Abstract
Objectives:
This study investigated the radio-opacity of commercially available glass ionomer cements (GICs), flowable resin composites (FRCs) and calcium hydroxide cements (CHCs) and compared this with the radio-opacity of enamel, dentine and aluminium stepwedge. 16 GICs, 8 FRCs and 4 CHCs were analysed.
Methods:
Three sets of three samples were prepared: 1 mm, 2 mm and 3 mm thickness for GIC and FRC and 1 mm thickness for CHC. Specimens of enamel and dentine with the same thicknesses were obtained. As a control, an aluminium stepwedge was used. Radiographs were taken with a digital Kodak RVG 5000 (0.32 s, 30 cm). The images were analysed using the Image Tool® program (v. 2.00; The University of Texas Health Science Center, San Antonio, TV) to obtain the mean grey values.
Results:
Analysis of variance was used to investigate the significance of differences among the groups. For pairwise comparisons, the Tukey test was applied (p < 0.05). The GICs Ionomaster (Wilcos, Petrópolis, Brazil), Maxxion (FGM, Joinville, Brazil), Bioglass R (Biodinâmica, Ibiporã, Brazil), Bioglass F (Biodinâmica), Vidrion R (SS White, Rio de Janerio, Brazil) and Vidrion F (SS White), presented radio-opacity lower than that of dentine. All FRCs and CHCs studied showed radio-opacity higher than that of dentine. Vitro Fil (DFL, Rio de Janeiro, Brazil), Magic Glass (Vigodent, Rio de Janeiro, Brazil), Vitrebond (3M, Sumaré SP, Brazil), Riva Self Cure (SDI, Victoria, Australia), Riva Light Cure (SDI), Fill Magic (Vigodent), Opallis (FGM, Joinville, Brazil), Surefil SDR (Dentsply, Milford, DE), Tetric N (Ivoclar Vivadent, Schaan, Lichtenstein), Tetric (Ivoclar Vivadent), Hydro C (Dentsply, Petrópolis, Brazil), Hydcal (Technew, Madalena, Portugal) and Liner (Vigodent) showed radio-opacity similar to or greater than that of enamel for all thicknesses.
Conclusions:
The increased thickness of the materials studied increases their radio-opacity. Some commercially available GICs used as a base and liner for restorations have a very low radio-opacity (Ionomaster, Maxxion, Bioglass R, Bioglass F, Vidrion R and Vidrion F).
Keywords: calcium hydroxide, digital radiography, composite resins, glass ionomer cements
Introduction
The radio-opacity of dental materials used for restorations is extremely important for the radiographic diagnosis, especially when assessing posterior teeth.1 A material with adequate radio-opacity allows the detection of secondary caries and distinguishes it from the restorative material and surrounding tooth structure. Moreover, the proximity of pulp can be easily visualized, just like marginal defects, overhangs and open margins.2 Currently, three types of base and liner material are recommended for restorations: glass ionomer cement (GIC), flowable resin composite (FRC) and calcium hydroxide cement (CHC). Manufacturers of restorative materials are responsible for improving the degree of radio-opacity of their products, by incorporating fillers or by using radio-opaque compounds.3 Elements with high atomic numbers, such as barium, strontium, zinc, yttrium and ytterbium, can improve radio-opacity for optimal diagnostics.4
The ideal radio-opacity for base and liner materials has been discussed by several authors. Studies of radio-opacity are usually evaluated and compared with enamel, dentine or aluminium (Al).5 The International Standards Organization (ISO) has developed standards for the radio-opacity of dental materials. According to ISO 40496 (resin composite) and ISO 99177 (GIC), if a manufacturer claims their product to be radio-opaque, its radio-opacity must be equal to or greater than that of Al with the same thickness. It has been demonstrated that the radio-opacity of dentine is approximately equivalent to that of Al with the same thickness, and enamel has approximately twice the radio-opacity of Al with the same thickness.8 On the other hand, some research4,9–12 suggests that the radio-opacity of a material that will be used as a base or liner should be equal to or slightly greater than that of enamel, once it provides an optimum contrast, ideal for the detection of secondary caries in radiographs. Digital imaging systems have been used in dental practice, providing advantages over conventional radiographic systems, such as involving a shorter exposure time to X-rays, being faster and easier to use and enabling accurate evaluation of radio-opacity.13 These advantages can also be employed in laboratory research to evaluate the radio-opacity of dental materials. With this system, the radiograph is available for computer image software to determine the mean grey values (MGVs) of each material or structure, which are represented within a scale ranging between 0 (black) and 255 (white).
GICs, FRCs and CHCs with inadequate radio-opacity have been found in clinical practice, and it has been reported that the radio-opacity of dental materials is highly variable.5 Pedrosa et al14 suggested that continuity in the study of the radio-opacity of materials is important, in order to evaluate new materials that come on to the market and prevent the occurrence of interpretation errors during image diagnosis. Thus, the objective of this current study was to investigate the radio-opacity of 16 commercially available GICs, 8 FRCs and 4 CHCs by using digital radiographs and image analysis to determine the MGVs of materials and compare them with those of with enamel and dentine in different thicknesses.
