Abstract
The V600E mutation in the kinase BRAF is frequently detected in melanomas and results in constitutive activation of BRAF, which then promotes cell proliferation by the mitogen-activated protein kinase (MAPK) signaling pathway. Although the BRAFV600E kinase inhibitor vemurafenib has remarkable antitumor activity in patients with BRAFV600E-mutated melanoma, its effects are limited by the onset of drug resistance. We found that exposure of melanoma cell lines with the BRAFV600E mutation to vemurafenib decreased the abundance of anti-apoptotic proteins and induced intrinsic mitochondrial apoptosis. Vemurafenib-treated melanoma cells showed increased cytosolic concentration of calcium, a potential trigger for endoplasmic reticulum (ER) stress, which can lead to apoptosis. Consistent with an ER stress-induced response, vemurafenib decreased the abundance of the ER chaperone protein GRP78, increased the abundance of the spliced isoform of the transcription factor X-box protein 1 (XBP1) (which transcriptionally activates genes involved in ER stress responses), increased the phosphorylation of the translation initiation factor eIF2α (which would be expected to inhibit protein synthesis), and induced the expression of ER stress-related genes. Knockdown of the ER stress response protein ATF4 significantly reduced vemurafenib-induced apoptosis. Moreover, the ER stress inducer thapsigargin prevented invasive growth of tumors formed from vemurafenib-sensitive melanoma cells in vivo. In melanoma cells with low sensitivity or resistance to vemurafenib, combination treatment with thapsigargin augmented or induced apoptosis. Thus, thapsigargin or other inducers of ER stress may be useful in combination therapies to overcome vemurafenib resistance.
Introduction
The estimated median survival for patients with stage IV melanoma is 8 ± 2 months (1), with classical chemotherapy and immunotherapy regimens conferring little survival benefit. The mitogen-activated protein kinase (MAPK, also known as RAF-MEK-ERK) and the phosphoinositide 3 kinase (PI3K, also known as the PI3K-AKT-mTOR or AKT) signaling pathways play major roles in melanoma initiation, progression and therapy resistance (2-5). About 50% of melanomas have activating BRAFV600E kinase mutations. Although the BRAFV600E kinase inhibitor vemurafenib induces tumor regression and improves survival in the majority of individuals with BRAFV600E-mutant melanomas, nearly all responses are partial (6-8), with a subpopulation of patients showing primary resistance in metastases. Moreover, the acquisition of secondary resistance leading to relapse was observed in nearly every patient. The overall aim of the present study was to elucidate the mechanisms that underlie the antitumor activity of vemurafenib and to identify strategies to enhance its antitumor effects and overcome mechanisms of resistance.
The mechanisms of vemurafenib resistance are complex and multi-faceted. The available data suggests that resistance involves reactivation of the MAPK pathway or activation of other survival pathways, such as the AKT pathway, which may occur through several means: BRAFV600E amplification (9, 10), increased abundance of splice isoforms of BRAFV600E that dimerize in a RAS-independent manner (10), secondary mutations in NRAS (11) or MAPK kinase (MEK) (12), increased abundance of the mitogen-activated protein kinase 8 (MAP3K8 or COT) which activates the extracellular signal-regulated kinase (ERK) through MEK (13), switching among the three RAF isoforms (14) and increased abundance of receptor tyrosine kinases (14, 15).
Bim (Bcl-2 interacting mediator of cell death), a BH3-only proapoptotic Bcl-2 family member, plays a key role in BRAF inhibitor-induced apoptosis in BRAFV600E melanoma cells (16, 17). Bim is activated by endoplasmic reticulum (ER) stress and is essential for ER stress-induced apoptosis in various cell types (18). ER stress is caused by disturbances in the structure and function of the ER and can result from hypoxia, nutrient deprivation, calcium (Ca2+) imbalance, or perturbation of protein glycosylation, leading to the accumulation of unfolded proteins in the ER and activation of the unfolded protein response (UPR) pathway (19-21) (Fig. S1A). The UPR pathway is triggered through three sensors: activating transcription factor 6 (ATF6), PKR-like ER kinase (PERK), and inositol-requiring enzyme 1 (IRE1). Under normal conditions, these sensors are maintained in an inactive state bound to the chaperone protein GRP78 (glucose-regulated protein 78). Upon ER stress, misfolded and unfolded proteins bind to GRP78, releasing it from the UPR sensors, which trigger the UPR by inducing the transcription of genes encoding proteins involved in the UPR, reducing global protein synthesis, and stimulating ER-associated protein degradation. These activities serve to restore normal ER function or, when normal ER function cannot be restored, trigger apoptosis (21-23). Δ9-tetrahydrocannabinol (THC) induces ER stress-mediated apoptosis in brain tumor cells through PERK-mediated phosphorylation and activation of the eukaryotic translation initiation factor eIF2α and an increase in the abundance of ER stress-related proteins, including nuclear protein 1 (NUPR1; also known as p8), ATF3, ATF4, DNA-damage-inducible transcript 3 [DDIT3; also known as growth arrest and DNA-damage-inducible protein (GADD153), or CCAAT enhancer binding protein homolog (CHOP)], and Tribble 3 (TRB3) (24, 25) (Fig. S1B). In the present study, we demonstrate that vemurafenib-induced apoptosis in melanoma cells proceeds through the induction of ER stress. Furthermore, we show that vemurafenib resistance may be overcome by combination therapies that augment ER stress.
Results
Vemurafenib induces intrinsic mitochondrial apoptosis specifically in BRAFV600E melanoma cells
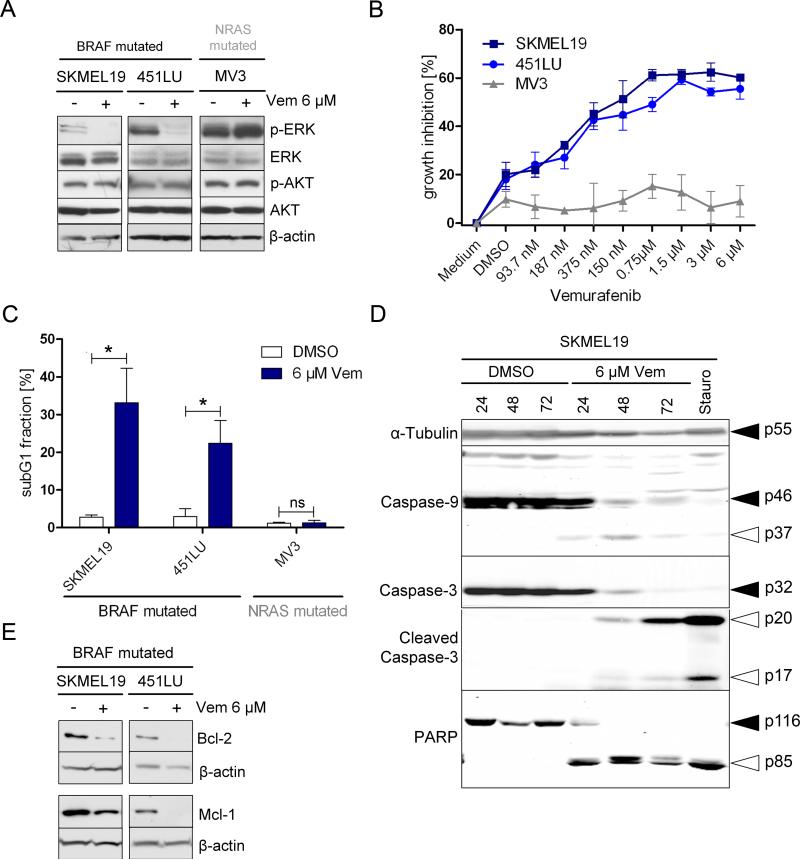
As expected, vemurafenib inhibited phosphorylation of ERK in BRAFV600E melanoma cells, but it did not affect phosphorylation of AKT (Fig. 1A). Vemurafenib caused greater growth inhibition in BRAF-mutated melanoma cells than in NRAS-mutated melanoma cells (Fig. 1B). Accordingly, vemurafenib caused a G1 cell cycle arrest in BRAF-mutated but not NRAS-mutated cells and likewise increased apoptosis only in BRAF-mutated cells, as indicated by a five to seven-fold increase in the percentage of cells in the subG1 fraction (Fig. 1C). Apoptotic cell death was confirmed by the enrichment of nucleosomes in the cytoplasm of BRAF-mutated melanoma cells using a cell-death detection ELISA (Fig. S2), and was caspase-dependent, because pre-treating cells with the pan-caspase inhibitor Q-VD-OPH abrogated the accumulation of subG1 cells (Fig. S3). Western blot analysis confirmed extensive processing of caspase-3 and its substrate PARP by 24 hours after treatment (Fig. 1D). Processing of the initiator caspase-9 (Fig. 1D) plus a decrease in the abundance of anti-apoptotic Bcl-2 and Mcl-1 proteins (Fig. 1E), indicated that activation of the intrinsic apoptosis pathway played a crucial role in vemurafenib-induced cyotoxicity.
