Abstract
Background. H5 DNA priming was previously shown to improve the antibody response to influenza A(H5N1) monovalent inactivated vaccine (MIV) among individuals for whom there was a 24-week interval between prime and boost receipt. This study defines the shortest prime-boost interval associated with an improved response to MIV.
Methods. We administered H5 DNA followed by MIV at intervals of 4, 8, 12, 16, or 24 weeks and compared responses to that of 2 doses of MIV (prime-boost interval, 24 weeks).
Results. H5 DNA priming with an MIV boost ≥12 weeks later showed an improved response, with a positive hemagglutination inhibition (HAI) titer in 91% of recipients (geometric mean titer [GMT], 141–206), compared with 55%–70% of recipients with an H5 DNA and MIV prime-boost interval of ≤8 weeks (GMT, 51–70) and 44% with an MIV-MIV prime-boost interval of 24 weeks (GMT, 27).
Conclusion. H5 DNA priming enhances antibody responses after an MIV boost when the prime-boost interval is 12–24 weeks.
Clinical Trials Registration. NCT01086657.
Keywords: Avian influenza, DNA vaccine, H5N1, boost interval, hemagglutination inhibition
(See the Brief Report by Khurana et al on pages 413–7.)
Influenza is a worldwide public health burden, and despite the availability of vaccines, improved strategies for inducing durable and broad immunity remain a high priority. Highly pathogenic strains, such as influenza A(H5N1), present an additional challenge in that transmissibility is variable, the H5 antigen is not highly immunogenic, and the severity of disease and mortality rate are significant when human infection does occur [1]. The World Health Organization maintains data on human H5N1 cases [2], and although, to date, there have been <1000 documented human cases and none of the H5N1 circulating viruses have been highly transmissible in humans, 2 studies performed by independent teams suggest there is potential for increased transmissibility between mammals through genetic alterations that could also occur in the wild [3, 4].
Although the epidemiologic and clinical characteristics of H5N1 infection present a difficult challenge for public health policy makers, the absence of the confounding effect of baseline immunity to H5N1 in the population allows researchers to evaluate novel influenza vaccine strategies utilizing the H5 antigen. DNA influenza vaccines have been shown to induce cross-neutralizing antibodies, with some of those directed against the conserved region of the hemagglutinin (HA) stem in clinical trials [5], and are protective against infection from multiple strains of influenza in animal models [6]. Additionally, H5 DNA priming enhances the overall humoral immune response to inactivated influenza vaccine, specifically when the boost interval is increased from 4 to 24 weeks [5]. We describe further evaluation of H5 DNA priming for an H5N1 monovalent inactivated vaccine (MIV) boost with prime-boost intervals of 4, 8, 12, 16, and 24 weeks.
METHODS
Vaccines
H5 DNA vaccine (VRC-AVIDNA036-00-VP) was manufactured at the National Institute of Allergy and Infectious Diseases (NIAID) Vaccine Research Center's (VRC's) Vaccine Pilot Plant, operated by SAIC (Frederick, MD). The vaccine consists of a closed-circular plasmid DNA macromolecule (VRC-9123) that expresses an A/Indonesia/5/2005(H5N1) HA sequence derived from a human isolate (Influenza Sequence Database no. 125873; Los Alamos National Laboratory database). The plasmid contained a CMV/R promoter [7]. Vaccine was prepared under good manufacturing practices at 4 mg/mL in phosphate-buffered saline.
Subvirion A(H5N1) MIV (A/Indonesia/05/2005), at a concentration of 90 µg/0.5 mL, was produced by Sanofi Pasteur (Swiftwater, PA) in accordance with the methods used to manufacture the licensed influenza virus vaccine Fluzone. Vaccines lacked preservative or adjuvant.
Study Design
VRC 310 was a single-site, phase 1, open-label, randomized clinical trial conducted at the National Institutes of Health (NIH) Clinical Center by the NIAID VRC (Bethesda, MD; clinical trials registration NCT01086657). The study was approved by the NIAID Intramural Institutional Review Board. US Department of Health and Human Services guidelines for conducting clinical research were followed.
Healthy adults aged 18–60 years with no history of H5 influenza vaccination were eligible for the study. Subjects were randomly assigned in equal numbers into 6 groups defined by the prime-boost vaccination schedule. One schedule was an MIV prime with an MIV boost 24 weeks later, while 5 schedules involved an H5 DNA prime with an MIV boost. Two of the H5 DNA–MIV groups (4- and 24-week prime-boost intervals) were designed to further validate the findings in a prior trial, VRC 306 [5]. Three of the groups receiving H5 DNA and MIV were designed to evaluate alternate boost intervals of 8, 12, and 16 weeks. Study end points were safety, tolerability, and immunogenicity of the vaccine schedules.
