Abstract
Biliary stenting has evolved dramatically over the past 30 years. Advancements in stent design have led to prolonged patency and improved efficacy. However, biliary stenting is still affected by occlusion, migration, anatomical difficulties, and the need for repeat procedures. Multiple novel plastic biliary stent designs have recently been introduced with the primary goals of reduced migration and improved ease of placement. Self-expandable bioabsorbable stents are currently being investigated in animal models. Although not US Food and Drug Administration approved for benign disease, fully covered self-expandable metal stents are increasingly being used in a variety of benign biliary conditions. In malignant disease, developments are being made to improve ease of placement and stent patency for both hilar and distal biliary strictures. The purpose of this review is to describe recent developments and future directions of biliary stenting.
Keywords: plastic stents, self-expandable metal stents, drug eluting stents, bioabsorbable stents, malignant biliary strictures, benign biliary strictures
Introduction
Biliary obstruction results from various malignant and benign conditions including but not limited to pancreatic cancer, cholangiocarcinoma, gallbladder cancer, malignant lymphadenopathy, choledocholithiasis, chronic pancreatitis, and postoperative strictures. For patients with distal or hilar malignant strictures, fewer than 20% are candidates for curative resection because they are poor surgical candidates or because of unresectable disease secondary to local spread and distant metastases.1–5 Biliary obstruction develops in 70% to 90% of these patients, resulting in jaundice, cholangitis, pruritis, malabsorption, coagulopathy, and hepatocellular dysfunction.1,2 Thus, in the vast majority of patients with distal or hilar malignant biliary strictures, palliation with endoscopic methods becomes the primary goal. Patients with benign biliary strictures can develop pain, jaundice, cholangitis, and secondary benign biliary cirrhosis. Endoscopic therapy is the preferred approach to treat benign strictures because of its less invasive nature and lower morbidity as compared with surgery.6
Endoscopic placement of the first plastic biliary stent was described in 1980.7,8 Since then, many developments have been made to improve the function of biliary stents. In 1982, the first large bore (10F) plastic stent was endoscopically placed into the bile duct. Instead of pigtails, these stents used single or double flaps to prevent migration.9 Speer et al demonstrated that larger gauge stents (10F) performed better than smaller 8F stents in malignant obstruction.10 Early experiences with self-expandable metal stents (SEMS) were described in the late 1980s.11–13 SEMS have been shown to have prolonged patency as compared to plastic stents. Although SEMS are more expensive than plastic stents, SEMS become more cost effective in patients with distal malignant strictures from unresectable cancer who are expected to survive more than 4 to 6 months.14–19 For malignant hilar strictures, SEMS have also been shown to have improved patency and lower rates of cholangitis as compared to plastic stents.20 Stent designs continue to evolve in order to improve stent patency and to reduce complications of stenting which can include occlusion, cholangitis, and migration. Here we discuss future developments in plastic and metal stents in both malignant and benign conditions. A summary of the key human studies discussed in this article are illustrated in Table 1A and B.
Table 1A.
Human studies of future developments in biliary stenting – malignant disease
| Author | Year | Study type | Subject | Stent | N | Outcomes |
|---|---|---|---|---|---|---|
| Haber et al27 | 2001 | Prospective feasibility | Bioabsorbable stents | Bioabsorbable biliary wallstent | 50 | Safely deployed in 48 of 50 patients |
| Park et al43 | 2009 | Prospective cohort | Bilateral hilar stenting | Bonastent M-hilar | 35 | Technical success 94.3% |
| Kogure et al46 | 2011 | Pilot study | Bilateral hilar stenting | Niti-S large cell D-type | 5 | Technical success 100% |
| Song et al53 | 2011 | Prospective randomized pilot study | Drug-eluting stents | Paclitaxel-eluting covered metal stents versus standard covered metal stents | 49 | No difference in stent patency or survival |
| Hu et al62 | 2012 | Prospective randomized trial | Anti-reflux stents | Anti-reflux stent versus standard uncovered metal stent | 104 | Improved mean patency with anti-reflux stents |
| Cheon et al22,* | 2012 | Prospective randomized pilot study | Novel plastic materials | Cotton-Leung Sof-flex versus standard stent | 46 | Reduced migration with Sof-flex |
| Kim et al42 | 2013 | Retrospective cohort | Bilateral hilar stenting | Niti-S Y stent new design versus old design | 97 | Improved technical success (87.9%) with new design |
Notes:
This study included both benign and malignant strictures.
Table 1B.
Human studies of future developments in biliary stenting – benign disease
| Authors | Year | Study type | Subject | Stent | N | Outcomes |
|---|---|---|---|---|---|---|
| Mahajan et al69 | 2009 | Prospective cohort | Various benign biliary strictures | Viabil | 44 | Overall stricture resolution 83%, 65% for chronic pancreatitis |
| Perri et al70 | 2012 | Prospective cohort | Chronic-pancreatitis related strictures | Niti-S | 10 | 90% resolution at stent removal, 80% sustained resolution |
| Deviere et al71 | 2012 | Prospective trial | Various benign biliary strictures | Wallflex | 187 | Resolution 86% for chronic pancreatitis, 68% post-liver transplant, and 100% post-operative |
Note: FCSEMS for benign biliary strictures.
Abbreviation: FCSEMS, fully covered self-expandable metal stents.
Plastic stents
Materials and designs
Several recent developments have been made to improve the performance of standard plastic biliary stents. Migration remains a problem with plastic stents. A retrospective review of 322 biliary stent placements revealed a distal migration rate of 5.9% and a proximal migration rate of 4.9%.21
Cheon et al performed a prospective randomized trial comparing a new polyurethane (PU) stent (Cotton-Leung Sof-flex stent; Cook Endoscopy, Winston-Salem, NC, USA) with a standard 10F polyethylene (PE) stent (Cook Endoscopy).22 The PU stent was made of a new pliable and radiopaque material (pellethane) with the intent that it would more easily conform to the curvature of the bile duct, especially in cases requiring stent placement into the left hepatic duct. Both stents have similar stent flaps. Forty-six patients with malignant and benign hilar strictures were included. Overall, migration was significantly lower with the PU stents as compared with the PE stents (4.5% versus 29%, P = 0.032). There was a trend towards lower migration with PU stents versus PE stents when placed in the left hepatic duct (4.8% versus 25%, P = 0.07). There was no difference in median stent patency between the two groups.
