Abstract
Daily preexposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) is a novel strategy for preventing human immunodeficiency virus infection. We investigated in macaques whether FTC/TDF prevents transmission of a tenofovir-resistant simian/human immunodeficiency virus (SHIV) containing the K65R mutation. Six macaques received weekly a dose of FTC/TDF 3 days before rectal SHIV exposures and a second dose 2 hours after. Six untreated animals were controls. Animals were exposed rectally to escalating virus doses weekly for up to 28 weeks. PrEP significantly delayed infection with SHIVK65R (P = .028), although 4 of 6 FTC/TDF-treated macaques were infected at the end of the challenges. These findings highlight the need to closely monitor PrEP efficacy in areas with prevalent K65R.
Keywords: Preexposure prophylaxis, HIV-1 drug resistance, tenofovir disoproxil fumarate, emtricitabine
Daily preexposure prophylaxis (PrEP) with oral emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF) is a novel strategy for preventing Human Immunodeficiency virus type 1 (HIV) infection. Three human clinical trials with daily FTC/TDF among men who have sex with men and heterosexually active men and women have provided proof of concept that daily PrEP can prevent sexual HIV transmission [1–3]. These results prompted the Food and Drug Administration to approve the use of FTC/TDF for PrEP to prevent sexual HIV transmission in high-risk populations [4].
In areas with widespread access to antiretroviral therapy, drug-resistant viruses are prevalent and frequently transmitted [5]. Resistance to tenofovir (TFV) and FTC are primarily associated with the K65R and M184V mutations, respectively, in the gene encoding reverse transcriptase. Exposure to an HIV type 1 (HIV-1) strain that is resistant to TFV and/or FTC is a potential threat to the success of PrEP with FTC/TDF. However, PrEP failures due to infection with a drug-resistant virus are difficult to document in humans and to distinguish from acquired resistance because of the need for sampling and testing early during infection, which is often not feasible.
Simian/human immunodeficiency virus (SHIV) infection of macaques is a well-established model of HIV transmission that can be used to assess the impact of drug-resistant viruses on the efficacy of FTC/TDF. We recently used a repeat low-dose rectal SHIV transmission model to investigate whether exposure to an FTC-resistant SHIV isolate containing the M184V mutation diminishes the efficacy of PrEP with FTC/TDF [6]. We found that FTC/TDF maintained full prophylactic efficacy against this mutant despite the >100-fold resistance to FTC conferred by M184V. The hypersusceptibility to TFV conferred by M184V and, possibly, other factors, such as residual FTC activity and reduced virus replicative fitness, may have played a role in the observed efficacy [6]. Here, we explored whether the same FTC/TDF regimen prevents transmission of a TFV-resistant mutant containing K65R.
METHODS
Generation of a TFV-Resistant SHIV162P3K65R Mutant
Plasmids pVP1 and pSHIVp3gp160 were kindly provided by Dr Cecilia Cheng-Mayer (Aaron Diamond AIDS Research Center). We used the SHIV162p3 background since this isolate and the isogenic FTC-resistant SHIV162p3M184V background have been extensively used to investigate the prophylactic efficacy of FTC/TDF in macaques [6, 7]. K65R was introduced in pVP1 by site-directed mutagenesis; we introduced 2 nucleotide changes (AAA→CGA) to minimize reversion of K65R in vitro and in vivo [8]. Reverse-transcriptase mutations N69S and I118V were also introduced in pVP1K65R. N69S and I118V are commonly seen in wild-type simian immunodeficiency virus (SIV), do not increase resistance to TFV, and improve fitness of SIVK65R mutants in vivo [8]. Infectious SHIV162P3K65R was generated in HEK-293T cells and expanded in CD8-depleted rhesus macaque peripheral blood mononuclear cells (PBMCs).
In Vitro Characterization of SHIV162P3K65R
The susceptibility of SHIV162P3K65R to TFV and FTC was evaluated using TZM-bl cells [8]. The impact of K65R on virus fitness was evaluated by using a competitive SHIV162p3 replication assay involving PBMCs [6]. Briefly, an 80:20 mixture of SHIV162P3K65R and SHIV162P3WT was used to infect 5 × 105 rhesus macaque PBMCs at a multiplicity of infection of 0.002. Changes in the relative proportion of the 2 competing variants were monitored over time by sequence analysis of SHIV RNA from culture supernatants [6].
