Abstract
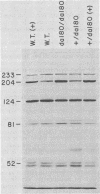
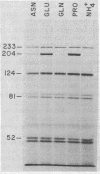
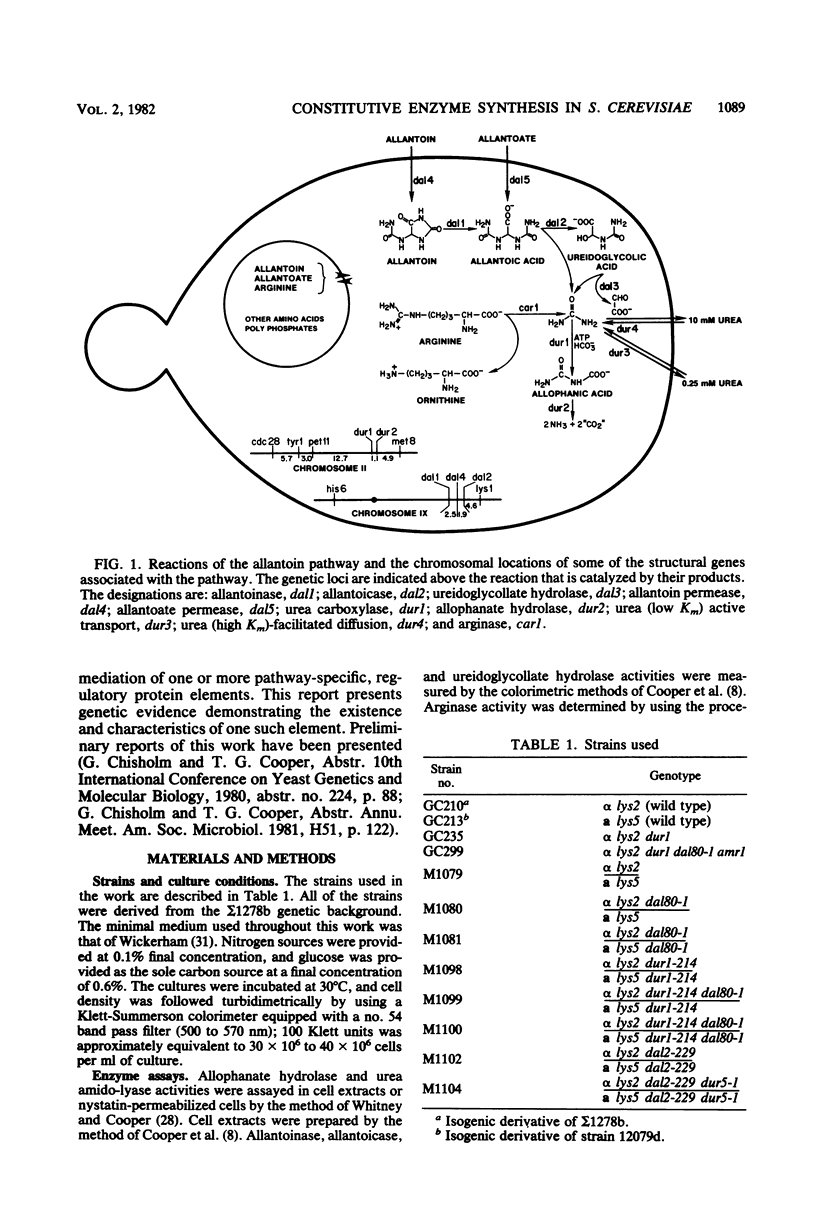
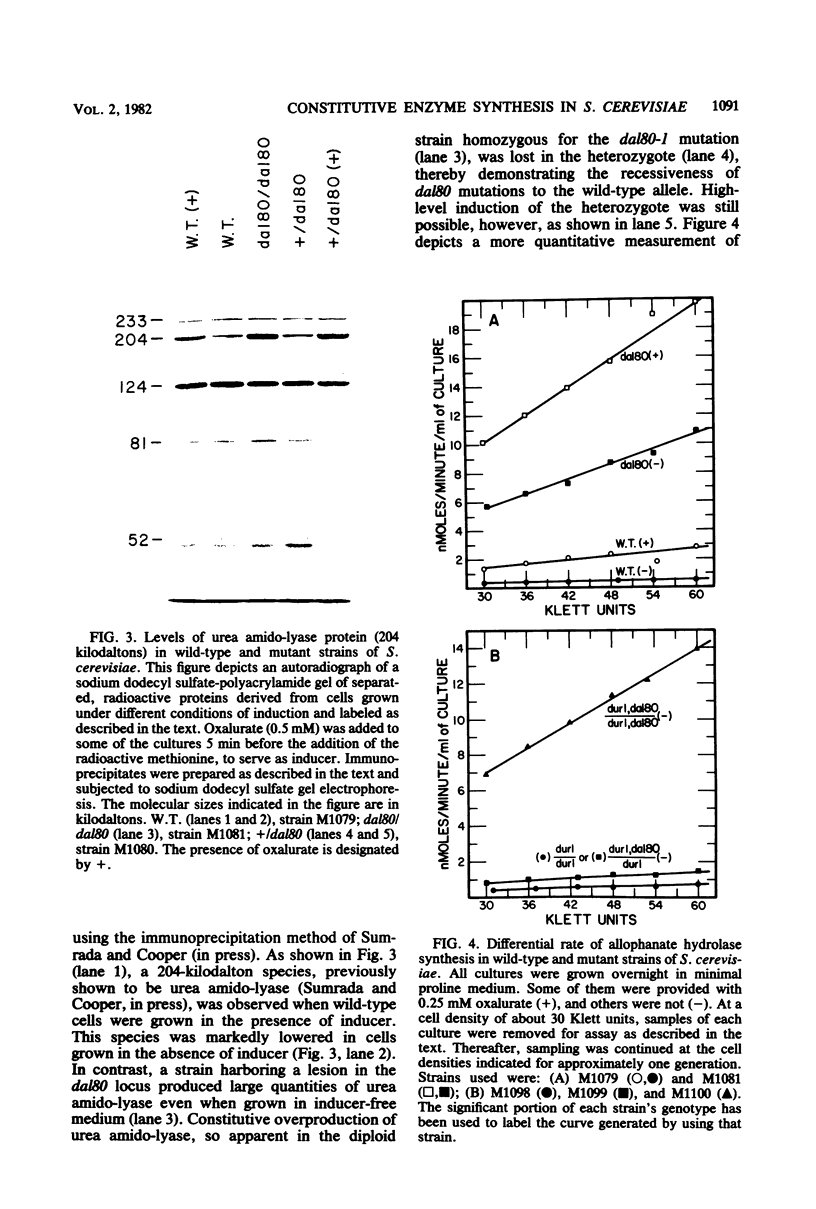
Degradation of allantoin, allantoate, or urea by Saccharomyces cerevisiae requires the participation of four enzymes and four transport systems. Production of the four enzymes and one of the active transport systems is inducible; allophanate, the last intermediate of the pathway, functions as the inducer. The involvement of allophanate in the expression of five distinct genes suggested that they might be regulated by a common element. This suggestion is now supported by the isolation of a new class of mutants (dal80). Strains possessing lesions in the DAL80 locus produce the five inducible activities at high, constitutive levels. Comparable constitutive levels of activity were also observed in doubly mutant strains (durl dal80) which are unable to synthesize allophanate. This, with the observation that arginase activity remained at its uninduced, basal level in strains mutated at the DAL80 locus, eliminates internal induction as the basis for constitutive enzyme synthesis. Mutations in dal80 are recessive to wild-type alleles. The DAL80 locus has been located and is not linked to any of the structural genes of the allantoin pathway. Synthesis of the five enzymes produced constitutively in dal80-1-containing mutants remains normally sensitive to nitrogen repression even though the dal80-1 mutation is present. From these observations we conclude that production of the allantoin-degrading enzymes is regulated by the DAL80 gene product and that induction and repression of enzyme synthesis can be cleanly separated mutationally.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossinger J., Cooper T. G. Execution times of macromolecular synthetic processes involved in the induction of allophanate hydrolase at 15 degrees C. J Bacteriol. 1976 Oct;128(1):498–501. doi: 10.1128/jb.128.1.498-501.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger J., Cooper T. G. Sequence of molecular events involved in induction of allophanate hydrolase. J Bacteriol. 1976 Apr;126(1):198–204. doi: 10.1128/jb.126.1.198-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossinger J., Lawther R. P., Cooper T. G. Nitrogen repression of the allantoin degradative enzymes in Saccharomyces cerevisiae. J Bacteriol. 1974 Jun;118(3):821–829. doi: 10.1128/jb.118.3.821-829.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Bossinger J. Selective inhibition of protein synthesis initiation in Saccharomyces cerevisiae by low concentrations of cycloheximide. J Biol Chem. 1976 Nov 25;251(22):7278–7280. [PubMed] [Google Scholar]