Another flexible pellethane multiperforated plastic stent was evaluated in a retrospective review of 19 patients with both malignant and benign strictures (Johlin stent-JPWS; Cook Endoscopy).23 This stent was originally designed for pancreatic duct placement and has a tapered proximal tip with modifiable length. The migration rate was low with partial migration occurring with one stent. Four of five patients with benign strictures had resolution of the strictures. Four stents became occluded after a mean of 57 days. More data are needed to evaluate the performance of this stent design.
A new 7F double-pigtail, dual tapered-tip, PE stent (Compass BDS stent; Cook Endoscopy) was described in a retrospective review of 232 endoscopic retrograde cholangiopancreatographies (ERCPs) with placement of 346 stents.24 This stent was designed to be softer with less memory than traditional plastic stents and additionally has an increased number of drainage holes. Forty-eight percent of patients had bilateral stents. Subjectively, the stents were more easily placed than standard pigtail stents. There were no complications, stent migration, or malfunction.
New plastic stent designs appear to focus on more pliable materials intended to reduce migration rates and perhaps ease insertion. Additional studies are needed to evaluate the performance of these stents.
Magnetic stents
Magnetic tipped plastic stents have also been developed to provide a non-endoscopic method of stent removal. Ryou et al performed a proof-of-principle study in a porcine model.25 They used plastic Geenen pancreatic stents (Cook Endoscopy) tipped with a 9.5 mm neodymium-iron-boron magnet extension (KJ Magnetics, Jamison, PA, USA). The stents were successfully placed endoscopically in the bile ducts of five pigs. Under fluoroscopy, the stents were removed in a mean of 33 seconds using a 3.8 pound external hand-held neodymium-iron-boron disk magnet (KJ Magnetics). Endoscopic visualization confirmed dislodgement of the stents into the duodenum with no visible tissue damage. The authors address the concern that an improperly handled external magnet could cause proximal movement of the stent into the duct. Additionally, they are working to develop a magnetic catheter that could pass through the duodenoscope to retrieve proximally migrated stents.
Magnetically removable stents could prove useful in situations such as postoperative bile leaks where a repeat endoscopy may not always be necessary. Further study into the safety and functionality of these stents is needed.
Bioabsorbable stents
Self-expandable bioabsorbable stents have been studied for potential use in malignant and benign disease. The use of polylactic acid as a potential bioabsorbable material was initially described in 1966, when Kulkarni et al showed that polylactic acid implants were safe and biodegradable when surgically implanted into a porcine model.26
A prospective multicenter study was conducted of a 10 mm × 74 mm bioabsorbable poly-L-lactide (PLLA) stent (Bioabsorbable Biliary Wallstent; Microvasive, Natick, MA, USA) in 50 patients with malignant biliary obstruction.27 The stent was safely deployed in 48 of 50 patients demonstrating feasibility. However, this stent exhibited only 60% of the radial force as compared with the standard Wallstent.
Another feasibility study for biliary stenting was performed in a porcine model using a self-expanding 10 mm × 50 mm stent composed of polylactide (PLA) filaments loaded with barium for radiopacity (BioStent; Bionx Implants, Blue Bell, PA, USA).28 Follow-up cholangiography confirmed stent patency in seven of eight available pigs at 2 months, six of six at 4 months, and four of four at 6 months. On histologic exam, there was no evidence of inflammation, tissue hyperplasia, or endothelialization. Due to the incorporation of non-absorbable PU runners, this stent is only partially bioabsorbable.
In another study, a novel self-expanding 6–7 mm × 50 mm PLA-barium sulphate stent (PLA-BaSO4) was compared with a 10F PE stent in a porcine model of cystic duct leakage with 12 pigs.29 The PLA-BaSO4 group had significantly lower total bile output and reduced time with a percutaneous drain in place (median 5 days versus 7 days). By radiography, all stents were found to be in place at 1 and 3 months. At 6 months, no PLA-BaSO4 stents were seen, and one PE stent was identified.
More recently, a feasibility study was performed by Yamamoto et al using a biodegradable 6 mm × 15 mm PLLA Z stent with a platinum marker.30 The stents were placed in the bile ducts of 12 dogs. Laparotomy and cholangiography were performed in three dogs at each time point of 1, 3, 6, and 9 months. The stents were patent in all dogs at all time points. Endothelial proliferation or imbedding in the bile duct wall was seen in nine of 12 dogs. No macroscopic stent degradation was seen at 1, 3, or 6 months; however, moderate fragmentation was seen in all stents removed at 9 months.
There are multiple potential uses for self-expanding bioabsorable stents in biliary applications; for example, in bile duct leaks, a bioabsorbable stent could prevent the need for an additional endoscopy to remove the stent. However, bioabsorable stents still need additional study and outcome data before clinical use.
Metal stents
Malignant disease
Bilateral hilar stenting
Infectious complications after ERCP and stent insertion occur in 5%–38% of patients, usually resulting from contrast injection above the stricture without adequate drainage of all opacified ducts.31–33 Placement of unilateral versus bilateral stents for hilar malignant biliary obstruction remains a controversial topic because it is thought that unilateral drainage alone may not completely relieve jaundice and actually may induce cholangitis. There are five major studies that have compared unilateral and bilateral plastic stenting (Table 2); these studies have shown conflicting results.34–38
Table 2.
Comparison of unilateral and bilateral stenting in hilar malignant obstruction
| Study | Year | Study design | N | Stent type | Favor |
|---|---|---|---|---|---|
| Deviere et al34 | 1988 | Retrospective | 48 | Plastic | Bilateral stenting |
| Polydorou et al35 | 1989 | Retrospective | 151 | Plastic | Unilateral stenting |
| Polydorou et al36 | 1991 | Retrospective | 132 | Plastic | Unilateral stenting |
| Chang et al37 | 1998 | Retrospective | 98 | Plastic, three SEMS | Bilateral stenting |
| De Palma et al38 | 2001 | Prospective, randomized | 157 | Plastic | Unilateral stenting |
Abbreviation: SEMS, self-expandable metal stents.
