Abstract
Some children with uncomplicated malaria progress to cerebral malaria despite appropriate treatment; identifying them in advance might improve their care. The objective of this study was to determine if plasma concentrations of a malaria protein, HRP2 (histidine-rich protein 2) would serve this purpose. Cases and controls were children presenting with uncomplicated malaria; the cases (n = 25) developed cerebral malaria, and the controls (n = 125) did not. Mean plasma HRP2 concentrations were significantly higher in the cases, and an HRP2 cutoff was identified that could predict disease progression (sensitivity and specificity, 88% for each). Quantitative measurements of HRP2 may be a useful screening tool.
Keywords: cerebral malaria, HRP2, malaria diagnostics
Malaria is a disease with an enormous impact on humanity. African children bear 90% of the mortality burden, with estimates of 1.2 million deaths every year [1]. The number of children with Plasmodium falciparum infections greatly outnumbers those with malarial illness, and of those with a malarial illness, only a small proportion progress to severe and complicated malaria. The determinants of disease progression and severity remain elusive. If clinicians could identify which children with apparently uncomplicated malaria are likely to deteriorate, these children could be referred for more comprehensive care. Currently, there is no way to distinguish this high-risk subset from those who will recover following routine treatment with oral antimalarials.
Cerebral malaria (CM), one of the most deadly forms of severe malaria, is defined as a Blantyre Coma Score of ≤2, with peripheral P. falciparum parasitemia of any density and no other discernible causes of coma (eg, hypoglycemia-associated coma reversed by glucose infusion, meningitis, or postictal state) [2]. The specificity of this definition can be improved from 61% to 100% by including, as a criterion, presence of malaria retinopathy on ocular funduscopic examination [3].
HRP2 is a protein released from parasite-infected erythrocytes in vivo and in vitro. The function of the protein is unknown, but it is produced throughout the 48-hour life cycle of the parasite. As the parasite matures, more HRP2 is excreted, and most HRP2 (a median of 89%) is released at the moment of schizont rupture, when the parasite is sequestered in deep tissues [4]. Semiquantitative assessments of HRP2 concentrations in adults show a positive correlation between HPR2 and disease severity [5]. In African children presenting with severe febrile illness, quantitative measures of admission HRP2 values predict mortality and are correlated with disease severity [6, 7]. Quantitative measures of HRP2 can also accurately distinguish retinopathy-positive CM from retinopathy-negative CM [8].
The objective of this study was to evaluate plasma concentrations of HRP2 in children with uncomplicated malaria at the time of arrival to hospital as a predictor of clinical deterioration during the hospitalization. The ability to identify patients who are more likely to progress to severe malaria would be very helpful in the clinical triage of symptomatic parasitemic patients in malaria-endemic areas.
METHODS
Study Population
All children presenting to the Accident and Emergency (A&E) area at Queen Elizabeth Central Hospital, Blantyre, Malawi, with signs and symptoms of malaria undergo routine diagnostics including a thick blood film for malaria parasites and determination of packed cell volume. From February 2010 to June 2011, the capillary tubes used to measure packed cell volume in patients with evidence of malaria infection on microscopy were labeled and stored at 4°C after centrifugation.
Case Subjects
Children who were admitted to the hospital with uncomplicated malaria (P. falciparum parasitemia of any density and Blantyre Coma Score of 5) were included as case subjects (“cases”) if they deteriorated clinically, were admitted to the pediatric research ward, and satisfied a stringent clinical case definition of CM (World Health Organization classification plus retinopathy). All cases received standard treatment with intramuscular quinine (10 mg/kg at 0 hours, 4 hours, and then twice daily) prior to their transfer to the research ward, after which quinine was administered via intravenous infusion.
Control Subjects
Control subjects were selected from the children who were discharged from A&E or who were admitted to the hospital for further care and recovered without clinical deterioration. Five controls for each case were selected randomly from the capillary tubes of parasitemic children who presented to A&E on the same day as each case.