- Cooper T. G., Gorski M., Turoscy V. A cluster of three genes responsible for allantoin degradation in Saccharomyces cerevisiae. Genetics. 1979 Jun;92(2):383–396. doi: 10.1093/genetics/92.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Lam C., Turoscy V. Structural analysis of the dur loci in S. cerevisiae: two domains of a single multifunctional gene. Genetics. 1980 Mar;94(3):555–580. doi: 10.1093/genetics/94.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Lawther R. P. Induction of the allantoin degradative enzymes in Saccharomyces cerevisiae by the last intermediate of the pathway. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2340–2344. doi: 10.1073/pnas.70.8.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Marcelli G., Sumrada R. Factors influencing the observed half-lives of specific synthetic capacities in Saccharomyces cerevisiae. Biochim Biophys Acta. 1978 Feb 16;517(2):464–472. doi: 10.1016/0005-2787(78)90213-7. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Sumrada R. Urea transport in Saccharomyces cerevisiae. J Bacteriol. 1975 Feb;121(2):571–576. doi: 10.1128/jb.121.2.571-576.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois E. L., Wiame J. M. Catabolic synergism: a cooperation between the availability of substrate and the need for nitrogen in the regulation of arginine catabolism in Saccharomyces cerevisiae. Mol Gen Genet. 1978 Sep 8;164(3):275–283. doi: 10.1007/BF00333157. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Effects of inducer addition and removal upon the level of allophanate hydrolase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1100–1104. doi: 10.1016/s0006-291x(73)80008-7. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Cooper T. G. Kinetics of induced and repressed enzyme synthesis in Saccharomyces cerevisiae. J Bacteriol. 1975 Mar;121(3):1064–1073. doi: 10.1128/jb.121.3.1064-1073.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Phillips S. L., Cooper T. G. Lomofungin inhibition of allophanate hydrolase synthesis in Saccharomyces cerevisiae. Mol Gen Genet. 1975;137(2):89–99. doi: 10.1007/BF00341675. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Riemer E., Chojnacki B., Cooper T. G. Clustering of the genes for allantoin degradation in Saccharomyces cerevisiae. J Bacteriol. 1974 Aug;119(2):461–468. doi: 10.1128/jb.119.2.461-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine Y., Dubois E., Wiame J. M. The regulation of urea amidolyase of Saccharomyces cerevisiae: mating type influence on a constitutivity mutation acting in cis. Mol Gen Genet. 1978 Nov 9;166(3):251–258. [PubMed] [Google Scholar]
