Abstract
AIM: To investigate the effect of glycyrrhizic acid (GA) on carbon tetrachloride (CCl4)-induced hepatocyte apoptosis in rats via a p53-dependent mitochondrial pathway.
METHODS: Forty-five male Sprague-Dawley rats were randomly and equally divided into three groups, the control group, the CCl4 group, and the GA treatment group. To induce liver fibrosis in this model, rats were given a subcutaneous injection of a 40% solution of CCl4 in olive oil at a dose of 0.3 mL/100 g body weight biweekly for 8 wk, while controls received the same isovolumetric dose of olive oil by hypodermic injection, with an initial double-dose injection. In the GA group, rats were also treated with a 40% solution of CCl4 plus 0.2% GA solution in double distilled water by the intraperitoneal injection of 3 mL per rat three times a week from the first week following previously published methods, with modifications. Controls were given the same isovolumetric dose of double distilled water. Liver function parameters, such as alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined. Pathologic changes in the liver were detected by hematoxylin and eosin staining. Collagen fibers were evaluated by Sirius red staining. Hepatocyte apoptosis was investigated using the terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate nick end labeling (TUNEL) assay and the cleaved caspase-3 immunohistochemistry assay. The expression levels of p53 and apoptosis-related proteins were evaluated by immunohistochemistry or Western blotting analysis.
RESULTS: After 8 wk of treatment, GA significantly reduced serum activity of ALT (from 526.7 ± 57.2 to 342 ± 44.8, P < 0.05) and AST (from 640 ± 33.7 to 462.8 ± 30.6, P < 0.05), attenuated the changes in liver histopathology and reduced the staging score (from 3.53 ± 0.74 to 3.00 ± 0.76, P < 0.05) in CCl4-treated rats. GA markedly reduced the positive area of Sirius red and the ratio of the hepatic fibrotic region (from 7.87% ± 0.66% to 3.68% ± 0.32%, P < 0.05) compared with the CCl4 group. GA also decreased the expression level of cleaved caspase-3 compared to the CCl4 group. TUNEL assay indicated that GA significantly diminished the number of TUNEL-positive cells compared with the CCl4 group (P < 0.05). GA treatment clearly decreased the level of p53 (P < 0.05) detected by immunohistochemistry and Western blotting analysis. Compared with the CCl4 group, we also found that GA reduced the Bax/Bcl-2 ratio (P < 0.05), the expression of cleaved caspase-3 (P < 0.05), cleaved caspase-9 (P < 0.05), and inhibited cytochrome C and second mitochondria-derived activator of caspases (Smac) release from mitochondria to cytoplasm, i.e., GA reduced the expression level of Smac, which inhibited c-IAP1 activity (P < 0.05), ultimately inhibiting the activity of caspase-3, according to Western blotting analysis. As a result, GA suppressed activation of the caspase cascades and prevented hepatocyte apoptosis.
CONCLUSION: GA can inhibit CCl4-induced hepatocyte apoptosis via a p53-dependent mitochondrial pathway to retard the progress of liver fibrosis in rats.
Keywords: P53, Apoptosis, Liver fibrosis, Glycyrrhizic acid, Mitochondria
Core tip: This study is the first to investigate the effects of glycyrrhizic acid (GA) on p53-dependent apoptosis in carbon tetrachloride (CCl4)-induced hepatic injury. The results indicated that GA can attenuate hepatocyte apoptosis via a p53-mediated mitochondrial pathway and retard the progression of liver fibrosis induced by CCl4 in rats.
INTRODUCTION
Liver fibrosis, induced by various pathological factors, is a common outcome in many chronic liver diseases, and is a serious threat to human health. It is known that the foundation of liver fibrosis is the imbalance between synthesis and degradation of extracellular matrix (including collagen, glycoproteins, polysaccharides, amines, etc.).