The study statistician and pharmacist maintained the randomization code, and subjects and clinicians were blinded to group assignment until enrollment was completed, on day 0. Subjects received the prime dose on day 0.
H5 DNA vaccine was administered at 4 mg via a needle-free Biojector device (Bioject; Tualatin, OR). MIV was administered at 90 µg by needle and syringe. The doses of the vaccines were based on findings of previous clinical trials [1, 7]. Injections were given intramuscularly in the deltoid muscle.
Local and systemic reactogenicity was assessed for 5 days after prime vaccination and for 7 days following boost vaccination. Adverse events were recorded for each subject by using the Medical Dictionary for Regulatory Activities and were assessed for severity by using a scale (0–5) developed by the Division of AIDS, NIAID, and adapted for healthy volunteer studies.
Laboratory Analyses
H5 neutralizing antibodies were evaluated by the ability of sera to prevent infection of 293A cells by replication-incompetent HA-pseudotyped virus [8]. Pseudotyped virus expressed the H5 antigen and luciferase reporter gene. Neutralization activity was quantified by the relative decrease in luciferase activity as compared to infection of 293A cells in the absence of sera, as described elsewhere [6]. The 80% inhibition serum titer (ID80) was calculated relative to the signal in the absence of sera, using 5-parameter curve fitting.
Binding antibodies, assessed by ELISA, directed against H5 antigen (Immune Technologies, New York, NY) were analyzed with 96-well Immulon2 (Dynex Technologies) plates coated with purified recombinant proteins (A/Indonesia/05/2005[H5N1] H5 or A/Vietnam/1203/2004[H5N1] H5), using methods adapted from those described elsewhere [7]. The end point titer was calculated as the most dilute serum concentration that gave an optical density reading of >0.2 above background.
Hemagglutination inhibition (HAI) assays were performed in V-bottomed 96-well plates, using 4 hemagglutinating units of virus and 1% horse red blood cells, as described elsewhere [9]. The virus strain used for the HAI assay was a low-pathogenic H5N1-PR8 reassortant (clade 2.1; A/Indonesia/5/2005[H5N1]/PR8-IBCDC-RG2) obtained from Ruben Donis at the Centers for Disease Control and Prevention Influenza Branch (Atlanta, GA) [9].
Cell absorption/ELISA assays were performed as described elsewhere [6]. End point titers of antibodies directed against the A/Indonesia/5/2005(H5N1) HA stem were determined by an enzyme-linked immunosorbent assay (ELISA) with recombinant wild-type (WT) or Δstem A/Indonesia/5/2005(H5N1) HA trimers, using adaptations of previously reported methods [6]. Trimeric HA proteins were purified as described by Wei et al [6], and the stem mutant trimer (Δstem) showed minimal reactivity with the previously defined CR6261 monoclonal antibody directed to this region, in contrast to the WT A/Indonesia/5/2005(H5N1) HA trimer [6]. End point titers were calculated as the most dilute serum concentrations that gave optical density readings of >0.2 above background [5, 6, 10].
CD4+ and CD8+ T-cell responses to H5 were assessed 2 weeks following the MIV boost by intracellular cytokine staining (ICS) for interleukin 2 (IL-2), tumor necrosis factor α (TNF-α), or interferon γ (IFN-γ), as described elsewhere [11, 12].
Statistical Methods
We reported positive response rates with exact 95% confidence intervals (CIs) computed by the Pearson-Clopper method. We reported the magnitude of antibody response by geometric mean and 95% CIs. Comparisons between any 2 groups were based on the Fisher exact test (for the positive response rate) and the Wilcoxon test (for the response magnitude). Statistical computations were done by the statistical software SAS and R.
RESULTS
Demographic Characteristics and Vaccine Safety
A total of 64 healthy adult subjects were enrolled into VRC 310 between 8 March and 13 May 2010. The study included 34 men and 30 women, with a mean age (±SD) of 34 ± 11 years and a mean body mass index (±SD) of 26.8 ± 5.4. Supplementary Table 1 shows study demographic data. Supplementary Figure 1 shows random assignment of the subjects to the study groups and the disposition by group through study completion. One subject randomly assigned to group 1 chose to not receive any study injections. All other subjects received study injections according to schedule, and 62 completed follow-up through 24 weeks after boosting. Vaccines were well tolerated, and there were no vaccine-related serious adverse events. When reactogenicity was present, the severity was mild to moderate and similar among all groups (Supplementary Tables 2 and 3).
