Abstract
Unhealthy lifestyle habits are a major contributor to coronary artery disease. The purpose of the present study was to investigate the associations of smoking, weight maintenance, physical activity, and diet with coronary calcium, cardiovascular events, and mortality. US participants who were 44–84 years of age (n = 6,229) were followed in the Multi-Ethnic Study of Atherosclerosis from 2000 to 2010. A lifestyle score ranging from 0 to 4 was created using diet, exercise, body mass index, and smoking status. Coronary calcium was measured at baseline and a mean of 3.1 (standard deviation, 1.3) years later to assess calcium progression. Participants who experienced coronary events or died were followed for a median of 7.6 (standard deviation, 1.5) years. Participants with lifestyle scores of 1, 2, 3, and 4 were found to have mean adjusted annual calcium progressions that were 3.5 (95% confidence interval (CI): 0.0, 7.0), 4.2 (95% CI: 0.6, 7.9), 6.8 (95% CI: 2.0, 11.5), and 11.1 (95% CI: 2.2, 20.1) points per year slower, respectively, relative to the reference group (P = 0.003). Unadjusted hazard ratios for death by lifestyle score were as follows: for a score of 1, the hazard ratio was 0.79 (95% CI: 0.61, 1.03); for a score of 2, the hazard ratio was 0.61 (95% CI: 0.46, 0.81); for a score of 3, the hazard ratio was 0.49 (95% CI: 0.32, 0.75); and for a score of 4, the hazard ratio was 0.19 (95% CI: 0.05, 0.75) (P < 0.001 by log-rank test). In conclusion, a combination of regular exercise, healthy diet, smoking avoidance, and weight maintenance was associated with lower coronary calcium incidence, slower calcium progression, and lower all-cause mortality over 7.6 years.
Keywords: coronary artery disease, CT and MRI, diet, epidemiology, exercise, primary prevention, risk factors, weight reduction
Coronary heart disease (CHD) remains the leading cause of death worldwide (1). There has been much attention paid to the role of lifestyle modification in CHD risk reduction. As part of their 2020 Impact Goals, the American Heart Association (AHA) developed a model of ideal cardiovascular health that included avoiding smoking, maintaining a body mass index (BMI, measured as weight (kg)/height (m)2) less than 25, being physically active, and adhering to a healthy diet (2). A recent study from the National Health and Nutrition Examination Survey (NHANES) showed an inverse correlation between the AHA's ideal cardiovascular health parameters and all-cause mortality rates (3). Another study of over 80,000 women showed that individuals who did not smoke, maintained a BMI of less than 25, exercised for 30 minutes/day, and consumed a Mediterranean-style diet had a 92% lower risk of sudden cardiac death (4).
To build a causal argument in epidemiologic studies, it is critical to show that these behaviors are associated with intermediate measures of disease, as well as with hard endpoints. The purpose of the present study was to investigate the associations of healthy diet, regular exercise, smoking avoidance, and normal weight maintenance with coronary artery calcium (CAC) incidence, CAC progression, CHD events, and all-cause mortality in a single longitudinal evaluation.
MATERIALS AND METHODS
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective study of the risk factors for and prevalence and progression of cardiovascular disease. The MESA design methods have been published previously (5). Briefly, 6,814 participants who were 44–84 years of age and self-identified as white, African American, Hispanic, or Chinese were recruited at 6 academic centers (Columbia University, New York, New York; Johns Hopkins University, Baltimore, Maryland; Northwestern University, Chicago, Illinois; University of California-Los Angeles, Los Angeles, California; University of Minnesota, Minneapolis, Minnesota; and Wake Forest University, Winston-Salem, North Carolina) from 2000 to 2002. All participants were free from clinical cardiovascular disease at the time of enrollment. The study protocol was approved by the institutional review board at each site. All participants gave informed consent before enrollment. Anthropometric measurements, lifestyle behaviors, medical history, and laboratory data were all assessed, as described previously (5).
Coronary artery calcium
CAC was measured using electron beam or multidetector computed tomography. All participants were scanned twice consecutively at baseline, and scans were read by an expert physician-reader at Harbor-UCLA Medical Center, California. Results from each participant's 2 scans were averaged to provide a more accurate point estimate of his or her Agatston calcium score. More information on the methodology of CAC measurement and interpretation is available in the Web Appendix (available at http://aje.oxfordjournals.org/) and prior reports (6, 7).
To quantify CAC progression, we randomly selected 2,954 participants to have a follow-up computed tomography examination between September 2002 and February 2004. There were 2,805 women selected between March 2004 and October 2005 and 1,406 selected between October 2005 and February 2008. The mean time between the baseline scan and the last follow-up scan was 3.1 (standard deviation (SD), 1.3) years.
CHD events
CHD events consisted of nonfatal myocardial infarction, resuscitated cardiac arrest, angina, coronary revascularization, and death due to CHD. Event criteria are available in the Web Appendix and online (http://www.mesa-nhlbi.org). Participants were contacted at intervals of 9–12 months, and information was collected about interim hospitalizations, outpatient CHD diagnoses, and deaths. Death certificates and medical records were requested. Next-of-kin interviews were conducted for participants with out-of-hospital cardiovascular deaths. Medical records were obtained for 98% of participants with hospitalized CHD events and 95% of participants with outpatient cardiovascular diagnoses. Follow-up telephone interviews were completed for 92% of living participants. The last follow-up was in June of 2011.