It has been shown that hepatocyte apoptosis can induce liver fibrosis[1-3]. Hepatocyte apoptosis is a major form of cell death which is primarily triggered by activation of the caspase family of cysteine proteases during the progression of chronic liver disease[4]. Many reports have shown that p53 is accumulated in hepatocytes in several fibrotic liver diseases[5-7]. The protein p53 can lead to apoptosis predominantly through p53-regulated genes such as P21, PUMA, NOXA and Bax[8]. The intensity of inflammation induces pro-apoptotic protein p53 with inhibition of anti-apoptotic Bcl-2 in non-alcoholic fatty liver disease[5]. Thioacetamide activates p53, increases caspase-3, Bax and Bad protein contents, and possibly causes the release of cytochrome C from mitochondria and the disintegration of membranes, eventually leading to apoptosis of cells in thioacetamide (TAA)-induced liver fibrosis and cirrhosis[9]. The pro-apoptotic protein, Bax, is a positive regulator and the anti-apoptotic protein, Bcl-xL, is a negative regulator that regulates the release of cytochrome C from mitochondria to the cytoplasm[10,11]. The presence of Bax protein is a direct result of the release of cytochrome C from mitochondria and activation of caspase-9[12]. Inhibitors of apoptosis proteins (IAPs), which regulate apoptosis through various factors, play a vital role in inhibition of the apoptotic process[13]. c-IAP1, c-IAP2 and Survivin, as key members of IAPs, can inhibit the activity of caspase-3 and -7, thus blocking cell apoptosis[14,15]. During the apoptotic process, second mitochondria-derived activator of caspases (Smac), released from mitochondria into the cytoplasm, bind and antagonize IAPs, subsequently reducing the inhibition of caspases by IAPs resulting in apoptosis[16-18]. p53 activation enhances X-IAP inhibition-induced cell death by promoting mitochondrial release of Smac[19]. Therefore, inhibiting p53-dependent hepatocyte apoptosis may be an effective therapeutic strategy for the treatment and prevention of hepatic fibrosis.
Chinese herbal medicine has been widely used to cure diseases for thousands of years in China, especially chronic liver diseases. In recent years, the efficacy of Chinese herbal medicine has been appraised by modern biological technology[20,21]. Glycyrrhizic acid (GA), also known as Glycyrrhizin[22], is the major bioactive component of licorice root extract. GA, a glycosylated saponin, which has one molecule of glycyrrhetinic acid and two molecules of glucuronic acid, has adrenal cortex hormone-like effects[23,24]. GA has numerous pharmacologic effects, such as anti-inflammatory, anti-viral, anti-tumor and hepatoprotective activities[25]. GA also exerts an anti-apoptotic effect through the inhibition of hepatocyte apoptosis[26,27]. Recent findings indicate that GA significantly inhibits hepatocyte apoptosis by down-regulating the expression of caspase-3 and inhibiting the release of cytochrome C from mitochondria into the cytoplasm[28].
It has been reported that carbon tetrachloride (CCl4) can induce hepatocyte apoptosis and liver fibrosis in animal models[29-33]. The damage responses, induced by CCl4 injection in rat and mouse models, are similar to liver cirrhosis in humans[34]. Thus, we presumed here that GA treatment started from the early stage of chronic liver disease could effectively attenuate hepatocyte apoptosis, consequently inhibit liver fibrosis and retard disease progression in rats. This study sought to investigate the effects of GA on p53-dependent apoptosis in CCl4-induced hepatic injury.
MATERIALS AND METHODS
Materials
GA was purchased from Sigma (St Louis, MO, United States). Anti-caspase-3, anti-caspase-9, anti-c-IAP1, anti-cytochrome C, anti-Smac, anti-Bcl-2, anti-Bax and anti-COXIV antibodies were purchased from Cell Signaling Technology (Beverly, MA, United States). Anti-GADPH and anti-p53 antibodies were bought from Abcam (Cambridge, United Kingdom), horseradish peroxidase-conjugated anti-mouse and anti-rabbit immunoglobin G antibodies were purchased from Cell Signaling Technology. The chemiluminescence reaction kit (ECL Plus) was purchased from Millipore (Billerica, MA, United States). Anti-cleaved-caspase-3 antibody and the mitochondria/cytoplasm fractionation kit were purchased from Beyotime Biotechnology (Haimen, Jiangsu Province, China).