Methods and materials
Sample preparation
The GICs, FRCs and CHCs evaluated in the current study are presented in Table 1.
Table 1.
Product, type, manufacturer and radio-opaque filler of studied materials
| Product | Type of material | Manufacturer | Radio-opaque filler type |
| Magic Glass | Glass ionomer cement | Vigodent, Rio de Janeiro, Brazil | Radio-opaque fluoraluminium silicate |
| Maxxion | Glass ionomer cement | FGM, Joinville, Brazil | Fluoraluminium silicate |
| Maxxion Radiopaco (Maxxion R) | Glass ionomer cement | FGM | Strontium |
| Riva Self Cure (SC) | Glass ionomer cement | SDI, Victoria, Australia | Strontium, fluoraluminium silicate |
| Ionomaster | Glass ionomer cement | Wilcos, Petrópolis, Brazil | Fluoraluminium silicate |
| Vitrofil | Glass ionomer cement | DFL, Rio de Janeiro, Brazil | Strontium, aluminium |
| Bioglass R | Glass ionomer cement | Biodinâmica, Ibiporã, Brazil | Aluminium, barium |
| Bioglass F | Glass ionomer cement | Biodinâmica | Aluminium, barium |
| Vidrion F | Glass ionomer cement | SS White, Rio de Janeiro, Brazil | Aluminium, barium |
| Vidrion R | Glass ionomer cement | SS White | Aluminium, barium |
| Ketac Molar Easymix | Glass ionomer cement | 3M ESPE, Seefeld, Germany | Strontium, lanthanum |
| Riva Light Cure (LC) | Glass ionomer cement | SDI | Strontium, fluoraluminium silicate |
| Vitremer | Glass ionomer cement | 3M, Sumaré SP, Brazil | Strontium, fluoraluminium silicate |
| Vitrebond | Glass ionomer cement | 3M | Strontium, zinc, fluoraluminium silicate |
| Ionoseal | Glass ionomer cement | VOCO, Cuxhaven, Alemanha | Strontium, zinc |
| Ionosit | Glass ionomer cement | DMG, Hamburg, Germany | Zinc |
| Surefil SDR | Flow composite | Dentsply, Milford, USA | Barium, aluminium strontium |
| Wave | Flow composite | SDI | Strontium |
| Fill Magic | Flow composite | Vigodent | Barium, aluminium |
| Natural Flow | Flow composite | DFL | Barium, aluminium |
| Tetric | Flow composite | Ivoclar Vivadent, Schaan, Lichtenstein | Barium, ytterbium, aluminium |
| Te-Econom Plus | Flow composite | Ivoclar Vivadent | Barium, aluminium |
| Tetric N | Flow composite | Ivoclar Vivadent | Barium, ytterbium |
| Opallis | Flow composite | FGM | Barium, aluminium. |
| Liner | Calcium hydroxide cement | Vigodent | Barium, zinc |
| Hydro C | Calcium hydroxide cement | Dentsply, Petrópolis, Brazil | Zinc |
| Hydcal | Calcium hydroxide cement | Technew, Madalena, Portugal. | Barium, zinc |
| Dycal | Calcium hydroxide cement | Dentsply, Petrópolis, Brazil | Zinc |
Plastic ring moulds with a 4 mm internal diameter and 1 mm, 2 mm and 3 mm depths were used to prepare the specimens. The materials were prepared in accordance with the manufacturers' instructions. The mould was placed on a glass slab and the materials were placed in the mould until it was overfilled. For the insertion of GICs, a syringe was used (Centrix; 3M Dental Products, St. Paul, MN) to minimize bubbles. The application tip of the syringe of the FRC provided by the manufacturer was used, and CHCs were inserted using a specific instrument (10120; SS White, Rio de Janeiro, Brazil). A mylar matrix strip (Probem; Catanduva, São Paulo, Brazil) followed by a glass slab (Perfecta; São Paulo, Brazil) was placed over the plastic moulds of each material to flatten the surface. For chemically activated materials, the cure time followed the manufacturer's recommendation. The light-activating materials were cured with a light source for 40 s (Degulux® Soft Start; Degussa Dental, Dusseldorf, Germany). Both sides of the specimens were light cured. Three specimens of each thickness (1 mm, 2 mm and 3 mm) were prepared, resulting in nine samples for GICs and FRCs. For the CHCs, specimens with only 1 mm thickness were prepared, because they most closely resembled those used in dental restorations, resulting in three specimens for each material.
To obtain enamel and dentine specimens, three freshly extracted, human third molars were used in this study (ethical approval was obtained from the Research Ethics Committee of the University of São Paulo, São Paulo, Brazil). Each crown was cut transversally using a slow diamond saw and fragments 1 mm, 2 mm or 3 mm thick were obtained (Labcut 1010; EXTEC, Enfield, CT). For thickness adjustment, the specimens were ground flat with carbide paper (#600 grit) and checked using a digital calliper (Mitutoyo, São Paulo, Brazil). The slices were kept in distilled water until use.
