Abstract
Background. Shortening tuberculosis treatment could significantly improve patient adherence and decrease the development of drug resistance. Phosphodiesterase inhibitors (PDE-Is) have been shown to be beneficial in animal models of tuberculosis. We assessed the impact of PDE-Is on the duration of treatment in tuberculous mice.
Methods. We analyzed the time to death in Mycobacterium tuberculosis–infected mice receiving type 4 PDE-Is (rolipram and cilomilast) and the impact on bacterial burden, time to clearance, and relapse when types 3 and 5 PDE-Is (cilostazol and sildenafil, respectively) and rolipram were added to the standard treatment. We investigated pharmacokinetic interactions between PDE-Is (cilostazol and sildenafil) and rifampin.
Results. The type 4 PDE-Is rolipram and cilomilast accelerated the time to death in tuberculous mice. The addition of rolipram to standard tuberculosis treatment increased bacterial burden and did not decrease the time to bacterial clearance in the lung, while the addition of the cilostazol and sildenafil reduced the time to clearance by 1 month. Cilostazol and sildenafil did not have negative pharmacokinetic interactions with rifampin.
Conclusions. Type 4 PDE-Is may increase the severity of tuberculosis and should be carefully investigated for use in patients with latent or active tuberculosis. Cilostazol and sildenafil may benefit tuberculosis patients by shortening the duration of therapy.
Keywords: tuberculosis, mouse model, phosphodiesterase inhibitor, rolipram, cilomilast, cilostazol, sildenafil
The current standard treatment regimen for human tuberculosis consists of the daily administration of multiple drugs for 6 months: 2 months of rifampin, isoniazid, pyrazinamide, and ethambutol therapy, followed by 4 months of rifampin and isoniazid therapy [1]. Because previous tuberculosis treatment regimens ranged from 12–24 months in duration, the current standard is referred to as the short-course regimen. Successful treatment outcome is critically dependent on strict patient adherence to this drug regimen, and significant public health infrastructure and resources are necessary to ensure adherence to a 6-month treatment program. Thus, even shorter tuberculosis drug treatment strategies that effectively cure tuberculosis are desperately needed. A shortened standard regimen could significantly increase patient adherence, benefitting both the individual patient (because of a decreased burden of taking drugs and decreased risk of drug-associated toxicities) and the community (because of decreased transmission, emergence of drug resistance, and costs associated with drug distribution and adherence monitoring).
The pathology of pulmonary tuberculosis is highly associated with the host response. It is well appreciated that the inflammatory environment at the site of infection is critically linked to the tissue damage and remodeling that characterize the disease, as well as to the “success” of the infecting Mycobacterium tuberculosis bacilli. As such, investigators have long been interested in modulation of the host response as a mechanism to enhance, and possibly shorten, the antimicrobial-focused tuberculosis treatment regimen. In 2007, the World Health Organization published a report highlighting the potential benefits of immunotherapeutic interventions for the treatment of tuberculosis, including drug-resistant tuberculosis; this report recommended the fast-tracking of carefully designed studies of immunotherapy agents for adjunctive treatment of tuberculosis [2].
Recent work by our group and others has suggested that modulation of host cyclic nucleotide phosphodiesterase (PDE) activity may enhance killing of M. tuberculosis and decrease the time to culture negativity in animal models of tuberculosis [3–6]. PDEs catalyze the breakdown of key cellular signaling molecules, namely 3′, 5′-cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP). The PDEs are classified into 11 families (PDE1–11) primarily on the basis of amino acid sequence and substrate preference, and many of these families are further divided into isoform subfamilies composed of related but different genes, with many of the individual messenger RNAs processed into different splice variants. Thus, an animal host can express a wide range of functional PDEs, with each unique PDE differing in its structure, kinetic properties, cellular expression and localization, regulation, and sensitivity to PDE inhibitors (PDE-Is) [7]. Several PDE-Is have been shown to be beneficial for the treatment of tuberculosis in both mouse and rabbit models. Koo et al reported that administration of the PDE4-I thalidomide analogue CC-3052 in combination with isoniazid to M. tuberculosis–infected mice increased bacterial clearance in the lungs and decreased lung pathology as compared to mice treated with isoniazid alone [4]. Subbian et al characterized the impact of administration of isoniazid with and without CC-3052 to M. tuberculosis–infected rabbits and found similar results: compared with isoniazid treatment alone, CC-3052 plus isoniazid therapy decreased lung pathology and bacterial burden, reduced macrophage activation, and altered host and bacterial gene expression patterns [5, 6]. Previous work in our laboratory demonstrated that addition of the US Food and Drug Administration (FDA)–approved PDE-Is cilostazol and sildenafil (PDE3-I and PDE5-I, respectively) to standard tuberculosis treatment reduced the time to lung sterilization by 1 month in a mouse model of tuberculosis chemotherapy [3]. Thus, host-directed PDE-Is appear extremely promising for use as potential adjunctive drugs for use in combination with antimycobacterial compounds for the accelerated treatment of tuberculosis.