HRP2 Determination
Capillary tubes from the cases and controls were identified and retrieved from the stored samples, and the plasma from each was extracted and frozen. Aliquots were thawed in batches and analyzed for HRP2 value by enzyme-linked immunosorbent assay (ELISA).
Serum was diluted at a ratio of either 1:100 or 1:500 in phosphate-buffered saline (PBS). These samples, as well as a titration of a stock of recombinant HRP2, were plated in duplicate onto a plate precoated with anti-HRP2 antibody (Cellabs, Brookvale, Australia). The manufacturer's protocol was followed, except for the modification of all incubations being carried out at 37°C in a humidified chamber. In brief, a 1-hour sample incubation step was followed with extensive washing with PBS/0.1% Tween. One hundred microliters of the second conjugated antibody was plated and allowed to incubate for 1 hour. The conjugate was subsequently washed off and 100 μL of substrate was added for 15 minutes, during which color change was observed. This reaction was stopped and the plate was analyzed at optical density (OD) 450. A standard curve was generated from the recombinant protein, and the readings from the diluted unknown samples were compared to this curve to calculate HRP2 levels. When the initial dilution of the sample resulted in an OD reading that did not lie within the linear range of the recombinant protein, the sample was rediluted at either 1:1000 or 1:10. Samples that remained off scale (low), despite redilution, were assigned a value equivalent to the lowest possible detection value.
Statistical Analyses
Descriptive statistics including means and standard deviations were computed with Excel software (Microsoft, Redmond, WA). Statistical differences in continuous variables between groups were determined using a t test with an α level of <.05 to determine statistical significance. To quantify the discriminative ability of HRP2 levels to distinguish CM cases from uncomplicated controls, receiver