Lifestyle score
We selected 4 lifestyle-related variables for the scoring system: diet, BMI, smoking status, and physical activity level. For each variable, a binary score was defined (Mediterranean-style diet vs. unhealthy diet, optimal BMI vs. suboptimal BMI, never smoker vs. ever smoker, and regular physical activity vs. sedentary lifestyle), with a score of 1 awarded for each healthy behavior.
Dietary health was categorized based on a previously published scale quantifying adherence to a Mediterranean diet (8). The scale has been associated with mortality in over 20,000 adults and is easily reproducible. Per this method, participants were awarded points for consuming more healthy foods (vegetables, legumes, fruits, nuts, cereal/grains, and fish) and fewer detrimental foods (full-fat dairy, meat, poultry, and saturated fat) than the median intake and for having an optimal alcohol intake. This amounted to a possible diet score of 11 points. Participants with a diet score above the median received 1 point toward their comprehensive lifestyle score. (For complete dietary methodology, see the Web Appendix.)
BMI was categorized as optimal (≥18.5 and ≤24.9) or suboptimal (≥25 or ≤18.4) based on current World Health Organization and National Institutes of Health guidelines (9, 10). Participants with an optimal BMI received 1 point toward their lifestyle score. Smoking status was categorized as never smoker or previous/current smoker, with 1 point awarded for never smoking.
Physical activity data were obtained from the MESA Typical Week Physical Activity Survey (11). Participants who averaged more than 150 minutes/week of moderate-intensity physical activity or more than 75 minutes/week of vigorous-intensity activity were considered active based on current AHA guidelines and were awarded 1 point (12). The complete survey methodology is available in the Web Appendix and online (11). The addition of points for diet, physical activity, smoking status, and BMI amounted to a comprehensive lifestyle score ranging from 0 (least healthy) to 4 (healthiest) for each participant.
Statistical analysis
The variance of baseline descriptive variables was analyzed across lifestyle scores. Given the non-normality of the data, variance was measured using the Kruskal-Wallis equality-of-populations rank test.
CAC incidence was calculated for baseline zero CAC scores, and CAC progression was calculated for baseline nonzero scores. Annual CAC progression was calculated using the following equation: (mean phantom-adjusted CACfollow-up − mean phantom-adjusted CACbaseline)/(years between scans). Participants were excluded if coronary stents, which make scans uninterpretable, were placed during follow-up. For participants with multiple follow-up scans (n = 1,118), the final scan was used for the progression analysis.
The odds ratio for CAC incidence by lifestyle score was calculated using a generalized linear model with the logit link function. Annual CAC progression was calculated using median regression analysis because of the non-normality of CAC distribution. Hierarchical multivariable models were used: model 1 was unadjusted; model 2 was adjusted for age, sex, race, study site, and income; and model 3 was additionally adjusted for intermediate variables on the atherosclerosis pathway, including hypertension, use of medications to control hypertension, elevated fasting plasma glucose, use of medications for diabetes, elevated high-density lipoprotein cholesterol (HDL-C), elevated non–HDL-C, elevated triglycerides, use of lipid-lowering medications, and C-reactive protein level.
Time to CHD event or all-cause death was plotted using Kaplan-Meier statistics. Cox proportional hazards models were used to calculate the hazard ratio for suffering a CHD event or death based on lifestyle score in separate analyses. Models 1–3 were performed as above. An additional model (model 4) was then included to adjust for baseline CAC and determine whether lifestyle score added predicted value to baseline atherosclerosis. Hazard ratios and 95% confidence intervals were plotted by lifestyle score for each model. All statistical analyses were performed using STATA, version 12 (StataCorp LP, College Station, Texas). This data analysis plan was peer reviewed and approved by the MESA Publications and Presentations committee. A complete copy of the a priori statistical analysis plan is available at www.mesa-nhlbi.org.
RESULTS
Descriptive data
Of 6,814 total MESA participants, 585 were excluded because of missing lifestyle variable data. The final study population included 6,229 participants; their characteristics are shown in Tables 1 and 2. The median and mean diet scores were both 5 out of 11 points (interquartile range (IQR), 4–7). Of the participants included in our analyses, 2,311 (37%) were taking medications for hypertension, 1,013 (16%) were taking lipid-lowering medications, 594 (10%) were taking medications for diabetes, and 878 (14%) were taking more than 1 medication.
Table 1.