Our objective was to build on these initial studies and to further evaluate the impact of PDE3-, PDE4-, and PDE5-Is as adjunctive therapy agents in mouse models of tuberculosis. We hypothesized that the addition of a PDE4-I to the standard 6-month short-course treatment for tuberculosis would reduce the time to sterilization in the acute mouse model of tuberculosis chemotherapy; however, on the basis of results of previous studies, we could not predict how PDE4-I adjunctive therapy would compare to PDE3- and PDE5-I treatment. We used the commercially available PDE4-Is rolipram and cilomilast. Our data again demonstrated that the addition of cilostazol and sildenafil to the standard tuberculosis drug regimen decreased the time to culture conversion by 1 month in our model, and we have further characterized the pharmacokinetics of these drugs in the context of tuberculosis treatment. In contrast to our hypothesis, our data indicate that the addition of PDE4-Is to the standard tuberculosis chemotherapy regimen was not beneficial and, in fact, was detrimental in our mouse model. Thus, additional studies are needed to understand the impact of adjunctive PDE4-Is in the treatment of tuberculosis.
MATERIALS AND METHODS
Animals
This study was performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All described procedures have been approved by the Johns Hopkins University Animal Care and Use Committee. Female BALB/c mice approximately 6 weeks old (Charles River Laboratories) were used for all experiments.
Bacterial Stock
M. tuberculosis strain H37Rv was obtained from the Johns Hopkins Center for Tuberculosis Research stocks. For each infection, an aliquot was cultured in Middlebrook 7H9 broth supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase, 0.5% (vol/vol) glycerol, and 0.05% (vol/vol) Tween-80.
Aerosol Infection
In all experiments, mice were infected by the aerosol route, using the Glas-Col inhalation exposure system as previously described [3]. Immediately following infection, the mice were randomly assigned to treatment groups. Five mice were killed using isoflurane vapor the day after infection to determine the numbers of colony-forming units (CFUs) implanted in the lungs, as previously described [3].
Drug Preparation and Administration
Isoniazid and rifampin were obtained from Sigma–Aldrich, pyrazinamide was obtained from Fisher Scientific, cilomilast was purchased from LGM Pharma, and rolipram was purchased from MP Biomedicals. Cilostazol is manufactured by Otsuka Pharmaceutical, and sildenafil is manufactured by Pfizer. Stock solutions were prepared weekly by using distilled water and were stored at 4°C. The solutions were prepared such that the desired concentration would be delivered in a 0.2-mL total volume, and all drugs were administered to mice by oral gavage daily, 5 days per week. Rifampin was given 1 hour after administration of the other drugs to avoid an adverse pharmacokinetic reaction [8].
Time-to-Death Mouse Model
We performed a time-to-death experiment to evaluate the impact of PDE4-Is in this mouse model of tuberculosis. The day after aerosol infection with M. tuberculosis, treatment was initiated with the commercially available PDE4-Is cilomilast, at 10 mg/kg (a dose known to have antiinflammatory properties in mice [9, 10]), and rolipram, at 10 mg/kg (a dose known to have antiinflammatory properties in mice [11, 12]), as well as isoniazid, at 25 mg/kg (positive control; mice in this group should not die) and distilled water (sham, negative control; mice in this group should start to die by 4 weeks after infection). Treatment was continued for 55 days after infection, and deaths were recorded.