SEMS have been shown to have improved patency and lower rates of cholangitis as compared with plastic stents in hilar tumors.20 However, there are very limited data comparing unilateral with bilateral SEMS for hilar obstruction. A retrospective study showed improved patency with bilateral SEMS as compared to the unilateral group; however, there was no difference in complications or survival.39 Bilateral stenting may be preferred in situations where both hepatic systems are injected with contrast in attempts to reduce the risk of cholangitis in contaminated, but undrained areas.37 Bilateral SEMS stenting may also allow for future endoscopic contralateral access. In hilar strictures, SEMS are generally uncovered to prevent occlusion of the contralateral system. The stent-within-stent deployment technique, where the wire mesh of a SEMS is dilated for the introduction of a second stent, was first described in 1996 by Silverman and Slivka.40 This can be a challenging technique, which has led to the introduction of multiple novel stent designs.41
Many stents designed specifically for bilateral hilar stenting feature a central portion with a more open weave of the wire mesh to facilitate placement of the contralateral stent through this central portion. Recent developments include extending the central open-weave portion of the primary stent from 10 mm to 25 mm and tightening the weave of this central portion (Niti-S Biliary Y-stent; MI Tech, Seoul, Korea). The longer open-weave portion allows more flexibility in aligning this area at the hilum; tightening the weave increases radial force to maintain the original spacing of the mesh for easier contralateral cannulation. In a retrospective study of 97 patients with unresectable hilar malignant obstruction, Kim et al showed that these stent changes improved technical success of bilateral stent-in-stent placement from 58.1% to 87.9% (P = 0.001).42 Median stent patency was 159 days.
The Bonastent M-Hilar stent (Standard Sci Tech, Seoul, Korea) has a cross wired nitinol mesh structure with smaller cell size (1.6 mm × 1.6 mm) in the 25 mm central section intended to reduce tumor ingrowth (Figure 1). Park et al performed a prospective study of this stent which included 35 patients with unresectable malignant hilar obstruction.43 Overall technical success for stent-in-stent placement was 94.3%. Median stent patency was 150 days. Other recent reports of the Bonastent M-hilar stent describe technical success rates of 94.6% in 56 patients and 95.4% in 44 patients with median patency of 238 and 114 days respectively.44,45 In cases of stent occlusion, endoscopic revision was successful in 15/17 or 88.2%.44
Figure 1.
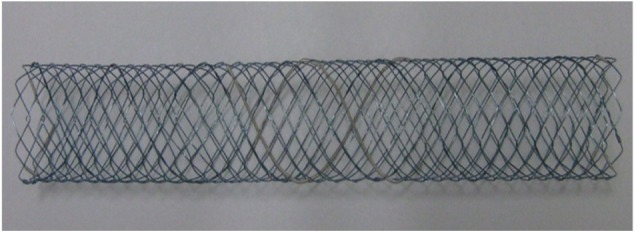
Bonastent M-hilar stent (Standard Sci Tech, Seoul, Korea).
Note: The more loosely woven center portion is centered at the hilum to facilitate placement of the second (contralateral) stent through the mesh.
Another newly described stent features a uniform large cell (7 mm) mesh throughout the entire length of the stent (Niti-S large cell D-type biliary stent; Taewoong Medical, Seoul, Korea). This design allows more flexibility in stent placement as there is no central open-weave portion which must be aligned with the hilum. Thicker nitinol wire is used to provide adequate radial force even with the larger diameter of the mesh cells. Technical success for bilateral stent-in-stent placement was reported as 100% for five patients in a pilot study.46 During a median follow-up of 152 days, stent occlusion occurred in one patient at 43 days from tumor ingrowth, a second patient at 96 days from sludge, and a third patient at 202 days from tumor ingrowth.
An alternative technique for bilateral metal stent placement in malignant hilar obstruction involves sequential insertion of bilateral side-by-side small caliber (6F) stents (Zilver 635; Cook Endoscopy). The stents are deployed in a simultaneous/alternating fashion. Technical success was reported as 100% in a study of ten patients.47
Hilar stent designs have continued to evolve, resulting in improved technical success rates. However, additional data are needed to determine the optimal stent design to ensure high technical success rates along with long duration of stent patency and ease of revision.
Drug eluting stents
SEMS are frequently used for malignant biliary obstruction, but occlusion from stent overgrowth and ingrowth occurs in approximately one third of cases.48 Drug eluting stents may improve stent patency and provide a method for local delivery of chemotherapeutic agents. In vitro, paclitaxel has been shown to exert inhibitory effects on pancreatic adenocarcinoma cells, gallbladder epithelial cells, and fibroblasts.49 Several studies have shown that paclitaxel-eluting stents are safe in porcine and canine models of the bile duct.50,51 A pilot study of 10% (weight/volume) paclitaxel-eluting stents (Niti-S Mira-Cover stent; Taewoong Medical) in 21 human subjects with unresectable malignant biliary strictures produced a mean stent patency of 429 days.52 Of the six patients who agreed to blood sampling, all exhibited low levels of paclitaxel, emphasizing local drug delivery with limited systemic exposure. A prospective, randomized pilot study was performed by Song et al comparing 20% (weight/volume) paclitaxel-eluting covered metal stents and conventional covered metal stents in 49 patients with malignant biliary obstruction.53 There was no statistically significant difference between the two groups in terms of stent patency or survival.
The method of drug delivery is another potential area of investigation in drug eluting stents which may provide improved performance. A porcine model has shown that paclitaxel can be safely delivered in a medium composed of the surfactant Pluronic F-127 with improved local drug delivery.54,55 More recently, gemcitabine-eluting stents have been shown to be safe in porcine models, in particular at a concentration of 10% (weight/volume).56,57 Human studies are needed to evaluate safety and efficacy. An alternative approach, brachytherapy, using holmium-166 incorporated covered metal stents (Taewoong Medical), has been shown to be safe in a canine model.58 Currently, there is no definitive evidence showing an advantage for drug-eluting stents, but this is an area with tremendous potential.