Figure 1. Vemurafenib induces intrinsic mitochondrial apoptosis in BRAF-mutant melanoma cells.
(A) Western blot analysis for phosphorylated (p)-ERK, ERK, p-AKT, and AKT was performed on melanoma cells with BRAF (SKMEL19, 451LU) or NRAS mutation (MV3) that were treated with vemurafenib (Vem) or DMSO (control) for 24 hours (h). (B) Growth assessment using 4-methylumbelliferyl heptanoate of melanoma cells treated with vemurafenib for 72 h. One representative experiment of four is shown (mean ± SD). (C) Apoptosis (as assessed by the subG1 fraction) was quantified in melanoma cells treated with vemurafenib or DMSO for 72 h. Bar graphs show the quantification of results from 4 independent experiments (mean ± SD, Mann-Whitney test, 95% CI; * P < 0.05; ns=not significant). (D) Western blot analysis for caspase-9, caspase-3, cleaved caspase-3, and PARP was performed on melanoma cells treated with vemurafenib or DMSO for the indicated time periods or with staurosporine (Stauro, 1 μM) for 15 h. Black arrow: uncleaved protein. White arrow: cleaved protein. (E) Western blot analysis for Bcl-2 and Mcl-1 was performed on melanoma cells treated with vemurafenib or DMSO for 72 h. Western blots in (A, D, E) show a representative experiment (n=3); β-actin or α-tubulin served as the loading control.
Vemurafenib triggers ER stress
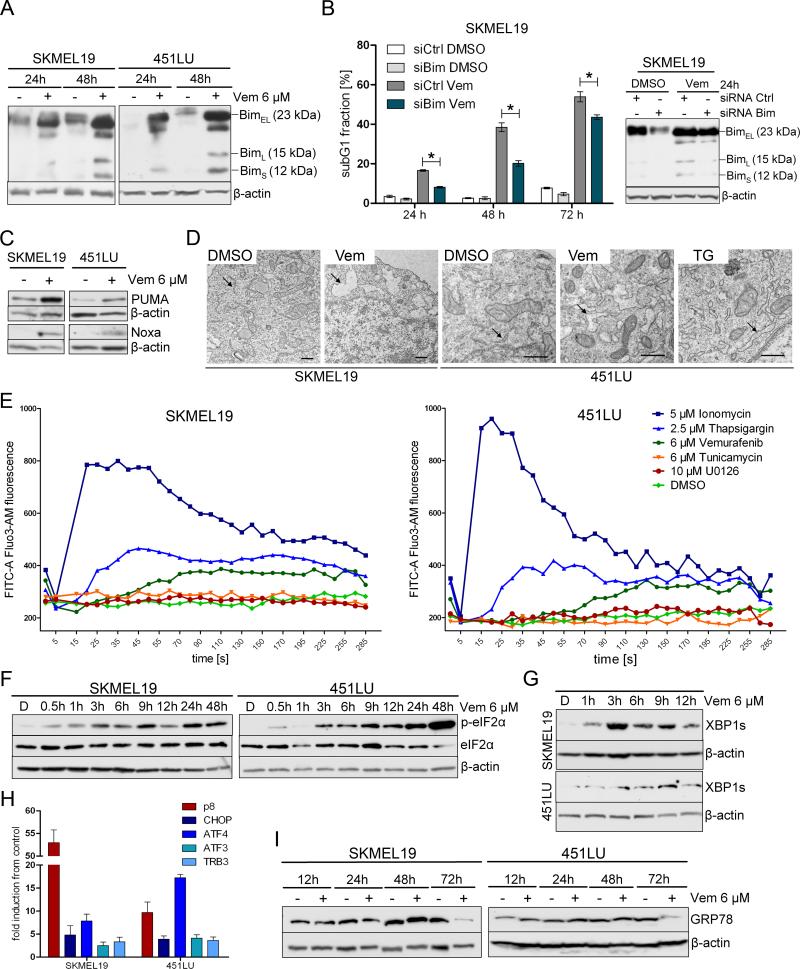
Both BRAF inhibitors and ER stress increase the abundance of the proapoptotic protein BIM (16, 18). Indeed, vemurafenib increased the abundance of the three BIM isoforms, BIMS (12 kD), BimL (15 kD), and BimEL (23 kD), in BRAF-mutated melanoma cells (Fig. 2A). Bim knockdown by siRNA impaired vemurafenib-induced apoptosis demonstrating the relevance of Bim induction in this mechanism (Fig. 2B). We also evaluated other BH3-only pro-apoptotic Bcl-2 family members and found that vemurafenib also increased the abundance of PUMA and Noxa (Fig. 2C).
Figure 2. Vemurafenib triggers ER stress.
(A) Western blot analysis to detect BIM isoforms was performed on BRAF-mutated (SKMEL19, 451LU) melanoma cells treated with vemurafenib (+) or DMSO (-). (B) Western blot analysis for BIM isoforms was performed on BRAF-mutated melanoma cells (SKMEL19) transfected with control siRNA or siRNA directed against BIM prior to treatment with vemurafenib or DMSO. Apoptosis (subG1 fraction) was quantified by propidium iodide staining. Bar graphs show the quantification of results from 3 independent experiments (mean ± SD, Mann-Whitney test, 95% CI; * P < 0.05). (C) Western blot analysis for PUMA and Noxa was performed on BRAF-mutated (SKMEL19, 451LU) melanoma cells treated with vemurafenib or DMSO for 24 h. (D) EIectron microscopy of melanoma cells treated with vemurafenib, DMSO, or thapsigargin (TG) for 6 h. DMSO-treated cells show a normal-sized endoplasmic reticulum (arrows). Vemurafeniband thapsigargin-treated cells show dilation of the endoplasmic reticulum (arrows). Scale bar = 0.5 μm, n=2 experiments. (E) Melanoma cells loaded with Fluo3-AM were exposed to the indicated treatments and monitored by flow cytometry. Changes in fluorescence intensity over time reflect changes in cytosolic Ca2+ concentrations. As a positive control, melanoma cells were treated with ionomycin, a selective Ca2+ ionophore. One representative experiment is shown (n=3). (F, G) Western blot analysis for p-eIF2α, eIF2α, and spliced XBP1 (XBP1s) was performed at the indicated time points on BRAF-mutated (SKMEL19, 451LU) melanoma cells treated with vemurafenib or DMSO (D). (H) Real-time PCR to detect the expression of p8, ATF4, ATF3, CHOP, and TRB3 relative to the DMSO control was performed in melanoma cells treated with vemurafenib or DMSO for 12 h. Mean ± SD; n=3 experiments. (I) Western blot analysis for GRP78 was performed on melanoma cells treated with vemurafenib or DMSO for the indicated times. (A, B, C, F, G, I) Western blots show one representative experiment (n=3); β-actin served as the loading control.
ER stress can be triggered by various stimuli, including disturbances in Ca2+ homeostasis. Like the classical ER stress inducer thapsigargin, vemurafenib treatment induced a morphology typical of ER stress in BRAF-mutated cells, particularly ER lumen swelling (Fig. 2D), and increased cytosolic Ca2+ concentration (26), whereas tunicamycin, another ER stress inducer, and the MEK inhibitor U0126 did not affect cytosolic Ca2+ concentration (Fig. 2E).
Upon ER stress, the sensor PERK is activated and phosphorylates eIF2α (Fig. S1A). To determine the onset of ER stress in response to vemurafenib, we examined the phosphorylation of eIF2α by Western blotting and found that it was rapidly phosphorylated following vemurafenib treatment (Fig. 2F). IRE1 is another ER transmembrane protein that serves as an ER stress sensor and mediates the UPR (Fig. S1A). Activated IRE1 splices the mRNA for X-box binding protein 1 (XBP1) such that the encoded protein is a potent transcriptional activator. In addition to phosphorylation of eIF2α, vemurafenib increased the abundance of the spliced form of XBP1 (XBP1s) in SKMEL19 and 451LU cells, as shown by Western blot analysis (Fig. 2G). Additionally, vemurafenib treatment increased the expression of the ER stress-related genes, including p8, ATF4, ATF3, CHOP, and TRB3 (Fig. S1B) in BRAF-mutated melanoma cells, particularly in the expression of p8, which encodes a co-transcription factor, and ATF4 (Fig. 2H).