An aluminium stepwedge with nine 1 mm incremental steps was used (#90-951; Margraf Corporation, Jenkintown, PA). The composition of this stepwedge is 98.3% aluminium, 0.08% copper, 0.5% silicon, 0.9% magnesium and 0.22% chromium.
Radiographic procedures
The specimens were placed on a digital sensor (RVG 5000 Kodak; Eastman Kodak Company, Vincennes, France) which was fixed to an aluminium support to hold it parallel to the horizontal plane and to allow radiography in the perpendicular direction to the horizontal plane. The same procedure was used to take digital radiography of the aluminium stepwedge, specimens of enamel and dentine. All specimens were placed at a 30 cm distance for 0.32 s in a dental X-ray unit (70 kVp/7 mA; Gnatus, Ribeirão Preto, Brazil) connected to a digital system (Kodak RVG 5000 Trophy for Eastman Kodak Company). The X-ray unit was kept in the same position throughout the experiment. The radiographic images were saved in JPEG format and stored directly on a computer (Samsung SyncMaster 753DFX; Samsung, Manaus, Brazil) connected to the digital system through its software (KDIS 6.8 Patient File; Eastman Kodak Company).
Digital analysis procedures
The radio-opacity (in pixels) of the samples was determined in the digital radiographs, which were exported to Image Tool® image software (v. 2.00, The University of Texas Health Science Center, San Antonio, CA). This software is able to provide an MGV by delimiting an area on the centre of the image (0.79 × 0.79 cm) with the software cursor. This software shows data concerning the highest and the lowest radio-opacity of the sample, and average values, which were considered to be the sample's initial radio-opacity (MGV). As each