Anti-reflux stents
Metal stents with an antireflux valve may theoretically reduce the incidence of cholangitis and potentially prolong stent patency. Initial investigations into anti-reflux stents involved plastic biliary stents. In order to demonstrate how stents become occluded, clinically patent plastic biliary stents were evaluated 3 months after placement using confocal laser scanning microscopy and scanning electron microscopy.59 The interior wall of the stents was covered in a biofilm composed of living and dead bacteria along with yeasts and sometimes crystals. Large numbers of dietary plant fibers were found in all stents forming a network resembling a filter. Dua et al conducted a prospective randomized trial with 48 patients comparing a 10F plastic biliary stent (10F Tannenbaum stent; Cook Endoscopy) to the same stent affixed with a 4 cm windsock styled anti-reflux valve.60 Median patency was longer with the anti-reflux stent (145 days versus 101 days, P = 0.002).
After initial study involving plastic anti-reflux stents, more recent investigations have focused on metal anti-reflux stents. A feasibility study utilizing both covered and uncovered nitinol stents was performed in 23 patients with unresectable malignant biliary obstruction.61 A hemispheric anti-reflux valve made of silicone was attached to the luminal end of the stent. All patients had improvement in jaundice. Five patients had elective repeat endoscopy performed between 1 and 5 months; in each case, the anti-reflux valve appeared functional without any food particles inside the stents. Median patency was 14 months with a 3-month patency rate of 95.1%, 6-month patency rate of 74.2%, and 12-month patency rate of 55.9%.
More recently, a prospective randomized trial was performed by Hu et al in 104 patients with non-hilar malignant biliary obstruction.62 Fifty-two patients received an anti-reflux stent, and 52 patients received a standard uncovered metal stent. Although there was no difference in survival, mean patency was better with the anti-reflux stents (16.6 months versus 9.9 months, P = 0.031), and there were fewer episodes of fever prior to stent occlusion in the patients with anti-reflux stents (P = 0.031).
Anti-reflux stents may be valuable in reducing the incidence of cholangitis while potentially increasing stent patency; however, more studies are needed to confirm the effectiveness of these stents.
Benign disease
Fully covered self-expandable metal stents
The current standard of care for treatment of benign biliary strictures involves endoscopic placement of multiple plastic stents with stent exchanges every 3 months for a year; frequently, the number of stents placed is increased at each stent exchange.63,64 This approach results in long term resolution >80% for postoperative strictures, but long term resolution rates for chronic pancreatitis are much lower at 20% to 30%.65,66 The primary limitation of current stent treatment is the need for multiple sessions, on average between 3 to 5 sessions.
Due to significantly higher patency rates as compared to plastic stents, SEMS have been evaluated in the management of benign biliary strictures. The uncovered design of initial SEMS precluded use in benign disease due to problems with removability; however, fully covered self-expandable metal stents (FCSEMS) are a promising development. FCSEMS are not US Food and Drug Administration (FDA) indicated for benign disease, but are increasingly being used for various benign conditions. Use of SEMS for benign disease requires reliable removability in all cases. FCSEMS have been shown to be removable after a range of 6–355 days and with few complications.67 Covered SEMS have been successfully used for benign biliary strictures of multiple etiologies including chronic pancreatitis, postoperative, posttransplant anastomotic, choledocholithiasis, and various others.68 Figure 2A–C shows the placement and removal of a fully covered metal stent in a patient with a post-liver transplant anastomotic stricture.
Figure 2.
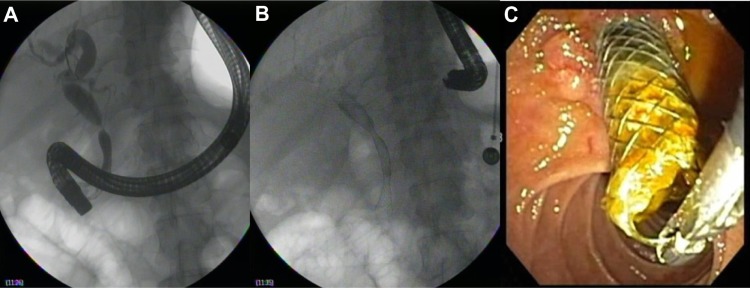
Placement (A and B) and then removal (C) of a fully covered metal stent after 6-month indwell time in a liver transplant patient with an anastomotic stricture.
An early prospective study of 44 patients with placement of FCSEMS (Viabil; Conmed, Utica, NY, USA) for benign biliary strictures of various etiologies was performed by Mahajan et al.69 After a median stent placement time of 3.3 months, overall stricture resolution was 83%. The resolution rate for chronic pancreatitis related strictures was 65%.
A prospective study of 17 patients with FCSEMS (Niti-S; Taewoong Medical) for benign biliary strictures secondary to chronic pancreatitis was performed between 2007 and 2009.70 Initial stents had unflared ends and an unacceptable migration rate (100%), but later patients received stents with flared ends resulting in a 40% complete distal migration rate. For the ten patients with stents using flared ends, the stricture resolution rate at the time of stent removal at 6 months was 90%; at 12 months of follow-up, the resolution rate was 80%.
In a large prospective multicenter trial of FCSEMS for benign biliary strictures by Deviere et al, preliminary results were reported for 187 patients with various etiologies of their strictures.71 Stricture resolution was 86% for chronic pancreatitis, 68% for post-liver transplant, and 100% for postoperative after a mean follow-up of 209 days after removal.
FCSEMS have also been used successfully for post-sphincterotomy bleeding, post-sphincterotomy perforation, refractory bile leaks, and bleeding after endoscopic papillary large balloon dilation.72–74 Covered stents have traditionally been avoided in hilar strictures due to concern about occluding the contralateral biliary system; however, two cases have been reported using 10 mm × 12 cm FCSEMS for benign hilar strictures with placement of a contralateral 10F plastic stent to ensure drainage of the contralateral system.75 This technique provides a valuable option for patients with refractory hilar strictures that have not responded to traditional plastic stent therapy. Several studies have described novel FCSEMS that have retrieval sutures attached to either the proximal, distal, or both ends of the stents.76–78 The end of the suture is accessible outside of the papilla and in some cases allows placement of the stent completely inside of the bile duct without crossing the ampulla.
Though not FDA approved for benign disease, the use of FCSEMS for benign conditions is rapidly expanding. They have been shown to be quite effective in biliary strictures that are often difficult to resolve with placement of multiple plastic stents. Additionally, FCSEMS appear to be useful in situations that previously required aggressive endoscopic or surgical interventions such as iatrogenic bleeding or perforation. Although FCSEMS look promising for benign biliary disease, there are no long-term data, and these stents may have their own set of complications (Figure 3).