operating characteristic curves and area under the curve values were calculated using SPSS software (version 18.0, IBM Corporation, Somers, NY). Interval likelihood ratios and 95% confidence intervals (CIs) were generated using the CIA software package (Confidence Interval Analysis, version 2.2.0, University of Southampton, UK).
Institutional Review Board Approval
The study was approved by the institutional review boards at the University of Malawi College of Medicine, Michigan State University, and the University of Washington.
RESULTS AND DISCUSSION
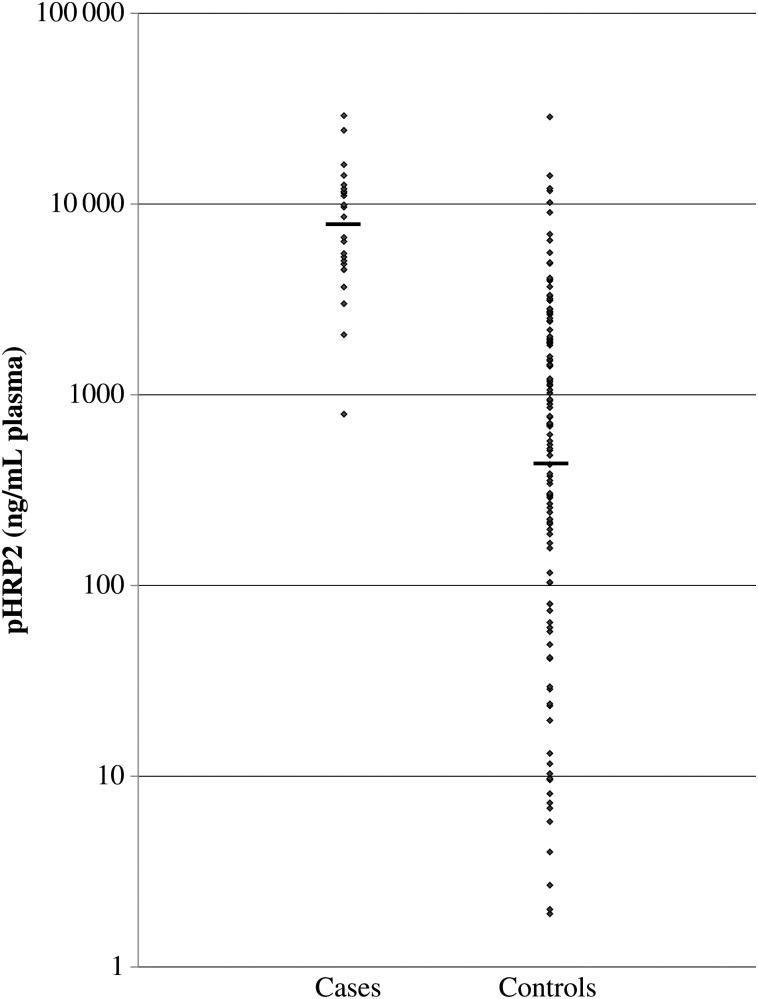
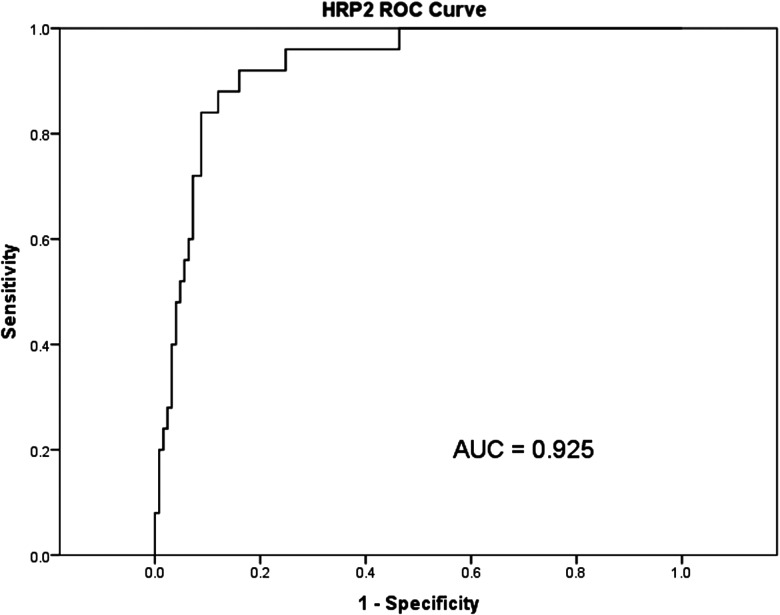
Twenty-five cases were identified between February 2010 and June 2011 at Queen Elizabeth Central Hospital in Blantyre, Malawi, and were matched, by date, with 125 controls. The geometric mean plasma HRP2 concentration was higher in children who progressed to CM than in children who continued to have an uncomplicated malarial illness (7838 ng/mL [SD, 2.5 ng/mL] vs 421 ng/mL [SD, 9.0 ng/mL], P < .001; Figure 1). An HRP2 concentration of 3500 ng/mL of plasma predicted subsequent clinical deterioration with a sensitivity of 88% and a specificity of 88%. The area under the receiver operating characteristic curve (AUROC) was 0.925 (95% CI, .88–.97; Figure 2). The positive likelihood ratio, given a cutoff of 3500 ng/mL, was 7.33, meaning that patients above the cutoff were 7 times more likely to progress to CM. Conversely, the negative likelihood ratio was 0.14, meaning that patients below the cutoff value of 3500 ng/mL were about one-seventh as likely to undergo disease progression.
Figure 1.
Histidine-rich protein 2 (HRP2) concentrations in case and control subjects. The mean concentration in cases (n = 25) was 7838 ng/mL (SD, 2.5 ng/mL). In controls (n = 125), the mean concentration was 421 ng/mL (SD, 9.0 ng/mL). The difference between the 2 was significant (P < .001). Abbreviation: HRP2, histidine-rich protein 2.
Figure 2.