sample was submitted to three exposures, the sample's final radio-opacity was considered to be the mean of those values. These values were also converted into millimetres of aluminium (mmAl), using an adaptation of the following equation proposed by Vivan et al15:
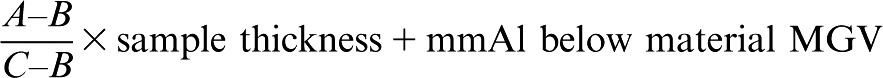 |
(1) |
where A is the material's MGV; B is the MGV of the aluminium stepwedge increment immediately below the material's MGV; and C is the MGV of the aluminium stepwedge increment immediately above the material's MGV.
Analysis of variance was used to compare the radio-opacity in millimetres of aluminium of each type of material studied, and the Tukey test was applied to determine the pairwise comparisons at p < 0.05 for both tests.
Results
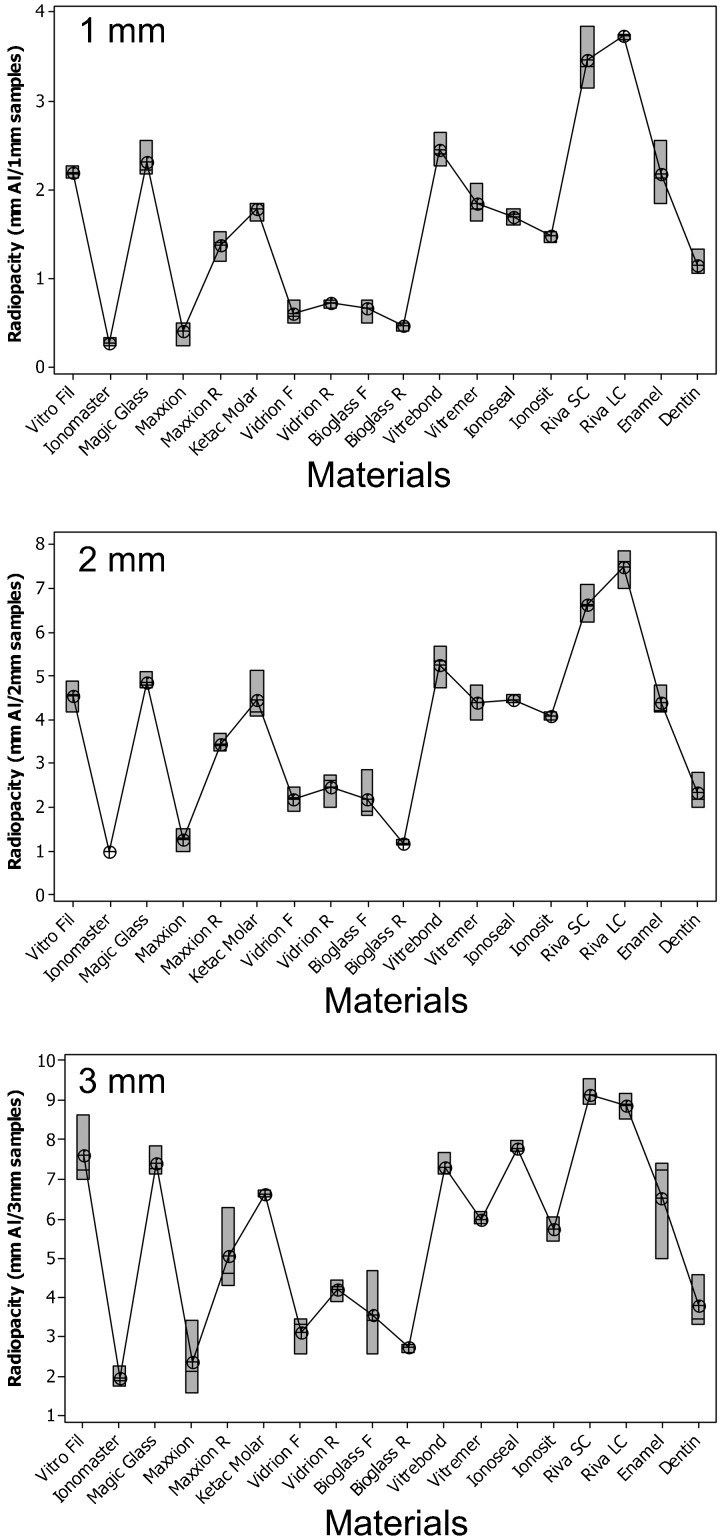
The radio-opacity means of GICs, enamel and dentine are presented in Figure 1. Considering all the evaluated thicknesses, Ionomaster, Maxxion, Bioglass R, Bioglass F, Vidrion R and Vidrion F presented radio-opacity lower (p < 0.05) or similar to dentine. Riva Light Cure (LC) and Riva Sure Cure (SC) were more radio-opaque than enamel for all thicknesses (p < 0.05). Figure 2 shows the radiographic images of the GICs, enamel, dentine and aluminium stepwedge. There was a large variation among the radio-opacities: some GICs showed a radio-opacity similar to dentine, while others exhibited a higher radio-opacity. It was also observed that the radio-opacity increased with specimen thickness.
Figure 1.
Radio-opacity (in mmAl) and differences (p = 0.00) between glass ionomer cements, enamel and dentine. See Table 1 for product details
Figure 2.
Radiographic images of glass ionomer cements, enamel, dentine and aluminium for 1 mm, 2 mm and 3 mm