Standard Tuberculosis Chemotherapy Model
We evaluated the effect of the PDE4-I rolipram when administered as adjunctive therapy to the standard drug regimen, using the 6-month acute mouse model for tuberculosis chemotherapy. This model is used to create a bacillary burden in the mouse lungs that is representative of that found in human cavitary tuberculosis by infecting the mice with a relatively high inoculum and allowing the bacilli to multiply for 2 weeks. At this point, treatment is initiated with human-equivalent doses of the standard first-line drug regimen for the 6-month duration used for the treatment of human tuberculosis. This model has been well established for the assessment of treatment-shortening regimens as compared to the standard regimen [13–15]. We used rolipram because it is a prototypic, commercially available, oral PDE4-I that has been tested in humans as an antidepressant and antiinflammatory agent [16, 17]. BALB/c mice were infected by aerosol with M. tuberculosis, and treatment was initiated 14 days after infection, with randomly assigned treatment groups. The standard mouse regimen consists of 2 months of daily treatment with rifampin (10 mg/kg), isoniazid (25 mg/kg), and pyrazinamide (150 mg/kg), followed by 4 months of daily treatment with rifampin (10 mg/kg) and isoniazid (25 mg/kg). We analyzed the bactericidal effect (by means of CFU counts) and sterilizing effect (by means of time to culture-negative status) of adding rolipram (10 mg/kg) and a cilostazol/sildenafil combination (both at 10 mg/kg), administered daily for the entire 6-month (ie, 24-week) treatment period (Table 1). Five mice from each treatment group were killed every 4 weeks after treatment initiation. After dissection, lungs were homogenized for plating as described. To assess the impact of discontinuation of treatment on relapse, drug administration was ended for 10 mice in each treatment group after 16 and 20 weeks; these mice, as well as 10 mice that completed the entire 24 weeks of treatment, were maintained for an additional 12 weeks after treatment completion, at which point the mice were killed ,and the proportion with culture-positive lungs (indicating relapse) was determined.
Table 1.
Experimental Scheme for the Addition of Phosphodiesterase Inhibitors to the Standard Tuberculosis Treatment Regimen
| Mice Euthanized, No., by Time Point |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regimen | D–13 | D0 | Mo1 | Mo2 | Mo3 | Mo4 | Mo5 | Mo6 | Mo 4 + 3a | Mo 5 + 3b | Mo 6 + 3c |
| Untreated | 5 | 5 | … | … | … | … | … | … | … | … | … |
| Standard (2RHZ/4RH) | … | … | 5 | 5 | 5 | 5 | 5 | 5 | 10 | 10 | 10 |
| Standard + rolipram | … | … | 5 | 5 | 5 | 5 | 5 | 5 | 10 | 10 | 10 |
| Standard + cilostazol/sildenafil | … | … | 5 | 5 | 5 | 5 | 5 | 5 | 10 | 10 | 10 |
| Total | 5 | 5 | 15 | 15 | 15 | 15 | 15 | 15 | 30 | 30 | 30 |
Abbreviations: H, isoniazid; R, rifampin; Z, pyrazinamide.
a Treatment was stopped after 4 months, and mice were euthanized 3 months later to assess relapse.
b Treatment was stopped after 5 months, and mice were euthanized 3 months later to assess relapse.
c Mice were euthanized 3 months after the full treatment course to assess relapse.
Pharmacokinetic Study
In mice, coadministration of rifampin with isoniazid and pyrazinamide is associated with negative pharmacokinetic interactions [8]. To study possible drug-drug interactions of cilostazol and sildenafil with rifampin, we conducted a pharmacokinetic study in mice following administration of cilostazol (10 mg/kg), sildenafil (10 mg/kg), rifampin (10 mg/kg), cilostazol/rifampin combination (10 mg/kg each), and sildenafil/rifampin combination (10 mg/kg each). Following oral gavage, blood samples (volume, around 0.3 mL) were drawn from mice (3 mice per group per time point) 0.5, 1, 2, 4, 8, 12, and 24 hours after administration. Serum samples were separated, stored at −80°C, and analyzed using a validated high-performance liquid chromatography assay at the University of Florida [14, 18, 19].
Data Analysis
Lung CFU counts (x) were log-transformed as log10 (x + 1) before analysis. The Student t test, the Bonferroni multiple comparison test, or the log-rank test were used as required to determine statistical significance. A P value of <.05 was considered statistically significant for all statistical analyses.
RESULTS
Administration of PDE4-Is Decreases Survival Time in M. tuberculosis-Infected Mice
We performed a time-to-death experiment to evaluate the impact of PDE4-Is in this mouse model of tuberculosis. As expected, none of the mice treated with isoniazid died over the entire 55-day course of the experiment, while more than half of the sham-treated mice died within 30 days after infection (Figure 1). Treatment with the PDE4-Is accelerated the time to death for the infected mice, with median survival times of 25.5 and 27.5 days after infection for rolipram and cilomilast treatment, respectively. The median survival of the rolipram-treated mice was significantly less than that of the sham-treated mice (29 days after infection; P < .01, by the log-rank test). These data indicated that inhibition of PDE4s intensified, rather than mitigated, tuberculosis in this mouse model.
Figure 1.
