Abstract
Early age at the natural final menstrual period (FMP) or menopause has been associated with numerous health outcomes and might be a marker of future ill health. However, potentially modifiable factors affecting age at menopause have not been examined longitudinally in large, diverse populations. The Study of Women's Health Across the Nation (SWAN) followed 3,302 initially premenopausal and early perimenopausal women from 7 US sites and 5 racial/ethnic groups, using annual data (1996–2007) and Cox proportional hazards models to assess the relation of time-invariant and time-varying sociodemographic, lifestyle, and health factors to age at natural FMP. Median age at the FMP was 52.54 years (n = 1,483 observed natural FMPs). Controlling for sociodemographic, lifestyle, and health factors, we found that racial/ethnic groups did not differ in age at the FMP. Higher educational level, prior oral contraceptive use, and higher weight at baseline, as well as being employed, not smoking, consuming alcohol, having less physical activity, and having better self-rated health over follow-up, were significantly associated with later age at the FMP. These results suggest that age at the natural FMP reflects a complex interrelation of health and socioeconomic factors, which could partially explain the relation of late age at FMP to reduced morbidity and mortality.
Keywords: age, education, ethnicity, menopause, oral contraceptives, race, smoking, weight
The age at the final menstrual period (FMP), natural menopause, holds intrinsic public health interest because it is associated with numerous health outcomes and might be a marker of aging and health (1–3). Later age at FMP has been associated with longer survival; greater life expectancy (4); reduced rates of all-cause mortality (5), cardiovascular disease (4, 6–12), cardiovascular death (13, 14), atherosclerosis (15), stroke (16), angina after myocardial infarction (17), low bone density (18), osteoporosis (19), and fracture (20); but increased breast (21, 22), endometrial, and ovarian cancer risk (4, 23, 24).
Physiological changes marking the onset of perimenopause (i.e., declining estradiol levels, rising follicle-stimulating hormone levels, and menstrual cycle irregularity) begin in women's mid-40s (25, 26). The median age at onset of the late perimenopause (defined as no periods in the prior 3 months but having period(s) in the prior 12 months) is 47.5 years (27–31), and age at FMP (followed by 12 months of amenorrhea) in white women from industrialized countries is 50–52 years, with slight evidence of increasing age at FMP in more recent cohorts (31–35). Age at FMP could vary by race/ethnicity (36–39) and demographic and lifestyle factors, particularly smoking (28, 29, 33, 35–37, 40–50). Age at FMP has been positively associated with maternal age at menopause (41, 51–55), but few longitudinal studies have investigated this. One twin study indicated genetic control of age at menopause (56), but potentially modifiable factors that might affect age at menopause, including weight, calorie and alcohol intake, and passive smoke exposure, have not been examined longitudinally in large, diverse populations, nor has the time-varying effect of such factors been assessed longitudinally.
We therefore examined these research hypotheses in the multiracial/multiethnic sample of midlife women in the Study of Women's Health Across the Nation (SWAN) cohort: 1) Previously established risk factors for early age at FMP that do not change meaningfully over time (e.g., smoking) would be related to early age at FMP in longitudinal analyses, consistent with prior cross-sectional analyses; 2) higher weight and caloric intake would be associated with later age at FMP, whereas passive smoke exposure and higher alcohol intake would be related to earlier age at FMP; and 3) age at FMP would be correlated with maternal age at menopause.
MATERIALS AND METHODS
Study population
We screened 16,065 community-based women aged 40–55 years (screener response rate = 46.6%) during 1995–1997 for eligibility for a longitudinal cohort in 7 sites in the United States (57). Each site screened 1 minority sample (African Americans in Pittsburgh, Boston, the Detroit area, and Chicago; Japanese in Los Angeles; Chinese in the Oakland, California, area; and Hispanics in New Jersey) and 1 Caucasian sample. Women who spoke English or Spanish, Cantonese, or Japanese (at New Jersey, Oakland, and Los Angeles, respectively) were eligible. The institutional review boards at all sites approved the protocol; all cohort participants provided signed, written informed consent.
Eligibility criteria for the cohort included age 42–52 years, having an intact uterus and at least 1 ovary, no use in the prior 3 months of exogenous hormones affecting ovarian function, not pregnant or lactating, had a menstrual period in the previous 3 months, and self-identification with each site's designated racial/ethnic groups. We recruited 3,302 eligible participants (50.7% response rate among eligible women). The present analyses included data through annual visit 10 (1996–2007), with 68% overall retention. Excluding the New Jersey site, which did not collect data for visits 6–10, retention was 78% through visit 10 (ranging from 71% for African-American women to 88% for Japanese women). We censored New Jersey participants after visit 5.
Data collection
Annual visits included an in-person interview, self-administered questionnaires, and measurement of weight and height (with calibrated scales and a stadiometer). All questionnaires were translated into Cantonese, Japanese, and Spanish and back-translated; translation discrepancies were resolved by 2 translators.
Outcome
Our primary outcome was age at the natural FMP, determined from annual interviews indicating 12 months of amenorrhea since the last menstrual period for no other cause (e.g., hysterectomy, bilateral oophorectomy). For 159 women missing this date at the visit before the first visit at which 12 months of amenorrhea was established, we interpolated the FMP date on the basis of interview date and reported number of months of amenorrhea at the prior visit.
Independent variables
Time-independent variables included primary race/ethnicity (self-defined as black or African American, non-Hispanic Caucasian, Chinese, Japanese, or Hispanic) and educational attainment from the screening questionnaire. Time-varying demographic variables included annual self-reported employment, marital status, and difficulty paying for basics such as food, shelter, and heat.
Time-varying lifestyle variables included annual self-reported active (58) and passive (59) smoke exposure and physical activity (60, 61), as well as diet (total calories and alcohol) from baseline and annual visits 5 and 9. Information on diet was obtained through the use of a modified Block Food Frequency Questionnaire (62–64), with added foods for the Hispanic, Chinese, and Japanese versions (65, 66).
Baseline time-invariant health-related variables included self-reported parity, prior oral contraceptive and other exogenous hormone use, and maternal age at menopause (reported at visit 4), as well as measured weight and height. Time-varying variables included self-assessed health (excellent, very good, good, or fair/poor); diabetes (use of diabetes medications or elevated fasting serum glucose); and changes in weight, serum estradiol level, and follicle-stimulating hormone level. Blood was drawn annually on days 2–5 of the menstrual cycle for women who were still cycling regularly and on any day for other women. SWAN's central laboratory at the University of Michigan assayed all annual blood samples for glucose, estradiol, and follicle-stimulating hormone (67, 68).
Data analyses
To test baseline racial/ethnic differences, we computed χ2 statistics for categorical variables and Kruskal–Wallis tests for continuous variables. We censored a participant's data at the visit at which she reported initiating hormone therapy if no subsequent hormone therapy-free bleeding occurred, at the date of hysterectomy or bilateral oophorectomy, or at the last menstrual period at the end of data collection if it occurred before 12 months of amenorrhea, either because of attrition or because of ending data collection at visit 10. Time-varying covariate information was included in analyses if it occurred ≤3 months after the date of FMP or censoring, to maximize use of observed data while avoiding inclusion of predictors that could be influenced by the FMP. We omitted 49 participants whose last menstrual period at initial eligibility screening occurred more than 3 months before baseline. Of the remaining 3,253 participants, 1,483 had an observed date at natural FMP, and 1,770 were censored because of one of the following: being postmenopausal but missing FMP date (n = 30); hysterectomy or bilateral oophorectomy before having ≥12 consecutive months of untreated amenorrhea (n = 192); hormone therapy initiation without hormone therapy-free bleeding after a 6-month washout period, followed by ≥12 consecutive months of amenorrhea (n = 590); missing data or attrition before visit 10 and before ≥12 consecutive months of amenorrhea (n = 652); or end of data collection at visit 10 before ≥12 consecutive months of amenorrhea (n = 306).
To identify predictors of age at natural FMP, we used Cox proportional hazards modeling (69). We used age, rather than time since baseline, as the time scale (70). In our final model, we did not adjust for estradiol or follicle-stimulating hormone because they probably constitute the underlying biological process that determines concurrent menopause status and the pathway to age at FMP. For time-invariant covariates, we applied the Kaplan–Meier approach to generate survival graphs and median age at FMP. Variables were retained in Cox models at P ≤ 0.05, applying backward elimination. Race/ethnicity was included as a predictor, and study site was included as a stratifying factor to account for nonproportional hazards by site (71).
We assessed the validity of the proportional hazards assumption for each predictor by testing the significance of its interaction with age (71) and determining whether its inclusion improved model fit, indicated by the Akaike Information Criterion statistic (72). The data were subject to left truncation or late entry, because participants had “survived” (as not yet postmenopausal) to at least age at screening minus 90 days. To account for this, participants were excluded from all risk sets at ages younger than this age (71).
For time-varying covariates, to distinguish cross-sectional (between-woman) from longitudinal (within-woman) effects, we included both the baseline value and change since baseline (for continuous predictors) or follow-up (for categorical predictors) value as separate predictors in the multivariate model. Variables indicating change since baseline were treated as linearly related to the log hazard; thus, positive coefficients are presented as hazard ratios >1 for increase over time (e.g., increase in weight since baseline). Because the coefficients for baseline and follow-up diabetes were not different, we combined these into a single time-varying predictor, “ever had diabetes,” to increase statistical power. For covariates with intermittent missing values, we compared unadjusted hazard ratios using 2 approaches: carrying the last value forward and omitting observations with missing covariate data. All results were very similar (data not shown). Within-woman correlation was high for all time-varying covariates except anxiety, life events, and depressive symptoms scores; thus, we carried the last value forward for all missing covariate data except for these variables, although these 3 variables were not preserved in the final model.
