, Rodríguez-Millán E, Frades I, Torres L, Luka Z, Wagner C, Esteller M, Lu SC, Martínez-Chantar ML, Mato JM. Fatty liver and fibrosis in glycine N-methyltransferase knockout mice is prevented by nicotinamide. Hepatology. 2010;52:105–114. doi: 10.1002/hep.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Vázquez-Chantada M, Ariz U, Varela-Rey M, Embade N, Martínez-Lopez N, Fernández-Ramos D, Gómez-Santos L, Lamas S, Lu SC, Martínez-Chantar ML, Mato JM. Evidence for LKB1/AMP-activated protein kinase/endothelial nitric oxide synthase cascade regulated by hepatocyte growth factor, S-adenosylmethionine, and nitric oxide in hepatocyte proliferation. Hepatology. 2009;49:608–617. doi: 10.1002/hep.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Vázquez-Chantada M, Fernández D, Embade N, Woodhoo A, Martinez N, Varela-Rey M, Luka Z, Wagner C, Caballería J, Gorospe M, Lu SC, Mato JM, Martínez-Chantar ML. HuR/Methylated-HuR and AUF1 regulate the expression of methionine adenosyltransferase during liver proliferation, differentiation and carcinogenesis. Gastroenterology. 2010;138:1943–1953. doi: 10.1053/j.gastro.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Vinci CR, Clarke SG. Recognition of age-damaged (R,S)-adenosyl-l-methionine by two methyltransferases in the yeast Sacharomyces cerevisiae. J Biol Chem. 2007;282:8604–8612. doi: 10.1074/jbc.M610029200. [DOI] [PubMed] [Google Scholar]
- 256.Vinci CR, Clarke SG. Homocysteine methyltransferases Mht1 and Sam4 prevent the accumulation of age-damaged (R,S)-AdoMet in the yeast Saccharomyces cerevisiae. J Biol Chem. 2010;285:20526–20531. doi: 10.1074/jbc.M110.113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Vinci CR, Clarke SG. Yeast, plants, worms and flies use a methyltransferase to metabolize age-damaged (R,S)-AdoMet, but what do mammals do? Rejuvenation Res. 2010;13:362–364. doi: 10.1089/rej.2009.0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Vugrek O, Beluzić R, Nakić N, Mudd SH. S-adenosylhomocysteine hydrolase (AHCY) deficiency: two novel mutations with lethal outcome. Hum Mutat. 2009;30:E555–565. doi: 10.1002/humu.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, Vance DE, Tzoneva M, Hart AC, Näär AM. A conserved SREBP1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang S, Fusaro G, Padmanabhan J, Chellappan SP. Prohibitin co-localizes with Rb in the nucleus and recruits N-CoR and HDAC1 for transcriptional repression. Oncogene. 2002;21:8388–8396. doi: 10.1038/sj.onc.1205944. [DOI] [PubMed] [Google Scholar]
- 261.Watson WH, Zhao Y, Chawla RK. S-adenosylmethionine attenuates the lipopolysaccharide-induced expression of the gene for tumour necrosis factor alpha. Biochem J. 1999;342:21–25. [PMC free article] [PubMed] [Google Scholar]
- 262.Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153:109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 263.Woodard RW, Tsai MD, Floss HG, Crooks PA, Coward JK. Stereochemical course of the transmethylation catalyzed by catechol O-methyltransferase. J Biol Chem. 1980;255:9124–9127. [PubMed] [Google Scholar]
- 264.Woodcock DM, Adams JK, Allan RG, Cooper IA. Effect of several inhibitors of enzymatic DNA methylation on the in vivo methylation of different classes of DNA sequences in a cultured human cell line. Nucleic Acids Res. 1983;11:489–499. doi: 10.1093/nar/11.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wu SE, Huskey WP, Borchardt RT, Schowne REL. Chiral instability at sulfur of S-adenosylmethionine. Biochemistry. 1983;22:2828–2832. doi: 10.1021/bi00281a009. [DOI] [PubMed] [Google Scholar]