Efficacy of FTC/TDF in Preventing Rectal Transmission of SHIV162P3K65R
The efficacy of FTC/TDF in preventing rectal transmission of SHIV162p3K65R was investigated using a macaque model consisting of weekly virus exposures. Six macaques received a weekly dose of FTC/TDF (20 and 22 mg/kg, respectively) by gavage 3 days before each virus exposure and a second dose 2 hours after [7]. Six untreated macaques were used as controls. Virus exposures were done by a traumatic inoculation of 1 mL of virus into the rectal vault [7]. We used a repeat-exposure, virus-challenge dose-escalation design because of the low transmissibility of K65R. Exposures were initiated at low doses of virus (12 tissue culture infective doses [TCID50]) for up to 14 weeks and were then escalated to high doses (120 TCID50) in uninfected macaques for up to 14 additional weeks after an 8-week rest period with no drug treatment, to control for potential residual infectious virus from low challenge doses of virus. Animals in the FTC/TDF arm were considered protected if they remained seronegative and had undetectable SHIV RNA in plasma and SHIV DNA in PBMCs during the virus challenges and 8 additional weeks. All procedures were done under anesthesia, using standard doses of ketamine hydrochloride. The Centers for Disease Control and Prevention Institutional Animal Care and Use Committee approved this study.
Plasma SHIV RNA was quantified by reverse transcription polymerase chain reaction (PCR) analysis [7]. Proviral DNA in PBMCs was quantified using a quantitative double-stranded primer PCR/nonprobe-based method that uses RNAse P as an internal standard. Virus-specific serologic responses were measured using an enzyme immunoassay (BioRad, Genetic Systems HIV-1/HIV-2, Redmond, WA).
Statistical Analysis
The risk of infection was estimated using the Cox proportional hazards model while controlling for differences in infectivity by dose. The protective effect of FTC/TDF at low and high challenge doses of virus was compared by inclusion of a 2-way interaction term in the Cox proportional hazards model. By study design, the challenge dose of virus was escalated for animals that remained uninfected after the maximum 14 weeks of low-dose exposures. Techniques for analysis of studies with interim monitoring require adjustment of the type I error rate [9]. The critical value for concluding the presence of a statistically significance difference in this 2-phase study design was fixed at a P value of < .0295 [10]. Acute RNA viremias were compared using the Wilcoxon rank-sum test.
RESULTS
Characteristics of SHIV162P3K65R
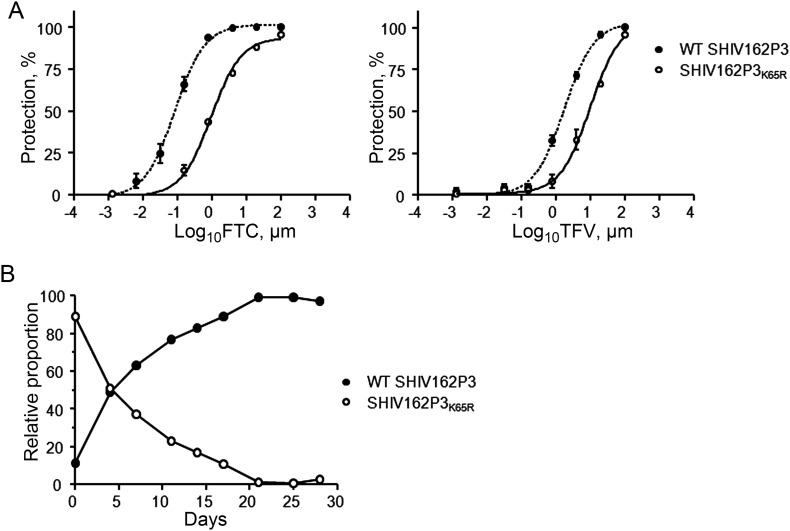
We generated a high-titer SHIV162P3K65R stock as measured by RNA concentration (1.48 × 109 copies/mL) and virus infectivity level (21 206 TCID50/mL). Similar to HIV-1, SHIV162P3K65R had moderate resistance to both FTC (11-fold) and TFV (5.6-fold) (Figure 1A). SHIV162P3K65R was rapidly outcompeted by SHIV162P3WT in a competitive replication assay, demonstrating the high fitness cost of K65R in SIV (Figure 1B). Thus, as in HIV-1, K65R in SIV reduces replicative fitness and susceptibility to both TFV and FTC.