Baseline Descriptive Characteristics of the Population by Lifestyle Score, Multi-Ethnic Study of Atherosclerosis, 2000–2010
| Characteristic | Total Population (n = 6,229) |
Lifestyle Score |
P Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (n = 1,096) |
1 (n = 2,363) |
2 (n = 1,947) |
3 (n = 694) |
4 (n = 129) |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
| Sex | <0.001 | ||||||||||||
| Female | 3,295 | 53 | 485 | 44 | 1,227 | 52 | 1,113 | 57 | 406 | 59 | 64 | 50 | |
| Male | 2,934 | 47 | 611 | 56 | 1,136 | 48 | 844 | 43 | 288 | 41 | 65 | 50 | |
| Race | <0.001 | ||||||||||||
| White | 2,471 | 40 | 405 | 37 | 861 | 36 | 792 | 41 | 331 | 48 | 82 | 64 | |
| Asian | 790 | 13 | 45 | 4 | 254 | 11 | 328 | 17 | 142 | 20 | 21 | 16 | |
| Black | 1,609 | 26 | 331 | 30 | 655 | 28 | 470 | 24 | 137 | 20 | 16 | 12 | |
| Hispanic | 1,359 | 22 | 315 | 29 | 593 | 25 | 357 | 18 | 84 | 12 | 10 | 8 | |
| Study site | <0.001 | ||||||||||||
| Wake Forest University | 972 | 16 | 177 | 16 | 372 | 16 | 305 | 16 | 87 | 13 | 31 | 24 | |
| Columbia University | 909 | 15 | 163 | 15 | 367 | 16 | 270 | 14 | 91 | 13 | 18 | 14 | |
| Johns Hopkins University | 950 | 15 | 183 | 17 | 362 | 15 | 289 | 15 | 100 | 14 | 16 | 12 | |
| University of Minnesota | 1,005 | 16 | 251 | 23 | 398 | 17 | 269 | 14 | 76 | 11 | 11 | 9 | |
| Northwestern University | 1,119 | 18 | 118 | 11 | 379 | 16 | 387 | 20 | 196 | 28 | 39 | 30 | |
| University of California- Los Angeles | 1,274 | 20 | 204 | 19 | 485 | 21 | 427 | 22 | 144 | 21 | 14 | 11 | |
| Regular exercisea | 1,127 | 18 | 0 | 0 | 175 | 7 | 438 | 22 | 385 | 55 | 129 | 100 | <0.001 |
| Healthy dietb | 2,822 | 45 | 0 | 0 | 785 | 33 | 1,292 | 66 | 616 | 89 | 129 | 100 | <0.001 |
| Family history premature CHD | 969 | 16 | 184 | 17 | 385 | 16 | 283 | 15 | 97 | 97 | 16 | 12 | 0.16 |
Abbreviation: CHD, coronary heart disease.
a Defined as more than 150 hours/week of moderate physical activity or more than 75 hours/week of vigorous physical activity.
b Defined as scoring above the median on total Mediterranean diet score.
Table 2.
Medians and Interquartile Ranges for Baseline Descriptive Statistics by Lifestyle Score,a Multi-Ethnic Study of Atherosclerosis, 2000–2010
| Characteristic | Total Population (n = 6,229) | Lifestyle Score |
P Value | ||||
|---|---|---|---|---|---|---|---|
| 0 (n = 1,096) | 1 (n = 2,363) | 2 (n = 1,947) | 3 (n = 694) | 4 (n = 129) | |||
| Age, years | 62 (53–70) | 61 (53–69) | 62 (54–70) | 64 (54–71) | 63 (53–71) | 62 (54–71) | <0.001 |
| Body mass indexb | 27 (24–31) | 30 (27–33) | 29 (26–31) | 26 (23–30) | 24 (22–26) | 23 (22–24) | <0.001 |
| Pack-years of smoking | 0 (0–15) | 17 (6–35) | 1 (0–18) | 0 (0–2) | 0 (0) | 0 (0) | <0.001 |
| Fasting glucose level, mg/dL | 90 (83–99) | 92 (85–103) | 91 (83–101) | 89 (82–97) | 87 (81–94) | 85 (79–91) | <0.001 |
| Systolic blood pressure, mm Hg | 124 (111–140) | 125 (112–140) | 125 (113–141) | 123 (109–139) | 120 (109–138) | 117 (107–136) | <0.001 |
| Diastolic blood pressure, mm Hg | 72 (65–79) | 73 (66–80) | 72 (66–79) | 71 (64–78) | 70 (64–77) | 70 (63–76) | <0.001 |
| Non–HDL-C level, mg/dL | 143 (121–167) | 143 (121–165) | 141 (117–164) | 136 (114–159) | 132 (114–155) | 141 (119–164) | <0.001 |
| HDL-C level, mg/dL | 45 (38–53) | 47 (40–57) | 51 (42–62) | 53 (43–64) | 54 (46–69) | 48 (40–59) | <0.001 |
| Triglyceride level, mg/dL | 126 (86–177) | 116 (81–168) | 106 (76–155) | 101 (70–145) | 83 (67–122) | 112 (78–162) | <0.001 |
Abbreviation: HDL-C, high-density lipoprotein cholesterol.
a Participants with lifestyle scores of 0–2 had a median gross family income of $35,000–$40,000 per year and a median educational level of “some college.” Participants with scores of 3–4 had a median gross family income of $50,000–$75,000 per year and median educational level of “bachelor's degree.”
b Weight (kg)/height (m)2.
Only 129 (2%) participants satisfied all 4 healthy lifestyle criteria. Age, race, sex, BMI, physical activity, diet, pack-years of cigarette smoking, fasting plasma glucose level, blood pressure, non–HDL-C level, HDL-C level, and triglyceride level all showed significant variance across lifestyle scores (Tables 1 and 2).
Over the follow-up period, 208 participants developed angina, 142 suffered a myocardial infarction, 20 experienced resuscitated cardiac arrest, 150 underwent percutaneous coronary intervention, 94 underwent coronary artery bypass grafting, and 41 died from CHD. Several participants suffered more than 1 CHD event; 305 unique participants suffered CHD events. A total of 374 participants died from any cause over the course of the study. Of the 374 deaths, 41 were due to CHD, 15 were due to stroke, 1 was due to noncardiac atherosclerosis, 19 were due to other cardiac causes, 294 were due to noncardiac causes, and 4 were due to unknown causes. The median time to last follow-up or death was 7.6 (SD, 1.5) years.
Coronary artery calcium
All participants underwent baseline computed tomography scans for CAC scoring. A few participants (182) did not have follow-up scans and were excluded from CAC analysis, leaving 6,047 participants. Approximately 41% of all follow-up scans were at the second examination (median, 1.6 (SD, 0.3) years later), 39% were at the third examination (median, 3.1 (SD, 0.3) years later), and 19% were at the fourth examination (median, 4.8 (SD, 0.5) years later). The mean time between baseline and the last scan was 3.1 (SD, 1.3) years. Participants with scores of 3 and 4 had a greater prevalence of no baseline CAC than did those with a score of 0 (P < 0.001) (Web Figure 1).