Animal model of liver fibrosis and treatment
Male SD rats weighing 150-200 g were purchased from the Experimental Animal Center of Zhongshan Hospital, Fudan University. Rats were kept in a temperature-controlled room with an alternating 12-h dark and light cycle. Forty-five rats were randomly and equally divided into three groups, the control group, the CCl4 group, and the GA treatment group. To induce liver fibrosis in this model, rats were given a subcutaneous injection of a 40% solution of CCl4 (Wako Pure Chemical, Osaka, Japan) in olive oil at a dose of 0.3 mL/100 g body weight biweekly for 8 wk, while controls received the same isovolumetric dose of olive oil by hypodermic injection, with an initial double-dose injection. In the GA group, rats were also treated with a 40% solution of CCl4 plus 0.2% GA solution in double distilled water by the intraperitoneal injection of 3 mL per rat three times a week from the first week following previously published methods[35,36], with modifications. Controls were given the same isovolumetric dose of double distilled water. Animals were sacrificed 24 h after the last injection. Blood was obtained from the left ventricular apex for measurements of aminotransferases and the samples were stored at -20 °C. The liver was removed and rinsed with 0.9% saline, some liver sections were fixed in 10% buffered formaldehyde and embedded in paraffin for, and the remaining liver was stored at -70 °C for protein experiments.
Liver function
Blood was centrifuged at 3500 g at 4 °C for 10 min to separate the plasma. The activity of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were detected using a Siemens Advia 1650 automatic analyzer.
Sirius-red and hematoxylin and eosin staining
The thick sections (5 μm) were stained with hematoxylin and eosin (HE) and Sirius-red. HE staining was performed to assess pathologic changes in the liver. The standard of pathological grade was according to consensus on evaluation of the diagnosis and severity of hepatic fibrosis[37]. Sirius-red staining was performed to detect hepatic fibrosis. The Sirius red-positive areas were assessed in four different fields for each section by Image J Software (National Institutes of Health, Bethesda, MD, United States) and were in accordance with the following expression (collagen area/total area-vascular lumen area) × 100[38].
Immunohistochemical staining
Liver tissue sections were subjected to dewaxing, hydration and thermal induction antigen retrieval. Slices were blocked and incubated with anti-p53 antibody (1:50) and anti-cleaved-caspase-3 antibody (1:100) which were diluted in TBS-5% bovine serum albumin (BSA) at 4 °C overnight. Negative-control antibody was species-matched. The following day, the slices were washed and incubated with secondary antibodies. The slices were then incubated with 3, 3’-diaminobenzidine tetrachloride for 5-10 min to develop the color, and staining was observed under light microscopy (Olympus, Japan).
Terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate nick end labeling assay
The terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate nick end labeling (TUNEL) assay (Roche, Germany) was performed in accordance with the manufacturer’s protocol. Nuclei were redyed with 4,6-diamidino-2-phenylindole (DAPI) staining. Cells marked by TUNEL were evaluated using fluorescence microscopy (Olympus, Japan).
Protein preparation
Mitochondria were isolated with a tissue mitochondria isolation kit according to the manufacturer’s instructions. During mitochondria preparation, all samples were placed on ice. Eighty mg liver tissue was cut into pieces, tissue mitochondria isolation reagent A with phenylmethylsulfonyl fluoride (PMSF) was added, and then homogenized in an ice bath approximately 10 times. The homogenate was centrifuged at 600 rpm at 4 °C for 5 min. The supernatant was then collected and centrifuged at 11000 g at 4 °C for 10 min. The supernatant contained the cytoplasmic protein, and the precipitate contained the mitochondria. The cytoplasmic and mitochondrial fractions of the lysate were estimated by Western blotting. Liver tissues were homogenized in RIPA Lysis Buffer with PMSF and then centrifuged at 12000 g for 15 min at 4 °C, and the supernatant was the total protein.
Western blotting analysis
Proteins were separated by 10% or 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes (Millipore). The membranes were blocked with 5% BSA for 2 h, and then incubated overnight at 4 °C with rabbit anti-caspase-9, anti-caspase-3, anti-Smac, anti-cytochrome C, anti-c-IAP1, anti-Bcl-2, anti-Bax antibodies and mouse anti-p53, anti-GAPDH and anti-COXIV antibodies. The membranes were then incubated with HRP-conjugated goat anti-rabbit IgG and goat anti-mouse IgG (1:5000, diluted) at room temperature for 2 h, and then washed again and detected by the enhanced chemiluminescence (ECL) reaction. The intensities of the bands were analyzed by Image J software.
Statistical analysis
Each experiment was repeated at least 3 times. Data were estimated using analysis of variance and all values are expressed as mean ± SD. A P value < 0.05 was considered significant. All analyses in the study were implemented by SPSS 11.5 software for Windows (Chicago, IL, United States).