Figure 3.
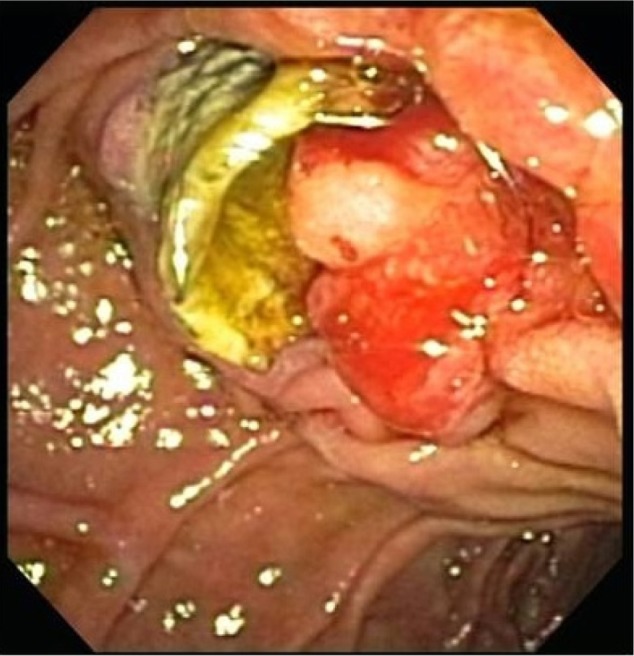
Epithelial hyperplasia surrounding a fully covered metal stent after 3-month indwell time in a patient with benign biliary stricture secondary to chronic pancreatitis.
Conclusion
Many advancements have been made in biliary stenting since it was first described in 1980.7,8 In terms of plastic stents, multiple designs have been introduced attempting to improve ease of placement and reduce migration rates. Magnetic stents have been examined in an animal model and show potential for benefit in cases such as postoperative bile leaks. The development of self-expandable bioabsorbable biliary stents is clearly a challenging area but may eventually prove to be helpful in situations such as bile leaks or benign strictures. For patients with unresectable malignant biliary disease, new metal stent designs have been developed and tested for both hilar and distal biliary obstruction. Variants of the Y-stent design continue to be introduced with improvements in ease of placement and stent patency. Drug eluting stents are still in preliminary stages of development with mixed results thus far; however, with so many variables including the drug and delivery medium, significant opportunities for future research are present. Anti-reflux stents have shown promising initial results, but still need further study. The use of FCSEMS for benign biliary disease is rapidly expanding and data continues to accumulate regarding their use for benign strictures. Challenging clinical situations such as strictures from chronic pancreatitis have the potential for significant benefit and possible avoidance of surgery in some cases. FCSEMS are also being used in other benign biliary indications such as iatrogenic bleeding and perforation. Advancements in biliary stenting continue to evolve. Enhancements in stent design which lead to improved patency and functionality will ultimately guide the future of biliary stenting.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Levy MJ, Baron TH, Gostout CJ, Petersen BT, Farnell MB. Palliation of malignant extrahepatic biliary obstruction with plastic versus expandable metal stents: an evidence-based approach. Clin Gastoenterol Hepatol. 2004;2(4):273–285. doi: 10.1016/s1542-3565(04)00055-2. [DOI] [PubMed] [Google Scholar]
- 2.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165(1):68–72. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 3.Guthrie CM, Haddock G, De Beaux AC, Garden OJ, Carter DC. Changing trends in the management of extrahepatic cholangiocarcinoma. Br J Surg. 1993;80(11):1434–1439. doi: 10.1002/bjs.1800801128. [DOI] [PubMed] [Google Scholar]
- 4.Smith AC, Dowsett JF, Russel RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344(8938):1655–1660. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 5.Prat F, Chapat O, Ducot B, et al. Predictive factors for survival of patients with inoperable malignant distal biliary strictures: a practical management guideline. Gut. 1998;42(1):76–80. doi: 10.1136/gut.42.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davids PH, Tanka AK, Rauws EA, et al. Benign biliary strictures. Surgery or endoscopy? Ann Surg. 1993;217(3):237–243. doi: 10.1097/00000658-199303000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soehendra N, Reynders-Frederix V. Palliative bile duct drainage – a new endoscopic method of introducing a transpapillary drain. Endoscopy. 1980;12(1):8–11. doi: 10.1055/s-2007-1021702. [DOI] [PubMed] [Google Scholar]
- 8.Laurence BH, Cotton PB. Decompression of malignant biliary obstruction by duodenoscopic intubation of bile duct. Br Med J. 1980;280(6213):522–523. doi: 10.1136/bmj.280.6213.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huibregtse K, Tytgat GN. Palliative treatment of obstructive jaundice by transpapillary introduction of large bore bile duct endoprosthesis. Gut. 1982;23(5):371–375. doi: 10.1136/gut.23.5.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Speer AG, Cotton PB, MacRae KD. Endoscopic management of malignant biliary obstruction: stents of 10 French gauge are preferable to stents of 8 French gauge. Gastrointest Endosc. 1988;34(5):412–417. doi: 10.1016/s0016-5107(88)71407-8. [DOI] [PubMed] [Google Scholar]
- 11.Huibregtse K, Cheng J, Coene PPLO, Fockens P, Tytgat GNJ. Endoscopic placement of expandable metal stents for biliary strictures – a preliminary report on experience with 33 patients. Endoscopy. 1989;21(6):280–282. doi: 10.1055/s-2007-1012969. [DOI] [PubMed] [Google Scholar]
- 12.Neuhaus H, Hagenmüller F, Classen M. Self-expanding biliary stents: preliminary clinical experience. Endoscopy. 1989;21(5):225–228. doi: 10.1055/s-2007-1012954. [DOI] [PubMed] [Google Scholar]
- 13.Irving JD, Adam A, Dick R, Dondelinger RF, Lunderquist A, Roche A. Gianturco expandable metallic biliary stents: results of a European clinical trial. Radiology. 1989;172(2):321–326. doi: 10.1148/radiology.172.2.2664861. [DOI] [PubMed] [Google Scholar]
- 14.Davids PH, Groen AK, Rauws EA, Tytgat GN, Huibregtse K. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340(8834–8835):1488–1492. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- 15.Carr-Locke DL, Ball TJ, Connors PJ, et al. Multicenter, randomized trial of wallstent biliary endoprosthesis versus plastic stent. Gastrointest Endosc. 1993;39:310A. [Google Scholar]