Figure 1.
Characteristics of simian/human immunodeficiency virus (SHIV) 162P3K65R in vitro. A, Susceptibility of SHIV162P3K65R to tenofovir (TFV) and emtricitabine (FTC). Values denote the inhibition of the percentage of protection seen in TZM-bl cells at increasing concentrations of drug. Data represent 2 separate experiments done in triplicate. B, Competition dynamics among wild-type (WT) SHIV162P3 and SHIV162P3K65R. The experiment was initiated at an 80:20 mutant to WT ratio. The two lines denote the proportion of K65R and WT SHIV, over time.
Efficacy of FTC/TDF Against K65R SHIV
To determine whether FTC/TDF could prevent infection with SHIV162P3K65R, we administered to 6 macaques 2 weekly doses of FTC/TDF and exposed them rectally once per week to SHIV162P3K65R. Drug doses were given 3 days before and 2 hours after each virus exposure. Virus challenges were initiated with 12 TCID50 of SHIV162P3K65R, a dose that equates to the dose of SHIV162P3WT commonly used in this macaque model [7]. The specific study design is shown in Figure 2A. Figure 2B shows that at 12 TCID50, only 3 of 6 untreated controls and 1 of 6 treated animals were infected after 14 virus challenges. We therefore increased the virus dose to 120 TCID50 after an 8-week resting period. At the 120 TCID50 dose, the remaining 3 controls were infected, as were 3 of the 5 remaining FTC/TDF-treated animals. Infection of PrEP-treated animals was delayed, compared with infection of untreated controls; the temporal risk of infection was reduced 5.2–fold (95% confidence interval, 1.2–22.2) by FTC/TDF (P = .0280). The protective effect of FTC/TDF was similar at low and high challenge doses of virus (P = .90). Analysis of SHIV reverse-transcriptase sequences at infection confirmed the presence of K65R in all infected animals (data not shown).
Figure 2.
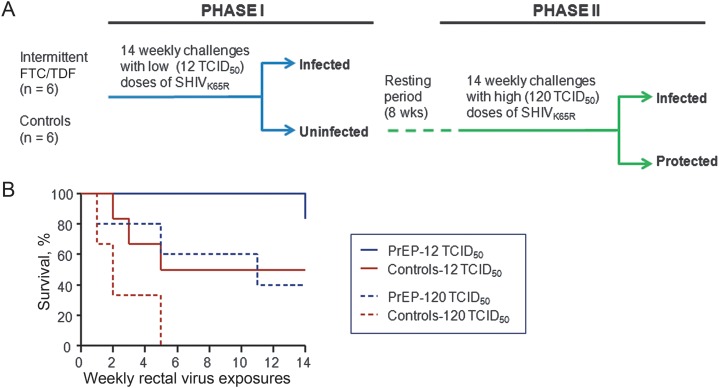
Prophylactic efficacy of emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) against tenofovir-resistant simian/human immunodeficiency virus (SHIV). A, Experimental design. Macaques were exposed to low (12 median tissue culture infective doses [TCID50]) doses of SHIV162P3K65R (phase I). After an 8-week rest period, the remaining uninfected animals were exposed to a 10-fold higher dose (120 TCID50) of SHIV162P3K65R (phase II). B, Efficacy of FTC/TDF against SHIV162P3K65R. Each survival curve represents the dose-specific cumulative percentage of uninfected macaques as a function of weekly rectal exposures. Protected animals remained seronegative and viral RNA/DNA negative during the 28 exposures and an additional follow-up period of 10 weeks.