Baseline current smokers were overrepresented in the 49 excluded women, perhaps because of earlier FMPs and higher early attrition. The reasons for the disproportionate exclusion of New Jersey (including Hispanic) participants could have been similar, plus greater error in self-reported date of last menstrual period at screening, consistent with their higher proportion of “don't know” responses. In sensitivity analyses, we omitted an additional 411 women whose baseline data collection occurred on or after their reported last menstrual period (i.e., women with no time-varying covariates measured before FMP/censoring), yielding additional losses of smokers, Hispanics, and New Jersey participants and a slight increase in the median age at FMP (52.79 years). The resulting Cox model, however, was very similar, except baseline weight was no longer retained in the model (P = 0.065), probably because of reduced power with the reduced sample size.
RESULTS
Baseline sample characteristics
The median baseline age was 46.3 years (Table 1). About half of the women were premenopausal, with a significantly higher proportion among the Chinese and Japanese participants. Most women had at least some college education, did not report difficulty paying for basics, were employed, and reported oral contraceptive use but not other exogenous hormone use before baseline—all with significant racial/ethnic differences. Less than half of the women had ever smoked; the highest proportion of current smokers and the greatest passive smoke exposure were among African-American women, again with significant racial/ethnic variation.
Table 1.
Baseline (1996–1997) Characteristics of SWAN Cohort by Race/Ethnicity, United States (n = 3,253)
| Baseline Characteristic | African American (n = 916) |
Caucasian (n = 1,533) |
Chinese (n = 248) |
Hispanic (n = 277) |
Japanese (n = 279) |
P Valuea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | ||
| Age, years | 46.0 (44.0–48.2) | 46.0 (44.0–48.3) | 46.6 (44.3–48.1) | 46.0 (44.3–48.0) | 46.7 (44.4–48.7) | 0.1154 | ||||||||||
| Menopause status | 0.0001 | |||||||||||||||
| Premenopausal | 454 | 50.2 | 788 | 52.7 | 152 | 62.0 | 150 | 58.4 | 173 | 62.7 | ||||||
| Early perimenopausal | 450 | 49.8 | 706 | 47.3 | 93 | 38.0 | 107 | 41.6 | 103 | 37.3 | ||||||
| Educational level | <0.0001 | |||||||||||||||
| High school or less | 243 | 26.9 | 245 | 16.1 | 72 | 29.0 | 190 | 70.9 | 51 | 18.3 | ||||||
| Some college | 368 | 40.7 | 466 | 30.6 | 54 | 21.8 | 51 | 19.0 | 97 | 34.8 | ||||||
| College degree | 294 | 32.5 | 811 | 53.3 | 122 | 49.2 | 27 | 10.1 | 131 | 47.0 | ||||||
| Financial strain | <0.0001 | |||||||||||||||
| Very hard | 114 | 12.5 | 88 | 5.8 | 13 | 5.2 | 69 | 25.8 | 10 | 3.6 | ||||||
| Somewhat hard | 307 | 33.7 | 399 | 26.2 | 57 | 23.0 | 152 | 56.7 | 74 | 26.6 | ||||||
| Not hard | 490 | 53.8 | 1,039 | 68.1 | 178 | 71.8 | 47 | 17.5 | 194 | 69.8 | ||||||
| Employed | 718 | 79.1 | 1,285 | 85.3 | 218 | 88.6 | 145 | 55.3 | 203 | 73.0 | <0.0001 | |||||
| Prior oral contraceptive use | 735 | 81.0 | 1,206 | 80.2 | 156 | 63.4 | 117 | 44.8 | 147 | 52.9 | <0.0001 | |||||
| Prior hormone therapy use | 78 | 8.6 | 222 | 14.9 | 23 | 9.4 | 22 | 8.6 | 26 | 9.4 | <0.0001 | |||||
| Smoking | <0.0001 | |||||||||||||||
| Never | 483 | 53.9 | 780 | 51.1 | 233 | 94.0 | 183 | 67.0 | 178 | 64.3 | ||||||
| Past | 197 | 22.0 | 495 | 32.4 | 11 | 4.4 | 46 | 16.9 | 63 | 22.7 | ||||||
| Current | 216 | 24.1 | 253 | 16.6 | 4 | 1.6 | 44 | 16.1 | 36 | 13.0 | ||||||
| Passive smoking, person-hours/week | <0.0001 | |||||||||||||||
| 0 | 313 | 34.6 | 602 | 39.5 | 194 | 78.2 | 174 | 63.0 | 170 | 61.6 | ||||||
| 1–4 | 236 | 26.1 | 480 | 31.5 | 38 | 15.3 | 32 | 11.6 | 62 | 22.5 | ||||||
| ≥5 | 357 | 39.4 | 443 | 29.1 | 16 | 6.5 | 70 | 25.4 | 44 | 15.9 | ||||||
| Parity | <0.0001 | |||||||||||||||
| 0 | 82 | 9.0 | 369 | 24.2 | 33 | 13.3 | 18 | 6.7 | 46 | 16.5 | ||||||
| 1 | 159 | 17.4 | 259 | 17.0 | 37 | 14.9 | 42 | 15.7 | 39 | 14.0 | ||||||
| 2 | 269 | 29.4 | 487 | 31.9 | 128 | 51.6 | 81 | 30.3 | 120 | 43.0 | ||||||
| 3 | 202 | 22.1 | 254 | 16.6 | 40 | 16.1 | 75 | 28.1 | 57 | 20.4 | ||||||
| ≥4 | 203 | 22.2 | 157 | 10.3 | 10 | 4.0 | 51 | 19.1 | 17 | 6.1 | ||||||
| Marital status | <0.0001 | |||||||||||||||
| Never married | 195 | 21.5 | 180 | 12.0 | 22 | 8.9 | 13 | 5.0 | 20 | 7.2 | ||||||
| Currently married/ partnered | 430 | 47.3 | 1,076 | 71.5 | 199 | 80.9 | 196 | 74.8 | 225 | 80.9 | ||||||
| Previously married/ partnered | 284 | 31.2 | 248 | 16.5 | 25 | 10.2 | 53 | 20.2 | 33 | 11.9 | ||||||
| Current diabetes | 74 | 8.1 | 50 | 3.3 | 3 | 1.2 | 19 | 7.0 | 0 | 0.0 | <0.0001 | |||||
| Body mass index, kg/m2 | 30.2 (26.1–36.3) | 26.0 (22.9–31.3) | 22.4 (20.8–24.7) | 28.3 (25.4–32.2) | 22.1 (20.4–24.6) | <0.0001 | ||||||||||
| <25 | 169 | 18.8 | 655 | 43.2 | 189 | 76.8 | 65 | 23.6 | 219 | 79.1 | ||||||
| 25–29.9 | 268 | 29.9 | 393 | 25.9 | 46 | 18.7 | 108 | 39.1 | 45 | 16.3 | ||||||
| ≥30 | 460 | 51.3 | 470 | 31.0 | 11 | 4.5 | 103 | 37.3 | 13 | 4.7 | ||||||
| Total calories | 1,818 (1,344–2,409) | 1,704 (1,375–2,157) | 1,659 (1,353–2,161) | 1,547 (1,275–1,949) | 1,798 (1,342–2,140) | <0.0001 | ||||||||||
| Dietary fiber, g | 10.7 (7.8–14.7) | 11.3 (8.4–14.9) | 13.6 (10.6–18.0) | 11.5 (8.4–14.7) | 11.3 (8.7–14.8) | <0.0001 | ||||||||||
| Physical activity score (excluding work) | 7.3 (6.1–8.4) | 8.1 (6.9–9.3) | 7.3 (5.9–8.4) | 6.6 (5.8–7.6) | 7.8 (6.8–9.0) | <0.0001 | ||||||||||
| Maternal age at menopause, years | <0.0001 | |||||||||||||||
| Unknown type or age | 283 | 36.6 | 365 | 26.8 | 95 | 40.4 | 54 | 32.0 | 103 | 38.6 | ||||||
| Medical,b <40 | 52 | 6.7 | 105 | 7.7 | 3 | 1.3 | 5 | 3.0 | 9 | 3.4 | ||||||
| Medical, 40–44 | 42 | 5.4 | 84 | 6.2 | 2 | 0.9 | 5 | 3.0 | 10 | 3.8 | ||||||
| Medical, 45–49 | 39 | 5.1 | 90 | 6.6 | 10 | 4.3 | 4 | 2.4 | 10 | 3.8 | ||||||
| Medical, 50–54 | 21 | 2.7 | 43 | 3.2 | 3 | 1.3 | 2 | 1.2 | 5 | 1.9 | ||||||
| Medical, ≥55 | 15 | 1.9 | 26 | 1.9 | 3 | 1.3 | 3 | 1.8 | 1 | 0.4 | ||||||
| Natural, <45 | 25 | 3.2 | 61 | 4.5 | 9 | 3.8 | 6 | 3.6 | 5 | 1.9 | ||||||
| Natural, 45–49 | 73 | 9.4 | 134 | 9.9 | 21 | 8.9 | 18 | 10.7 | 28 | 10.5 | ||||||
| Natural, 50–54 | 142 | 18.4 | 337 | 24.8 | 60 | 25.5 | 48 | 28.4 | 69 | 25.8 | ||||||
| Natural, ≥55 | 81 | 10.5 | 116 | 8.5 | 29 | 12.3 | 24 | 14.2 | 27 | 10.1 | ||||||
| Follicle-stimulating hormone, mIU/mL | 16.4 (11.2–27.8) | 15.3 (10.7–25.3) | 16.4 (11.2–27.5) | 15.6 (10.1–28.4) | 14.5 (10.6–23.8) | 0.2746 | ||||||||||
| Estradiol, pg/mL | 55.0 (34.2–89.3) | 56.5 (34.3–89.2) | 49.0 (27.7–81.3) | 59.3 (27.6–98.8) | 51.9 (31.0–84.9) | 0.0247 | ||||||||||
| Number of alcohol servings per week | <0.0001 | |||||||||||||||
| None | 479 | 56.9 | 581 | 39.1 | 191 | 78.9 | 133 | 50.8 | 147 | 57.9 | ||||||
| <1 | 25 | 3.0 | 40 | 2.7 | 4 | 1.7 | 15 | 5.7 | 7 | 2.8 | ||||||
| 1–7 | 226 | 26.8 | 499 | 33.6 | 37 | 15.3 | 102 | 38.9 | 65 | 25.6 | ||||||
| >7 | 112 | 13.3 | 365 | 24.6 | 10 | 4.1 | 12 | 4.6 | 35 | 13.8 | ||||||
| Self-reported health | <0.0001 | |||||||||||||||
| Excellent | 140 | 15.4 | 438 | 29.1 | 42 | 17.1 | 13 | 5.0 | 54 | 19.4 | ||||||
| Very good | 299 | 32.9 | 638 | 42.4 | 73 | 29.7 | 59 | 22.5 | 102 | 36.7 | ||||||
| Good | 322 | 35.5 | 330 | 21.9 | 78 | 31.7 | 120 | 45.8 | 72 | 25.9 | ||||||
| Fair/poor | 147 | 16.2 | 100 | 6.6 | 53 | 21.5 | 70 | 26.7 | 50 | 18.0 | ||||||
Abbreviations: IQR, interquartile range; SWAN, Study of Women's Health Across the Nation.