The abundance of the ER chaperone protein GRP78 is increased in various cancers including melanoma (27, 28). GRP78 plays a key role in protecting cells against ER stress-induced apoptosis and its abundance is rapidly increased by ER stress inducers such as thapsigargin (29). However, vemurafenib treatment decreased the abundance of GRP78 in both cell lines after 72 hours (Fig. S4, Fig. 2I).
ER stress plays a role in vemurafenib-induced apoptosis
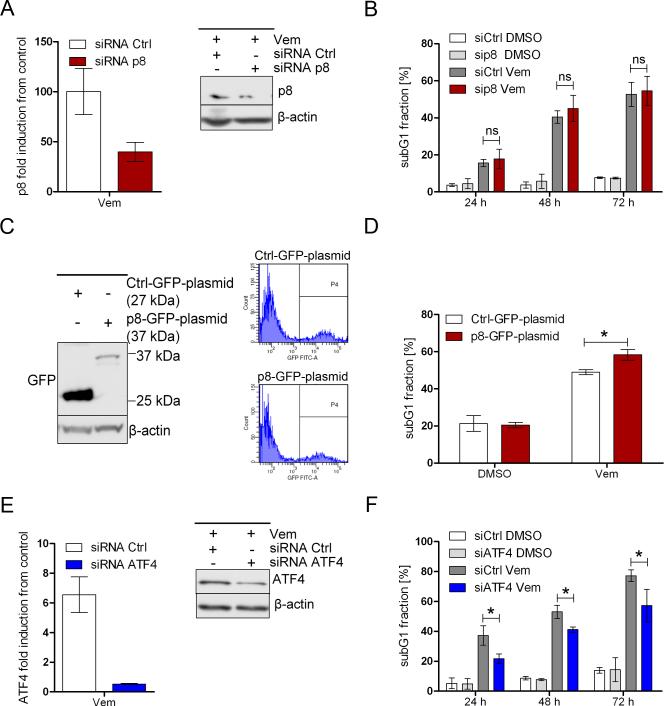
Because vemurafenib treatment led to increased expression of p8 and ATF4, we next used siRNA-mediated knockdown to assess their roles in vemurafenib-induced apoptosis. Knocking down p8 in BRAFV600E melanoma cells yielded conflicting results; we observed either diminished or no effect on the pro-apoptotic effects of vemurafenib. For example, in control-siRNA transfected SKMEL19 melanoma cells, vemurafenib treatment increased the abundance of p8 by about 100-fold compared with untreated cells (Fig. 3A), whereas in p8-siRNA transfected cells, vemurafenib treatment increased the abundance of p8 by about 40-fold, indicating that the p8-siRNA suppressed the vemurafenib-induced increase in p8 abundance by only about 60% (Fig. 3A), suggesting that p8 knockdown was insufficient in melanoma cells with high vemurafenib-induced p8 expression. However, reduced p8 expression did not translate into a reduced apoptotic response to vemurafenib (Fig. 3B).
Figure 3. ER stress plays a role in vemurafenib-induced apoptosis.
(A, B) BRAF-mutated melanoma cells (SKMEL19) transfected with control or p8-targeted siRNA were treated with vemurafenib (6 μM) or DMSO. After 15 h, real-time PCR analysis (A) was performed to measure p8 mRNA expression in vemurafenib-treated cells relative to DMSO-treated (control) cells. Abundance of p8 protein were detected by Western blot analysis 18 h after treatment (one representative experiment of three is shown). 24, 48, and 72 h after treatment, apoptosis (subG1 fraction) was quantified (B). Bar graphs show the quantification of results from 4 independent experiments (mean ± SD). (C, D) Melanoma cells (SKMEL19) were transfected with Ctrl-GFP-plasmid (pEGFP-N1, 27kDa) or p8-GFP-plasmid (pEGFP-C2-p8, 37kDa). After 24 h, Western blot analysis for GFP (C) was performed (one representative experiment of three is shown). Apoptosis (subG1 fraction) was quantified in GFP-positive cells treated with vemurafenib (6 μM) or DMSO for 48 h (D). Bar graphs show the quantification of results from 4 independent experiments (mean ± SD). (E, F) Real-time PCR to detect ATF4 mRNA expression was performed in melanoma cells (SKMEL19) transfected with control or ATF4 siRNA and treated with vemurafenib (3 μM) or DMSO (E). Western blot analysis for ATF4 was performed 48 h after treatment (one representative experiment of three is shown). 24, 48, and 72 h after treatment, apoptosis (as assessed by the subG1 fraction) was quantified (F). Bar graphs show the quantification of results from 4 independent experiments (mean ± SD). P values for (B, D, F) were determined by Mann-Whitney test, 95% CI; * P < 0.05; ns=not significant.
We further investigated the role of p8 by transfecting BRAFV600E melanoma cells with p8 tagged with green fluorescent protein (GFP) (Fig. 3C). FACS-sorted p8-GFP-positive melanoma cells (Fig. 3C) were treated with vemurafenib and subjected to cell cycle analysis. Overexpression of p8 in melanoma cells did not induce apoptosis on its own but rather augmented the pro-apoptotic effects of vemurafenib, suggesting that high p8 expression may increase the sensitivity of BRAFV600E melanoma cells to vemurafenib (Fig. 3D). In contrast, the siRNA-mediated knockdown of ATF4 efficiently reduced both vemurafenib-induced increase in ATF4 expression and apoptosis (Fig. 3E,F). In particular, at 24 hours after treatment with ATF4-targeted siRNA and vemurafenib, the reduction in apoptosis achieved was statistically significant (Fig. 3F). Thus, ATF4 appeared to play a pro-apoptotic role in vemurafenib-induced, ER-stress mediated apoptosis.
Conventional inducers of ER stress induce apoptosis and inhibit invasive tumor growth of melanoma cells
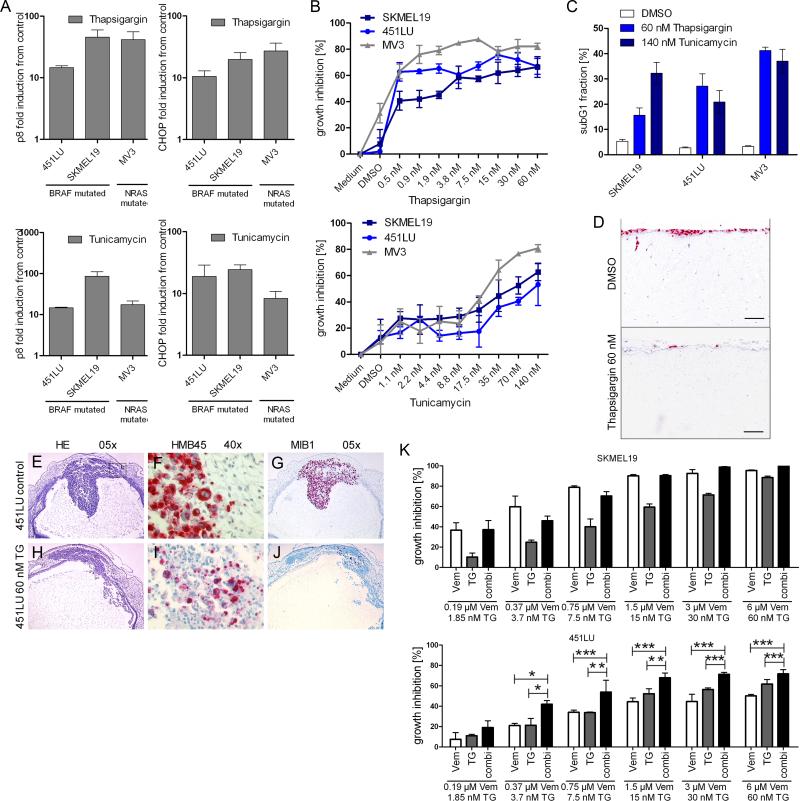
Having shown that melanoma cells were sensitive to vemurafenib-induced ER stress-mediated apoptosis, we next asked whether conventional ER stress inducers, such as thapsigargin and tunicamycin, might elicit similar antitumor effects. Both thapsigargin and tunicamycin increased the expression of ER stress-related genes (Fig. 4A), inhibited growth (Fig. 4B), and induced apoptosis (Fig. 4C) in both BRAF- and NRAS-mutated melanoma cell lines. These effects were specific to melanoma cells because both thapsigargin and tunicamycin did not appear to affect the survival of human fibroblasts at concentrations that were pro-apoptotic in melanoma cells (Fig. S5).