SHIV-infected PrEP recipients with breakthrough infection continued to receive FTC/TDF for at least 6 weeks after the first RNA detection. The median peak RNA levels were similar in PrEP recipients with breakthrough infection and untreated controls (5.88 and 6.28 log10 viral RNA copies/mL, respectively; P = .75), as were the median peak proviral DNA levels (4.98 and 4.49 DNA copies/106 PBMCs, respectively; P = .34). Areas under the curve over the first 6 weeks of infection were also similar between the 2 groups for both RNA and DNA (P = .75 and P = .47, respectively). These values were also similar when the animals were stratified according to the virus dose required for infection (not shown).
DISCUSSION
TDF and FTC are important components of first-line therapy and have been extensively used for treatment. The M184V mutation associated with FTC resistance is one of the most prevalent nucleoside reverse-transcriptase inhibitor resistance mutations seen in patients who do not respond to treatment [11]. Consequently, M184V-containing viruses are frequently transmitted and commonly seen among drug-naive, recently infected persons. The overall prevalence of K65R among patients with no response to antiretroviral treatment has remained low (3%), although a long duration of suboptimal therapy with TDF or didanosine led to high frequencies of K65R, particularly in sub-Saharan Africa [12, 13]. The high prevalence of K65R in these areas raises concerns about secondary transmission of K65R and its potential impact on PrEP effectiveness.
Using a SHIV isolate with the K65R mutation that reproduces the resistance and replicative fitness profile of HIV-1 containing K65R, we showed that macaques exposed to this virus and treated with PrEP are not fully protected from rectal infection, suggesting that K65R has the potential to reduce the prophylactic efficacy of FTC/TDF in humans. These results are consistent with those of a previous study involving newborn macaques that demonstrated partial prophylactic efficacy of subcutaneous TFV against oral infection with SIVmac055K65R [14]. Although we found that transmission of K65R was significantly delayed by PrEP, 4 of the 6 animals were infected at the end of the challenge series. These findings differ from our earlier observations involving macaques exposed to SHIV162p3WT, which showed that the same FTC/TDF regimen and doses protected most (5/6) of the animals [6]. For SHIV162p3WT, the temporal risk for infection was reduced 16-fold by FTC/TDF as compared to the 5.2-fold reduction seen for SHIV162p3K65R [7]. Although our study lacked statistical power to draw statistically rigorous conclusions about differences in the FTC/TDF effect according to the virus strain (wild type or drug resistant), this observation suggests that the efficacy of FTC/TDF against K65R mutants is somewhat reduced, compared with that against wild-type virus.
Our results with K65R also differ from those of our earlier study, which showed full protection by this regimen against an isogenic FTC-resistant SHIV162p3M184V isolate [6]. The protection against M184V viruses conferred by FTC/TDF was most likely due to the 2-fold hypersusceptibility to TFV conferred by M184V that might have rendered TFV more effective in blocking transmission. In contrast to M184V, K65R confers 5- and 11-fold resistance to TFV and FTC, respectively, and thus reduces the effective drug concentrations for both drugs at the rectal mucosa and systemically. Since oral FTC/TDF results in lower TFV-diphosphate concentrations in vaginal tissues as compared to rectal tissues, our data suggest that the efficacy of FTC/TDF against vaginal infection with K65R mutants may be further reduced [15].
The partial efficacy against K65R seen with only 2 weekly FTC/TDF doses is encouraging and indicates that drug levels were near the protection threshold required for K65R. It is possible that daily FTC/TDF could have had a slightly better prophylactic efficacy against this mutant because of intracellular FTC-TP and TFV-DP accumulation after daily dosing. Partial protection with FTC/TDF against K65R, along with the high efficacy against SHIV162p3M184V noted in an earlier study, also suggests a less important contribution of FTC to rectal protection [6]. However, TDF delayed but did not prevent rectal infection in macaques, while FTC and FTC/TDF were each protective, suggesting that both drugs are important to maximize rectal efficacy [16]. A direct comparison of TDF, FTC, or FTC/TDF against wild-type and mutant SHIV would be needed to fully define relative roles for FTC or TDF in rectal protection.