RESULTS
Function of GA on serum parameters of hepatic fibrosis induced by CCl4
The activities of ALT and AST were significantly increased in the CCl4 treated group compared with those in the control group (P < 0.05). In the GA group, the activities of ALT and AST were markedly decreased compared with those in rats with liver fibrosis not treated with GA (P < 0.05) (Table 1).
Table 1.
Effect of glycyrrhizic acid on plasma alanine aminotransferase and aspartate aminotransferase activity in CCl4-induced rats
| Group | ALT (U/L) | AST (U/L) |
| Control | 42.4 ± 6.0 | 70.2 ± 2.3 |
| CCl4 | 526.7 ± 57.2 | 640 ± 33.7 |
| GA | 342 ± 44.8a | 462.8 ± 30.6a |
P < 0.05 vs the carbon tetrachloride (CCl4) group. GA: Glycyrrhizic acid; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
Role of GA in the improvement of liver fibrosis induced by CCl4
After 8 wk of CCl4 administration, liver histopathology was significantly changed in the CCl4 group. The livers, in the control group, showed an integrated lobular structure with central venous and hepatic cord radiation (Figure 1). The staging score was 0 (Table 2). The positive area of Sirius red staining in the control group was around the central vein rather than in the hepatic parenchyma. There were numerous Steatosis and ballooning of hepatocytes in the GA and CCl4 groups. In the CCl4 group, the liver showed fibrous connective tissue proliferation, fiber interval formation which was associated with disorder of lobular structure in the portal area, and most rat livers appeared to have pseudo lobules (Figure 1). The score of hepatic fibrosis in the CCl4 group increased to 3.53 ± 0.74 (Table 2). The positive areas of Sirius red staining in the CCl4 group were in the boundaries of the hepatic lobules and the ratio of the hepatic fibrotic region was 7.87% ± 0.66%. In the GA group, livers appeared to have fibrous connective tissue proliferation, the formation of a few fiber intervals in the portal area, and the occasional pseudo lobule (Figure 1). The score was 3.00 ± 0.76 (P < 0.05) in the GA group (Table 2). The positive area of Sirius red staining in the GA group was decreased, and the ratio of the hepatic fibrotic region (3.68% ± 0.32%, P < 0.05) was reduced compared with the CCl4 group (Figure 1).