Figure 4. Inducers of ER stress trigger apoptosis and inhibit invasive tumor growth of melanoma cells.
(A) Real-time PCR to detect p8 and CHOP mRNA expression was performed in BRAF-mutated (SKMEL19, 451LU) and NRAS-mutated (MV3) melanoma cells treated with DMSO (control), 60 nM thapsigargin, or 595 nM tunicamycin for 12 h. One representative experiment of three is shown (means ± SD). (B) Growth assessment using 4-methylumbelliferyl heptanoate of melanoma cells treated with the indicated concentrations of thapsigargin or tunicamycin for 72 h. One representative experiment of three is shown (mean ± SD). (C) Apoptosis (subG1 fraction) was quantified in melanoma cells treated with thapsigargin or tunicamycin for 72 h. Bar graphs show the quantification of results from 3 independent experiments (mean ± SD). (D) NRAS-mutated melanoma cells (MV3) seeded onto dermal reconstructs were treated with DMSO (control) or thapsigargin. Red, Ki67 (n=3 experiments). Scale bar = 100 μm. (E to J) Melanoma cells were injected into the rhombencephalic brain vesicle of stage 13/14 HH chick embryos. After 48 and 72 h, embryos were treated with DMSO (control, top row) or thapsigargin (TG, bottom row). HE: hematoxylin and eosin stain at 5× magnification (E and H). HMB45: immunohistochemistry with the melanoma-specific marker HMB45, at 40× magnification(F and I). MIB1: immunohistochemistry for the proliferation marker Ki67 with the MIB1 antibody at 5× magnification (G and J). n=2 experiments, 8 embryos per treatment. (K) Growth assessment of melanoma cells treated with the indicated concentrations of vemurafenib, thapsigargin, or both for 72 h. Bar graphs show the quantification of results from 3 independent experiments (mean ± SD, Mann-Whitney test, 95% CI; * P < 0.05, ** P < 0.001, *** P < 0.0001).
We then tested whether ER stress inducers also affected invasive tumor growth of melanoma cells in a more physiological context by treating MV3 melanoma cells seeded onto human dermal reconstructs with thapsigargin. Thapsigargin reduced the number of proliferating melanoma cells and abolished invasive tumor growth of melanoma cells into the dermis compared with DMSO-treated controls (Fig. 4D). Impaired invasive tumor growth is likely due to the fact that melanoma cells undergo apoptosis in response to thapsigargin (Fig. 2D).
We then tested effects of thapsigargin on in vivo tumor formation and invasion using a chick embryo model (30) in which melanoma cells were injected into the rhombencephalic brain vesicle. The DMSO-treated control embryos showed massive solid tumor formation in the roof plate of the rhombencephalic brain vesicle and the adjacent mesenchyme (Fig. 4E), invasion of melanoma cells into the neural and mesenchymal tissues, as indicated by the melanoma-specific marker HMB45 (Fig. 4F), and a greater than 90% proliferation index in melanoma cells 96 hours after injection, as detected by immunohistochemistry with the Ki67-detecting antibody MIB1 (Fig. 4G). In contrast, the thapsigargin-treated embryos developed tumors comprised of loosely packed melanoma cells (Fig. 4H) which invaded into the mesenchyme surrounding the primary tumor to a lesser extent (Fig. 4I) and a lower than 10% proliferation index as detected by immunohistochemistry for Ki67 (Fig. 4J), demonstrating the effectiveness of thapsigargin in preventing the growth and invasion of melanoma cells with BRAFV600E.
We next determined whether the anti-tumor effects of vemurafenib were enhanced by ER stress inducers. In SKMEL19 melanoma cells, which were sensitive to vemurafenib, thapsigargin did not enhance the anti-proliferative and pro-apoptotic effects of vemurafenib. However, in 451LU cells, which were less sensitive to vemurafenib, combination treatment with thapsigargin significantly increased growth inhibition (Fig. 4K) and the percentage of cells undergoing apoptosis (Fig. S6). This indicated that ER stress might boost vemurafenib-mediated antitumor activity, and suggested that this combination approach may abrogate intrinsic resistance. In agreement with previously published studies we also noted that the heat shock protein 90 (HSP90) inhibitor XL888 also overcame intrinsic BRAF inhibitor resistance (31) (Fig. S7A).
ER stress inducers overcome acquired resistance to vemurafenib
We next explored whether melanoma cells with acquired vemurafenib resistance retained sensitivity to other ER stress inducers, such as thapsigargin. For this, we established vemurafenib-resistant cell lines by chronically treating BRAF inhibitor-sensitive melanoma cell lines with increasing concentrations of vemurafenib or DMSO (control) for 6 months. In the following experiments, we used the vemurafenib-sensitive parental cell line, SKMEL19-Vem-S and the vemurafenib-resistant cell line, SKMEL19-Vem-R.
Sequencing of the BRAF, NRAS, and C-KIT genes in SKMEL19-Vem-S and SKMEL19-Vem-R cells did not reveal the acquisition of additional mutations in these genes (Table S1). Vemurafenib did not inhibit growth in SKMEL19-Vem-R cells, thus demonstrating the acquisition of drug resistance in these cells, whereas it inhibited growth in SKMEL19-Vem-S cells (Fig. 5A). Likewise, vemurafenib did not induce apoptosis in SKMEL19-Vem-R cells whereas it triggered a 6-fold higher rate of apoptosis in SKMEL19-Vem-S cells (Fig. 5B).
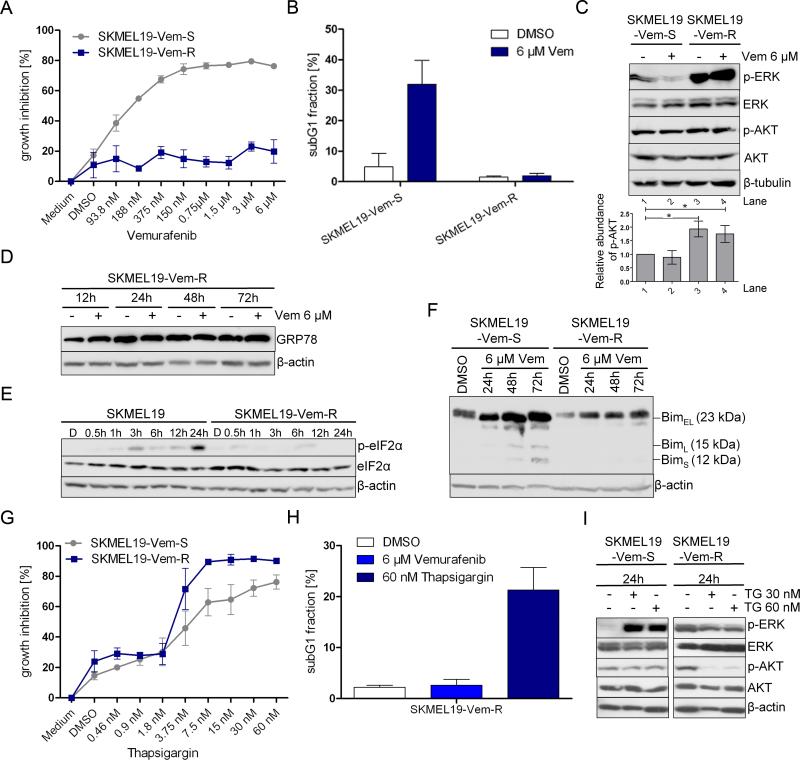
Figure 5. ER stress inducers overcome acquired resistance to vemurafenib.