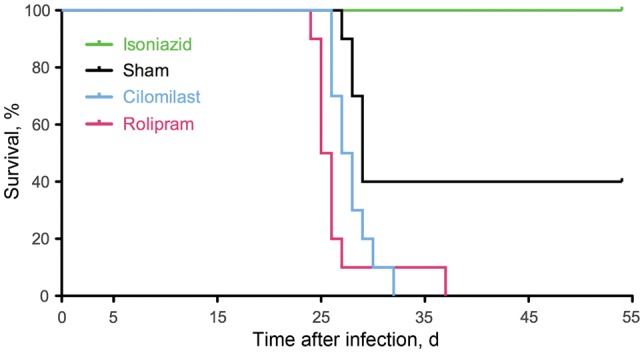
Time to death for Mycobacterium tuberculosis–infected mice treated with type 4 phosphodiesterase inhibitors. The median survival duration for the rolipram-treated mice was significantly shorter than for the sham-treated mice (P < .01, by the log-rank test).
Addition of Rolipram to Standard Treatment Is Not Beneficial in a Mouse Model of Tuberculosis Chemotherapy
We used the acute mouse model of tuberculosis chemotherapy to assess the impact of the addition of rolipram to the standard tuberculosis drug regimen. BALB/c mice were infected by aerosol, achieving a mean day 1 lung implantation of (±SD) of 3.630 ± 0.076 log10 CFUs, and 2 weeks after infection the mean bacterial lung burden (±SD) was 7.095 ± 0.294 log10 CFUs. As expected, all of the sham-treated mice died within 6 weeks of infection, and administration of the standard regimen resulted in culture negativity after 5 months of treatment (Figure 2 and Supplementary Table 1). In agreement with our previous results [3], adjunctive therapy with cilostazol and sildenafil led to bacterial clearance 1 month earlier than with standard treatment alone. However, the addition of rolipram to the standard regimen was not beneficial; throughout the time course, rolipram-treated mice consistently exhibited a higher bacterial burden in the lungs (statistically significant differences at months 1, 2, and 3 after treatment initiation, by the Student t test, Supplementary Table 1), and the time to culture negativity did not differ from that for mice receiving only the standard regimen. Thus, in this standard mouse model, rolipram did not contribute to enhanced killing of M. tuberculosis.
Figure 2.
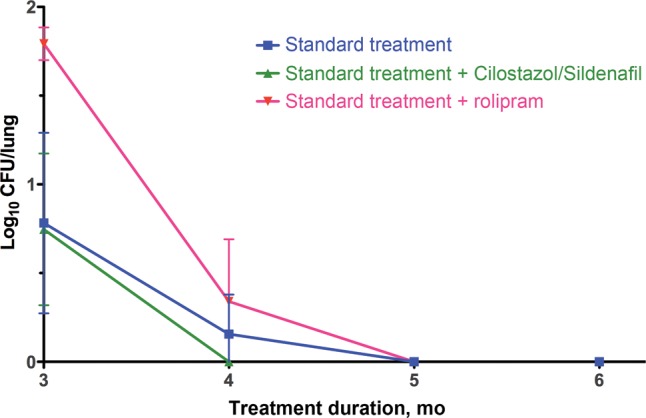
Bacterial burden in the lungs of Mycobacterium tuberculosis–infected mice treated with adjunctive phosphodiesterase inhibitors. Error bars represent SDs. Colony-forming units for all time points are presented in Supplementary Table 1.
In addition to analysis of the bactericidal activity of adjuvant PDE-I therapy, we also evaluated the influence of these host-directed compounds on posttreatment relapse. After completion of 4, 5, or 6 months of therapy, treatment was discontinued for 10 mice from each group; these mice were maintained for an additional 3 months after treatment termination and then analyzed for culturable M. tuberculosis in their lungs. The addition of cilostazol and sildenafil to the standard regimen did not alter relapse rates as compared to the rate for mice receiving only standard therapy, regardless of the duration of treatment (Table 2). However, administration of adjunctive rolipram for 4 or 5 months resulted in relapse rates that were higher than those in the other treatment groups. These data indicate that the use of rolipram may have detrimental effects in the absence of treatment adherence.
Table 2.
Relapse 3 Months After Stopping Treatment
| Mice With No Detectable Bacilli in Lungs, Proportion |
|||
|---|---|---|---|
| Treatment Duration | Standard Treatment (2RHZ/4RH) | Standard Treatment + Rolipram | Standard Treatment + Cilostazol and Sildenafil |
| 4 mo (mo 4 + 3a) | 5/10 | 9/10 | 6/10 |
| 5 mo (mo 5 + 3b) | 1/10 | 4/10 | 2/10 |
| 6 mo (mo 6 + 3c) | 0/10 | 0/10 | 0/10 |
Relapse was defined as detection of culturable Mycobacterium tuberculosis.