- 16.Knyrim K, Wagner HJ, Pausch J, Vakil N. A prospective, randomized, controlled trial of metal stents for malignant obstruction of the common bile duct. Endoscopy. 1993;25(3):207–212. doi: 10.1055/s-2007-1010294. [DOI] [PubMed] [Google Scholar]
- 17.Prat F, Chapat O, Ducot B, et al. A randomized trial of endoscopic drainage methods for inoperable malignant strictures of the common bile duct. Gastrointest Endosc. 1998;47(1):1–7. doi: 10.1016/s0016-5107(98)70291-3. [DOI] [PubMed] [Google Scholar]
- 18.Kaassis M, Boyer J, Dumas R, et al. Plastic or metal stents for malignant stricture of the common bile duct? Results of a randomized prospective study. Gastrointest Endosc. 2003;57(2):178–182. doi: 10.1067/mge.2003.66. [DOI] [PubMed] [Google Scholar]
- 19.Moses PL, Alan BN, Gordon SR, Mitty RD, Branch MS, Kowalski TE. A randomized multicenter trial comparing plastic to covered metal stents for the palliation of lower malignant biliary obstruction. Gastrointest Endosc. 2006;63:AB289. [Google Scholar]
- 20.Wagner HJ, Knyrim K, Vakil N, Klose KJ. Plastic endoprostheses versus metal stents in the palliative treatment of malignant hilar biliary obstruction a prospective and randomized trial. Endoscopy. 1993;25(3):213–218. doi: 10.1055/s-2007-1010295. [DOI] [PubMed] [Google Scholar]
- 21.Johanson JF, Schmalz MJ, Geenen JE. Incidence and risk factors for biliary and pancreatic stent migration. Gastrointest Endosc. 1992;38(3):341–346. doi: 10.1016/s0016-5107(92)70429-5. [DOI] [PubMed] [Google Scholar]
- 22.Cheon YK, Oh HC, Cho YD, Lee TY, Shim CS. New 10F soft and pliable polyurethane stents decrease the migration rate compared with conventional 10F polyethylene stents in hilar biliary obstruction: results of a pilot study. Gastrointest Endosc. 2012;75(4):790–797. doi: 10.1016/j.gie.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Sethi A, Gonzalez S, Gonda TA, Poneros JM, Stevens PD. Evaluation of a flexible multiperforated plastic stent in biliary diseases: an initial single center experience. Gastrointest Endosc. 2011;73(4):AB193. [Google Scholar]
- 24.Osterhoff RA, Valadao RM, Bagatelos KC, Ostroff JW. One-year experience with the successful use of a novel plastic double-pigtail biliary stent. Gastrointest Endosc. 2012;75(4):AB312. [Google Scholar]
- 25.Ryou M, Cantillon-Murphy P, Shaikh SN, et al. Magnetic pancreaticobiliary stents and retrieval system: obviating the need for repeat endoscopy (with video) Gastrointest Endosc. 2012;75(4:):888–892. doi: 10.1016/j.gie.2011.09.051. e1. [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni RK, Pani KC, Neuman C, Leonard F. Polylactic acid for surgical implants. Arch Surg. 1966;93(5):839–843. doi: 10.1001/archsurg.1966.01330050143023. [DOI] [PubMed] [Google Scholar]
- 27.Haber G, Freeman ML, Bedford R, Raijman I, Slivka A, Dumot JA. A prospective multi-center study of a bioabsorbable biliary wallstent (BAS) in 50 patients with malignant obstructive jaundice (MOJ) Gastrointest Endosc. 2001;53(5):AB121. [Google Scholar]
- 28.Ginsberg G, Cope C, Shah J, et al. In vivo evaluation of a new bioabsorbable self-expanding biliary stent. Gastrointest Endosc. 2003;58(5):777–784. doi: 10.1016/s0016-5107(03)02016-9. [DOI] [PubMed] [Google Scholar]
- 29.Laukkarinen J, Nordback I, Mikkonen J, Karkkäinen P, Sand J. A novel biodegradable biliary stent in the endoscopic treatment of cystic-duct leakage after cholecystectomy. Gastrointest Endosc. 2007;65(7):1063–1068. doi: 10.1016/j.gie.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto K, Yoshioka T, Furuichi K, et al. Experimental study of poly-L-lactic acid biodegradable stents in normal canine bile ducts. Cardiovasc Intervent Radiol. 2011;34(3):601–608. doi: 10.1007/s00270-010-0045-2. [DOI] [PubMed] [Google Scholar]
- 31.Dowsett JF, Vaira D, Hatfield AR, et al. Endoscopic biliary therapy using the combined percutaneous and endoscopic technique. Gastroenterology. 1989;96(4):1180–1186. doi: 10.1016/0016-5085(89)91639-9. [DOI] [PubMed] [Google Scholar]
- 32.Freeman ML, Overby C. Selective MRCP and CT-targeted drainage of malignant hilar biliary obstruction with self expanding metallic stents. Gastrointest Endosc. 2003;58(1):41–49. doi: 10.1067/mge.2003.292. [DOI] [PubMed] [Google Scholar]
- 33.Singh V, Singh G, Verma GR, Singh K, Gulati M. Contrast-free unilateral endoscopic palliation in malignant hilar biliary obstruction: new method. J Gastroenterol Hepatol. 2004;19(5):589–592. doi: 10.1111/j.1440-1746.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 34.Deviere J, Baize M, de Toeuf J, Cremer M. Long-term followup of patients with hilar malignant stricture treated by endoscopic internal biliary drainage. Gastrointest Endosc. 1988;34(2):95–101. doi: 10.1016/s0016-5107(88)71271-7. [DOI] [PubMed] [Google Scholar]
- 35.Polydorou AA, Chisholm EM, Romanos AA, et al. A comparison of right versus left hepatic duct endoprosthesis insertion in malignant hilar biliary obstruction. Endoscopy. 1989;21(6):266–271. doi: 10.1055/s-2007-1012966. [DOI] [PubMed] [Google Scholar]
- 36.Polydorou AA, Cairns SR, Dowsett JF, et al. Palliation of proximal malignant biliary obstruction by endoscopic endoprosthesis insertion. Gut. 1991;32(6):685–689. doi: 10.1136/gut.32.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang WH, Kortan P, Haber GB. Outcome in patients with bifurcation tumors who undergo unilateral versus bilateral hepatic duct drainage. Gastrointest Endosc. 1998;47(5):354–362. doi: 10.1016/s0016-5107(98)70218-4. [DOI] [PubMed] [Google Scholar]