Receiver operating characteristic curve comparing histidine-rich protein 2 concentrations to clinical deterioration. The area under the curve was 0.925. Abbreviations: AUC, area under the curve; HRP2, histidine-rich protein 2; ROC, receiver operating characteristic curve.
Currently there are no clinical or laboratory features that predict the risk of clinical deterioration when patients present with uncomplicated malaria. Peripheral blood parasitemia is frequently used in the clinical setting to assess disease severity in malaria, but is neither a reliable indicator of disease severity nor a reliable predictor of fatal outcome [9, 10]. The parasites in circulation are the young ring stages. The more mature trophozoites, sequestered in capillary beds, are thought to be crucial to the pathology of severe malaria, but this stage is only rarely seen in the peripheral blood. In one study, an estimate of parasite biomass based on a statistical model fitted to longitudinal patterns of peripheral parasitemia was compared to 5 putative markers of parasite biomass, including HRP2. Concentrations of HRP2 correlated with peripheral parasitemia, but the relationship with the estimated sequestered load was weak [11]. Dondorp et al developed a model of total body parasite burden based on observed in vitro HRP2 production rates [10]. The model assumptions include the half-life of HRP2 in vivo, parasite multiplication rates, and the amount of HRP2 released per individual parasite, each of which is highly variable. Overall, the calculated total parasite biomass correlated directly with disease severity in adults; HRP2 concentrations were not compared to disease severity [10]. Three studies have shown a positive correlation between HRP2 and severe malaria. Desakorn et al measured HRP2 in plasma and whole blood of 55 adult patients, and the concentrations were considerably higher in patients with severe malaria than in those with uncomplicated malaria [4]. A similar association was seen in Tanzanian children with traditionally defined CM, where higher HRP2 levels were associated with coma depth and mortality [12]. A large study of African children presenting with febrile illness has shown that HRP2 admission levels correlate with disease severity [6].
A weakness of the study reported here is that the control patients were selected from an anonymized list; additional clinical and laboratory data on the control cases are not available. The 2 groups are likely to show differences in some other parameters as well. A larger study incorporating more extensive clinical and laboratory parameters on both groups is under way. It is possible that control patients developed CM after being discharged; if so, this would not have been captured. However, misclassifying these possible cases as controls would skew our data toward the null hypothesis, diminishing the observed differences between the 2 groups.
The practical application of this information is complicated by the relatively low incidence of CM. Subsequent surveillance in the pediatric A&E in Blantyre revealed that only 1 in 190 children progressed from the initial uncomplicated malaria illness to stringently defined CM (data not shown). The relative infrequency of disease progression decreases the positive predictive value of high HRP2 concentrations. Even with a highly sensitive diagnostic test, disease progression is so rare that the number of true positives is overshadowed by the number of false-positive tests.
One approach to improving the predictive value of a positive test is to narrow the patient population that undergoes the test. Refining the population from “all parasitemic children” to a subset of children who are more likely to progress to severe disease (eg, parasitemic children with history of convulsions) might improve the positive predictive value of the test. Another approach is to broaden the definition of severe malaria beyond stringently defined CM to include other clinical features that, if present, would be better handled in higher acuity settings (eg, severe anemia, seizures, hypoxemia, shock). Studies incorporating both of these approaches are currently under way.
Conversely, the predictive value of a negative test is very high; a negative test reliably identifies patients who are extremely unlikely to develop CM. The calculations of interval likelihood ratios (Supplementary Table 1) illustrate this: the interval likelihood ratio for a cutoff value of <1000 ng/mL is 0.07. This essentially excludes the possibility of an eventual clinical deterioration. For example, even if the pretest probability of disease progression was as high as 20%, a value <1000 ng/mL results in a posttest probability of <2%. With such a low posttest probability, those patients with uncomplicated malaria could confidently be treated in the more peripheral health centers without referral to central hospitals.