Figure 1.
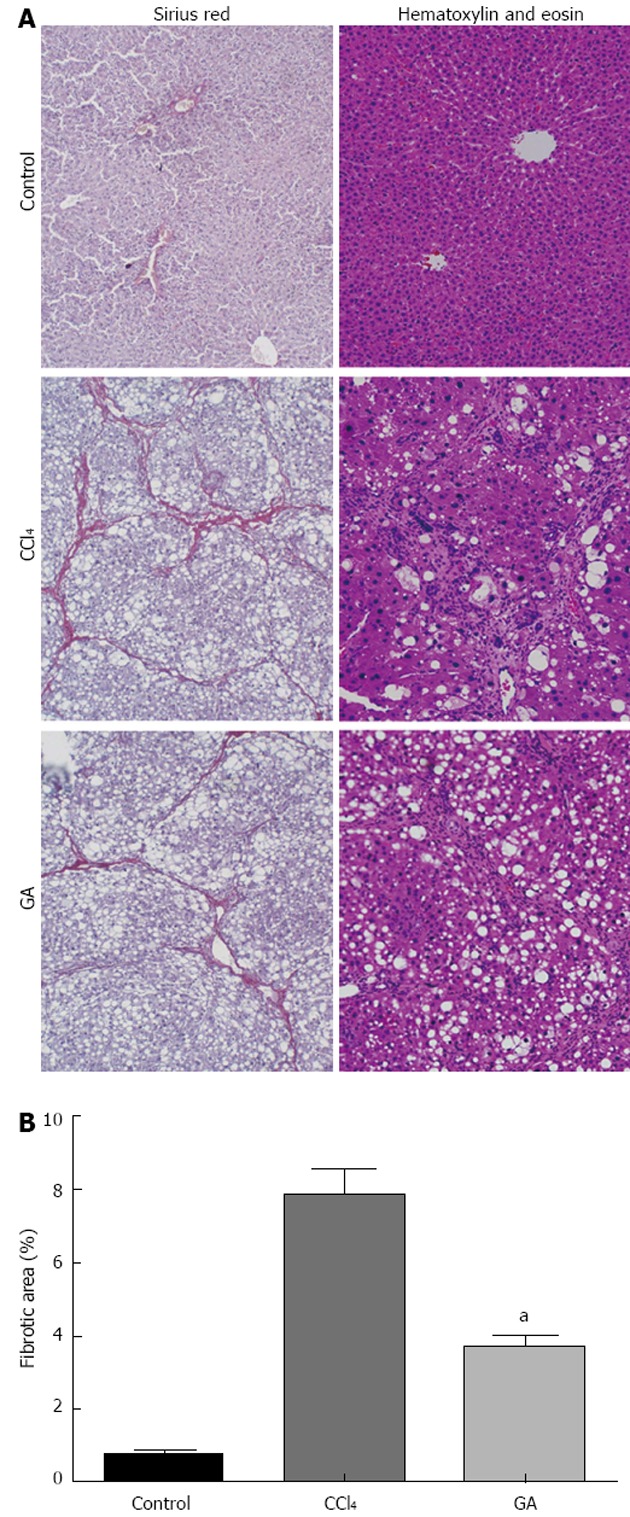
Histological examination of liver by hematoxylin and eosin and Sirius red staining. A: Histological examination. Rats were treated with carbon tetrachloride (CCl4) and/or glycyrrhizic acid (GA). Liver tissue sections were stained with hematoxylin and eosin or Sirius red (original magnification, × 100); B: Quantitative analysis of liver fibrosis by Sirius red staining. Results are represented as fibrotic area (%), which signifies the proportion of area stained red/area of total area-vascular lumen. Values are mean ± SD. aP < 0.05 vs CCl4.
Table 2.
Histopathological semiquantitative scores in the liver
| Group | n | 0 | + 1 | + 2 | + 3 | + 4 | Staging scores |
| Control | 15 | 15 | 0 | 0 | 0 | 0 | 0 |
| CCl4 | 15 | 0 | 0 | 2 | 3 | 10 | 3.53 ± 0.74 |
| GA | 15 | 0 | 10 | 1 | 10 | 3 | 3.00 ± 0.76a |
P < 0.05 vs the carbon tetrachloride (CCl4) group. GA: Glycyrrhizic acid.
Impact of GA on hepatic apoptosis induced by CCl4
The expression level of cleaved caspase-3 was high in the livers of rats in the CCl4 group. Interestingly, this level was reduced in the GA-treated group as detected by immunohistochemistry (Figure 2A). Under fluorescence microscopy, the TUNEL assays showed no stain and non-apoptotic nuclei in the normal liver tissue. High quantities of TUNEL cells were observed in the livers of the CCl4 group and numerous condensed and fragmented nuclei. In the GA-treated group, there were few TUNEL cells, and less DAPI staining was observed in the same slice. The merged images indicated that TUNEL-positive cells were different, as numerous fused cells were observed in the CCl4 group, while a significant reduction in these cells was detected in the GA-treated group (Figure 2B). Overall, these findings indicated that GA reduced apoptosis in liver lesion progression.
Figure 2.