Abbreviations: H, isoniazid; R, rifampin; Z, pyrazinamide.
a Treatment was stopped after 4 months, and mice were euthanized 3 months later to assess relapse.
b Treatment was stopped after 5 months, and mice were euthanized 3 months later to assess relapse.
c Mice were euthanized 3 months after the full treatment to assess relapse.
Cilostazol, Sildenafil, and Rifampin Levels Are Not Affected by Coadministration
Our objective was to evaluate serum concentration levels of rifampin and the PDE-Is when administered alone or in combination. Coadministration of either sildenafil or cilostazol with rifampin slightly altered the serum concentration levels of the drugs (Figure 3), but the overall pharmacokinetic parameters (ie, maximum plasma concentration of the drug [Cmax] and area under the concentration curve [AUC] values) remained within the human equivalent effective levels (Table 3) [20–22]. Thus, the pharmacokinetic parameters of the PDE-Is were not significantly modified by coadministration of rifampin; likewise, the similar parameters of rifampin were not significantly impacted by coadministration of cilostazol or sildenafil.
Figure 3.
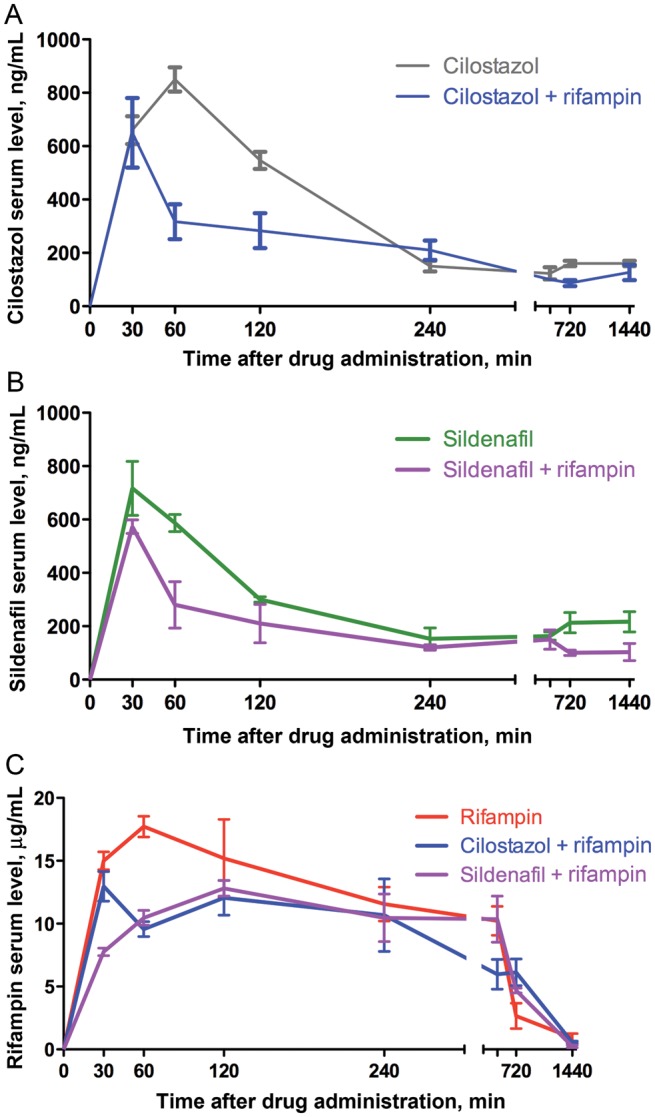
Pharmacokinetic profiles of cilostazol (A), sildenafil (B), and rifampin (C), alone or in combination. Error bars represent SDs.
Table 3.
Pharmacokinetic Parameters of Cilostazol, Sildenafil, and Rifampin
| Regimen | Cilostazol |
Sildenafil |
Rifampin |
|||
|---|---|---|---|---|---|---|
| Cmax, ng/mL | AUC0–24h, ng/mL × h | Cmax, ng/mL | AUC0–24h, ng/mL × h | Cmax, μg/mL | AUC0–24h, µg/mL × h | |
| Cilostazol | ||||||
| 100 mg (humans) | 625 | 5340 | … | … | … | … |
| 2 mg (mice) | 850 | 4971 | … | … | … | … |
| 2 mg + 2 mg rifampin (mice) | 650 | 3471 | … | … | 13.0 | 140 |
| Sildenafil | ||||||
| 50 mg (humans) | … | … | 159 | 530 | … | … |
| 2 mg (mice) | … | … | 717 | 5368 | … | … |
| 2 mg + 2 mg rifampin (mice) | … | … | 573 | 3192 | 12.8 | 143 |
| Rifampin | ||||||
| 600 mg (humans) | … | … | … | … | 11.6 | 76.7 |
| 2 mg (mice) | … | … | … | … | 17.7 | 146 |
Drug doses in mice were based on 20-g mice receiving 10 mg/kg. Human parameters for cilostazol were obtained from Bramer et al [22]; for sildenafil, from Nichols et al [20]; and for rifampin, from Acocella et al [21].