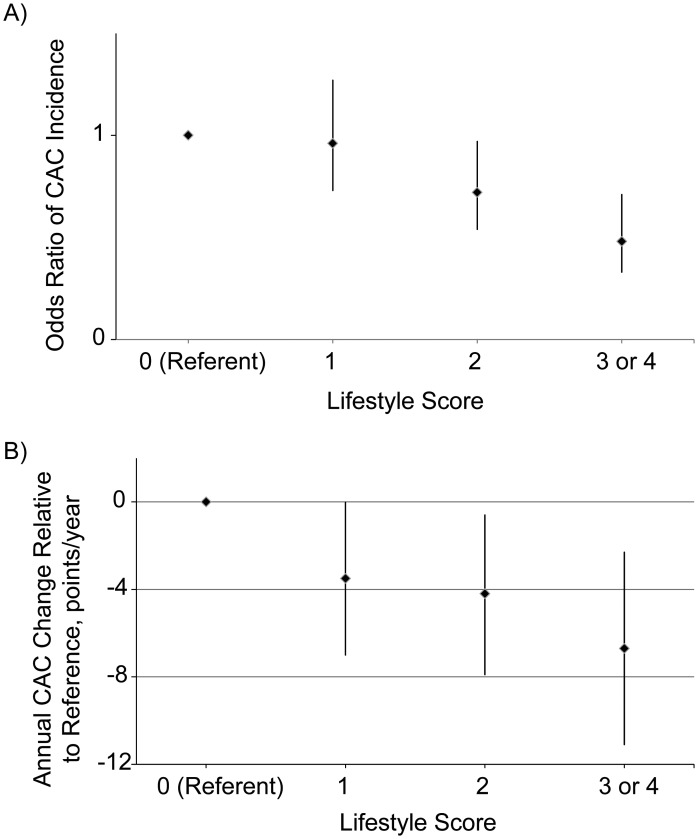
Over the follow-up period, 100 of 446 participants with a lifestyle score of 0 developed new CAC compared with 240 of 1,057 with a score of one, 167 of 911 with a score of two, 46 of 346 with a score of three, and 9 of 58 with a score of four. The odds ratios for incident CAC in model 2 (adjusted for years between scans, age, race, sex, study site, and income) were 0.96 (95% confidence interval (CI): 0.73, 1.27) for a score of one, 0.72 (95% CI: 0.54, 0.97) for a score of two, 0.47 (95% CI: 0.31, 0.71) for a score of three, and 0.54 (95% CI: 0.25, 1.16) for a score of four. The P value was 0.001 for the lifestyle score trend in both models 1 and 2. Because only a small number of participants satisfied all lifestyle criteria and had a score of 4 (n = 129), those with lifestyle scores of 3 and 4 were combined in subgroup analyses. The adjusted odds ratio for the combined scores in model 2 was 0.48 (95% CI: 0.33, 0.71), with P value less than 0.001 for the lifestyle score (Figure 1A). Adjustment for additional variables in model 3 resulted in no change in overall conclusions (P = 0.003).
Figure 1.
Coronary artery calcium (CAC) incidence and progression, Multi-Ethnic Study of Atherosclerosis, 2000–2010. A) Odds ratio of CAC incidence by lifestyle score, adjusted for follow-up time, age, race, sex, study site, and income (P < 0.001). B) Annual CAC progression relative to reference group, adjusted for age, race, sex, study site, and income (P = 0.002).
The median annual CAC progressions for participants with scores of 0–4 were as follows: 25 points/year (IQR, 7–73), 20 points/year (IQR, 6–58), 18 points/year (IQR, 6–53), 18 points/year (IQR, 6–54), and 14 points/year (IQR, 5–53), respectively. After adjustment for variables in model 2, participants with lifestyle scores of 1, 2, 3, and 4 had mean adjusted annual progressions of CAC that were slower by 3.5 points/year (95% CI: 0.0, 7.0), 4.2 points/year (95% CI: 0.6, 7.9), 6.8 points/year (95% CI: 2.0, 11.5), and 11.1 points/year (95% CI: 2.2, 20.1), respectively, relative to the reference group. The P value for the lifestyle score trend was 0.01 in model 1 and 0.003 in model 2. When lifestyle scores 3 and 4 were combined in model 2, the adjusted annual progression of CAC was 6.7 points/year (95% CI: 2.3, 11.1) slower than that of the reference group (P = 0.002) (Figure 1B). Adjustment for additional risk markers that may be on the causal pathway between lifestyle and atherosclerosis in model 3 resulted in a loss of significance (P = 0.20).