Vaccine-Induced Antibodies
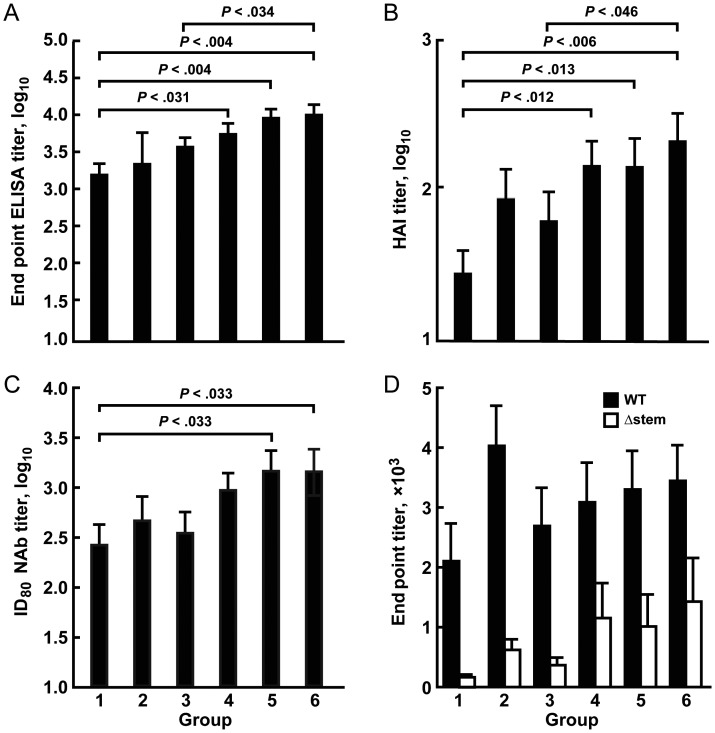
Antibody responses were assessed at the primary immunogenicity time point of 2 weeks after the MIV boost. The magnitude (GMT) of H5-binding antibodies as assessed by ELISA was highest in H5 DNA–primed subjects who received the MIV boost 12–24 weeks after priming (groups 4–6), with the highest responses in those with a boost interval of 24 weeks (Figure 1A). All subjects tested negative for H5 antibodies by HAI at baseline. The frequency of positive H5 HAI responses was greatest when the boost interval was at least 12 weeks (groups 4–6; Table 1). The HAI GMT was highest in subjects receiving the H5 DNA prime and MIV boost at the longer intervals (12, 16, and 24 weeks; Figure 1B). Neutralizing antibody responses were highest in subjects who received the MIV boost at 16 or 24 weeks (groups 5 and 6; Figure 1C). Cross-reactive antibody responses were also assessed against A/Vietnam/1203/2004(H5N1) by ELISA and neutralization assays. There was a trend toward higher-magnitude responses among the H5 DNA groups with the longest prime-boost intervals, compared with the MIV-MIV group (P < .085) or the H5 DNA–primed groups with shorter intervals. In an exploratory assessment, we normalized the HAI and neutralizing antibody responses against the overall ELISA antibody response. We found a trend toward greater HAI/ELISA and neutralizing antibody/ELISA titers in H5 DNA–primed groups 4–6, but only the HAI/ELISA comparison between group 6 and group 1 was statistically significant (P < .029).
Figure 1.
Antibody responses elicited by prime-boost vaccination with influenza H5N1 monovalent inactivated vaccine (MIV) on day 0 and at week 24 (group 1), H5 DNA on day 0 and MIV at week 4 (group 2), H5 DNA on day 0 and MIV at week 8 (group 3), H5 DNA on day 0 and MIV at week 12 (group 4), H5 DNA on day 0 and MIV at week 16 (group 5), and H5 DNA on day 0 and MIV at week 24 (group 6). A–C, Mean (±standard error of the mean) enzyme-linked immunosorbent assay (ELISA) end point titer (A), hemagglutination inhibition (HAI) titer (B), and 80% inhibition serum (ID80) neutralizing antibody (NAb) titer (C) 2 weeks after MIV boosting. P values are shown for statistically significant differences. D, Stem-directed antibody responses after MIV boosting. Postvaccination sera were preabsorbed with 293A cells expressing the stem mutant (Δstem) of A/Indonesia/5/2005(H5N1) hemagglutinin (HA) to remove non–stem-reactive HA antibodies. Analysis of binding of preabsorbed sera to wild-type (WT) or (Δstem) A/Indonesia/5/2005(H5N1) HA was performed by ELISA. Detection of human antibodies was performed with an anti-human secondary antibody. Mean titers (±SD) are shown. P values between WT and Δstem binding for each group are 0.0071, 0.0001, 0.0019, 0.0406, 0.0129, and 0.0448, respectively. Statistical analyses were performed with a 2-tailed unpaired t test, using the Prism 5 program (GraphPad Software).