- 38.De Palma GD, Galloro G, Siciliano S, Iovino P, Catanzano C. Unilateral versus bilateral endoscopic hepatic duct drainage in patients with malignant hilar biliary obstruction: results of a prospective, randomized, and controlled study. Gastrointest Endosc. 2001;53(6):547–553. doi: 10.1067/mge.2001.113381. [DOI] [PubMed] [Google Scholar]
- 39.Naitoh I, Ohara H, Nakazawa T, et al. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24(4):552–557. doi: 10.1111/j.1440-1746.2008.05750.x. [DOI] [PubMed] [Google Scholar]
- 40.Silverman W, Slivka A. New technique for bilateral metal mesh stent insertion to treat hilar cholangiocarcinoma. Gastrointest Endosc. 1996;43(1):61–63. doi: 10.1016/s0016-5107(96)70263-8. [DOI] [PubMed] [Google Scholar]
- 41.Kogure H, Isayama H, Kawakubo K, et al. Endoscopic bilateral metallic stenting for malignant hilar obstruction using newly designed stents. J Hepatobiliary Pancreat Sci. 2011;18(5):653–657. doi: 10.1007/s00534-011-0407-4. [DOI] [PubMed] [Google Scholar]
- 42.Kim DU, Kang DH, Kim GH, et al. Bilateral biliary drainage for malignant hilar obstruction using the ‘stent-in-stent’ method with a Y-stent: efficacy and complications. Eur J Gastroenterol Hepatol. 2013;25(1):99–106. doi: 10.1097/MEG.0b013e3283590a2a. [DOI] [PubMed] [Google Scholar]
- 43.Park do H, Lee SS, Moon JH, et al. Newly designed stent for endoscopic bilateral stent-in-stent placement of metallic stents in patients with malignant hilar biliary strictures: multicenter prospective feasibility study (with videos) Gastrointest Endosc. 2009;69(7):1357–1360. doi: 10.1016/j.gie.2008.12.250. [DOI] [PubMed] [Google Scholar]
- 44.Lee YH, Moon JH, Kim HI, et al. Usefulness of cross wired metalic stents for endoscopic bilateral stent-in-stent placement and revision in patients with high-grade malignant hilar strictures. Gastrointest Endosc. 2012;75:AB381. [Google Scholar]
- 45.Yoo KS, Kim JH, Kim KO, et al. Endoscopic nested Y-shaped self-expanding metal stent placement for advanced hilar cholangiocarcinoma: a study of a novel stent with a unique mesh structure. Gastrointest Endosc. 2012;75:AB388. [Google Scholar]
- 46.Kogure H, Isayama H, Nakai Y, et al. Newly designed large cell Niti-S stent for malignant hilar biliary obstruction: a pilot study. Surg Endosc. 2011;25(2):463–467. doi: 10.1007/s00464-010-1194-8. [DOI] [PubMed] [Google Scholar]
- 47.Chennat J, Waxman I. Initial performance profile of a new 6F self-expanding metal stent for palliation of malignant hilar biliary obstruction. Gastrointest Endosc. 2010;72(3):632–636. doi: 10.1016/j.gie.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 48.Kim HS, Lee DK, Kim HG, et al. Features of malignant biliary obstruction affecting the patency of metallic stents: a multicenter study. Gastrointest Endosc. 2002;55(3):359–365. doi: 10.1067/mge.2002.121603. [DOI] [PubMed] [Google Scholar]
- 49.Kalinowski M, Alfke H, Kleb B, Dürfeld F, Wagner HJ. Paclitaxel inhibits proliferation of cell lines responsible for metal stent obstruction: possible topical application in malignant bile duct obstructions. Invest Radiol. 2002;37(7):399–404. doi: 10.1097/00004424-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Lee DK, Kim HS, Kim KS, et al. The effect on porcine bile duct of a metallic stent covered with a paclitaxel-incorporated membrane. Gastrointest Endosc. 2005;61(2):296–301. doi: 10.1016/s0016-5107(04)02570-2. [DOI] [PubMed] [Google Scholar]
- 51.Lee SS, Shin JH, Han JM, et al. Histologic influence of paclitaxel-eluting covered metallic stents in a canine biliary model. Gastrointest Endosc. 2009;69(6):1140–1147. doi: 10.1016/j.gie.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Suk KT, Kim JW, Kim HS, et al. Human application of a metallic stent covered with a paclitaxel-incorporated membrane for malignant biliary obstruction: multicenter pilot study. Gastrointest Endosc. 2007;66(4):798–803. doi: 10.1016/j.gie.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 53.Song TJ, Lee SS, Yun SC, et al. Paclitaxel-eluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: a prospective comparative pilot study. Gastrointest Endosc. 2011;73(4):727–733. doi: 10.1016/j.gie.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 54.Kim JH, Kim M, Yang S, et al. The effect of multiplex paclitaxel emission stent using pluronic mixture membrane in biliary tract. Gastrointest Endosc. 2011;73(4):AB344. [Google Scholar]
- 55.Jang SI, Kim JH, Kim M, et al. Porcine feasibility and safety study of a new paclitaxel-eluting biliary stent with a pluronic-containing membrane. Endoscopy. 2012;44(9):825–831. doi: 10.1055/s-0032-1309881. [DOI] [PubMed] [Google Scholar]
- 56.Chung MJ, Kim KS, Park JY, et al. The effect on porcine bile duct with metallic stent covered with a gemcitabine-incorporated membrane. Gastrointest Endosc. 2010;71(5):AB162. doi: 10.1016/s0016-5107(04)02570-2. [DOI] [PubMed] [Google Scholar]
- 57.Chung MJ, Kim H, Kim KS, Park S, Chung JB, Park SW. Safety evaluation of self-expanding metallic biliary stents eluting gemcitabine in a porcine model. J Gastroenterol Hepatol. 2012;27(2):261–267. doi: 10.1111/j.1440-1746.2011.06866.x. [DOI] [PubMed] [Google Scholar]