Coronary heart disease
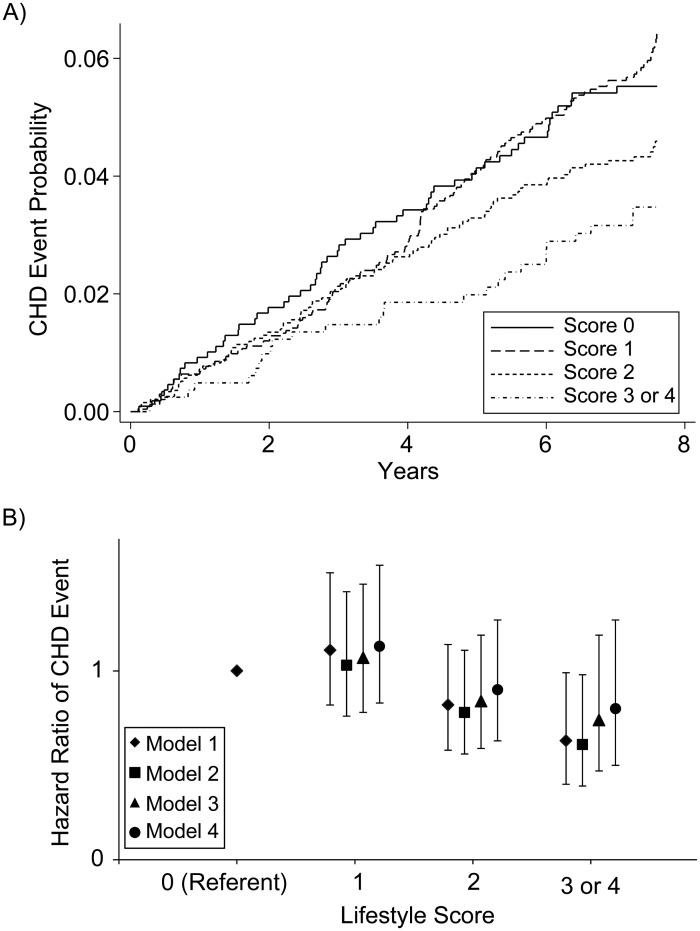
We used Kaplan-Meier hazard plots to display the unadjusted hazard function for time to CHD event by lifestyle score over 7.6 years (Figure 2A). The P value for the hazard function plot was 0.03 by log-rank test and 0.02 when scores of 3 and 4 were combined. Among participants with a score of 0, there were 56 CHD events (17% of total person-years); among those with a score of 1, there were 137 events (38% of total person-years); among those with a score of 2, there were 84 events (32% of total person-years); and among those with a score of 3 or 4, there were 28 events (13% of total person-years). In Cox models, the unadjusted hazard ratios for suffering a CHD event over 7.6 years for participants with scores of 1–4 were 1.11 (95% CI: 0.82, 1.52), 0.82 (95% CI: 0.58, 1.14), 0.61 (95% CI: 0.38, 1.00), and 0.72 (95% CI: 0.29, 1.80), respectively, compared with the reference group. The unadjusted hazard ratio for scores of 3 and 4 combined was 0.63 (95% CI: 0.40, 0.99) (Figure 2B). The absolute risk of having a CHD event in the reference group was 5.4%, compared with 3.5% in participants with a score of 3 or 4 (1.9% absolute risk reduction).
Figure 2.
Probability and hazard ratio of coronary heart disease (CHD), Multi-Ethnic Study of Atherosclerosis, 2000–2010. A) Unadjusted probability of CHD by lifestyle score over 7.6 years (P = 0.02 by log-rank test). B) Hazard ratio of CHD by lifestyle score over 7.6 years (P = 0.008 for model 1 trend).
The Cox models were repeated in a hierarchical fashion, as described in the methodology. After adjustment for intermediary variables in model 3 and also baseline CAC in model 4, there was a progressive increase in the P value and a loss of statistical significance (Web Table 1). There was no significant interaction between lifestyle score and sex (P = 0.94) or lifestyle score and race (P = 0.93) in the CHD analysis.
Mortality
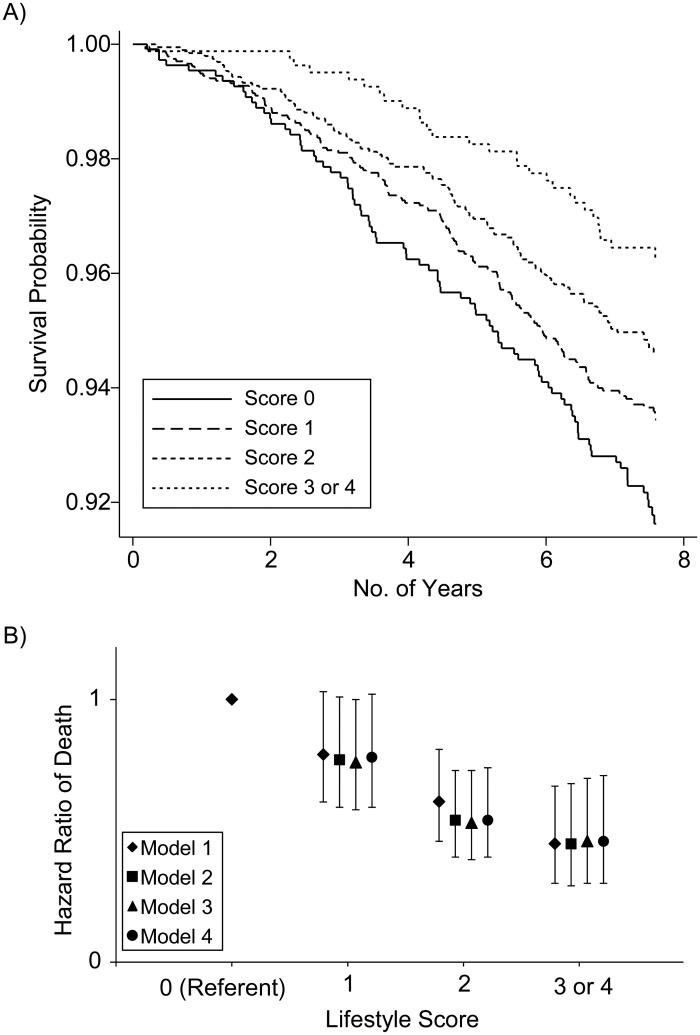
We used a Kaplan-Meier curve to display the unadjusted probability of survival by lifestyle score over 7.6 years (Figure 3A). The P value for the lifestyle score was <0.001 by log-rank test. There were 89 deaths among participants with a score of 0 (17% of total person-years), 155 deaths among those with a score of 1 (38% of total person-years), 99 deaths among those with a score of 2 (31% of total person-years), and 31 deaths among those with scores of 3 or 4 (13% of total person-years). In Cox models, participants with a score of 1 had an unadjusted hazard ratio 0.79 (95% CI: 0.61, 1.03), those with a score of 2 had a hazard ratio of 0.61 (95% CI: 0.46, 0.81), those with a score of 3 had a hazard ratio of 0.49 (95% CI: 0.32, 0.75), and those with a score of 4 had a hazard ratio of 0.19 (95% CI: 0.05, 0.75) for the risk of dying over 7.6 years compared with the reference group (P < 0.001 for the lifestyle score). When scores of 3 and 4 were combined, the unadjusted hazard ratio was 0.45 (95% CI: 0.30, 0.67; P < 0.001) (Figure 3B). The absolute risk of death in the reference group was 8.8%, compared with 3.9% in participants with score 3 or 4 (4.9% absolute risk reduction).