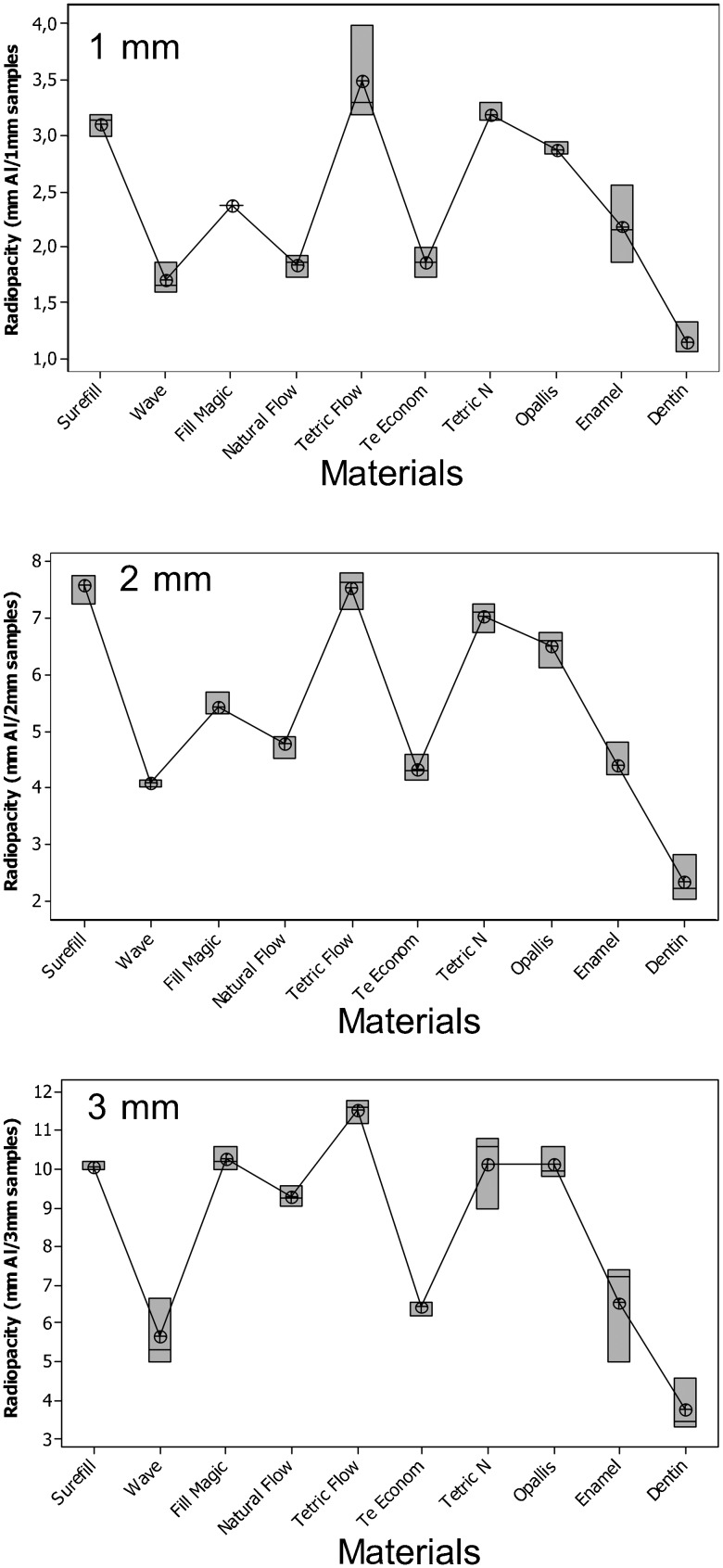
The radio-opacity means of FRCs, enamel and dentine are presented in Figure 3. All flowable composites studied were more radio-opaque than dentine (p < 0.05) and had a radio-opacity similar to or greater than enamel. Figure 4 shows the radiographic images of the FRCs, enamel and dentine of the same thickness and aluminium stepwedges. There was a variation among the radio-opacities for samples of the same thickness, which was not so clear among the GICs. It can also be observed that an increased thickness corresponded to an increase in radio-opacity.
Figure 3.
Radio-opacity (in mmAl) and differences (p = 0.00) between flowable resin composites, enamel and dentine. Product details are given in Table 1
Figure 4.
Radiographic images of flowable resin composites, enamel, dentine and aluminium for 1 mm, 2 mm and 3 mm
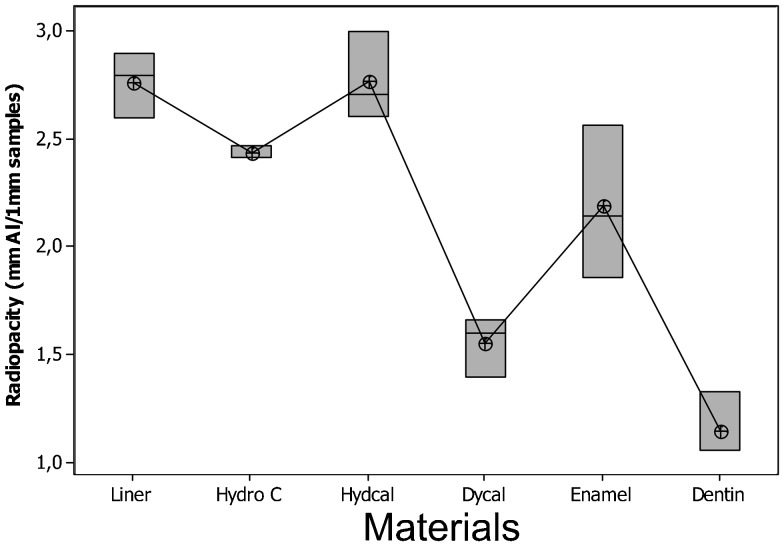
The radio-opacity means of CHCs, enamel and dentine are presented in Figure 5. All the CHCs had radio-opacity greater than dentine (p < 0.05) but only Dycal showed a radio-opacity lower than enamel. Hydro C, Hydcal and Liner presented similar or greater radio-opacities than enamel. Figure 6 shows the radiographic images of the CHCs, enamel and dentine of 1 mm thickness and aluminium stepwedges.
Figure 5.
Radio-opacity (in mmAl) and differences (p = 0.00) between calcium hydroxide cements, enamel and dentine for 1 mm
Figure 6.
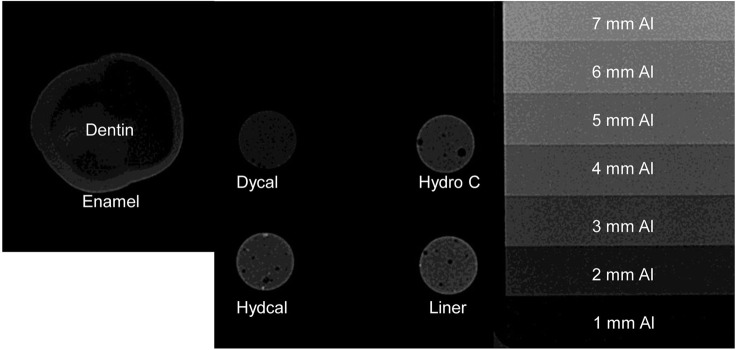
Radiographic image of calcium hydroxide cement, enamel, dentine and aluminium for 1 mm
Table 2 shows that the increased thickness of the studied materials corresponds to a significant increase in their radio-opacity.
Table 2.