(A) Growth assessment of SKMEL19-Vem-S and SKMEL19-Vem-R melanoma cells treated with the indicated concentrations of vemurafenib for 72 h. One representative experiment of 4 is shown (mean ± SD). (B) Apoptosis (subG1 fraction) was quantified in SKMEL19-Vem-S and SKMEL19-Vem-R cells treated with vemurafenib or DMSO for 72 h. Bar graphs show the quantification of results from 3 independent experiments (mean ± SD). (C, D, E, F) Western blot analysis for p-ERK, ERK, p-AKT, AKT, GRP78, p-eIF2α, eIF2α, and BIM isoforms was performed at the indicated time points in SKMEL19-Vem-S or SKMEL19-Vem-R cells treated with vemurafenib (6 μM) or DMSO, β-tubulin or β-actin served as loading control. One representative experiment of three is shown. (C) The ratio of the amount of p-AKT to that of AKT for each lane from three experiments is indicated in the accompanying bar graph (mean ± SD, Mann-Whitney test, 95% CI; * P < 0.05). (G) Growth assessment of SKMEL19-Vem-S and SKMEL19-Vem-R cells treated with the indicated concentrations of thapsigargin for 72 h. One representative experiment of 3 is shown (mean ± SD). (H) Apoptosis (subG1 fraction) was quantified in SKMEL19-Vem-R cells treated with vemurafenib, DMSO, or thapsigargin for 72 h. Bar graphs show the quantification of results from 3 independent experiments (mean ± SD). (I) Western blot analysis for p-ERK, ERK, p-AKT, AKT, and β-actin was performed in SKMEL19-Vem-S and SKMEL19-Vem-R cells treated with thapsigargin (TG) or DMSO for 24 h. One representative experiment of 3 is shown.
As expected, vemurafenib did not appear to affect the phosphorylation of ERK or AKT in SKMEL19-Vem-R cells (Fig. 5C), and the extent of basal phosphorylation of ERK and AKT in SKMEL19-Vem-R cells was greater than in SKMEL19-Vem-S cells (Fig. 5C). In contrast to its activity in SKMEL19-Vem-S cells (Fig. 2I), vemurafenib did not suppress the abundance of the ER chaperone protein GRP78 in SKMEL19-Vem-R cells after 72 hours (Fig. 5D), induce the phosphorylation of eIF2α (Fig. 5E), or increase the abundance of pro-apoptotic Bim isoforms (Fig. 5F).
We then investigated whether vemurafenib-resistant melanoma cells might be sensitive to apoptosis induction by other ER stress inducers. SKMEL19-Vem-S and SKMEL19-Vem-R cells treated with increasing concentrations of thapsigargin were growth inhibited (Fig. 5G) and underwent apoptosis (Fig. 5H). Thapsigargin increased phosphorylation of ERK in SKMEL19-Vem-S cells (Fig. 5I), whereas vemurafenib decreased phosphorylation of ERK in these cells (Fig. 1A). In SKMEL19-Vem-R cells, thapsigargin inhibited phosphorylation of AKT but not that of ERK (Fig. 5I), suggesting the two drugs operate through different mechanisms. Taken together, these data indicate that metastatic melanoma cells with acquired resistance to vemurafenib-induced ER stress-mediated apoptosis may remain sensitive to agents that induce ER stress-mediated apoptosis through a different mechanism. In agreement with previously published studies, we also observed that the HSP90 inhibitor XL888, which restores the abundance of Bim and suppresses the anti-apoptotic protein Mcl-1, also overcame acquired vemurafenib resistance (31) (Fig. S7B).
Discussion
Previous studies have demonstrated that some cancer types, including melanoma, may adapt to ER stress and become resistant to ER stress-induced apoptosis through the activation of pathways and expression of proteins that promote survival (32). In melanoma, it has been suggested that resistance to ER stress-mediated apoptosis may be mediated through the activation of the RAF-MEK-ERK or PI3K-AKT-mTOR pathways as well as increased abundance of the chaperone protein GRP78 and anti-apoptotic proteins, like Mcl-1 (32, 33). Previous studies show that inhibition of MEK signaling, either through siRNA knockdown or the MEK inhibitor U0126, sensitizes melanoma cells to ER stress-mediated apoptosis (33). In addition, inhibition of Mcl-1 function through the BH3 mimetic obatoclax also sensitizes melanoma cells to ER stress-mediated apoptosis (32). Consistent with these reports, we show here that the BRAFV600E inhibitor vemurafenib induces apoptosis by inhibiting the RAF-MEK-ERK pathway, decreasing the abundance of Bcl-2 and Mcl-1, and increasing the expression of ER stress-related genes. We propose a model for the mechanism of action of vemurafenib based on our findings (Fig. 6).
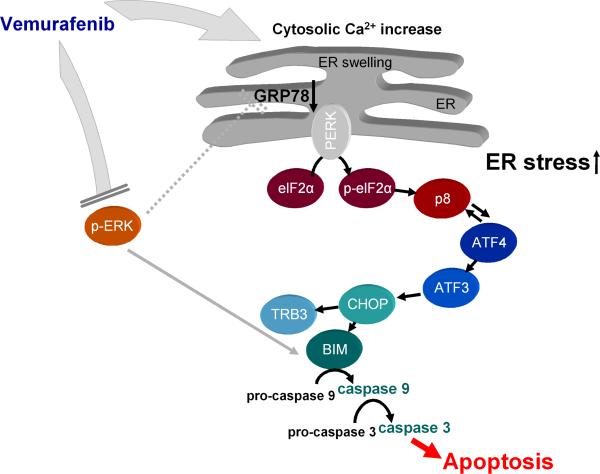
Figure 6. Proposed mechanism of vemurafenib action.
Vemurafenib induces ER stress by increasing the concentration of cytosolic Ca2+. eIF2α is phosphorylated, thereby globally inhibiting translation. The expression of ER stress-related genes and the abundance of the pro-apoptotic protein Bim are increased. Furthermore, vemurafenib inhibits phosphorylation of ERK, which enhances downstream pro-apoptotic signaling. Dashed lines represent indirect effects.
Our results showed that vemurafenib treatment led to a rapid increase in the concentration of cytosolic Ca2+ in a similar manner to thapsigargin, but unlike the MEK inhibitor U0126. The pan-RAF inhibitor sorafenib, which also inhibits the kinase activity of BRAFV600E, also increases the concentration of cytosolic Ca2+ through a MEK-independent mechanism that involves depletion of Ca2+ stores from the ER (34). Depletion of Ca2+ from the ER is a major factor in thapsigargin-induced ER stress and apoptosis (26). The mechanism that underlies sorafenib- or vemurafenib-induced Ca2+ mobilization remains to be determined.
Vemurafenib suppressed expression of the ER chaperone protein GRP78. Normally, upon ER stress expression of GRP78 is induced in an effort to restore normal ER function. Thus, the classical ER stress inducer thapsigargin rapidly induces GRP78 expression (34). However, in contrast to thapsigargin, the RAF inhibitor sorafenib and the MEK inhibitor PD184352 rapidly decrease the abundance of GRP78 (34). Thus, vemurafenib may induce ER stress and increase the abundance of GRP78 and simultaneously decrease the abundance of components in the MAPK pathway and GRP78. Diminishing the chaperone reserve and thus intensifying ER stress may contribute to the lethal actions of vemurafenib.
Vemurafenib increased the abundance of the spliced isoform of the transcription factor XBP1, a hallmark of ER stress, and triggered phosphorylation of the translation initiation factor eIF2α (Fig. S1A). Vemurafenib-induced ER stress was also associated with a marked increase in the expression of p8 and ATF4. p8 is a small DNA binding protein that acts as a co-transcription factor (35) through binding to proteins like p300, a transcriptional co-activator (36). p8 has been implicated in various biological functions that appear to be dependent on the tumor type and microenvironment (37), because contradictory reports show that p8 either promotes or suppresses tumor growth. In brain tumor cells, THC induces phosphorylation of eIF2α and increases the abundance of p8, which induces apoptosis by increasing the expression of the ER stress-related genes ATF4/3, CHOP, and TRB3 (24, 25). Furthermore, stimulation of the RAF-MEK-ERK pathway decreases the abundance of p8 and promotes proliferation of pancreatic cancer cells (38). In the current study, siRNA knockdown of p8 did not reliably affect vemurafenib-induced apoptosis. We hypothesized that p8 was not sufficiently knocked down in the presence of high vemurafenib-induced p8 expression, and indeed, p8 overexpression enhanced vemurafenib-induced apoptosis. Alternatively, other ER stress-related factors may have compensated for the p8 deficiency. ATF4 knockdown significantly reduced vemurafenib-induced apoptosis. Consistent with our data, a report has shown that ATF4 mediates ER-stress-induced cell death in neuroectodermal tumor cells treated with the chemotherapeutic agent, fenretinide, or the proteasome inhibitor, bortezomib (39). Together, these data indicate that ATF4 plays a relevant pro-apoptotic role in ER stress-mediated apoptosis.