Figure 3.
Probability and hazard ratio of death, Multi-Ethnic Study of Atherosclerosis, 2000–2010. A) Kaplan-Meier curve of survival probability (unadjusted) by lifestyle score over 7.6 years (P < 0.001 by log-rank test). B) Hazard ratio of death by lifestyle score over 7.6 years (P < 0.001 for model 1 trend).
The model was repeated in a hierarchical fashion as we did for CHD. Adjustment for intermediary variables in model 3 and also baseline CAC in model 4 did not significantly change the hazard ratios, and the P values remained <0.001 in all models (Web Table 2). There was no significant interaction between lifestyle score and sex (P = 0.75) or lifestyle score and race (P = 0.86) in the mortality analysis.
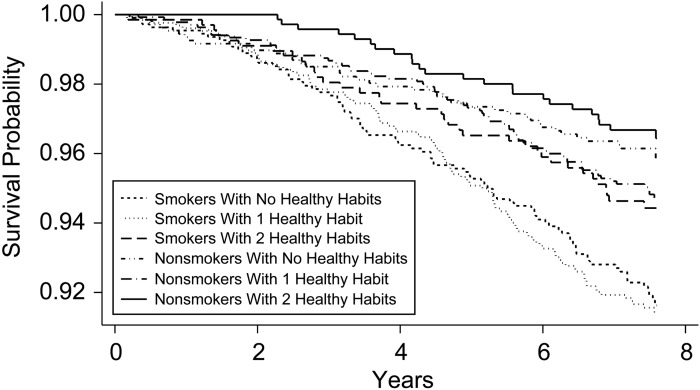
The hazard ratio of CHD and death was calculated for each lifestyle variable in Web Tables 3 and 4. Smoking was the lifestyle variable found to be associated with the greatest reduction in the risk of CHD and death in most models. Accordingly, Kaplan-Meier survival estimates were plotted for smokers and nonsmokers across various lifestyle scores (Figure 4). Survival estimates showed that smokers had lower survival rates than did nonsmokers, even if they adopted multiple healthy lifestyle variables.
Figure 4.
Kaplan-Meier survival estimates by smoking status across lifestyle scores (P < 0.001 by log rank test), Multi-Ethnic Study of Atherosclerosis, 2000–2010.
DISCUSSION
In the present study, we found that regular exercise, adherence to a Mediterranean-style diet, smoking avoidance, and normal weight maintenance were associated with lower CAC incidence, slower CAC progression, a trend toward lower CHD risk, and significantly lower all-cause mortality over 7.6 years. Participants who adopted all 4 of these behaviors had an approximately 80% lower death rate than did participants with no healthy behaviors. The lifestyle score predicted mortality despite adjustment for CAC and other intermediary variables on the atherosclerosis pathway in models 3 and 4. To our knowledge, this is the first study to connect the protective effects of healthy lifestyle across baseline subclinical vascular disease, atherosclerotic progression, clinical coronary heart disease, and death in a single longitudinal evaluation.
This study is timely given the AHA's recent efforts to reduce CHD risk through “Life's Simple Seven” (2), which include the 4 health behaviors of avoiding smoking, maintaining a BMI less than 25, being physically active, and adhering to a healthy diet. The correlations between these behaviors and mortality in the present study bolster the AHA's recommendations, and the additional measure of subclinical atherosclerosis lends biologic credibility to the outcomes investigated.
The results of this study are consistent with those of prior studies in which researchers investigated the impact of lifestyle on CHD and death independently. At least 4 large prospective studies have shown protective effects of smoking avoidance, weight maintenance, physical activity, and diet on mortality (3, 13–16). Ford et al. (14) showed that never smoking, adhering to a healthy diet, being physically active, and consuming moderate amounts of alcohol decreased the risk of all-cause mortality. Chiuve et al. (4) showed a 92% risk reduction in sudden cardiac death in a prospective study of 80,000 women who adopted the lifestyle modifications above.
Interestingly, the results of the present study showed that smoking played the largest role in CHD and mortality risk reduction compared with the other lifestyle variables (Web Tables 3 and 4). In fact, smokers who adopted 2 or more healthy behaviors still had lower survival rates at 7.6 years than did sedentary, obese nonsmokers (Figure 4). This underscores the importance of smoking avoidance as one of the strongest predictors of cardiovascular health and survival and confirms prior findings that smoking avoidance prolongs life (17–20).