Abbreviations: AUC0–24, area under the concentration curve in the first 24 hours after drug administration; Cmax, maximum plasma concentration of the drug.
DISCUSSION
Adjuvant, host-directed therapy for the treatment of tuberculosis has long been considered a possible mechanism for improving patient care [2]. Recent work by our laboratory and others has suggested that administration of host-directed PDE-Is in combination with antimycobacterial drugs may increase killing of M. tuberculosis and shorten the duration of tuberculosis chemotherapy [3–6]. Here, we have followed up on these recent reports and present 4 major findings. First, we demonstrated that administration of the PDE4-Is rolipram and cilomilast accelerates the time to death in tuberculous mice as compared to sham-treated mice. Second, we confirmed that the addition of the PDE3-I cilostazol and the PDE5-I sildenafil to the standard tuberculosis treatment regimen reduces the time to lung bacterial clearance by 1 month. Third, the addition of the PDE4-I rolipram to the standard tuberculosis regimen results in an increased bacterial burden in the lungs and an increased risk of relapse, compared with standard treatment alone. Fourth, the pharmacokinetic parameters of cilostazol, sildenafil, and rifampin are similar when these drugs are administered alone or in combination. Thus, our data support further investigation for the use of PDE3-Is and PDE5-Is but not PDE4-Is as possible treatment-shortening, host-directed adjuvant therapy for tuberculosis.
PDE-Is interfere with the PDE-driven hydrolysis of cAMP and cGMP, causing accumulation of these signaling molecules, leading to a number of downstream cellular effects. In humans and other mammals, a wide variety of PDEs exist (11 major families, with numerous subfamilies and splice variants), with each enzyme exhibiting specific substrate specificity and tissue expression. PDE4s are generally specific for cAMP, PDE5s hydrolyze cGMP, and PDE3s can break down both of these cyclic nucleotide substrates [23]. Several recent reports have indicated that the PDE4-I CC-3052 is beneficial when administered to tuberculous mice and rabbits [4–6]. In both of these models, administration of CC-3052 resulted in a modest increase in the M. tuberculosis CFUs in the lungs, compared with untreated controls; however, when coadministered with the mycobactericidal drug isoniazid, this PDE4-I increased bacterial killing. The researchers hypothesized that inhibition of PDE4 generated a local environment more favorable for M. tuberculosis replication, allowing the bacteria to remain metabolically active when the host response would otherwise impinge on bacterial growth. Thus, when CC-3052 was administered alone to the infected animals, an increase in the M. tuberculosis lung burdens was observed; however, as the mycobactericidal activity of isoniazid is known to be strongest against actively replicating bacilli, this increased multiplication paradoxically allowed for increased killing by isoniazid, ultimately resulting in more bacterial killing in the lungs. These intriguing findings indicated that PDE4-Is may be beneficial when coadministered with the standard tuberculosis drug regimen.
As CC-3052 is not commercially available, we investigated the impact of PDE4-Is on tuberculosis by using rolipram and cilomilast, and our initial experiment demonstrated that use of these PDE4-Is alone accelerated the time to death in M. tuberculosis–infected mice (Figure 1). Although these data raise serious concerns about administration of PDE4-Is to tuberculosis patients, this observation did not necessarily contradict what had been previously demonstrated with CC-3052. The acceleration in time to death indicated thriving M. tuberculosis growth in the PDE4-I–treated mice, which supports the hypothesis that PDE4-Is create an environment favorable for M. tuberculosis multiplication. Thus, addition of the PDE4-Is may set the stage for improved killing by antimycobacterial drugs. However, when we administered rolipram in combination with the standard tuberculosis treatment regimen, we did not observe an increase in bacterial clearance in infected mice but rather found that mice receiving rolipram exhibited an increased M. tuberculosis lung burden as compared to mice receiving standard therapy alone, with no impact on the time to bacterial clearance (Figure 2). Thus, our data indicate that PDE4-Is are not beneficial when administered alone or in combination with the standard antimycobacterial drug regimen in our mouse models of tuberculosis.