Our results show that patients who present with uncomplicated malaria but then progress to CM despite appropriate treatment have a significantly higher initial HRP2 concentration than patients whose clinical courses remain uncomplicated. The current format of this test, plasma measured in a 96-well ELISA plate, is not conducive to use in a remote, low-tech clinical setting where rapid results are required. Clinicians in these settings are often required to make disposition decisions with a limited amount of objective data. A rapid quantitative HRP2 diagnostic test would facilitate the identification of patients at greatest risk for progressing to complicated malaria. These patients could be referred to a center with capacity for higher acuity care, while those with minimal risk could be managed with oral or intramuscular antimalarials on an outpatient basis.
Developing a screening tool with predefined quantitative cutoffs, as well as refining the patient population to be screened, is likely to result in improved outcomes for children with malaria, especially if using the test led to earlier treatment with parenteral medications, and closer observation in a setting in which common complications (seizures, hypoglycemia, respiratory compromise, cerebral edema, and severe anemia) could be more easily managed.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank David Sullivan for providing recombinant HRP2; Cellabs for providing diagnostic tests; the Malawi-Liverpool-Wellcome Trust Clinical Research Programme for providing laboratory space and support; and the patients’ families for allowing their children to participate in these studies.
Financial support. This work was supported by the National Institutes of Health (RO1AI34969 to T. E. T. and K23AI079402 to K. B. S.) and the University of Washington Housestaff Association (grant to L. L. F.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Murray CJ, Rosenfeld LC, Lim SS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.[No authors listed]. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 3.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–7. [PMC free article] [PubMed] [Google Scholar]
- 4.Desakorn V, Dondorp AM, Silamut K, et al. Stage-dependent production and release of histidine-rich protein 2 by Plasmodium falciparum. Trans R Soc Trop Med Hyg. 2005;99:517–24. doi: 10.1016/j.trstmh.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Desakorn V, Silamut K, Angus B, et al. Semi-quantitative measurement of Plasmodium falciparum antigen PfHRP2 in blood and plasma. Trans R Soc Trop Med Hyg. 1997;91:479–83. doi: 10.1016/s0035-9203(97)90292-3. [DOI] [PubMed] [Google Scholar]
- 6.Hendriksen IC, Mwanga-Amumpaire J, von Seidlein L, et al. Diagnosing severe falciparum malaria in parasitaemic African children: a prospective evaluation of plasma PfHRP2 Measurement. PLoS Med. 2012;9:e1001297. doi: 10.1371/journal.pmed.1001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendriksen IC, White LJ, Veenemans J, et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis. 2013;207:351–61. doi: 10.1093/infdis/jis675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seydel KB, Fox LL, Glover SJ, et al. Plasma concentrations of parasite histidine-rich protein 2 distinguish between retinopathy-positive and retinopathy-negative cerebral malaria in Malawian children. J Infect Dis. 2012;206:309–18. doi: 10.1093/infdis/jis371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gravenor MB, van Hensbroek MB, Kwiatkowski D. Estimating sequestered parasite population dynamics in cerebral malaria. Proc Natl Acad Sci U S A. 1998;95:7620–4. doi: 10.1073/pnas.95.13.7620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dondorp AM, Desakorn V, Pongtavornpinyo W, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2:e204. doi: 10.1371/journal.pmed.0020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochola LB, Marsh K, Lowe B, Gal S, Pluschke G, Smith T. Estimation of the sequestered parasite load in severe malaria patients using both host and parasite markers. Parasitology. 2005;131:449–58. doi: 10.1017/S0031182005008085. [DOI] [PubMed] [Google Scholar]
- 12.Rubach MP, Mukemba J, Florence S, et al. Plasma Plasmodium falciparum histidine-rich protein-2 concentrations are associated with malaria severity and mortality in Tanzanian children. PLoS One. 2012;7:e35985. doi: 10.1371/journal.pone.0035985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.