Average radio-opacities (in mmAl) of glass ionomer cements, flowable resin composites, calcium hydroxide cements, enamel and dentine with 1 mm, 2 mm and 3 mm thicknesses.
| Material | 1 mm | 2 mm | 3 mm |
| Vitro fil | 2.20 (±0.07)a | 4.56 (±0.35)b | 7.62 (±0.87)c |
| Ionomaster | 0.27 (±0.04)a | 1.00 (±0.00)b | 1.96 (±0.26)c |
| Magic Glass | 2.33 (±0.20)a | 4.87 (±0.01)b | 7.42 (±0.37)c |
| Maxxion | 0.40 (±0.14)a | 1.26 (±0.25)b | 2.38 (±0.92)c |
| Maxxion R | 1.37 (±0.16)a | 3.47 (±0.21)b | 5.07 (±1.07)c |
| Ketac molar | 1.79 (±0.11)a | 4.48 (±0.57)b | 6.62 (±0.10)c |
| Vidrion F | 0.61 (±0.12)a | 2.18 (±0.28)b | 3.10 (±0.47)c |
| Vidrion R | 0.72 (±0.05)a | 2.44 (±0.38)b | 4.20 (±0.26)c |
| Bioglass F | 0.66 (±0.14)a | 2.18 (±0.58)b | 3.56 (±1.00)c |
| Bioglass R | 0.47 (±0.05)a | 1.17 (±0.07)b | 2.73 (±0.12)c |
| Vitrebond | 2.45 (±0.19)a | 5.25 (±0.48)b | 7.32 (±0.31)c |
| Vitremer | 1.85 (±0.21)a | 4.40 (±0.40)b | 5.99 (±0.17)c |
| Ionoseal | 1.71 (±0.10)a | 4.46 (±0.11)b | 7.80 (±0.16)c |
| Ionosit | 1.48 (±0.07)a | 4.10 (±0.10)b | 5.75 (±0.30)c |
| Riva SC | 3.46 (±0.35)a | 6.65 (±0.42)b | 9.12 (±0.37)c |
| Riva LC | 3.73 (±0.02)a | 7.49 (±0.44)b | 8.86 (±0.32)c |
| Fill Magic | 2.38 (±0.00)a | 5.42 (±0.21)b | 10.2 (±0.3)c |
| Natural Flow | 1.84 (±0.10)a | 4.77 (±0.21)b | 9.29 (±0.26)c |
| Tetric | 3.50 (±0.43)a | 7.52 (±0.34)b | 11.5 (±0.30)c |
| TE-Econom | 1.86 (±0.13)a | 4.33 (±0.25)b | 6.43 (±0.21)c |
| Tetric N | 3.20 (±0.08)a | 7.03 (±0.25)b | 10.1 (±0.98)c |
| Opallis | 2.88 (±0.05)a | 6.49 (±0.37)b | 10.1 (±0.41)c |
| Wave | 1.70 (±0.13)a | 4.06 (±0.08)b | 5.66 (±0.89)c |
| Surefil | 3.11 (±0.10)a | 7.58 (±0.28)b | 10.0 (±0.11)c |
| Liner | 2.76 (±0.15) | — | — |
| Hydro C | 2.43 (±0.02) | — | — |
| Hydcal | 2.77 (±0.20) | — | — |
| Dycal | 1.55 (±0.13) | — | — |
| Enamel | 2.19 (±0.35)a | 4.40 (±0.34)b | 6.55 (±1.34)c |
| Dentine | 1.15 (±0.15)a | 2.33 (±0.41)b | 3.77 (±0.69)c |
Different superscript letters demonstrate significant differences between thicknesses (analysis of variance and post-hoc Tukey test; p < 0.05).
Discussion
The presence of secondary caries is one of the main reasons for professionals to replace restorations.16 Base and liner materials must have an optimal radio-opacity to contrast with recurrent caries, enabling the correct diagnosis. The base material or liner should be sufficiently radio-opaque to be identifiable and to delimit the tooth–restoration interface from the tooth structure.2 The materials investigated in this study can be purchased by professionals and, in most cases, no information about their radio-opacity was found in the manufacturer's instructions.
The investigated materials presented highly variable radio-opacity. This result is similar to the findings of Williams and Billington.17 The literature demonstrates that there is difficulty in establishing an ideal standard material radio-opacity to be employed for restoration. Excessive radio-opacity may hide the diagnosis of caries adjacent to the restoration.4 The degree of radio-opacity interferes directly with the radiographic contrast, damaging the visual acuity and, consequently, diminishing the perception of detail, according to Espelid et al.18 These authors also suggest that material with a moderate radio-opacity is more appropriate, and that an optimal radio-opacity would be slightly greater than enamel. In this current study, evaluating the radio-opacity of the GICs Riva LC and Riva SC and FRCs Tetric and Tetric N it was observed that the radio-opacity of these materials by far exceeded the radio-opacity of enamel, making them less suitable because excessive radio-opacity may obscure the presence of a caries lesion. Moreover, a high radio-opacity near a less radio-opaque area can cause the Mach Band effect, which produces a visual illusion that enhances the contrast between a light and a darker area, making the dark borderline area darker. This effect might be misinterpreted as caries, and its perception can vary between observers.18 The radio-opacity presented by the FRC Te Econom or by Vitrebond and Vitrofil GIC seems to be sufficient and appropriate. However, a very low radio-opacity, such as that presented by the GICs Ionomaster, Maxxion, Bioglass R, Bioglass F, Vidrion R and Vidrion F, does not seem to be sufficient, and they can be mistaken for dentines or even for caries lesions.