a Kruskal–Wallis for continuous variables, χ2 for categorical variables.
b Medical includes menopause induced surgically or by medications.
Parity was significantly higher in African-American and Hispanic women and was lowest in Chinese women. A significantly greater proportion of Chinese and Japanese women were currently married or partnered, and a higher proportion of African-American women were previously or never married.
More African-American women and fewer Hispanic, Chinese, and Japanese women had diabetes or were obese. Calorie and fiber intake, physical activity score, and maternal age at menopause also differed significantly by race/ethnicity. No significant racial/ethnic differences were observed in serum follicle-stimulating hormone, but Chinese and Japanese women had significantly lower estradiol levels. Caucasians had the highest alcohol intake and best self-rated health.
Unadjusted results
Factors significantly related to reaching the FMP (i.e., earlier FMP) in unadjusted analyses were (in descending strength of association) reporting it was very or somewhat difficult to pay for basics; smoking during follow-up; maternal natural menopause under age 49 years; not being Caucasian or Japanese; ever having diabetes; never having been married; having poorer baseline self-rated health; and reporting more physical activity during follow-up (Table 2). Women had significantly later FMPs if they had a mother who had medically induced menopause at ≥55 years or at 45–49 years; had some college or had graduated college; had previous oral contraceptive or hormone therapy use; had higher alcohol consumption; were employed during follow-up; or were taller. No significant associations were observed for parity, baseline physical activity, passive smoke exposure during follow-up, calorie intake, baseline weight, or change in weight.
Table 2.
Unadjusted and Adjusted Hazard Ratios (95% Confidence Intervals) for Age at Natural Final Menstrual Period From Cox Proportional Hazards Modeling Accounting for Left-Truncation, All 7 SWAN Sites, United States, Baseline Through Follow-up Visit 10 (1996–2007)
| Characteristic | Unadjusted (n = 3,253) |
Adjusteda (n = 2,878) |
|||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | ||
| Race/ethnicity | 0.0009 | 0.624 | |||||
| African American | 1.24 | 1.10, 1.40 | 1.05 | 0.09, 1.21 | |||
| Caucasian | 1.00 | Referent | 1.00 | Referent | |||
| Chinese | 1.24 | 1.04, 1.48 | 1.14 | 0.85, 1.53 | |||
| Hispanic | 1.38 | 1.08, 1.76 | 0.81 | 0.51, 1.28 | |||
| Japanese | 1.04 | 0.87, 1.24 | 0.90 | 0.67, 1.20 | |||
| Financial strain | <0.0001 | ||||||
| Very hard | 1.57 | 1.30, 1.89 | |||||
| Somewhat hard | 1.22 | 1.09, 1.36 | |||||
| Not at all hard | 1.00 | Referent | |||||
| Baseline smokingb | 0.132 | 0.236 | |||||
| Never | 1.00 | Referent | 1.00 | Referent | |||
| Past | 0.98 | 0.86, 1.10 | 1.03 | 0.90, 1.19 | |||
| Current | 1.26 | 0.98, 1.62 | 1.26 | 0.97, 1.65 | |||
| Time-varying smokingb | 1.49 | 1.16, 1.91 | 0.0017 | 1.53 | 1.18, 2.00 | 0.002 | |
| Maternal type/age at FMP, yearsc | <0.0001 | ||||||
| Unknown | 1.05 | 0.91, 1.21 | |||||
| Medicald | |||||||
| Age <40 | 0.90 | 0.69, 1.15 | |||||
| Age 40–44 | 0.96 | 0.74, 1.25 | |||||
| Age 45–49 | 0.72 | 0.55, 0.93 | |||||
| Age 50–54 | 0.87 | 0.62, 1.21 | |||||
| Age ≥55 | 0.45 | 0.28, 0.73 | |||||
| Natural | |||||||
| Age <45 | 1.36 | 1.02, 1.81 | |||||
| Age 45–49 | 1.30 | 1.07, 1.58 | |||||
| Age 50–54 | 1.00 | Referent | |||||
| Age ≥55 | 0.83 | 0.68, 1.01 | |||||
| Marital statusb | |||||||
| Baseline | 0.115 | ||||||
| Never | 1.20 | 1.00, 1.43 | |||||
| Previously married/ partnered | 1.02 | 0.87, 1.20 | |||||
| Currently married/ partnered | 1.00 | Referent | |||||
| Time-varying married/ partnered | 1.01 | 0.87, 1.16 | 0.934 | ||||
| Ever diabetes | 1.24 | 1.02, 1.50 | 0.0297 | ||||
| Self-reported health, baseline | 1.17 | 1.11, 1.24 | <0.0001 | 1.11 | 1.04, 1.19 | 0.002 | |
| Educational level | <0.0001 | 0.003 | |||||
| High school or less | 1.00 | Referent | 1.00 | Referent | |||
| Some college | 0.83 | 0.72, 0.95 | 0.88 | 0.75, 1.03 | |||
| College degree | 0.67 | 0.59, 0.76 | 0.77 | 0.66, 0.90 | |||
| Baseline ever-use of oral contraceptives | 0.82 | 0.73, 0.92 | 0.0007 | 0.85 | 0.75, 0.97 | 0.015 | |
| Exogenous hormone therapyb | |||||||
| Ever use (baseline) | 0.83 | 0.69, 0.98 | 0.031 | ||||
| Time-varying | 0.93 | 0.78, 1.10 | 0.378 | ||||
| Alcohol, no. of servings/weekb,e | |||||||
| Baseline | 0.94 | 0.89, 0.996 | 0.0357 | 0.97 | 0.92, 1.04 | 0.395 | |
| Change since baseline | 0.88 | 0.81, 0.95 | 0.0018 | 0.90 | 0.83, 0.98 | 0.017 | |
| Current employmentb | |||||||
| Baseline | 0.92 | 0.80, 1.06 | 0.264 | 0.99 | 0.85, 1.17 | 0.941 | |
| Time-varying | 0.82 | 0.73, 0.92 | 0.0009 | 0.87 | 0.77, 0.98 | 0.026 | |
| Baseline height, 75th vs. 25th percentile | 0.91 | 0.84, 0.98 | 0.0134 | ||||
| Parity | 0.688 | ||||||
| 0 | 1.00 | Referent | |||||
| 1 | 1.04 | 0.88, 1.25 | |||||
| 2 | 0.99 | 0.85, 1.15 | |||||
| 3 | 1.01 | 0.85, 1.19 | |||||
| ≥4 | 1.11 | 0.93, 1.33 | |||||
| Physical activity scoreb | |||||||
| Baseline | 0.99 | 0.95, 1.02 | 0.348 | 1.03 | 0.99, 1.07 | 0.153 | |
| Change since baseline | 1.05 | 1.001, 1.10 | 0.0434 | 1.07 | 1.02, 1.12 | 0.007 | |
| Passive smoking, person-hours/weekb | |||||||
| Baseline | 0.0154 | ||||||
| 0 | 1.00 | Referent | |||||
| 1–4 | 0.86 | 0.74, 0.99 | |||||
| ≥5 | 1.09 | 0.92, 1.29 | |||||
| Time-varying | 0.470 | ||||||
| 0 | 1.00 | Referent | |||||
| 1–4 | 0.99 | 0.85, 1.15 | |||||
| ≥5 | 1.10 | 0.92, 1.31 | |||||
| Log total calories, 75th vs. 25th percentileb | |||||||
| Baseline | 0.94 | 0.30, 2.96 | 0.913 | ||||
| Change since baseline | 0.99 | 0.95, 1.03 | 0.610 | ||||
| Baseline weight, 75th vs. 25th percentilef | 1.00 | 0.93, 1.07 | 0.967 | 0.92 | 0.84, 0.996 | 0.039 | |
| Change in weight, 75th vs. 25th percentilef | 1.02 | 0.97, 1.07 | 0.431 | ||||
Abbreviations: CI, confidence interval; FMP, final menstrual period; SWAN, Study of Women's Health Across the Nation.
a Adjusted for all other variables with hazard ratios entered in this column.
b Baseline and time-varying values of the predictor are both included as predictors.
c Measured at first annual follow-up; sample size = 2,805 for unadjusted analyses.
d Medical includes menopause induced surgically or by medications.
e 0 = none, 1 = infrequent (<2), 2 = moderate (2–7), 3 = heavy (>7).
f Baseline height, baseline weight, and change in weight all are included as predictors.