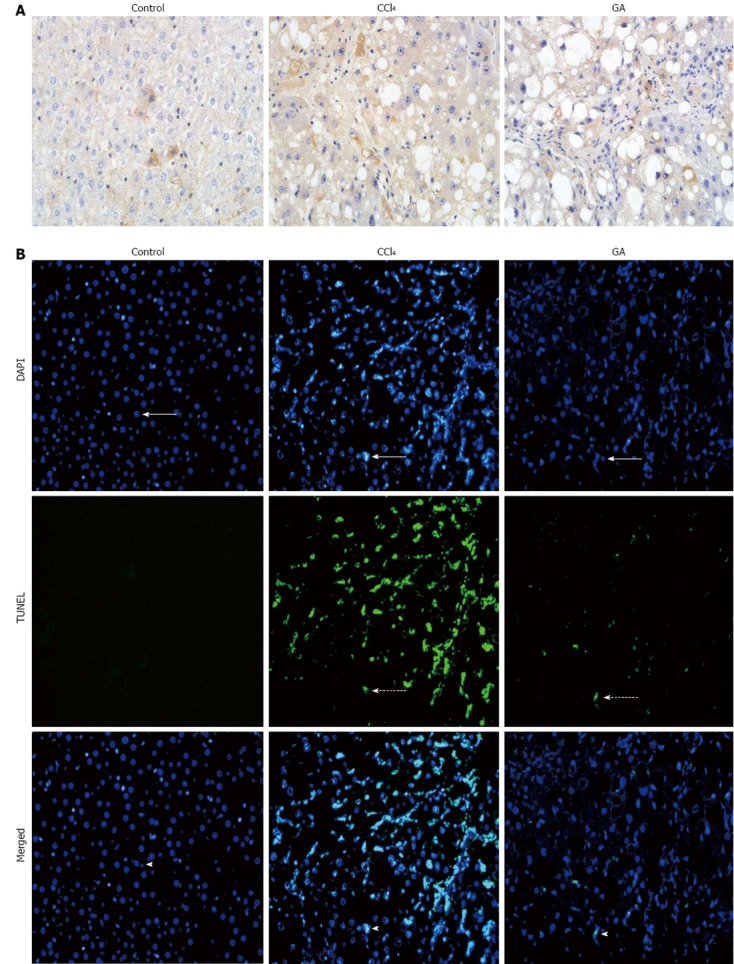
Impact of glycyrrhizic acid treatment on hepatic apoptosis induced by carbon tetrachloride in rats. A: Liver tissue sections from the different groups were subjected to immunohistochemistry to determine the expression level of cleaved caspase-3 (original magnification, × 400); B: Fluorescence microscopy image showing terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate nick end labeling (TUNEL) stain (dashed arrows), and the same tissue slices were respectively counterstained with 4’6-diamidino-2-phenylindole (DAPI) to localize the nuclei (arrows). Images of combined with DAPI, indicated TUNEL-positive cells (arrow heads) (original magnification, × 200). GA: Glycyrrhizic acid; CCl4: Carbon tetrachloride.
Effect of GA on the level of proteins induced by CCl4
The level of p53 was significantly higher in the livers of rats in the CCl4 group than in the other two groups as detected by immunohistochemical staining, while in the GA group, the expression level of p53 was reduced (Figure 3A). This is consistent with the Western blotting analysis (Figure 3B) which showed that p53 was activated in the CCl4 group and clearly reduced in the GA group.
Figure 3.
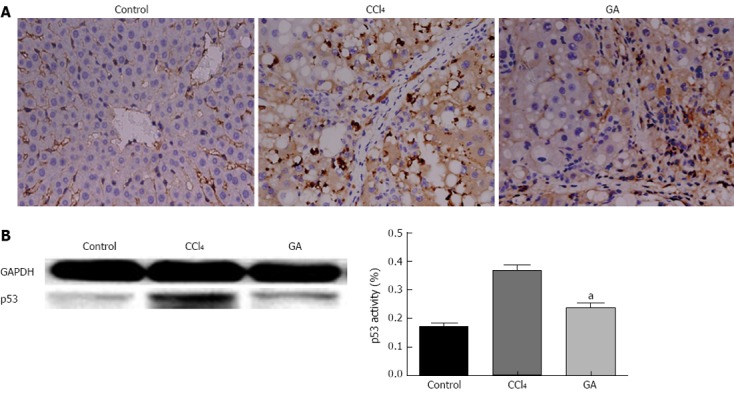
Effect of glycyrrhizic acid treatment on the expression level of p53 in the livers of rats injured by carbon tetrachloride. A: Liver tissue slices from the different groups were subjected to immunohistochemistry (original magnification, × 400). B: Total protein fractions prepared from livers were analyzed by Western blotting to assess the expression level of p53 and GAPDH to confirm the same sample loading. The results of Western blotting analysis were similar in at least three replicate independent experiments. All values are presented as mean ± SD. Statistical significance was defined as follows: aP < 0.05 vs the carbon tetrachloride (CCl4) group. GA: Glycyrrhizic acid.