The material's composition seems to be the most important factor that influences radio-opacity.10,11,13 The manufacturers include chemical elements such as barium, zinc, aluminium, strontium, silicon, yttrium, ytterbium and lanthanum in the products to increase radio-opacity.13,17,19 The higher the atomic number of the element added to the radio-opaque filler of the material composition, the higher the radio-opacity of the materials, because the absorption capacity of X-rays is increased.20 Therefore, ytterbium, which has the atomic number 70, is the element that provides the highest radio-opacity, followed by barium (Z = 56), yttrium (Z = 39), strontium (Z = 38), zinc (Z = 30), silicon (Z = 14) and aluminium (Z = 13). This importance can be demonstrated in this study by analysing the differences between the GIC Maxxion and its radio-opaque version Maxxion R. While the first one had a radio-opacity lower than dentine, for all thicknesses, the second one had a radio-opacity greater than this structure, meeting the ISO recommendation. This difference in radio-opacity is because the manufacturer has added strontium to Maxxion R; this has better radio-opacity than the silicon found in the composition of Maxxion. It has been demonstrated that the compound fluoraluminium silicate, which is present in the GICs' radio-opaque filler, does not provide sufficient radio-opacity.10
The percentage in which these elements are included in the composition of the materials also interferes with radio-opacity.17 This may be why materials containing barium did not show high radio-opacity, as with Bioglass F, Bioglass R, Vidrion F and Vidrion R.
The results presented in this study by the GIC Ketac Molar agree with those of Wenzel et al,21 who concluded that this material can be distinguished from the structure of the tooth without difficulty. Vitremer is also often included in studies of radio-opacity. Thus, the present study corroborates the results obtained by Hara et al10 and Turgut et al,12 in which this material presented radio-opacity higher than dentine.
The FRCs with the highest radio-opacities were Tetric, Tetric N and Surefil. Tetric showed radio-opacity greater than enamel, confirming results found by several authors.2,22–24
Generally, the increased thickness of GICs and FRCs in this current study improved their radio-opacity as also found by Pires de Souza et al.26
The radio-opacity of the CHCs studied was provided by zinc (Hydro C and Dycal) or barium and zinc (Liner and Hydcal). The radio-opacities of Dycal and Hydro C were lower than those of Liner and Hydcal, suggesting that the addition of barium improves the radio-opacity. All these materials showed radio-opacities greater than those of dentine, which confirms the results obtained by Devito et al25 and Pires de Souza et al.26 The clinician expects that a material such as CHC, when applied next to the pulp, is easily identified radiographically. Radio-opacity lower than enamel, as shown by Dycal, can lead the clinician to an incorrect diagnosis, mistaking this cement for dentine, caries, pulp or voids.
Therefore, it would be most appropriate to incorporate ytterbium and barium in materials, given that they provide suitable radio-opacity. The high cost of these elements and changes in the material's final colour could explain why manufacturers add chemical compounds with low radio-opacities, like fluoraluminium silicate.
The main advantage when a radiographic digital system is used is that development procedures are not required. Digital analysis provides the mean grey value, which is calculated directly by the computer software with the same standard as all specimens. The obtained mean grey value is used to convert the radio-opacity means to millimetres of aluminium.
In conclusion, there are some commercially available materials used as base and liner materials for restorations that have inadequate radio-opacity. Studies that evaluate the radio-opacity of base and liner materials should be undertaken periodically, since the manufacturers are constantly reformulating the composition of their products in order to achieve better properties and cost.