- Littlewood B. S., Chia W., Metzenberg R. L. Genetic control of phosphate-metabolizing enzymes in Neurospora crassa: relationships among regulatory mutations. Genetics. 1975 Mar;79(3):419–434. doi: 10.1093/genetics/79.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R., Cooper T. G. Allantoin transport in Saccharomyces cerevisiae. J Bacteriol. 1977 Sep;131(3):839–847. doi: 10.1128/jb.131.3.839-847.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R., Cooper T. G. Oxaluric acid: a non-metabolizable inducer of the allantoin degradative enzymes in Saccharomyces cerevisiae. J Bacteriol. 1974 Mar;117(3):1240–1247. doi: 10.1128/jb.117.3.1240-1247.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R., Gorski M., Cooper T. Urea transport-defective strains of Saccharomyces cerevisiae. J Bacteriol. 1976 Mar;125(3):1048–1056. doi: 10.1128/jb.125.3.1048-1056.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W., Hafner E. W. Convenient method for detecting 14CO2 in multiple samples: application to rapid screening for mutants. J Bacteriol. 1976 Oct;128(1):485–486. doi: 10.1128/jb.128.1.485-486.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turoscy V., Cooper T. G. Allantoate transport in Saccharomyces cerevisiae. J Bacteriol. 1979 Dec;140(3):971–979. doi: 10.1128/jb.140.3.971-979.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turoscy V., Cooper T. G. Pleiotropic control of five eucaryotic genes by multiple regulatory elements. J Bacteriol. 1982 Sep;151(3):1237–1246. doi: 10.1128/jb.151.3.1237-1246.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G., Magasanik B. The induction of urea carboxylase and allophanate hydrolase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6203–6209. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Requirement for HCO3- by ATP: urea amido-lyase in yeast. Biochem Biophys Res Commun. 1970 Aug 24;40(4):814–819. doi: 10.1016/0006-291x(70)90975-7. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Urea carboxylase and allophanate hydrolase. Two components of adenosine triphosphate:urea amido-lyase in Saccharomyces cerevisiae. J Biol Chem. 1972 Mar 10;247(5):1349–1353. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Urea carboxylase and allophanate hydrolase: two components of a multienzyme complex in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1972 Oct 6;49(1):45–51. doi: 10.1016/0006-291x(72)90007-1. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. Urea carboxylase from Saccharomyces cerevisiae. Evidence for a minimal two-step reaction sequence. J Biol Chem. 1973 Jan 10;248(1):325–330. [PubMed] [Google Scholar]
- Whitney P. A., Magasanik B. The induction of arginase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6197–6202. [PubMed] [Google Scholar]
- Zacharski C. A., Cooper T. G. Metabolite compartmentation in Saccharomyces cerevisiae. J Bacteriol. 1978 Aug;135(2):490–497. doi: 10.1128/jb.135.2.490-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]