Adjusted results
In multivariable analyses, racial/ethnic differences were no longer statistically significant (Table 2). Earlier age at FMP was related most strongly to smoking during follow-up (not at baseline) (hazard ratio (HR) = 1.53, 95% confidence interval (CI): 1.18, 2.00). The following variables were less strongly but significantly related to earlier age at FMP: reporting poorer health at baseline (HR = 1.11, 95% CI: 1.04, 1.19) and more physical activity during follow-up (HR = 1.07, 95% CI: 1.02, 1.12). The following were significantly related to later age at FMP: having graduated college (HR = 0.77, 95% CI: 0.66, 0.90), having used oral contraceptives before baseline (HR = 0.85, 95% CI: 0.75, 0.97), being employed during follow-up (HR = 0.87, 95% CI: 0.77, 0.98), having higher alcohol consumption during follow-up (HR = 0.90, 95% CI: 0.83, 0.98), and having higher baseline weight (interquartile range HR = 0.92, 95% CI: 0.84, 0.996). Financial strain, maternal age at menopause, marital status, ever having diabetes, hormone therapy use, parity, height, passive smoke exposure, weight change, and calorie intake were not statistically significantly associated with age at FMP and thus were not retained in the final multivariable model after adjustment for other variables.
Median age at natural FMP
The median age at FMP was 52.54 years. After adjustment for baseline covariates using Cox models (69), the median age at FMP was significantly higher in women who (in descending order of significance) did not smoke, reported better health at baseline, had more education, had higher baseline weight, or had used oral contraceptives previously (Table 3, Figures 1–4).
Table 3.
Median Age (Years) at Final Menstrual Period Accounting for Left-Truncation, Unadjusted and Adjusted for Baseline Covariates and Time-Invariant Predictors in Multivariate Cox Proportional Hazards Model, SWAN, United States, Baseline through Follow-up Visit 10 (1996–2007)
| Unadjusted | P Value | Adjusteda | P Value | |
|---|---|---|---|---|
| Baseline smoking | <0.0001 | <0.0001 | ||
| Neverb | 52.73 | 52.76 | ||
| Past | 52.88 | 52.83 | ||
| Current | 51.35 | 51.43 | ||
| Baseline self- reported health | <0.0001 | 0.0014 | ||
| Excellentb | 53.05 | 52.96 | ||
| Very good | 52.88 | 52.99 | ||
| Good | 52.00 | 52.36 | ||
| Fair/poor | 51.98 | 52.31 | ||
| Educational level | <0.0001 | 0.0021 | ||
| High school or less | 51.53 | 52.15 | ||
| Some college | 52.32 | 52.54 | ||
| College degree or higherb | 53.07 | 53.06 | ||
| Baseline weight | 0.178 | 0.0027 | ||
| 1st quartileb | 52.45 | 52.41 | ||
| 2nd quartile | 52.66 | 53.14 | ||
| 3rd quartile | 52.37 | 52.67 | ||
| 4th quartile | 52.70 | 53.07 | ||
| Prior oral contraceptive use | 0.0007 | 0.006 | ||
| No | 52.07 | 51.82 | ||
| Yesb | 52.72 | 52.59 | ||
| Baseline alcohol servings/ week | 0.857 | 0.245 | ||
| None | 52.53 | 52.54 | ||
| Light | 52.77 | 52.79 | ||
| Moderate | 52.59 | 52.52 | ||
| Heavy | 52.62 | 52.54 | ||
| Baseline diabetesc | 0.0947 | |||
| Nob | 52.62 | |||
| Yes | 48.65 | |||
| Baseline physical activity | 0.616 | 0.820 | ||
| 1st quartileb | 52.35 | 51.33 | ||
| 2nd quartile | 52.62 | 52.54 | ||
| 3rd quartile | 52.80 | 52.22 | ||
| 4th quartile | 52.82 | 52.42 | ||
| Race/ethnicity | 0.0009 | 0.653 | ||
| African American | 52.17 | 52.59 | ||
| Caucasianb | 52.88 | 52.85 | ||
| Chinese | 52.41 | 52.86 | ||
| Hispanic | 50.86 | 53.10 | ||
| Japanese | 53.14 | 53.24 | ||
| Financial strain | <0.0001 | |||
| Very hard | 51.51 | |||
| Somewhat hard | 52.04 | |||
| Not at all hard | 52.90 | |||
| Baseline employment | 0.0017 | 0.260 | ||
| No | 51.58 | 51.53 | ||
| Yesb | 52.75 | 52.44 | ||
| Baseline hormone therapy (ever) | 0.0234 | |||
| No | 52.48 | |||
| Yes | 53.18 | |||
| Baseline marital status | 0.0677 | |||
| Never married | 51.94 | |||
| Previously married/ partnered | 52.55 | |||
| Currently married/ partnered | 52.68 | |||
| Parityd | 0.688 | |||
| 0 | 52.35 | |||
| 1 | 52.48 | |||
| 2 | 52.54 | |||
| 3 | 52.76 | |||
| ≥4 | 52.32 |
Abbreviation: SWAN, Study of Women's Health Across the Nation.
a Adjusted for all variables in table except no. of children, as well as for site and baseline day of cycle.
b Reference group for covariate adjustment (i.e., apply their covariate distribution to all groups).
c P value omitted because hazards were not proportional.
d Adjusted for all variables in table, as well as for site and baseline day of cycle.
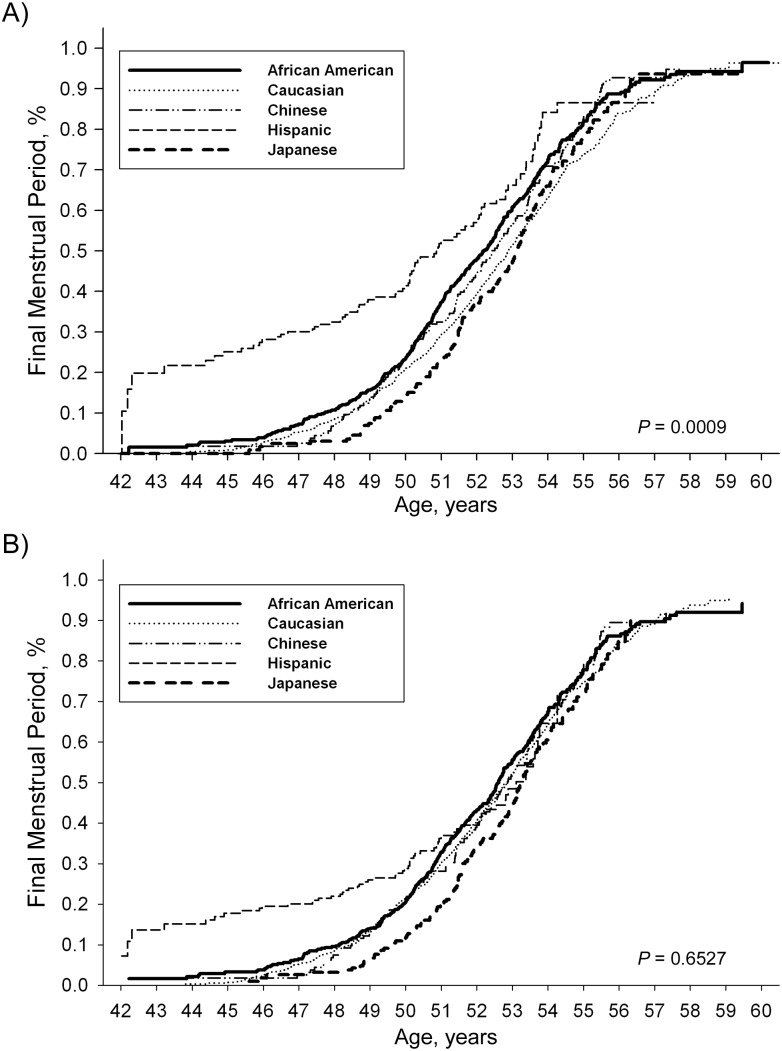
Figure 1.
Estimated distribution of age at final menstrual period, unadjusted and adjusted racial/ethnic differences, in the SWAN cohort, 1996–2007. For African Americans, unadjusted median age = 52.17 years and adjusted median age = 52.59 years; for Caucasians, unadjusted median age = 52.88 years and adjusted median age = 52.85 years; for Chinese, unadjusted median age = 52.41 years and adjusted median age = 52.86 years; for Hispanics, unadjusted median age = 50.86 years and adjusted median age = 53.10 years; and for Japanese, unadjusted median age = 53.14 years and adjusted median age = 53.24 years. SWAN, Study of Women's Health Across the Nation.