In melanoma cells, inhibition of the MEK-ERK pathway induces apoptosis and increases the abundance of Bim, a pro-apoptotic BH3-only member of the Bcl-2 family (40). Previous studies in breast carcinoma cell lines implicate Bim in the ER stress response. In these studies, ER stress increases the abundance of Bim through the induction of CHOP-mediated transcription and protein phosphatase 2A-mediated dephosphorylation, which prevents Bim ubiquitination and proteasomal degradation (18). Consistent with these studies, we found that vemurafenib inhibited phosphorylation of ERK, induced ER stress, and increased the abundance of all three Bim isoforms (BimS, BimL and BimEL) leading to increased apoptosis. The relevance of Bim induction was demonstrated by the impaired vemurafenib-induced apoptotic response after its knockdown by siRNA. In line with our observations, a study (16) shows that a key mechanism underlying apoptosis induction by the BRAFV600E inhibitor PLX4720 was induction of the short Bim isoform, BimS. Furthermore, we observed that vemurafenib increased the abundance of two other proteins of the BH3-only pro-apoptotic Bcl-2 family, PUMA and Noxa, both of which play a role in ER stress-mediated apoptosis (41).
Vemurafenib-resistant BRAFV600E melanoma cells exhibited enhanced basal phosphorylation of ERK and AKT compared to vemurafenib-sensitive cells. In resistant cells, vemurafenib did not inhibit phosphorylation of ERK, suppress the ER chaperone protein GRP78 after 72 hours, trigger phosphorylation of eIF2α, increase the abundance of pro-apoptotic Bim isoform, inhibit growth, or induce apoptosis. We found that thapsigargin inhibited growth and induced apoptosis in vemurafenib-resistant melanoma cells. Thapsigargin is currently being tested in a phase I study for treating patients with advanced solid tumors (ClinicalTrials.gov Identifier: NCTT01056029). We found that thapsigargin inhibited activation of the AKT pathway, but not that of the ERK pathway. AKT exerts multiple inhibitory effects on apoptosis by directly targeting and inhibiting proapoptotic proteins including forkhead transcription factors, the BH3-only protein BAD (Bcl-2-associated agonist of cell death), and caspase-9 (42). Furthermore, AKT activates members of the IAP (inhibitor of apoptosis) family, including XIAP (X-linked inhibitor of apoptosis) and cIAP2 (cellular inhibitor of apoptosis protein 2), which inhibit ER stress-mediated apoptosis at the post-mitochondrial phase. (43). Thus, suppression of AKT activation may sensitize vemurafenib-resistant melanoma cells to ER stress-mediated apoptosis. As described in the introduction, resistance to vemurafenib is mediated through multiple mechanisms, the diversity of which is a serious clinical challenge. Augmenting ER stress and blocking ER stress adaptation mechanisms may be one way to overcome vemurafenib resistance. Moreover, many signaling proteins involved in vemurafenib resistance are clients of HSP90 (31). Here, we showed that the HSP90 inhibitor XL888 overcomes both intrinsic and acquired resistance to vemurafenib.
Here, we demonstrate that vemurafenib is a potent inducer of ER stress in BRAFV600E mutant melanoma cells. Of clinical relevance, we found that the ER-stress inducer thapsigargin augmented or induced apoptosis in melanoma cells with low sensitivity or acquired resistance to vemurafenib. We suggest that the combination of vemurafenib with thapsigargin may be a strategy to manage both intrinsic and acquired resistance to BRAF inhibitors.
Materials and Methods
Isolation and culture of human cells
The use of human skin tissues was approved by the Medical Ethics committee of the University of Tuebingen. Experiments were performed in accordance with the Declaration of Helsinki. M. Herlyn kindly provided the metastatic melanoma cell lines 451LU, WM164 and 1205LU. SKMEL19 and MV3 metastatic melanoma cell lines were purchased (ATCC). Cell lines, keratinocytes, and fibroblasts were cultured and isolated as described previously (44-46).
Signaling pathway inhibitors and treatments
Signaling pathway regulators included the BRAFV600E kinase inhibitor, vemurafenib, (PLX4032, Plexxikon) and the MEK inhibitor, U0126, (Cell Signaling); ER stress inducers, thapsigargin (Sigma-Aldrich) and tunicamycin (Sigma-Aldrich); the selective Ca2+ ionophore ionomycin (Merck); the apoptosis inducer, staurosporine (Sigma-Aldrich); the caspase inhibitor, Q-VD-OPH and the inactive caspase inhibitor, Q-VD-OPH control (BioVision). The HSP90 inhibitor XL888 was provided by Exelixis. Inhibitors were dissolved in dimethylsulfoxide (DMSO), except for staurosporine (in water), and stored at -20 °C. Inhibitors were added directly to the melanoma cell culture medium. Controls were incubated with culture medium or culture medium and DMSO.
Western Blotting
Total protein was extracted from cell pellets, separated by electrophoresis on 4-20% SDS-polyacrylamide gels (15-120 μg/lane), and blotted onto PVDF membranes (Roche). Blots were probed with antibodies against AKT, AKT phosphorylated at Ser473, ERK, phosphorylated ERK, cleaved caspase-3, Mcl-1, Bcl-2, Bim, PUMA, Noxa, eIF2α, phosphorylated eIF2α, GRP78, GFP, β-tubulin, β-actin (Cell Signaling), PARP (Alexis Biochemicals/Enzo Life Sciences), caspase-9 (S. Wesselborg, Institute of Molecular Medicine, Duesseldorf), caspase-3 (BD Transduction Laboratories), ATF4 (R&D), p8 (J. L. Iovanna, Centre de Recherche INSERM, Marseille), spliced XBP1 (Biolegend) and α-tubulin (Sigma). Proteins were visualized with secondary peroxidase-conjugated antibodies (Cell Signaling) and the ECL system (Amersham) or with IRDye® secondary antibodies and the LI-COR® infrared imaging system (LI-COR).
Cell viability assay
Viability was measured using the 4-methylumbelliferyl heptanoate assay as described previously (47).
Apoptosis
Cell cycle
Cell cycle analyses using propidium idodide staining was performed as described previously (47).
Apoptotic cell death
Apoptosis estimated with the cell death detection ELISA kit (Roche) was performed according to manufacturer's instructions, or with propidium iodide staining. Apoptosis measured by Annexin V staining was carried out as described previously (17) with cells seeded at 60% confluency in 6-well tissue culture plates and left to grow overnight before being treated.
Organotypic skin culture
Organotypic cultures of human skin and melanoma were established as described previously (46). Inhibitors were tested for effects against invasive melanoma growth. MV3 melanoma cells were seeded onto human dermal reconstructs, cultured for 4 days, and then treated with thapsigargin (60 nM). Controls were treated with culture medium or culture medium with DMSO.
Chick embryo model
Chick embryo incubation, fenestration, injection procedures, and immunohistochemistry were described previously (30).
Gene expression
Quantitative real-time PCR (RT-PCR) was performed with the LightCycler® 480 SYBR Green System (Roche). RNA extraction (MACHERY-NAGEL) and reverse transcription (Invitrogen) were performed according to manufacturer's instructions. 1 μg cDNA was used for PCR analyses. Primer sequences are listed in Table S2. The expression of the genes was normalized to the expression of TATA-binding protein (TBP).
Experiments were performed in triplicate. PCR product specificity was verified by melting curve analysis.
Transmission electron microscopy
Cells were fixed in Karnovsky's fixative, embedded in agarose, coagulated, cut into small blocks, and fixed again in Karnovsky's solution. Blocks were embedded in glycid ether, and sectioned (30 nm) with an ultra microtome. Sections were mounted on copper grids, and analyzed with a Zeiss LIBRA 120 transmission electron microscope.
Transfection experiments
Genes were knocked down with prevalidated, small interfering RNAs (siRNAs) against ATF4 (Qiagen, SI03019345), Bim (Cell Signaling, 6518) or p8 (Qiagen, SI02664326). The validated negative control siRNA was a non-silencing, luciferase-specific siRNA (Biomers). J. L. Iovanna (Centre de Recherche INSERM, Marseille) kindly provided the p8-GFP expression vector (pEGFP-C2-p8). For siRNA knockdown, 2-2.5 × 105 cells were plated in 6-well plates overnight. Then, 25 nM siRNA was transfected with LipofectamineTM RNAiMAX (Invitrogen) according to manufacturer`s instructions. Cells were cultured in normal growth medium for 24 hours prior to drug treatment. Overexpression of p8 was achieved by transiently transfecting 1 μg of pEGFP-C2-p8 with Lipofectamine™ 2000 according to manufacturer`s instructions. Controls were transfected with empty vector (pEGFP-N1; Clonetech).