PDE4s are known to be encoded by 4 different genes, PDE4A-D. Because of splice variants, these genes encode >15 different isozymes, which are differentially expressed according to cell and tissue environment [16]. It is therefore possible that the specific PDE4 variants inhibited by rolipram, cilomilast, and CC-3052 may be different. PDE4-Is in general are known to be antiinflammatory, with CC-3052 documented to be a potent inhibitor of tumor necrosis factor α (TNF-α) [24]. TNF-α inhibition is highly associated with reactivation of latent tuberculosis; thus, the use of any drug that decreases the levels of this cytokine must be critically evaluated for use in patients with active or latent tuberculosis. The data presented here highlight this need for caution and suggest that studies that examine the risk of tuberculosis reactivation due to PDE4 inhibition should be conducted. Currently, only 1 PDE4-I, roflumilast, has received approval from the FDA for treatment of chronic obstructive pulmonary disease associated with chronic bronchitis and frequent exacerbations despite appropriate bronchodilator therapy [25]. The restrictions on the use of roflumilast are due to the psychiatric and gastrointestinal adverse events associated with this drug. These types of side effects are common to PDE4-Is and are largely the reason why PDE4-Is such as rolipram and cilomilast have been withdrawn from use in human studies. Thus, PDE4-Is in general may not be good candidates for long-term, adjunctive tuberculosis therapy. Importantly, our data also suggest that roflumilast should be evaluated for possible detrimental effects in patients with latent or active tuberculosis.
Although host-directed therapy for tuberculosis with PDE4-Is was not promising in our models, we have shown again that the addition of the PDE3-I cilostazol and the PDE5-I sildenafil to the standard tuberculosis treatment regimen resulted in bacterial clearance in the mouse lung 1 month earlier than standard therapy alone (Figure 2) [3]. As we have clearly shown that this treatment-shortening effect of the cilostazol/sildenafil combination does not alter relapse (Table 2), our data suggest that this PDE3-/PDE5-I combination should be investigated for use as adjunctive therapy in human tuberculosis cases, especially since our pharmacokinetic data indicate that coadministration of these drugs with rifampin does not significantly alter the pharmacokinetic parameters needed for adequate treatment (Figure 3 and Table 3). However, the interactions of PDE-Is with the drugs in the regimen other than rifampin have not been evaluated in our study. As cilostazol and sildenafil are already FDA approved and safe for long-term use in humans, our data have the potential for immediate translational use. Even a modest, 1-month shortening of tuberculosis treatment may have a significant impact on patient adherence, leading to increased cure rates and decreased emergence of drug resistance.
In summary, this work demonstrates that PDE4-Is are not beneficial and, in fact, are detrimental, alone or in combination with standard tuberculosis drugs, in our mouse models of tuberculosis. Thus, the use of PDE4-Is, especially the FDA-approved PDE4-I roflumilast, in tuberculosis patients should be investigated with extreme caution. In contrast, the combined use of the PDE3-I cilostazol and the PDE5-I sildenafil with the standard tuberculosis treatment regimen shortens the duration of therapy needed to achieve bacterial clearance in the mouse lungs. These FDA-approved PDE-Is should be investigated as possible adjunctive host-directed therapy for tuberculosis patients.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are very grateful to Clifford Lane of the National Institute of Allergy and Infectious Diseases Division of Clinical Research, for his critical review of the manuscript.
Financial support. This work was supported by the National Institutes of Health (grants AI30036, AI37856, and AI36973 to W. R. B.), the National Institute of Allergy and Infectious Diseases Division of Intramural Research (award to M. M.), the Fogarty International Center for the Advanced Study in the Health Sciences (D43TW007995 to R. L. M. and A. T.), and the Howard Hughes Medical Institute (to W. R. B.].