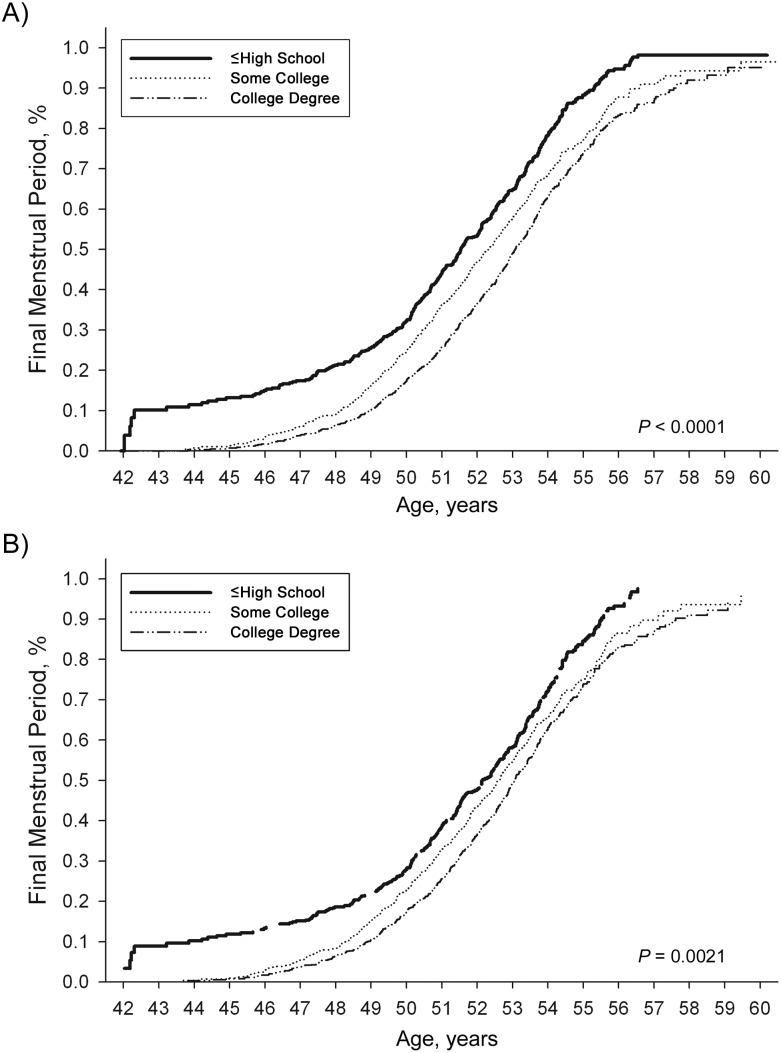
Figure 2.
Estimated distribution of age at final menstrual period, unadjusted and adjusted differences by educational level, in the SWAN cohort, 1996–2007. For ≤high school, unadjusted median age = 51.53 and adjusted median age = 52.13 years; for some college, unadjusted median age = 52.32 years and adjusted median age = 52.62 years; and for ≥college degree, unadjusted median age = 53.07 years and adjusted median age = 53.05 years. SWAN, Study of Women's Health Across the Nation.
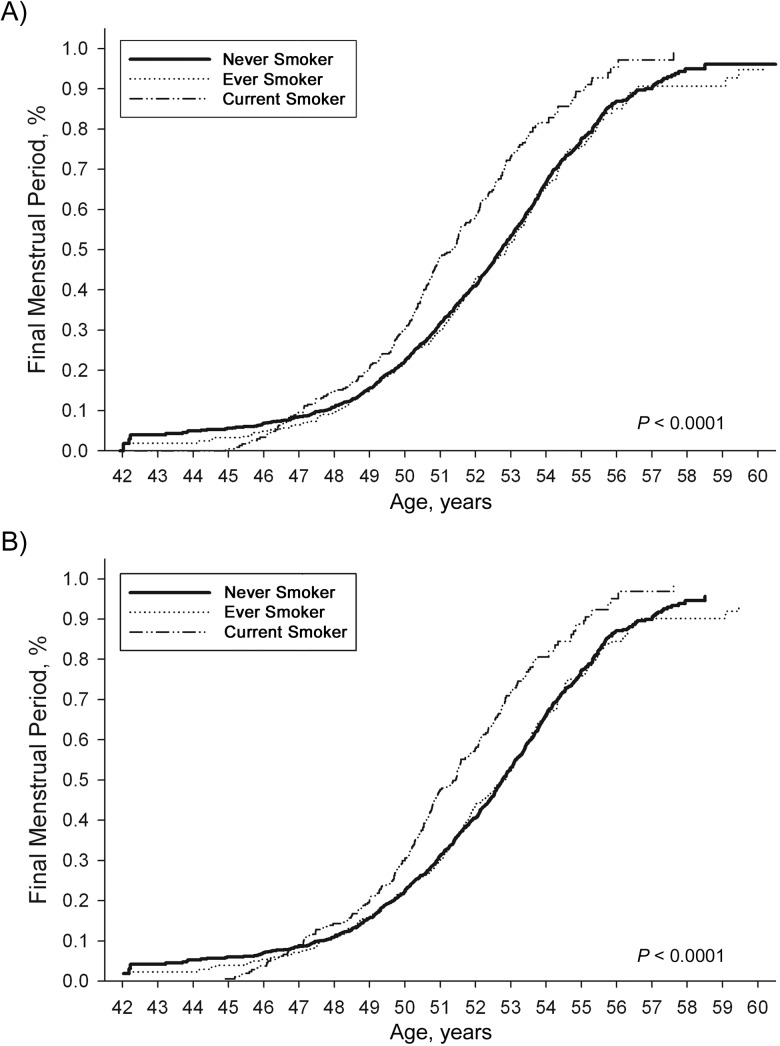
Figure 3.
Estimated distribution of age at final menstrual period, unadjusted and adjusted differences by baseline smoking status, in the SWAN cohort, 1996–2007. For never smokers, unadjusted median age = 52.73 years and adjusted median age = 52.76 years; for ever smokers, unadjusted median age = 52.88 years and adjusted median age = 52.83 years; and for current smokers, unadjusted median age = 51.35 years and adjusted median age = 51.43 years. SWAN, Study of Women's Health Across the Nation.
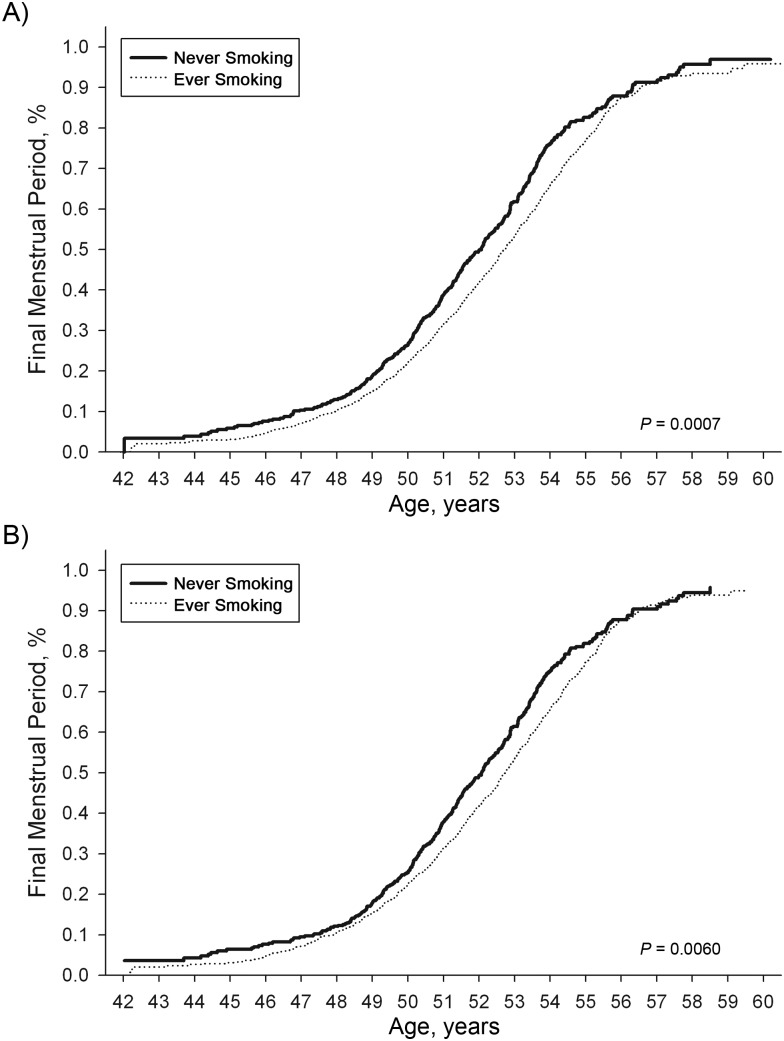
Figure 4.
Estimated distribution of age at final menstrual period, unadjusted and adjusted differences by oral contraceptive use, in the SWAN cohort, 1996–2007. For never users, unadjusted median age = 52.07 years and adjusted median age = 52.08 years; for ever users, unadjusted median = 52.72 years and adjusted median age = 52.75 years. SWAN, Study of Women's Health Across the Nation.