- 58.Won JH, Lee JD, Wang HJ, et al. Effects of a holmium-166 incorporated covered stent placement in normal canine common bile ducts. J Vasc Interv Radiol. 2005;16(5):705–711. doi: 10.1097/01.RVI.0000153113.87723.43. [DOI] [PubMed] [Google Scholar]
- 59.van Berkel AM, van Marle J, Groen AK, Bruno MJ. Mechanisms of biliary stent clogging: confocal laser scanning and scanning electron microscopy. Endoscopy. 2005;37(8):729–734. doi: 10.1055/s-2005-870131. [DOI] [PubMed] [Google Scholar]
- 60.Dua KS, Reddy ND, Rao VG, et al. Impact of reducing duodenobiliary reflux on biliary stent patency: an in vitro evaluation and a prospective randomized clinical trial that used a biliary stent with an antireflux valve. Gastrointest Endosc. 2007;65(6):819–828. doi: 10.1016/j.gie.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Hu B, Wang TT, Shi ZM, et al. A novel antireflux metal stent for the palliation of biliary malignancies: a pilot feasibility study (with video) Gastrointest Endosc. 2011;73(1):143–148. doi: 10.1016/j.gie.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 62.Hu B, Wu J, Gao DJ, Wang TT. The role of anti-reflux biliary stent: a prospective randomized study in 104 cases. Gastrointest Endosc. 2012;75(4):AB122. [Google Scholar]
- 63.Costamagna G, Pandolf M, Mutignani M, Spada C, Perri V. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54(2):162–168. doi: 10.1067/mge.2001.116876. [DOI] [PubMed] [Google Scholar]
- 64.Bergman JJ, Burgemeister L, Bruno MJ, et al. Long-term follow-up after biliary stent placement for postoperative bile duct stenosis. Gastrointest Endosc. 2001;54(2):154–161. doi: 10.1067/mge.2001.116455. [DOI] [PubMed] [Google Scholar]
- 65.Somogyi L, Chuttani R, Croffie J, et al. Biliary and pancreatic stents. Gastrointest Endosc. 2006;63(7):910–919. doi: 10.1016/j.gie.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Farnbacher MJ, Rabenstein T, Ell C, Hahn EG, Schneider HT. Is endoscopic drainage of common bile duct stenoses in chronic pancreatitis up-to-date? Am J Gastroenterol. 2000;95(6):1466–1471. doi: 10.1111/j.1572-0241.2000.02078.x. [DOI] [PubMed] [Google Scholar]
- 67.Kasher JA, Corasanti JG, Tarnasky PR, McHenry L, Fogel E, Cunningham J. A multicenter analysis of safety and outcome of removal of a fully covered self-expandable metal stent during ERCP. Gastrointest Endosc. 2011;73(6):1292–1297. doi: 10.1016/j.gie.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 68.Irani S, Akbar A, Kozarek RA, et al. Endoscopic treatment of benign biliary diseases using covered self-expandable metal stents (CSEMS) Gastrointest Endosc. 2012;75:AB390–AB391. [Google Scholar]
- 69.Mahajan A, Ho H, Sauer B, et al. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video) Gastrointest Endosc. 2009;70(2):303–309. doi: 10.1016/j.gie.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Perri V, Boškoski I, Tringali A, et al. Fully covered self-expandable metal stents in biliary strictures caused by chronic pancreatitis not responding to plastic stenting: a prospective study with 2 years of follow-up. Gastrointest Endosc. 2012;75(6):1271–1277. doi: 10.1016/j.gie.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Deviere JM, Reddy DN, Puspok A, et al. Preliminary results from a 187 patient multicenter prospective trial using metal stents for treatment of benign biliary strictures. Gastrointest Endosc. 2012;75(4):AB123. [Google Scholar]
- 72.Akbar A, Irani S, Baron TH, et al. Use of covered self-expandable metal stents for endoscopic management of benign biliary disease not related to stricture (with video) Gastrointest Endosc. 2012;76(1):196–201. doi: 10.1016/j.gie.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 73.Canena J, Liberato M, Horta D, Romão C, Coutinho A. Short-term stenting using fully covered self-expandable metal stents for treatment of refractory biliary leaks, postsphincterotomy bleeding, and perforations. Surg Endosc. 2013;27(1):313–324. doi: 10.1007/s00464-012-2368-3. [DOI] [PubMed] [Google Scholar]
- 74.Aslinia F, Hawkins L, Darwin P, Goldberg E. Temporary placement of a fully covered metal stent to tamponade bleeding from endoscopic papillary balloon dilation. Gastrointest Endosc. 2012;76(4):911–913. doi: 10.1016/j.gie.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 75.Poley JW, van Tilburg AJP, Kuipers EJ, Bruno MJ. Breaking the barrier: using extractable fully covered metal stents to treat benign hilar strictures. Gastrointest Endosc. 2011;74(4):916–920. doi: 10.1016/j.gie.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 76.Poley JW, Cahen DL, Metselaar HJ, et al. A prospective group sequential study evaluating a new type of fully covered self-expandable metal stent for the treatment of benign biliary strictures (with video) Gastrointest Endosc. 2012;75(4):783–789. doi: 10.1016/j.gie.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 77.Hu B, Gao DJ, Wu J. A novel dedicated metal stent for the resolution of benign bile duct strictures. Gastrointest Endosc. 2012;75(4:):AB375–AB376. [Google Scholar]
- 78.Hu B, Gao DJ, Yu FH, Wang TT, Pan YM, Yang XM. Endoscopic stenting for post-transplant biliary strictures: usefulness of a novel removable covered metal stent. J Hepatobiliary Pancreat Sci. 2011;18(5):640–645. doi: 10.1007/s00534-011-0408-3. [DOI] [PubMed] [Google Scholar]