- 266.Wu SM, Huang YH, Lu YH, Chien LF, Yeh CT, Tsai MM, Liao CH, Chen WJ, Liao CJ, Cheng WL, Lin KH. Thyroid hormone receptor-mediated regulation of the methionine adenosyltransferase 1 gene is associated with cell invasion in hepatoma cell lines. Cell Mol Life Sci. 2010;67:1831–1843. doi: 10.1007/s00018-010-0281-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xia M, Chen Y, Wang LC, Zandi E, Yang H, Bemanian S, Martinez-Chantar ML, Mato JM, Lu SC. Novel function and intracellular localization of methionine adenosyltransferase 2beta splicing variants. J Biol Chem. 2010;285:20015–20021. doi: 10.1074/jbc.M109.094821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yamada Y, Kirillova I, Peschon JJ, Fausto N. Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA. 1997;94:1441–1446. doi: 10.1073/pnas.94.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yang HP, Huang ZZ, Zeng ZH, Chen CJ, Selby RR, Lu SC. Role of promoter methylation in increased methionine adenosyltransferase 2A expression in human liver cancer. Am J Physiol Gastrointest Liver Physiol. 2001;280:G184–G190. doi: 10.1152/ajpgi.2001.280.2.G184. [DOI] [PubMed] [Google Scholar]
- 271.Yang HP, Huang ZZ, Zeng ZH, Chen CJ, Wang JH, Lu SC. The role of c-Myb and Sp1 in the up-regulation of methionine adenosyltransferase 2A gene expression in human hepatocellular carcinoma. FASEB J. 2001;15:1507–1516. doi: 10.1096/fj.01-0040com. [DOI] [PubMed] [Google Scholar]
- 272.Yang HP, Iglesias Ara A, Magilnick N, Xia M, Ramani K, Chen H, Lee TD, Mato JM, Lu SC. Expression pattern, regulation and function of methionine adenosyltransferase 2β alternative splicing variants in hepatoma cells. Gastroenterology. 2008;134:281–291. doi: 10.1053/j.gastro.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yang HP, Ko K, Xia M, Li TWH, Oh P, Li J, Lu SC. Induction of avian musculoaponeurotic fibrosarcoma proteins by toxic bile acid inhibits expression of GSH synthetic enzymes and contributes to cholestatic liver injury in mice. Hepatology. 2010;51:1291–1301. doi: 10.1002/hep.23471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yang HP, Ramani K, Xia M, Ko KS, Li TWH, Oh P, Li J, Lu SC. Dysregulation of glutathione synthesis during cholestasis in mice: molecular mechanisms and therapeutic implications. Hepatology. 2009;49:1982–1991. doi: 10.1002/hep.22908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Yang HP, Sadda MR, Li M, Zeng Y, Chen L, Bae W, Ou X, Runnegar MT, Mato JM, Lu SC. S-adenosylmethionine and its metabolite induce apoptosis in HepG2 cells: role of protein phosphatase 1 and Bcl-x(S) Hepatology. 2004;40:221–231. doi: 10.1002/hep.20274. [DOI] [PubMed] [Google Scholar]
- 276.Yang HP, Sadda MR, Yu V, Zeng Y, Lee TD, Ou XP, Chen LX, Lu SC. Induction of human methionine adenosyltransferase 2A expression by tumor necrosis factor alpha: role of NF-κB and AP-1. J Biol Chem. 2003;278:50887–50896. doi: 10.1074/jbc.M307600200. [DOI] [PubMed] [Google Scholar]
- 277.Zeng ZH, Huang ZZ, Chen CJ, Yang HP, Mao Z, Lu SC. Molecular cloning and functional characterization of the 5′-flanking region of human methionine adenosyltransferase 1A gene. Biochem J. 2000;346:475–482. [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang W, Wagner BJ, Ehrenman K, Schaefer AW, DeMaria CT, Crater D, DeHaven K, Long L, Brewer G. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol Cell Biol. 1993;13:7652–7665. doi: 10.1128/mcb.13.12.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Zhuge J, Cederbaum AI. Depletion of S-adenosyl-l-methionine with cycloleucine potentiates cytochrome P450 2E1 toxicity in primary rat hepatocytes. Arch Biochem Biophys. 2007;486:177–185. doi: 10.1016/j.abb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]