DISCUSSION
In our longitudinal study of 3,302 initially premenopausal and early perimenopausal women from 5 racial/ethnic groups followed up for 10 years, multivariable Cox models identified later age at natural FMP as significantly associated with greater educational attainment, prior oral contraceptive use, employment during follow-up, absence of smoking during follow-up, higher baseline weight, greater alcohol consumption during follow-up, better self-rated health, and lower physical activity. However, we found no significant racial/ethnic difference in age at natural menopause once socioeconomic, lifestyle, and health variables were controlled. Findings from previous studies are inconsistent with regard to racial/ethnic differences in age at FMP (36, 37, 40, 73–77). However, our large, well-controlled and analyzed data set provides evidence that social determinants are key factors related to age at menopause, a finding strengthened by other associations found that were consistent with previously published results.
Consistent with prior studies, our results showed that lower educational attainment (29, 30, 36, 41, 43, 50, 76) was significantly associated with an earlier menopause. A birth cohort study indicated that early life socioeconomic status was more strongly associated with age at FMP than adult status (77), although the former association was greatly attenuated when adjusted for childhood cognitive ability and having been breast-fed (52), factors not measured in our study.
Prior studies have consistently shown that current smoking (28, 29, 33, 35–37, 41, 43–48, 78, 79) and nonuse of oral contraceptives (30, 36, 41, 43, 80) are associated with an earlier FMP, as was observed in our study. Previous studies and ours have indicated that former smokers have an age at FMP similar to that of nonsmokers, which seems inconsistent with the polycyclic aromatic hydrocarbons in cigarette smoke being nonreversibly toxic to ovarian follicles (81, 82). Few studies have examined the relation of passive smoke exposure to age at FMP, although one early study showed that nonsmoking women whose spouses smoked had a similar age at FMP to that of smokers (83); we found no relation of time-varying passive smoke exposure.
Previous studies have reported that greater height was associated with later age at FMP (84–86), but we did not observe any such association in multivariable longitudinal models. However, although our multivariable model indicated that higher baseline weight was associated with later age at FMP, previous studies have been inconsistent, with some showing both increased body mass index and waist-to-hip ratio associated with later age at FMP (28, 35, 42, 49, 78, 85–88), but many showing no significant association (29, 30 36, 37, 89, 90), although most did not adjust for weight change. Inconsistent results could be due to differences in design (cross-sectional or retrospective vs. longitudinal) or analysis (inadequate or differing control of confounding and using survival analyses vs. comparing unadjusted mean ages at FMP). Also, our estimated association with baseline weight was small; thus, varying results across studies could be due to variations in samples or covariates included in the model.
We found that greater physical activity was modestly associated with earlier age at the FMP. Prior studies have been inconsistent, with one finding no relation (37) and one showing a later age at FMP associated with leisure-time physical activity (91). The inconsistency in results might be due at least partially to the weakness of the association, if it exists at all. We also found no significant relation of calorie intake to age at FMP. The literature is inconsistent with regard to the relation of dietary patterns to age at menopause, with some showing an earlier menopause among vegetarians (92), but one study showing a later age at natural menopause associated with higher green and yellow vegetable intake (93), and another study showing high intakes of fat, cholesterol, and coffee associated with earlier menopause (94). A large, longitudinal study found that high intakes of carbohydrates, vegetables, fiber, and cereal were related to earlier menopause, whereas higher intakes of fat, protein, and meat were associated with later menopause (95). A large, prospective study reported that higher calorie, fruit, and protein intakes were associated with later age at menopause but that vegetable, fat, soy, and fiber intakes were not related (91).
A few small or cross-sectional studies (51, 53–55) and one longitudinal study (52) have shown significant associations between mothers' and daughters' ages at menopause. Our analyses resulted in maternal age at menopause not being retained in adjusted models. However, this variable must be interpreted cautiously because mothers' age at menopause was reported by daughters.
Our study had several significant strengths. We provided longitudinal results on prospectively measured age at natural FMP from a large cohort of community-based midlife women from 5 racial/ethnic groups followed up for 10 years with high retention rates. Standard annual clinic visits provided relatively precise estimates of age at FMP. We also adjusted for multiple factors simultaneously in the Cox models, censoring at initiation of hormone therapy use or at hysterectomy or oophorectomy, thus providing hazard ratios for age at the natural FMP for the relations of all factors examined, using all available data over 10 years of follow-up.
The study also had limitations. First, the cohort included only women who had menstruated recently at baseline so that we could observe menstrually defined menopause, but this excluded women who at screening had had at least 3 months of amenorrhea (1.8%–2%) or hysterectomy or bilateral oophorectomy (8%–11%). Also, the age range for the cohort was restricted to 42–52 years. This left-truncation likely resulted in an overestimation of median age at FMP (96, 97) because women who experienced their FMP before age 42 years were not included in the cohort. However, the bias was likely to be small, inasmuch as the distribution of age at FMP from the cross-sectional screener shifted upward only slightly; for example, at age 52 years, 50.24% were postmenopausal in the full sample, versus 49.28% in the sample omitting 40–41-year-olds. Moreover, analyses accounted for left-truncation for entry at ages 42 years and higher. Second, over 10 years of follow-up, we tended to lose women who were less healthy, smokers, less educated, or Hispanic, which potentially could lead to overestimation of the median age at menopause because these factors are associated with an earlier FMP, although we adjusted for all of these factors in multivariable analyses. Third, we did not have exact dates of the FMP for all women, and age at maternal menopause was based on recall. This could have resulted in some inaccuracy or imprecision in estimating age at FMP, although was likely not differential and thus should not have produced markedly biased results. Fourth, self-reported race/ethnicity might have been misclassified, but this was unlikely to be differential with regard to age at menopause and thus might have resulted in underestimation of differences. Finally, censoring women who initiated hormone therapy use and had no bleeding after stopping use (17.9% of cohort) could have resulted in underestimation of the age at FMP because these women tended to have higher socioeconomic status and better health. However, we included their data up to hormone therapy initiation and included those who had bleeding after ceasing use, which should have reduced potential bias.
In conclusion, we found no significant racial/ethnic differences in age at natural FMP in our large cohort, representing 5 racial/ethnic groups, after controlling for several sociodemographic, lifestyle, and health factors. Our results suggest that the age at natural FMP reflects a complex interplay of host and environmental factors, many of which are related to social determinants and better health, which could partially explain the relation of late age at FMP to reduced morbidity and mortality for many health outcomes. Because the age at natural FMP is an important indicator of future morbidity, life expectancy, and mortality and affects women's reproductive capability, these results have clinical and public health implications for early identification of women who are at high risk for future morbidity and advising women about family planning as they approach midlife.
ACKNOWLEDGMENTS
Author affiliations: Department of Public Health Sciences, University of California Davis School of Medicine, Davis, California (Ellen B. Gold); Department of Obstetrics and Gynecology, University of California Davis School of Medicine, Sacramento, California (L. Elaine Waetjen); Division of Endocrinology, Clinical Nutrition and Vascular Medicine, Department of Internal Medicine, University of California Davis School of Medicine, Sacramento, California (Jennifer S. Lee); Biostatistics Research Group, Division of Preventive Medicine, Department of Medicine, University of Massachusetts Medical School, Worcester, Massachusetts (Sybil L. Crawford); Comprehensive Cancer Center, Wake Forest University School of Medicine, Winston-Salem, North Carolina (Nancy E. Avis); Department of General Internal Medicine, University of California Los Angeles David Geffen School of Medicine, Los Angeles, California (Carolyn J. Crandall); Departments of Psychiatry and Epidemiology, University of Pittsburgh School of Medicine and School of Public Health, Pittsburgh, Pennsylvania (Karen A. Matthews, Rebecca Thurston); Department of Pediatrics, University of Pittsburgh, Pittsburgh, Pennsylvania (Marike Vuga); and Department of Epidemiology, University of Michigan School of Public Health, Ann Arbor, Michigan (Siobán D Harlow).
SWAN has grant support from the National Institutes of Health, Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Health Office of Research on Women's Health (grants NR004061, AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495).
We thank the study staff at each site.