Table 1.
Hemagglutination Inhibition Response, by Study Group
| Response | Group 1 (n = 9) | Group 2 (n = 9) | Group 3 (n = 11) | Group 4 (n = 11) | Group 5 (n = 11) | Group 6 (n = 11) |
|---|---|---|---|---|---|---|
| 4-fold increase in titer, subjects, % | 44 | 70 | 55 | 91 | 91 | 91 |
| Postvaccination titer >1:40, subjects, % | 44 | 70 | 55 | 91 | 91 | 91 |
| Reciprocal GMT | ||||||
| Before vaccination | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 | <1:10 |
| After vaccination (95% CI) | 27 (12–63) | 70 (25–197) | 51 (19–141) | 141 (59–340) | 150 (60–376) | 206 (77–550) |
Group 1 received H5N1 monovalent inactivated vaccine (MIV) on day 0 and at week 24, group 2 received H5 DNA on day 0 and MIV at week 4, group 3 received H5 DNA on day 0 and MIV at week 8, group 4 received H5 DNA on day 0 and MIV at week 12, group 5 received H5 DNA on day 0 and MIV at week 16, and group 6 received H5 DNA on day 0 and MIV at week 24.
Abbreviations: CI, confidence interval; GMT, geometric mean titer.
Stem-Directed Antibodies Induced by H5 DNA and MIV Prime-Boost
We have previously shown that antibodies to the highly conserved epitope on the HA stem were elicited by DNA priming followed by MIV boosting with a 24-week interval [5]. Anti-stem antibodies in subjects with shorter prime-boost intervals were examined in this study. Immune sera from all subjects were preabsorbed with 293A cells expressing a stem mutant (Δstem) of A/Indonesia/5/2005(H5N1) HA that blocks binding of stem-specific monoclonal antibodies [6] Preabsorption removes all non–stem-directed antibodies, and the presence of anti-stem antibodies can be determined by their ability to bind WT A/Indonesia/5/2005(H5N1) HA but not Δstem HA. Similar to the MIV-MIV group, binding of the preabsorbed postvaccination sera to A/Indonesia/5/2005(H5N1) WT HA trimer was significantly higher than to Δstem HA in all groups primed with DNA (Figure 1D), thus demonstrating the ability of DNA priming to elicit stem-reactive antibodies following MIV boosting.
Vaccine-Induced T-Cell Responses
H5-specific CD4+ T-cell responses were more commonly detected than CD8+ T-cell responses. There were no H5-specific CD4+ or CD8+ T-cell responses detected in subjects who received 2 doses of MIV. Of patients in groups 2, 3, 4, 5, and 6, all of whom received the H5 DNA prime, 33%, 27%, 60%, 27%, and 45%, respectively, developed a detectable CD4+ T-cell response to IL-2, IFN-γ, or TNF-α.
DISCUSSION
We previously demonstrated that H5 DNA vaccine priming improves the response elicited by a MIV (H5N1) boost when the boost interval is 24 weeks [5]. Here, we further describe the boost-interval–dependent response to H5 DNA priming at prime-boost intervals of 4, 8, 12, 16, and 24 weeks, compared with a 2-dose MIV regimen with a 24-week prime-boost interval. All regimens were safe and well tolerated, consistent with previous DNA vaccine clinical trials [5, 7, 13, 14] and MIV studies [1].
We found that the length of the interval between priming and boosting significantly affects the magnitude and functional quality of the antibody response, and we have shown that DNA priming increases the potency and influences the specificity of the antibody responses. Overall, the frequency and magnitude of the antibody response was improved when the interval between H5 DNA and MIV receipt was 12, 16, or 24 weeks as compared to 4 or 8 weeks. However, the neutralizing antibody responses were greatest when the interval between DNA and MIV was 16 or 24 weeks.
As shown previously [5], the long boost interval improved the antibody responses seen in the H5 DNA–primed groups but did not improve the response seen in the MIV-MIV group, indicating that the specificity and functional properties induced by the prime differs between H5 DNA and MIV. DNA vaccines use different antigen-presentation pathways, facilitate CD4+ T-cell help, and increase the number and diversity of CD4+ T-cell clones [15], and this may lead to a greater expansion of antigen-specific B cells. Additionally, gene-based delivery and the expression of HA on transduced host cells may present a protein configuration and more authentic epitope structure. Work is needed to define the molecular and structural basis for the effects of gene-based priming and prime-boost intervals on immune response patterns.