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization (WHO) Treatment of tuberculosis: guidelines. 4th ed. Geneva, Switzerland: WHO Press; 2009. [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Report of the expert consultation on immunotherapeutic interventions for tuberculosis. Geneva, Switzerland: WHO Press; 2007. [Google Scholar]
- 3.Maiga M, Agarwal N, Ammerman NC, et al. Successful shortening of tuberculosis treatment using adjuvant host-directed therapy with FDA-approved phosphodiesterase inhibitors in the mouse model. PLoS One. 2012;7:e30749. doi: 10.1371/journal.pone.0030749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koo MS, Manca C, Yang G, et al. Phosphodiesterase 4 inhibition reduces innate immunity and improves isoniazid clearance of Mycobacterium tuberculosis in the lungs of infected mice. PLoS One. 2011;6:e17091. doi: 10.1371/journal.pone.0017091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Subbian S, Tsenova L, O'Brien P, et al. Phosphodiesterase-4 inhibition alters gene expression and improves isoniazid-mediated clearance of Mycobacterium tuberculosis in rabbit lungs. PLoS Pathog. 2011;7:e1002262. doi: 10.1371/journal.ppat.1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbian S, Tsenova L, O'Brien P, et al. Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am J Pathol. 2011;179:289–301. doi: 10.1016/j.ajpath.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omori K, Kotera J. Overview of PDEs and their regulation. Circ Res. 2007;100:309–27. doi: 10.1161/01.RES.0000256354.95791.f1. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon J, Dickinson JM, Sole K, Mitchison DA. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob Agents Chemother. 1996;40:552–5. doi: 10.1128/aac.40.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harada D, Tsukumo Y, Takashima Y, Manabe H. Effect of orally administered rolipram, a phosphodiesterase 4 inhibitor, on a mouse model of the dermatitis caused by 2,4,6-trinitro-1-chlorobenzene (TNCB)-repeated application. Eur J Pharmacol. 2006;532:128–37. doi: 10.1016/j.ejphar.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Strassmann G, Chan CC, et al. Protective effect of the type IV phosphodiesterase inhibitor rolipram in EAU: protection is independent of IL-10-inducing activity. Invest Ophthalmol Vis Sci. 1999;40:942–50. [PubMed] [Google Scholar]
- 11.Kobayashi M, Kubo S, Hirano Y, Kobayashi S, Takahashi K, Shimizu Y. Anti-asthmatic effect of ASP3258, a novel phosphodiesterase 4 inhibitor. Int Immunopharmacol. 2012;12:50–8. doi: 10.1016/j.intimp.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Leclerc O, Lagente V, Planquois JM, et al. Involvement of MMP-12 and phosphodiesterase type 4 in cigarette smoke-induced inflammation in mice. Eur Respir J. 2006;27:1102–9. doi: 10.1183/09031936.06.00076905. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal IM, Zhang M, Williams KN, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenthal IM, Tasneen R, Peloquin CA, et al. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother. 2012;56:4331–40. doi: 10.1128/AAC.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tasneen R, Li SY, Peloquin CA, et al. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother. 2011;55:5485–92. doi: 10.1128/AAC.05293-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalski JM, Golden G, Ikari J, Rennard SI. PDE4: a novel target in the treatment of chronic obstructive pulmonary disease. Clin Pharmacol Ther. 2012;91:134–42. doi: 10.1038/clpt.2011.266. [DOI] [PubMed] [Google Scholar]
- 17.Zhu J, Mix E, Winblad B. The antidepressant and antiinflammatory effects of rolipram in the central nervous system. CNS Drug Rev. 2001;7:387–98. doi: 10.1111/j.1527-3458.2001.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 19.Vats R, Varanasi KV, Arla R, Veeraraghvan S, Rajak S. Drug-drug interaction study to assess the effects of atorvastatin co-administration on pharmacokinetics and anti-thrombotic properties of cilostazol in male Wistar rats. Biopharm Drug Dispos. 2012;33:455–65. doi: 10.1002/bdd.1812. [DOI] [PubMed] [Google Scholar]
- 20.Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53(Suppl 1):5S–12. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acocella G, Nonis A, Gialdroni-Grassi G, Grassi C. Comparative bioavailability of isoniazid, rifampin, and pyrazinamide administered in free combination and in a fixed triple formulation designed for daily use in antituberculosis chemotherapy. I. Single-dose study. Am Rev Respir Dis. 1988;138:882–5. doi: 10.1164/ajrccm/138.4.882. [DOI] [PubMed] [Google Scholar]
- 22.Bramer SL, Forbes WP, Mallikaarjun S. Cilostazol pharmacokinetics after single and multiple oral doses in healthy males and patients with intermittent claudication resulting from peripheral arterial disease. Clin Pharmacokinet. 1999;37(Suppl 2):1–11. doi: 10.2165/00003088-199937002-00001. [DOI] [PubMed] [Google Scholar]
- 23.Savai R, Pullamsetti SS, Banat GA, et al. Targeting cancer with phosphodiesterase inhibitors. Expert Opin Investig Drugs. 2010;19:117–31. doi: 10.1517/13543780903485642. [DOI] [PubMed] [Google Scholar]
- 24.Marriott JB, Westby M, Cookson S, et al. CC-3052: a water-soluble analog of thalidomide and potent inhibitor of activation-induced TNF-alpha production. J Immunol. 1998;161:4236–43. [PubMed] [Google Scholar]
- 25.Taegtmeyer A, Leuppi J, Kullak-Ublick G. Roflumilast - a phosphodiesterase-4 inhibitor licensed for add-on therapy in severe COPD. Swiss Med Wkly. 2012;142 doi: 10.4414/smw.2012.13628. w13628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