Clinical centers: University of Michigan, Ann Arbor, Michigan—MaryFran Sowers, principal investigator (PI) 1994–2011, Siobán D. Harlow, PI 2011–present; Massachusetts General Hospital, Boston, Massachusetts—Robert Neer, PI 1994–1999, Joel Finkelstein, PI 1999–present; Rush University, Rush University Medical Center, Chicago, Illinois—Lynda Powell, PI; University of California, Davis/Kaiser, Davis, California—Ellen Gold, PI; University of California, Los Angeles, California—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, New York—Rachel Wildman, PI 2010–2011, Carol Derby, PI 2011–present; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry, New Jersey Medical School, Newark, New Jersey—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, Pennsylvania—Karen Matthews, PI. National Institutes of Health Program Office: National Institute on Aging, Bethesda, Maryland—Marcia Ory 1994–2001; Sherry Sherman 1994–present; National Institute of Nursing Research, Bethesda, Maryland—Program Officers. Central laboratory: University of Michigan, Ann Arbor, Michigan—Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating center: New England Research Institutes, Watertown, Massachusetts—Sonja McKinlay, PI 1995–2001; University of Pittsburgh, Pittsburgh, Pennsylvania—Kim Sutton-Tyrrell, PI 2001–present. Steering committee: Chris Gallagher, Chair; Susan Johnson, Chair.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the National Institute of Nursing Research, Office of Research on Women's Health, or the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1.Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol. 1998;8(4):229–235. doi: 10.1016/s1047-2797(97)00207-x. [DOI] [PubMed] [Google Scholar]
- 2.Wise AM, Krajnak KM, Kashon ML. Menopause: the aging of multiple pacemakers. Science. 1996;273(5271):67–70. doi: 10.1126/science.273.5271.67. [DOI] [PubMed] [Google Scholar]
- 3.Snowdon DA, Kane RL, Beeson WL, et al. Is early natural menopause a biologic marker of health and aging? Am J Public Health. 1989;79(6):709–714. doi: 10.2105/ajph.79.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ossewaarde ME, Bots ML, Verbeek ALM, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19,731 Norwegian women. Am J Epidemiol. 2003;157(10):923–929. doi: 10.1093/aje/kwg066. [DOI] [PubMed] [Google Scholar]
- 6.De Kleijn MJ, van der Schouw YT, Verbeek AL, et al. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155(4):339–345. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 7.van der Schouw YT, van der Graaf Y, Steyerberg EW, et al. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 8.Jacobsen BK, Nilssen S, Heuch I, et al. Does age at natural menopause affect mortality from ischemic heart disease? J Clin Epidemiol. 1997;50(4):475–479. doi: 10.1016/s0895-4356(96)00425-8. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 10.Atsma F, Bartelink ML, Grobbec DE, et al. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 11.Cui R, Iso H, Toyoshima H, et al. JACC Study Group. Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J Epidemiol. 2006;16(5):177–184. doi: 10.2188/jea.16.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lokkegaard E, Jovanovic Z, Heitmann BL, et al. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. 2006;53(2):226–233. doi: 10.1016/j.maturitas.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Jansen SC, Temme EH, Schouten EG. Lifetime estrogen exposure versus age at menopause as mortality predictor. Maturitas. 2002;43(20):105–112. doi: 10.1016/s0378-5122(02)00183-4. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52(4):303–307. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 15.Joakimsen O, Bonaa KH, Stensland-Bugge E, et al. Population-based study of age at menopause and ultrasound assessed carotid atherosclerosis: the Tromso Study. J Clin Epidemiol. 2000;53(5):525–530. doi: 10.1016/s0895-4356(99)00197-3. [DOI] [PubMed] [Google Scholar]
- 16.Lisabeth LD, Beiser AS, Brown DL, et al. Age at natural menopause and risk of ischemic stroke: The Framingham Heart Study. Stroke. 2009;40(4):1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parashar S, Reid KJ, Spertus JA, et al. Early menopause predicts angina after myocardial infarction. Menopause. 2010;17(5):938–945. doi: 10.1097/gme.0b013e3181e41f54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parazzini F, Bidoli E, Franceschi S, et al. Menopause, menstrual and reproductive history, and bone density in northern Italy. J Epidemiol Community Health. 1996;50(5):519–523. doi: 10.1136/jech.50.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kritz-Silverstein D, Barrett-Connor E. Early menopause, number of reproductive years, and bone mineral density in postmenopausal women. Am J Public Health. 1993;83(7):983–988. doi: 10.2105/ajph.83.7.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Der Voort DJ, Van Der Weijer PH, Barentsen R. Early menopause: increased fracture risk at older age. Osteoporos Int. 2003;14(6):525–530. doi: 10.1007/s00198-003-1408-1. [DOI] [PubMed] [Google Scholar]
- 21.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 22.Monninkhof EM, van der Schouw YT, Peeters PH. Early age at menopause and breast cancer: are leaner women more protected? A prospective analysis of the Dutch DOM cohort. Breast Cancer Res Treat. 1999;55(3):285–291. doi: 10.1023/a:1006277207963. [DOI] [PubMed] [Google Scholar]
- 23.De Graaff J, Stolte LA. Age at menarche and menopause of uterine cancer patients. Eur J Obstet Gynecol Reprod Biol. 1978;8(4):187–193. doi: 10.1016/0028-2243(78)90014-x. [DOI] [PubMed] [Google Scholar]
- 24.Franceschi S, La Vecchia C, Booth M, et al. Pooled analysis of 3 European case-control studies of ovarian cancer. II. Age at menarche and at menopause. Int J Cancer. 1991;49(1):57–60. doi: 10.1002/ijc.2910490111. [DOI] [PubMed] [Google Scholar]
- 25.Burger HG, Hale GE, Robertson DM, et al. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women's Midlife Health Project. Hum Reprod Update. 2007;13(6):559–565. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 26.Sowers MR, Zheng H, McConnell D, et al. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93(10):3958–3964. doi: 10.1210/jc.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103–115. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 28.Greendale GA, Hogan P, Kritz-Silverstein D, et al. Age at menopause in women participating in the Postmenopausal Estrogen/Progestins Interventions (PEPI) trial: an example of bias introduced by selection criteria. Menopause. 1995;2(1):27–34. [Google Scholar]
- 29.Luoto R, Laprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol. 1994;139(1):64–76. doi: 10.1093/oxfordjournals.aje.a116936. [DOI] [PubMed] [Google Scholar]
- 30.Stanford JL, Hartge P, Brinton LA, et al. Factors influencing the age at natural menopause. J Chron Dis. 1987;40(11):995–1002. doi: 10.1016/0021-9681(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 31.Magursky V, Mesko M, Sokolik L. Age at the menopause and onset of the climacteric in women of Martin district, Czechoslovakia. Int J Fertil. 1975;20(1):17–23. [PubMed] [Google Scholar]
- 32.Gold EB, Sternfeld B, Brown C, et al. The relation of demographic and lifestyle variables to symptoms in a multi-racial/ethnic population of women aged 40–55 years. Am J Epidemiol. 2000;152(5):463–473. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 33.van Noord PAH, Dubas JS, Dorland M, et al. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68(1):95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- 34.Flint M. Is there a secular trend in age of menopause. Maturitas. 1978;1(2):133–139. doi: 10.1016/0378-5122(78)90020-8. [DOI] [PubMed] [Google Scholar]
- 35.Rodstrom K, Bengtsson C, Milsom I, et al. Evidence for a secular trend in menopausal age: a population study of women in Gothenburg. Menopause. 2003;10(6):538–543. doi: 10.1097/01.GME.0000094395.59028.0F. [DOI] [PubMed] [Google Scholar]
- 36.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at menopause in a multi-ethnic population of women. Am J Epidemiol. 2001;153(9):865–874. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 37.Bromberger JT, Matthews KA, Kuller LH, et al. Prospective study of the determinants of age at menopause. Am J Epidemiol. 1997;145(2):124–133. doi: 10.1093/oxfordjournals.aje.a009083. [DOI] [PubMed] [Google Scholar]
- 38.Sievert LL, Hautaniemi SI. Age at menopause in Puebla, Mexico. Hum Biol. 2003;75(2):205–226. doi: 10.1353/hub.2003.0037. [DOI] [PubMed] [Google Scholar]
- 39.Garcia Vela A, Nava LE, Malacara JM. [The age of menopause in the urban population of the city of Leon, Guanajuato] Rev Invest Clin. 1987;39(4):329–332. [PubMed] [Google Scholar]
- 40.Alvarado G, Rivera R, Ruiz R, et al. Characteristicas del patron de sangrado menstrual en un grupo de mujeres normales de Durango. Ginecol Obstetr Mex. 1988;56:127–133. [PubMed] [Google Scholar]
- 41.Torgerson DJ, Avenell A, Russell IT, et al. Factors associated with onset of menopause in women aged 45–49. Maturitas. 1994;19(2):83–92. doi: 10.1016/0378-5122(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 42.MacMahon B, Worcester J. Age at menopause, United States 1960–1962. Vital Health Stat 11. 1966;(19):1–19. [PubMed] [Google Scholar]
- 43.Palmer JR, Rosenberg L, Wise LA, et al. Onset of natural menopause in African American women. Am J Public Health. 2003;93(2):299–306. doi: 10.2105/ajph.93.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med. 1985;103(3):350–356. doi: 10.7326/0003-4819-103-3-350. [DOI] [PubMed] [Google Scholar]
- 45.Andersen FS, Transbol I, Christiansen C. Is cigarette smoking a promoter of the menopause. Acta Med Scand. 1982;212(3):137–139. doi: 10.1111/j.0954-6820.1982.tb03185.x. [DOI] [PubMed] [Google Scholar]