References
- 1.Imperiano MT, Khoury HJ, Pontual MLA, Montes MAJR, Silveira MMF. Comparative radiopacity of four low-viscosity composites. Braz J Oral Sci 2007; 6: 1278–1282 [Google Scholar]
- 2.Bouschlicher MR, Cobb DS, Boyer DB. Radiopacity of compomers, flowable and conventional resin composites for posterior restorations. Oper Dent 1999; 24: 20–25 [PubMed] [Google Scholar]
- 3.Marouf N, Sidhu SK. A study on the radiopacity of different shades of resin-modified glass ionomer restorative materials. Oper Dent 1998; 23: 10–14 [PubMed] [Google Scholar]
- 4.Hara AT, Serra MC, Haiter- Neto F, Rodrigues AL., Jr Radiopacity of esthetic restorative materials compared with human tooth structure. Am J Dent 2001; 14: 383–386 [PubMed] [Google Scholar]
- 5.Ergücü Z, Türkün LS, Önem E, Güneri P. Comparative radiopacity of six flowable resin composites. Oper Dent 2010; 35: 436–440 [DOI] [PubMed] [Google Scholar]
- 6.ISO: DP. Dental resin based restorative materials. Geneva, Switzerland: International Standards Organization; 2009. [Google Scholar]
- 7.ISO: DP 9917. Glass ionomer cements materials. Geneva, Switzerland: International Standards Organization; 2009. [Google Scholar]
- 8.Van Dijken JWV, Wing KR, Ruyter IE. An evaluation of the radiopacity of composite restorative materials used in class I and class II cavities. Acta Odontol Scand 1989; 47: 401–407 [DOI] [PubMed] [Google Scholar]
- 9.Tveit AB, Espelid I. Radiographic diagnosis of caries and marginal defects in connection with radiopaque composite fillings. Dent Mater 1986; 2: 159–162 [DOI] [PubMed] [Google Scholar]
- 10.Hara AT, Serra MC, Rodrigues AL., Jr Radiopacity of glass ionomer/composite resin hybrid materials. Braz Dent J 2001; 12: 85–89 [PubMed] [Google Scholar]
- 11.Sidhu SK, Shah PMM, Chong BS, Pitt Ford TR. Radiopacity of resin-modified glass ionomer restorative cements. Quintessence Int 1996; 27: 639–643 [PubMed] [Google Scholar]
- 12.Turgut MD, Attar N, Önen A. Radiopacity of direct esthetic restorative materials. Oper Dent 2003; 28: 508–514 [PubMed] [Google Scholar]
- 13.Versteeg CH, Sanderink GC, van der Stelt PF. Efficacy of digital intra oral radiography in clinical dentistry. J Dent 1997; 25: 215–224 [DOI] [PubMed] [Google Scholar]
- 14.Pedrosa RF, Brasileiro IV, dos Anjos Pontual ML, dos Anjos Pontual A, Silveira MMF. Influence of materials radiopacity in the radiographic diagnosis of secondary caries: evaluation in film and two digital systems. Dentomaxillofac Radiol 2011; 40: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivan RR, Zapata RO, Bramante CM, Bernardineli N, Garcia RB, Duarte MAH, Moraes IG. Evaluation of the radiopacity of some commercial and experimental root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009; 108: e35–e38 [DOI] [PubMed] [Google Scholar]
- 16.Braga SRM, Vasconcelos BT, Macedo MRP, Martins VRG, Sobral MAP. Reasons for placement and replacement of direct restorative materials in Brazil. Quintessence Int 2007; 38: 1–6 [PubMed] [Google Scholar]
- 17.Williams JA, Billington RW. The radiopacity of glass ionomer dental materials. J Oral Rehab 1990; 17: 245–248 [DOI] [PubMed] [Google Scholar]
- 18.Espelid I, Tveit AB, Erickson RL, Keck SC, Glaspoole EA. Radiopacity of restorations and detection of secondary caries. Dent Mater 1991; 7: 114–117 [DOI] [PubMed] [Google Scholar]
- 19.Prévost AP, Forest D, Tanguay R, DeGrandmont P. Radiopacity of glass ionomer dental materials. Oral Surg Oral Med Oral Pathol 1990; 70: 231–235 [DOI] [PubMed] [Google Scholar]
- 20.Anusavice KJ., Chemistry of synthetic resins Phillip's science of dental materials. Philadelphia, PA: Saunders; 2003. pp. 211–235 [Google Scholar]
- 21.Wenzel A, Hintze H, Horsted-Bindslev P. Discrimination between restorative dental materials by their radiopacity measured in film radiography and digital images. J Forensic Odontostomatol 1998; 16: 8–13 [PubMed] [Google Scholar]
- 22.Attar N, Tam LF, McComb D. Flow, strength, stiffness and radiopacity of flowable resin composites. J Can Dent Assoc 2003; 69: 516–521 [PubMed] [Google Scholar]
- 23.Murchison DF, Charlton DG, Moore WS. Comparative radiopacity of flowable resin composites. Quintessence Int 1999; 30: 179–184 [PubMed] [Google Scholar]
- 24.Sabbagh J, Vreven J, Leloup G. Radiopacity of resin-based materials measured in film radiographs and storage phosphor plate (Digora). Oper Dent 2004; 29: 677–684 [PubMed] [Google Scholar]
- 25.Devito KL, Ortega AI, Haitner-Neto F. Radiopacity of calcium hydroxide cement compared with human tooth structure. J Appl Oral Sci 2004; 12: 290–293 [DOI] [PubMed] [Google Scholar]
- 26.Pires de Souza FCP, Pardini LC, Cruvinel DR, Hamida HM, Garcia LFR. In vitro comparison of the radiopacity of cavity lining materials with human dental structures. J Conserv Dent 2010; 13: 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]