Cytosolic Ca2+
Cytosolic Ca2+ was measured with Fluo3-AM (Invitrogen). Briefly, 5 × 106 cells were loaded with 2 μM Fluo3-AM for 20 min at room temperature, protected from light. Cells were washed, and then resuspended at 106 cells/ml in RPMI 1640 Medium, no Phenol Red (Gibco) with 10% fetal bovine serum. Changes in cytosolic Ca2+ concentrations were measured on a LSRII FACS flow cytometer (BD) at an excitation wavelength of 488 nm.
Mutation analysis
DNA for PCR and sequencing was isolated by proteinase K digestion of cell pellets from SKMEL19-Vem-S and SKMEL19-Vem-R cell lines (DNeasy Blood & Tissue Kit; Qiagen). PCR primer sets were designed to amplify the BRAF codon 600, KIT exons 11, 13, 17 and 18, and NRAS codon 61. Primer sequences are listed in Table S3. Purified PCR products were directly sequenced (Quintara Bio Sequencing Service), and traces were analyzed with Mutation Surveyor V3.23 (Softgenetics, State College, PA).
Statistical analysis
Statistical analysis was performed with GraphPad Prism version 5.01 (GraphPad Prism Software Inc.). The two groups were compared using the nonparametric Mann-Whitney Test (95%CI). P-values <0.05 were considered statistically significant. N values represent the number of independent experiments unless stated otherwise.
Supplementary Material
Acknowledgments
We would like to acknowledge Plexxikon for the donation of vemurafenib. We also thank Hannelore Bischof, Renate Nordin, and Birgit Fehrenbacher for outstanding assistance in electron microscopy and Cornelia Grimmel for excellent assistance in fluorescent cell sorting. Funding: DB, HN, SD, BS, SW, MS, CB, BS, CG and FM were supported by the Deutsche Forschungsgemeinschaft (SFB 773). TB was supported by the Deutsche Forschungsgemeinschaft (SFB 685, SPP1394, BI 696/10-1) and the University of Tuebingen, IZKF-Tuebingen, collaborative research program. KSMS was supported by NIH grant R01 CA161107-02. KHTP was supported by the Joanna M. Nicolay Foundation.
Footnotes
Author contributions: D.B and F.M. designed the study, interpreted the data, and wrote the paper; D.B., H.N., K.P., and T.S. performed the experiments; C.B. performed experiments in chick embryos; S.V. and J.L. provided the p8-GFP expression vector and contributed to the design of experiments; S.D., B.S., and S.W. contributed to apoptosis assays and performed Western blot analyses of caspase-9, caspase-3, cleaved caspase-3, and PARP; M.S. performed electron microscopy; T.B. performed fluorescent cell sorting; J.B. performed mutation analysis; K.L. contributed to RT-PCR; K.S. contributed to data analysis/interpretation and to wording/grammar; D.K. contributed to study design and analysis/interpretation of data; K.F. contributed to study design and interpretation of data; J.E. contributed to analysis/interpretation of ER stress and apoptosis assays and manuscript writing; C.G., D.S., B.S., K.L. and B.W. contributed to interpretation of data and manuscript writing.
Competing interests: The authors declare that they have no competing interests.
References
- 1.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr., Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199. doi: 10.1200/JCO.2009.23.4799. published online EpubDec 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949. doi: 10.1038/nature00766. published online EpubJun 27. [DOI] [PubMed] [Google Scholar]
- 3.Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, Van Belle P, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer research. 2003;63:756. published online EpubFeb 15. [PubMed] [Google Scholar]
- 4.Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473. doi: 10.1200/JCO.2005.07.168. published online EpubMar 1. [DOI] [PubMed] [Google Scholar]
- 5.Meier F, Schittek B, Busch S, Garbe C, Smalley K, Satyamoorthy K, Li G, Herlyn M. The RAS/RAF/MEK/ERK and PI3K/AKT signaling pathways present molecular targets for the effective treatment of advanced melanoma. Front Biosci. 2005;10:2986. doi: 10.2741/1755. [DOI] [PubMed] [Google Scholar]
- 6.Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, Hersey P, Kefford R, Lawrence D, Puzanov I, Lewis KD, Amaravadi RK, Chmielowski B, Lawrence HJ, Shyr Y, Ye F, Li J, Nolop KB, Lee RJ, Joe AK, Ribas A. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. The New England journal of medicine. 2012;366:707. doi: 10.1056/NEJMoa1112302. published online EpubFeb 23 (10.1056/NEJMoa1112302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. The New England journal of medicine. 2011;364:2507. doi: 10.1056/NEJMoa1103782. published online EpubJun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O'Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB. Inhibition of mutated, activated BRAF in metastatic melanoma. The New England journal of medicine. 2010;363:809. doi: 10.1056/NEJMoa1002011. published online EpubAug 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, Ng C, Chodon T, Scolyer RA, Dahlman KB, Sosman JA, Kefford RF, Long GV, Nelson SF, Ribas A, Lo RS. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun. 2012;3:724. doi: 10.1038/ncomms1727. 10.1038/ncomms1727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulikakos PI, Persaud Y, Janakiraman M, Kong X, Ng C, Moriceau G, Shi H, Atefi M, Titz B, Gabay MT, Salton M, Dahlman KB, Tadi M, Wargo JA, Flaherty KT, Kelley MC, Misteli T, Chapman PB, Sosman JA, Graeber TG, Ribas A, Lo RS, Rosen N, Solit DB. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature. 2011;480:387. doi: 10.1038/nature10662. published online EpubDec 15 (10.1038/nature10662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poulikakos PI, Rosen N. Mutant BRAF melanomas--dependence and resistance. Cancer cell. 2011;19:11. doi: 10.1016/j.ccr.2011.01.008. published online EpubJan 18. [DOI] [PubMed] [Google Scholar]
- 12.Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, Kehoe SM, Johannessen CM, Macconaill LE, Hahn WC, Meyerson M, Garraway LA. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3085. doi: 10.1200/JCO.2010.33.2312. published online EpubAug 1 (10.1200/JCO.2010.33.2312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, Emery CM, Stransky N, Cogdill AP, Barretina J, Caponigro G, Hieronymus H, Murray RR, Salehi-Ashtiani K, Hill DE, Vidal M, Zhao JJ, Yang X, Alkan O, Kim S, Harris JL, Wilson CJ, Myer VE, Finan PM, Root DE, Roberts TM, Golub T, Flaherty KT, Dummer R, Weber BL, Sellers WR, Schlegel R, Wargo JA, Hahn WC, Garraway LA. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968. doi: 10.1038/nature09627. published online EpubDec 16 (10.1038/nature09627) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, Wubbenhorst B, Xu X, Gimotty PA, Kee D, Santiago-Walker AE, Letrero R, D'Andrea K, Pushparajan A, Hayden JE, Brown KD, Laquerre S, McArthur GA, Sosman JA, Nathanson KL, Herlyn M. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer cell. 2010;18:683. doi: 10.1016/j.ccr.2010.11.023. published online EpubDec 14 (10.1016/j.ccr.2010.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, Chen Z, Lee MK, Attar N, Sazegar H, Chodon T, Nelson SF, McArthur G, Sosman JA, Ribas A, Lo RS. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973. doi: 10.1038/nature09626. published online EpubDec 16 (10.1038/nature09626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang CC, Lai F, Tay KH, Croft A, Rizos H, Becker TM, Yang F, Liu H, Thorne RF, Hersey P, Zhang XD. Apoptosis of human melanoma cells induced by inhibition of B-RAFV600E involves preferential splicing of bimS. Cell death & disease. 2010;1:e69. doi: 10.1038/cddis.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC, Wood E, Fedorenko IV, Sondak VK, Anderson AR, Ribas A, Palma MD, Nathanson KL, Koomen JM, Messina JL, Smalley KS. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer research. 2011;71:2750. doi: 10.1158/0008-5472.CAN-10-2954. published online EpubApr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337. doi: 10.1016/j.cell.2007.04.027. published online EpubJun 29. [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. The Journal of biological chemistry. 2004;279:25935. doi: 10.1074/jbc.R400008200. published online EpubJun 18. [DOI] [PubMed] [Google Scholar]