- 46.Hiatt RA, Fireman BH. Smoking, menopause, and breast cancer. J Natl Cancer Inst. 1986;76(5):833–838. [PubMed] [Google Scholar]
- 47.Hartz AJ, Kelber S, Borkowf H, et al. The association of smoking with clinical indicators of altered sex steroids—a study of 50,145 women. Public Health Rep. 1987;102(3):254–259. [PMC free article] [PubMed] [Google Scholar]
- 48.Brambilla DJ, McKinlay SM. A prospective study of factors affecting age at menopause. J Clin Epidemiol. 1989;42(11):1031–1039. doi: 10.1016/0895-4356(89)90044-9. [DOI] [PubMed] [Google Scholar]
- 49.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight and menopause. Am J Epidemiol. 1983;117(6):651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 50.Castelo-Branco C, Blümel JE, Chedraui P, et al. Age at menopause in Latin America. Menopause. 2006;13(4):706–712. doi: 10.1097/01.gme.0000227338.73738.2d. [DOI] [PubMed] [Google Scholar]
- 51.Torgerson DJ, Thomas RE, Reid DM. Mothers and daughters menopausal ages: is there a link? Eur J Obstet Gynecol Reprod Biol. 1997;74(1):63–66. doi: 10.1016/s0301-2115(97)00085-7. [DOI] [PubMed] [Google Scholar]
- 52.Mishra G, Hardy R, Kuh D. Are the effects of risk factors for timing of menopause modified by age? Results from a British birth cohort study. Menopause. 2007;14(4):717–724. doi: 10.1097/GME.0b013e31802f3156. [DOI] [PubMed] [Google Scholar]
- 53.Cramer DW, Xu H, Harlow BL. Family history as a predictor of early menopause. Fertil Steril. 1995;64(4):740–745. doi: 10.1016/s0015-0282(16)57849-2. [DOI] [PubMed] [Google Scholar]
- 54.De Bruin JP, Bovenhuis H, Van Noord PA, et al. The role of genetic factors in age at natural menopause. Hum Reprod. 2001;16(9):2014–2018. doi: 10.1093/humrep/16.9.2014. [DOI] [PubMed] [Google Scholar]
- 55.Murabito JM, Yang Q, Fox C, et al. Heritability of age at natural menopause in the Framingham Heart Study. J Clin Endocrinol Metab. 2005;90(6):3427–3430. doi: 10.1210/jc.2005-0181. [DOI] [PubMed] [Google Scholar]
- 56.Snieder H, MacGregor AJ, Spector ID. Genes control cessation of a woman's reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83(6):1875–1880. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 57.Sowers MF, Crawford S, Morgenstein D, et al. Design, survey sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobos RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. New York, NY: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 58.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118(6 pt 2):1–120. [PubMed] [Google Scholar]
- 59.Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol. 1989;130(4):696–704. doi: 10.1093/oxfordjournals.aje.a115391. [DOI] [PubMed] [Google Scholar]
- 60.Sternfeld B, Ainsworth BA, Quesenberry CP., Jr Physical activity patterns in a diverse population of women. Prev Med. 1999;28(3):313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 61.Baecke JAH, Burema J, Fritjers JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 62.Block G, Hartman AM, Dresser CM, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 63.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 64.Block G, Thompson F, Hartman AM, et al. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assn. 1992;92(6):686–693. [PubMed] [Google Scholar]
- 65.Block G, Norris JC, Mandel RM, et al. Sources of energy and six nutrients in diets of low-income Hispanic-American women and children: quantitative data from the HHANES survey, 1982–1984. J Am Diet Assoc. 1995;95(2):195–208. doi: 10.1016/S0002-8223(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 66.Reinli K, Block G. Phytoestrogen content of foods—a compendium of literature values. Nutr Cancer. 1996;26(2):123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 67.England BG, Parsons GH, Possley RM, et al. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clin Chem. 2002;48(9):1584–1586. [PubMed] [Google Scholar]
- 68.Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91(4):1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 69.Storer BE, Gooley TA, Jones MP. Adjusted estimates for time-to-event endpoints. Lifetime Data Anal. 2008;14(4):484–495. doi: 10.1007/s10985-008-9098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 71.Allison PD. Survival Analysis Using SAS: A Practical Guide. Cary, NC: SAS Institute Inc.; 1995. pp. 111–184. [Google Scholar]
- 72.Agresti A, Caffo B. Measures of relative model fit. Comput Stat Data Anal. 2002;39(2):127–136. [Google Scholar]
- 73.Otolorin EO, Adeyefa I, Osotimehin BO, et al. Clinical, hormonal and biochemical features of menopausal women in Ibadan, Nigeria. Afr J Med Sci. 1989;18(4):251–255. [PubMed] [Google Scholar]
- 74.Beyene Y. Cultural significance and physiological manifestations of menopause, a bicultural analysis. Culture Med Psychiat. 1986;10(1):47–71. doi: 10.1007/BF00053262. [DOI] [PubMed] [Google Scholar]
- 75.Chompootweep S, Tankeyoon M, Yamarat K, et al. The menopausal age and climacteric complaints in Thai women in Bangkok. Maturitas. 1993;17(1):63–71. doi: 10.1016/0378-5122(93)90124-z. [DOI] [PubMed] [Google Scholar]
- 76.Lawlor DA, Ebrahim S, Smith GD. The association of socio-economic position across the life course and age at menopause: the British Women's Heart and Health Study. Br J Obstet Gynaecol. 2003;110(12):1078–1087. [PubMed] [Google Scholar]
- 77.Hardy R, Kuh D. Social and environmental conditions across the life course and age at menopause in a British birth cohort study. BJOG. 2005;112(3):346–354. doi: 10.1111/j.1471-0528.2004.00348.x. [DOI] [PubMed] [Google Scholar]
- 78.Reynolds RF, Obermeyer CM. Age at natural menopause in Spain and the United States: results from the DAMES project. Am J Hum Biol. 2005;17(3):331–340. doi: 10.1002/ajhb.20121. [DOI] [PubMed] [Google Scholar]
- 79.Kinney A, Kline J, Levin B. Alcohol, caffeine and smoking in relation to age at menopause. Maturitas. 2006;54(1):27–38. doi: 10.1016/j.maturitas.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 80.van Keep PA, Brand PC, Lehert PH. Factors affecting the age at menopause. J Biosoc Sci. 1979;11(suppl S6):37–55. doi: 10.1017/s0021932000024299. [DOI] [PubMed] [Google Scholar]
- 81.Mattison DR, Thorgierssen SS. Smoking and industrial pollution and their effects on menopause and ovarian cancer. Lancet. 1978;1(8057):187–188. doi: 10.1016/s0140-6736(78)90617-7. [DOI] [PubMed] [Google Scholar]
- 82.Essenberg JM, Fagan L, Malerstein AJ. Chronic poisoning of the ovaries and testes of albino rats and mice by nicotine and cigarette smoke. West J Surg Obstet Gynecol. 1951;59(1):27–32. [PubMed] [Google Scholar]
- 83.Everson RB, Sandler DP, Wilcox AJ, et al. Effect of passive exposure to smoking on age at natural menopause. Br Med J (Clin Res Ed) 1986;293(6550):792. doi: 10.1136/bmj.293.6550.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brand PC, Lehert PH. A new way of looking at environmental variables that may affect the age at menopause. Maturitas. 1978;1(2):121–132. doi: 10.1016/0378-5122(78)90019-1. [DOI] [PubMed] [Google Scholar]
- 85.Pathak RK, Parashar P. Age at menopause and associated bio-social factors of health in Punjabi women. Open Anthropol J. 2010;3(2):172–180. [Google Scholar]
- 86.Hidayet NM, Sharaf SA, Aref SR, et al. Correlates of age at natural menopause: a community-based study in Alexandria. East Mediterr Health J. 1999;5(2):307–319. [PubMed] [Google Scholar]
- 87.Lindquist O, Bengtsson C. Menopausal age in relation to smoking. Acta Med Scand. 1979;205(1–2):73–77. doi: 10.1111/j.0954-6820.1979.tb06006.x. [DOI] [PubMed] [Google Scholar]
- 88.Daniell HWP. Smoking, obesity, and the menopause. Lancet. 1978;2(8085):373. doi: 10.1016/s0140-6736(78)92970-7. [DOI] [PubMed] [Google Scholar]
- 89.den Tonkelaar I, Seidell J, van Noord PA, et al. Fat distribution in relation to age, degree of obesity, smoking habits, parity and estrogen use: a cross-sectional study of 11,825 Dutch women participating in the DOM project. Int J Obesity. 1990;14(9):753–761. [PubMed] [Google Scholar]
- 90.Kaufman DW, Slone D, Rosenberg L, et al. Cigarette smoking and age at natural menopause. Am J Public Health. 1980;70(10):420–422. doi: 10.2105/ajph.70.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dorjgochoo T, Kallianpur A, Gao Y-T, et al. Dietary and lifestyle predictors of age at natural menopause and reproductive span in the Shanghai Women's Health Study. Menopause. 2008;15(5):924–933. doi: 10.1097/gme.0b013e3181786adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baird DD, Tarlavsky FA, Anderson JJB. Do vegetarians have earlier menopause? Am J Epidemiol. 1988;128(4):907–908. [Google Scholar]
- 93.Nagata C, Takatsuka N, Kawakami N, et al. Association of diet with the onset of menopause in Japanese women. Am J Epidemiol. 2000;152(9):863–867. doi: 10.1093/aje/152.9.863. [DOI] [PubMed] [Google Scholar]
- 94.Nagata C, Takatsuka N, Inaba S, et al. Association of diet and other lifestyle with onset of menopause in Japanese women. Maturitas. 1998;29(2):105–113. doi: 10.1016/s0378-5122(98)00012-7. [DOI] [PubMed] [Google Scholar]
- 95.Nagel G, Altenburg HP, Nieters A, et al. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas. 2005;52(3–4):337–347. doi: 10.1016/j.maturitas.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 96.Harlow SD, Cain K, Crawford S, et al. Evaluation of four proposed bleeding criteria for the onset of late menopausal transition. J Clin Endocrinol Metab. 2006;91(9):3432–3438. doi: 10.1210/jc.2005-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cain KC, Harlow SD, Little RJ, et al. Bias due to left truncation and left censoring in longitudinal studies of developmental and disease processes. Am J Epidemiol. 2011;173(9):1078–1084. doi: 10.1093/aje/kwq481. [DOI] [PMC free article] [PubMed] [Google Scholar]