As for subclinical disease, much attention has been directed toward CAC, given its link to risk of CHD and death (21–24). Numerous reports have found significant associations between physical activity level and CAC (12, 25–29). Several other lifestyle variables, including diet, smoking, and waist circumference, have also been associated with CAC (30, 31). Results of the present study further confirm the associations between these behavioral risk factors and subclinical disease.
Importantly, there were more striking differences in all-cause mortality than in CHD events with increasing lifestyle score in this study. Lifestyle factors likely take decades to take effect on atherosclerosis, so the more immediate effects on mortality suggest the importance of low-risk lifestyle in disease processes other than CHD alone. Indeed, Ford et al. (14) showed that low-risk behaviors in NHANES participants delayed cancer-specific mortality more than cardiovascular mortality. Also, a recent meta-analysis showed that adherence to a healthy diet reduced cancer-specific mortality by 22% (32). Similarly, several large studies have observed an association between obesity and cancer-specific mortality (20, 33–35). The current findings from MESA support these previous reports and stress the importance of behavioral risk factors in maintaining overall health.
Strengths and limitations
There are several strengths to the present study. First, the lifestyle score combines variables that are easily applied in clinical practice. Patients can understand this simple score and can be taught that establishment of the healthiest lifestyle is associated with an 80% lower rate of death over nearly 8 years. Second, all of the MESA participants were free of cardiovascular disease at enrollment, making this study applicable to the true primary prevention population. Third, MESA provides a multicenter, multi-ethnic, sex-balanced cohort, with some participants on contemporary prevention medications. This makes findings more generalizable across the clinical population.
There are a few limitations to acknowledge. First, only 129 of 6,229 participants satisfied all 4 healthy lifestyle criteria. Although this is likely a genuine representation of the US population behaviors, it may have limited the power to demonstrate differences in individual scores (particularly with regard to a score of 4) in the CHD event analysis. Studies with sufficient power are needed to investigate whether individual statistical significance for CHD events hold true for each lifestyle score.
Second, MESA did not measure cardiorespiratory fitness. Physical activity level does not always correlate with cardiorespiratory fitness because of comorbidities or musculoskeletal injuries that may decrease activity despite relative fitness (25). Including BMI in this score may help overcome this. Not surprisingly, participants who were both active and maintained normal BMI (score of 4) had a lower risk of death then did participants who only satisfied 1 of those criteria (Web Table 2). Further research that adds fitness to this score is needed to determine whether fitness plays an independent role in preventing CHD and death.
Third, the dietary score used in this study was based on communities’ median dietary habits for each food group. Although MESA participants were from 6 various communities across the United States, their dietary patterns may still vary significantly from participants in other countries. Future studies using this scale should include multinational participants to further improve the generalizability of the findings.
Conclusion
The present study showed that regular exercise, adherence to a Mediterranean-style diet, smoking avoidance, and maintenance of a normal weight were associated with lower CAC incidence, slower CAC progression, a trend toward lower CHD risk, and significantly lower all-cause mortality over 7.6 years. Of these behavioral variables, smoking avoidance was the one associated with the greatest reduction in the risk of CHD and death.
CAC is a potent measure of subclinical vascular disease and biologic aging. The association between the studied lifestyle variables and CAC, as well as a trend toward lower CHD risk in just 7.6 years, suggests that this impact will likely mature over time. The stronger association with mortality suggests the importance of lifestyle in noncardiac as well as cardiac disease processes. Further emphasis on education and counseling about these lifestyle modifications will be critical for improving future individual and community health.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Ciccarone Center for the Prevention of Heart Disease, Johns Hopkins University, Baltimore, Maryland (Haitham M. Ahmed, Michael J. Blaha, Khurram Nasir, Steven R. Jones, Juan J. Rivera, Roger S. Blumenthal); Center for Prevention and Wellness Research, Baptist Health Medical Group, Miami Beach, Florida (Khurram Nasir, Arthur Agatston); Department of Medicine, Herbert Wertheim College of Medicine, Florida International University, Miami, Florida (Khurram Nasir, Arthur Agatston); Department of Epidemiology, Robert Stempel College of Public Health, Florida International University, Miami, Florida (Khurram Nasir); South Beach Preventive Cardiology, Miami Beach, Florida (Juan J. Rivera, Arthur Agatston); Brigham and Women's Hospital, Boston, Massachusetts (Ron Blankstein); University of California-Irvine College of Medicine, Irvine, California (Nathan D. Wong); College of Medicine, University of Vermont, Burlington, Vermont (Susan Lakoski); School of Medicine, University of California-Los Angeles, Torrance, California (Matthew J. Budoff); School of Medicine, Wake Forest University, Winston-Salem, North Carolina (Gregory L. Burke); National Institutes of Health Clinical Center, Bethesda, Maryland (Christopher T. Sibley); and Johns Hopkins Bayview Medical Center, Johns Hopkins University, Baltimore, Maryland (Pamela Ouyang).
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (grant R01-HL071739 and contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169).
We thank the staff and other investigators of the Multi-Ethnic Study of Atherosclerosis for their valuable contributions. A full list of participating investigators is available at http://www.mesa-nhlbi.org.
Conflict of interest: none declared.