These findings suggest that, with an optimized prime-boost interval, DNA priming could significantly improve the responses seen with traditional influenza vaccines. As gene-based priming is further assessed in humans, a properly defined boost interval is essential to allow optimal development of the immune response. This may be useful in the search for universal influenza vaccine strategies and in approaches being sought to improve responses to influenza vaccines in individuals who are very young or elderly.
THE VRC 310 STUDY TEAM
Sarah Hubka, LaSonji Holman, Ingelise Gordon, Laura Novik, Pamela Costner, Floreliz Mendoza, Jamie Saunders, Brenda Larkin, Diane Johnson, Nina Berkowitz, Brandon Wilson, Tanya Clarke, Olga Vasilenko, Yesenia Merino, Joseph Casazza, Sheryl Young, Uzma Sarwar, Nicole L. Luongo Susan Leitman, Charla Andrews, Phillip Gomez, Becky Sheets, Judy Stein, Galina Yamshchikov, Hope Decederfelt, Judith Starling, LaChonne Stanford, Rhonda Washington-Lewis, Kathy Rhone, Hanne Andersen, Meghan Kunchai, Ly Diep, and Phyllis Zaia.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the vaccine trials volunteers, for their contribution and commitment to vaccine research; Brenda Hartman, for technical and graphical assistance; our NIH Clinical Center and NIAID colleagues, our VRC colleagues, Abraham Mittelman, Monique Young, Iris Pittman, the EMMES Corporation (Rockville, MD), Bioqual, The Biomedical Advanced Research and Development Authority (BARDA), the NIAID Institutional Review Board, the NIAID Office of Communications and Government Relations, the NIH Clinical Center Investigational New Drug Pharmacy, and the NIH Clinical Center Patient Recruitment and Public Liaison Office, for their contributions; and Linda Lambert and Robin Mason (DMID, NIAID) and Charles Whitaker (Sanofi Pasteur), for their assistance.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency or collaborators.
Financial support. This work was supported by The National Institutes of Health Intramural Program.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Confirmed human cases of avian influenza A(H5N1) http://www.who.int/csr/disease/avian_influenza/country/cases_table_2011_06_16/en/index.html. Accessed 14 August 2011. [Google Scholar]
- 3.Enserink M, Malakoff D. Biosecurity. Will flu papers lead to new research oversight? Science. 2012;335:20–22. doi: 10.1126/science.335.6064.20. –. [DOI] [PubMed] [Google Scholar]
- 4.Kawaoka Y. H5N1: Flu transmission work is urgent. Nature. 2012;482:155. doi: 10.1038/nature10884. [DOI] [PubMed] [Google Scholar]
- 5.Ledgerwood JE, Wei CJ, Hu Z, et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis. 2011;11:916–24. doi: 10.1016/S1473-3099(11)70240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei CJ, Boyington JC, McTamney PM, et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science. 2010;329:1060–4. doi: 10.1126/science.1192517. [DOI] [PubMed] [Google Scholar]
- 7.Martin JE, Sullivan NJ, Enama ME, et al. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol. 2006;13:1267–77. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei C-J, Boyington JC, Dai K, et al. Cross-Neutralization of 1918 and 2009 influenza viruses: role of glycans in viral evolution and vaccine design. Sci Transl Med. 2010;2:24ra1. doi: 10.1126/scitranslmed.3000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–5. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Wei CJ, Xu L, Kong WP, et al. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J Virol. 2008;82:6200–8. doi: 10.1128/JVI.00187-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catanzaro AT, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catanzaro AT, Roederer M, Koup RA, et al. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25:4085–92. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Ledgerwood JE, Graham BS. DNA vaccines: a safe and efficient platform technology for responding to emerging infectious diseases. Hum Vaccin. 2009;5:623–6. doi: 10.4161/hv.8627. [DOI] [PubMed] [Google Scholar]
- 14.Ledgerwood JE, Pierson TC, Hubka SA, et al. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203:1396–404. doi: 10.1093/infdis/jir054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu L, Kong WP, Nabel GJ. Enhanced breadth of CD4 T-cell immunity by DNA prime and adenovirus boost immunization to human immunodeficiency virus Env and Gag immunogens. J Virol. 2005;79:8024–31. doi: 10.1128/JVI.79.13.8024-8031.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.