Our macaque challenge experiment included a virus-dose-escalation design intended for evaluating PrEP efficacy at different virus doses. This experimental design also minimizes the number of animals used and adds statistical power by reusing animals at high virus doses and increasing the total number of per-animal exposures, although a statistical penalty is exacted by using a more stringent critical post hoc P value for determining the statistical significance of differences between treatment groups [9]. Using this novel approach, we demonstrate that the protective effect of FTC/TDF was similar at low and high doses of virus. While the transmissibility of SHIV162P3K65R was low at virus doses that efficiently infect macaques with SHIV162P3WT, we enhanced transmission efficiency by increasing the dose 10-fold on the basis of virus infectivity per virion particle ratio of K65R as compared to SHIV162P3WT [8]. For SHIV162P3M184V, this dose adjustment also enhanced transmissibility of this mutant to wild-type levels [6]. Our findings further demonstrate that, as with M184V, transmissibility of K65R virus is also lower than transmission of wild-type virus.
In summary, we show that FTC/TDF delays but does not fully prevent rectal infection with a K65R mutant in macaques. These results reiterate the need to closely monitor PrEP interventions in settings where the prevalence of circulating K65R viruses is high.
Notes
Acknowledgments. We thank Dr Michael Hendry, for his support and helpful discussions; Dr Gregory Langham, for serving as the attending veterinarian for this animal study protocol; and Dr David Garber, for maintaining our cohort of animals and coordinating animal studies. TDF and FTC were provided by Gilead Sciences through a material transfer agreement.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the Centers for Disease Control and Prevention (CDC) and an interagency agreement between the CDC and the National Institutes of Health (Y1-AI-0681-02).
Potential conflicts of interest. J. G. G.-L. and W. H. are named in a US government patent application related to methods for HIV prophylaxis. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. (10.1056/NEJMoa1110711) [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. FDA approves first medication to reduce HIV risk. 16 July 2012. http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm311821.htm . Accessed 10 May 2013. [Google Scholar]
- 5.Frentz D, Boucher CA, van de Vijver DA. Temporal changes in the epidemiology of transmission of drug-resistant HIV-1 across the world. AIDS Rev. 2012;14:17–27. [PubMed] [Google Scholar]
- 6.Cong ME, Youngpairoj AS, Zheng Q, et al. Protection against rectal transmission of an emtricitabine-resistant simian/human immunodeficiency virus SHIV162p3M184V mutant by intermittent prophylaxis with Truvada. J Virol. 2011;85:7933–6. doi: 10.1128/JVI.00843-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Lerma JG, Cong M, Mitchell J, et al. Intermittent prophylaxis with oral Truvada protects macques form rectal SHIV infection. Sci Transl Med. 2010;2:14ra4. doi: 10.1126/scitranslmed.3000391. [DOI] [PubMed] [Google Scholar]
- 8.Cong M, Youngpairoj AS, Aung WS, et al. Generation and mucosal transmissibility of emtricitabine- and tenofovir-resistant SHIV162p3 mutants in macaques. Virology. 2011;412:435–40. doi: 10.1016/j.virol.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Jennison C, Turnbull BW. Statistical approaches to interim monitoring of medical trials: a review and commentary. Statistical Sci. 1990;5:299–317. [Google Scholar]
- 10.Pocock SJ. Group sequential methods in the design and analysis of clinical trials . Biometrika. 1977;64:191–99. [Google Scholar]
- 11.Cheung PK, Wynhoven B, Harrigan PR. 2004: Which HIV-1 drug resistance mutations are common in clinical practice? AIDS Rev. 2004;6:107–16. [PubMed] [Google Scholar]
- 12.Sunpath H, Wu B, Gordon M, et al. High rate of K65R for antiretroviral therapy-naive patients with subtype C HIV infection failing a tenofovir-containing first-line regimen. AIDS. 2012;26:1679–84. doi: 10.1097/QAD.0b013e328356886d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamers RL, Sigaloff KC, Wensing AM, et al. PAStER. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54:1660–9. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 14.van Rompay KK, Miller MD, Marthas ML, et al. Prophylactic and therapeutic benefits of short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J Virol. 2000;74:1767–74. doi: 10.1128/jvi.74.4.1767-1774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]