REFERENCES
- 1.Mackay J, Mensah G. Geneva, Switzerland: World Health Organization; 2004. The Atlas of Heart Disease and Stroke. [Google Scholar]
- 2.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125(8):987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306(1):62–69. doi: 10.1001/jama.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 6.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 7.Nelson JC, Kronmal RA, Carr JJ, et al. Measuring coronary calcium on CT images adjusted for attenuation differences. Radiology. 2005;235(2):403–414. doi: 10.1148/radiol.2352040515. [DOI] [PubMed] [Google Scholar]
- 8.Trichopoulou A, Costacou T, Bamia C, et al. Adherence to a mediterranean diet and survival in a Greek population. NEJM. 2003;348(26):2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Geneva, Switzerland: World Health Organization; 2000. Obesity: Preventing and managing the global epidemic. WHO Technical Report Series 894. [PubMed] [Google Scholar]
- 10.National Institutes of Health. Bethesda, MD: National Institutes of Health; 1998. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. NIH Publication No. 98–4083. [Google Scholar]
- 11.Bertoni AG, Whitt-Glover MC, Chung H, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169(4):444–454. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 13.van Dam RM, Li T, Spiegelman D, et al. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford ES, Zhao G, Tsai J, et al. Low-risk lifestyle behaviors and all-cause mortality: findings from the National Health and Nutrition Examination Survey III Mortality Study. Am J Public Health. 2011;101(10):1922–1929. doi: 10.2105/AJPH.2011.300167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopinath B, Flood VM, Burlutsky G, et al. Combined influence of health behaviors on total and cause-specific mortality. Arch Intern Med. 2010;170(17):1605–1607. doi: 10.1001/archinternmed.2010.303. [DOI] [PubMed] [Google Scholar]
- 16.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292(12):1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 17.Daviglus ML, Stamler J, Pirzada A, et al. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292(13):1588–1592. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Dyer AR, Wang R, et al. Risk factor burden in middle age and lifetime risks for cardiovascular and non-cardiovascular death (Chicago Heart Association Detection Project in Industry) Am J Cardiol. 2007;99(4):535–540. doi: 10.1016/j.amjcard.2006.09.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boggs DA, Rosenberg L, Cozier YC, et al. General and abdominal obesity and risk of death among black women. NEJM. 2011;365(10):901–908. doi: 10.1056/NEJMoa1104119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. NEJM. 2008;359(20):2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 21.Newman AB, Naydeck BL, Ives DG, et al. Coronary artery calcium, carotid artery wall thickness, and cardiovascular disease outcomes in adults 70 to 99 years old. Am J Cardiol. 2008;101(2):186–192. doi: 10.1016/j.amjcard.2007.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budoff MJ, Hokansen JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3(12):1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Blaha M, Budoff MJ, Shaw LJ. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 24.McEvoy JW, Blaha MJ, DeFilippis AP, et al. Coronary artery calcium progression: an important clinical measurement? A review of published reports. J Am Coll Cardiol. 2010;56(20):1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed HM, Blaha MJ, Nasir K, et al. Effects of physical activity on cardiovascular disease. Am J Cardiol. 2012;109(2):288–295. doi: 10.1016/j.amjcard.2011.08.042. [DOI] [PubMed] [Google Scholar]
- 26.Bishop FK, Maahs DM, Snell-Bergeon JK, et al. Lifestyle risk factors for atherosclerosis in adults with type 1 diabetes. Diab Vasc Dis Res. 2009;6(4):269–275. doi: 10.1177/1479164109346359. [DOI] [PubMed] [Google Scholar]
- 27.Storti KL, Pettee Gabriel KK, Underwood DA, et al. Physical activity and coronary artery calcification in two cohorts of women representing early and late postmenopause. Menopause. 2010;17(6):1146–1151. doi: 10.1097/gme.0b013e3181e3a356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamer M, Kivimaki M, Lahiri A, et al. Walking speed and subclinical atherosclerosis in healthy older adults: the Whitehall II Study. Heart. 2010;96(5):380–384. doi: 10.1136/hrt.2009.183350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettee KK, Larouere BM, Kriska AM, et al. Associations among walking performance, physical activity, and subclinical cardiovascular disease. Prev Cardiol. 2007;10(3):134–140. doi: 10.1111/j.1520-037x.2007.06173.x. [DOI] [PubMed] [Google Scholar]
- 30.Diaz VA, Mainous AG, 3rd, Everett CJ, et al. Effect of healthy lifestyle behaviors on the association between leukocyte telomere length and coronary artery calcium. Am J Cardiol. 2010;106(5):659–663. doi: 10.1016/j.amjcard.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 31.Michos ED, Santos RD, Narla V, et al. Favorable cardiovascular risk factor profile is associated with reduced prevalence of coronary artery calcification and inflammation in asymptomatic nondiabetic white men. Prev Cardiol. 2008;11(4):189–194. doi: 10.1111/j.1751-7141.2008.00007.x. [DOI] [PubMed] [Google Scholar]
- 32.Balter K, Moller E, Fondell E. The effect of dietary guidelines on cancer risk and mortality. Curr Opin Oncol. 2012;24(1):90–102. doi: 10.1097/CCO.0b013e32834e0531. [DOI] [PubMed] [Google Scholar]
- 33.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospective studied cohort of US adults. NEJM. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 34.Jee SH, Sull JW, Park J, et al. Body-mass index and mortality in Korean men and women. NEJM. 2006;355(8):779–787. doi: 10.1056/NEJMoa054017. [DOI] [PubMed] [Google Scholar]
- 35.Zheng W, McLerran DF, Rolland B, et al. Association between body-mass index and risk of death in more than 1 million Asians. NEJM. 2011;364(8):719–729. doi: 10.1056/NEJMoa1010679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.