- 20.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annual review of biochemistry. 2005;74:739. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 21.Schoenthal AH. Targeting endoplasmic reticulum stress for cancer therapy. Frontiers in bioscience (Scholar edition) 2012;4:412. doi: 10.2741/s276. [DOI] [PubMed] [Google Scholar]
- 22.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. The Journal of clinical investigation. 2005;115:2656. doi: 10.1172/JCI26373. published online EpubOct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell death and differentiation. 2006;13:363. doi: 10.1038/sj.cdd.4401817. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 24.Carracedo A, Lorente M, Egia A, Blazquez C, Garcia S, Giroux V, Malicet C, Villuendas R, Gironella M, Gonzalez-Feria L, Piris MA, Iovanna JL, Guzman M, Velasco G. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer cell. 2006;9:301. doi: 10.1016/j.ccr.2006.03.005. published online EpubApr. [DOI] [PubMed] [Google Scholar]
- 25.Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, Gonzalez-Feria L, Iovanna JL, Guzman M, Boya P, Velasco G. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. The Journal of clinical investigation. 2009;119:1359. doi: 10.1172/JCI37948. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida I, Monji A, Tashiro K, Nakamura K, Inoue R, Kanba S. Depletion of intracellular Ca2+ store itself may be a major factor in thapsigargin-induced ER stress and apoptosis in PC12 cells. Neurochemistry international. 2006;48:696. doi: 10.1016/j.neuint.2005.12.012. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Current molecular medicine. 2006;6:45. doi: 10.2174/156652406775574523. published online EpubFeb. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang L, Scolyer RA, Lee CS, McCarthy SW, Cooper WA, Zhang XD, Thompson JF, Hersey P. Expression of glucose-regulated stress protein GRP78 is related to progression of melanoma. Histopathology. 2009;54:462. doi: 10.1111/j.1365-2559.2009.03242.x. published online EpubMar. [DOI] [PubMed] [Google Scholar]
- 29.Dong D, Ni M, Li J, Xiong S, Ye W, Virrey JJ, Mao C, Ye R, Wang M, Pen L, Dubeau L, Groshen S, Hofman FM, Lee AS. Critical role of the stress chaperone GRP78/BiP in tumor proliferation, survival, and tumor angiogenesis in transgene-induced mammary tumor development. Cancer research. 2008;68:498. doi: 10.1158/0008-5472.CAN-07-2950. published online EpubJan 15. [DOI] [PubMed] [Google Scholar]
- 30.Krochmann J, Sinnberg T, Meier F, Garbe C, Busch C. Melanoma cells in distinct growth phases retain specific invasive qualities during brain metastasis in vivo. Pigment cell & melanoma research. 2011;25:113. doi: 10.1111/j.1755-148X.2011.00914.x. published online EpubJan. [DOI] [PubMed] [Google Scholar]
- 31.Paraiso KH, Haarberg HE, Wood E, Rebecca VW, Chen YA, Xiang Y, Ribas A, Lo RS, Weber JS, Sondak VK, John JK, Sarnaik AA, Koomen JM, Smalley KS. The HSP90 inhibitor XL888 overcomes BRAF inhibitor resistance mediated through diverse mechanisms. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2502. doi: 10.1158/1078-0432.CCR-11-2612. published online EpubMay 1 (10.1158/1078-0432.CCR-11-2612) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang CC, Yang F, Thorne RF, Zhu BK, Hersey P, Zhang XD. Human melanoma cells under endoplasmic reticulum stress acquire resistance to microtubule-targeting drugs through XBP-1-mediated activation of Akt. Neoplasia (New York, N.Y. 2009;11:436. doi: 10.1593/neo.09208. published online EpubMay. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang CC, Chen LH, Gillespie S, Wang YF, Kiejda KA, Zhang XD, Hersey P. Inhibition of MEK sensitizes human melanoma cells to endoplasmic reticulum stress-induced apoptosis. Cancer research. 2007;67:9750. doi: 10.1158/0008-5472.CAN-07-2047. published online EpubOct 15. [DOI] [PubMed] [Google Scholar]
- 34.Rahmani M, Davis EM, Crabtree TR, Habibi JR, Nguyen TK, Dent P, Grant S. The kinase inhibitor sorafenib induces cell death through a process involving induction of endoplasmic reticulum stress. Molecular and cellular biology. 2007;27:5499. doi: 10.1128/MCB.01080-06. published online EpubAug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallo GV, Fiedler F, Calvo EL, Ortiz EM, Vasseur S, Keim V, Morisset J, Iovanna JL. Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth. The Journal of biological chemistry. 1997;272:32360. doi: 10.1074/jbc.272.51.32360. published online EpubDec 19. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmeister A, Ropolo A, Vasseur S, Mallo GV, Bodeker H, Ritz-Laser B, Dressler GR, Vaccaro MI, Dagorn JC, Moreno S, Iovanna JL. The HMG-I/Y-related protein p8 binds to p300 and Pax2 trans-activation domain-interacting protein to regulate the trans-activation activity of the Pax2A and Pax2B transcription factors on the glucagon gene promoter. The Journal of biological chemistry. 2002;277:22314. doi: 10.1074/jbc.M201657200. published online EpubJun 21. [DOI] [PubMed] [Google Scholar]
- 37.Chowdhury UR, Samant RS, Fodstad O, Shevde LA. Emerging role of nuclear protein 1 (NUPR1) in cancer biology. Cancer metastasis reviews. 2009;28:225. doi: 10.1007/s10555-009-9183-x. published online EpubJun. [DOI] [PubMed] [Google Scholar]
- 38.Malicet C, Lesavre N, Vasseur S, Iovanna JL. p8 inhibits the growth of human pancreatic cancer cells and its expression is induced through pathways involved in growth inhibition and repressed by factors promoting cell growth. Molecular cancer. 2003;2:37. doi: 10.1186/1476-4598-2-37. published online EpubNov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armstrong JL, Flockhart R, Veal GJ, Lovat PE, Redfern CP. Regulation of endoplasmic reticulum stress-induced cell death by ATF4 in neuroectodermal tumor cells. The Journal of biological chemistry. 2010;285:6091. doi: 10.1074/jbc.M109.014092. published online EpubFeb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934. doi: 10.1158/1078-0432.CCR-07-0665. published online EpubAug 15. [DOI] [PubMed] [Google Scholar]
- 41.Hersey P, Zhang XD. Adaptation to ER stress as a driver of malignancy and resistance to therapy in human melanoma. Pigment cell & melanoma research. 2008;21:358. doi: 10.1111/j.1755-148X.2008.00467.x. published online EpubJun (10.1111/j.1755-148X.2008.00467.x) [DOI] [PubMed] [Google Scholar]
- 42.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332. doi: 10.1038/nature02369. published online EpubMar 18. [DOI] [PubMed] [Google Scholar]
- 43.Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. The Journal of biological chemistry. 2004;279:49420. doi: 10.1074/jbc.M407700200. published online EpubNov 19. [DOI] [PubMed] [Google Scholar]
- 44.Lasithiotakis KG, Sinnberg TW, Schittek B, Flaherty KT, Kulms D, Maczey E, Garbe C, Meier FE. Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells. The Journal of investigative dermatology. 2008;128:2013. doi: 10.1038/jid.2008.44. published online EpubAug. [DOI] [PubMed] [Google Scholar]
- 45.Mancianti ML, Herlyn M, Weil D, Jambrosic J, Rodeck U, Becker D, Diamond L, Clark WH, Koprowski H. Growth and phenotypic characteristics of human nevus cells in culture. The Journal of investigative dermatology. 1988;90:134. doi: 10.1111/1523-1747.ep12462099. published online EpubFeb. [DOI] [PubMed] [Google Scholar]
- 46.Meier F, Nesbit M, Hsu MY, Martin B, Van Belle P, Elder DE, Schaumburg-Lever G, Garbe C, Walz TM, Donatien P, Crombleholme TM, Herlyn M. Human melanoma progression in skin reconstructs : biological significance of bFGF. The American journal of pathology. 2000;156:193. doi: 10.1016/S0002-9440(10)64719-0. published online EpubJan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinnberg T, Menzel M, Ewerth D, Sauer B, Schwarz M, Schaller M, Garbe C, Schittek B. beta-Catenin signaling increases during melanoma progression and promotes tumor cell survival and chemoresistance. PloS one. 2011;6:e23429. doi: 10.1371/journal.pone.0023429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.