We examined the impact of GA on Bcl-2 and Bax protein expression in CCl4-induced liver injury by Western blot analysis. As shown in Figure 4A, the expression level of the anti-apoptotic protein, Bcl-2, was decreased, while the expression of the pro-apoptotic protein, Bax, was increased in mitochondrial fraction of CCl4-induced hepatic injury, and the Bax/Bcl-2 ratio was elevated in the CCl4 group. In contrast, GA reversed the expression levels of Bcl-2 and Bax, and improved the Bax/Bcl-2 ratio. Both pro- and anti-apoptotic Bcl-2 proteins regulate cytochrome C from mitochondria to cytoplasm. The cytoplasmic fraction in the control group contained a negligible amount of cytochrome C. However, cytochrome C accumulated in the cytoplasm of liver tissue in the CCl4 group, and GA inhibited the release of cytochrome C (Figure 4B). Caspase activation plays an important role in apoptosis, and caspase-3 cleavage is a typical feature of apoptosis[39]. In the current study, we found that there was increased cleavage of caspase-9 (37 kDa) and caspase-3 (17 kDa) in the CCl4 group, suggesting severe apoptosis. Intriguingly, the levels of caspase-9 and caspase-3 cleavage diminished in the GA group (Figure 4C and D).
Figure 4.
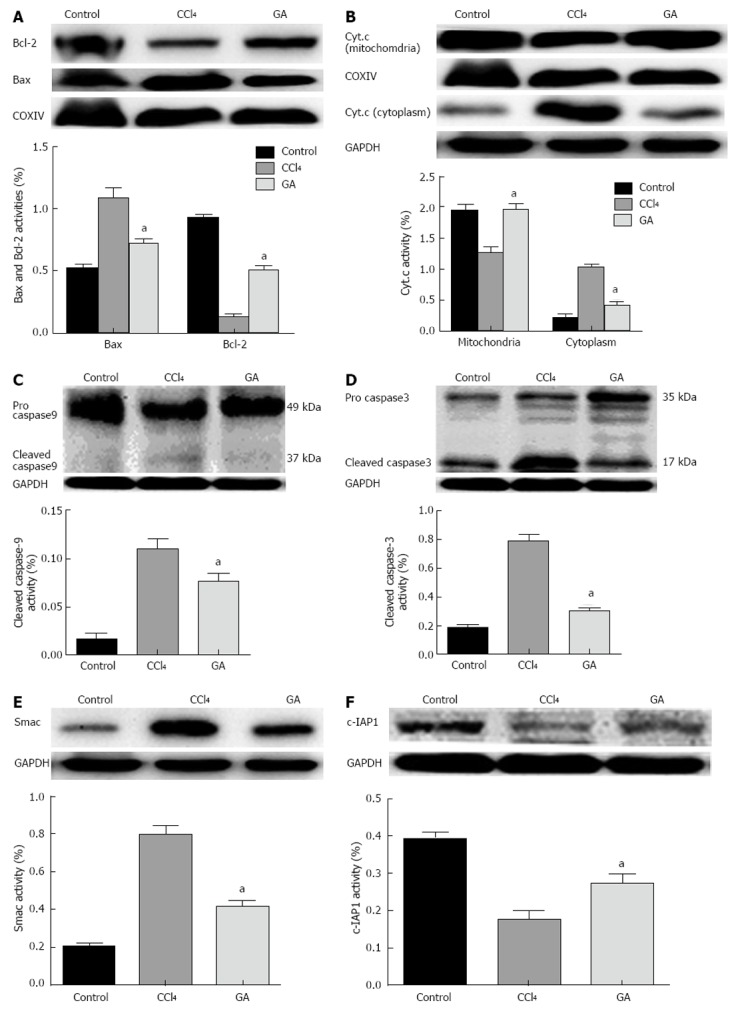
Impact of glycyrrhizic acid on CCl4-treated hepatocyte apoptosis signal cascades. Protein extracts from livers in the different groups were subjected to Western blotting. A: Expression levels of Bax and Bcl-2 in the mitochondria; B: Expression levels of cytochrome C (Cyt.c) in the cytoplasm and mitochondria; C: Expression level of caspase-9 in the total protein; D: Expression level of caspase-3 in the total protein; E: Expression level of Smac in the cytoplasm; F: Expression level of c-IAP1 the total protein. In all these experiments glyceraldehyde-3-phosphate dehydrogenase (GAPDH), COXIV were used to ensure equal sample loading. The Western blotting results represent three independent tests. The bar graph represents the value of in the different proteins via the density of bands from at least three independent tests. All values are presented as mean ± SD. Statistical significant was defined as follows: aP < 0.05 vs the CCl4 group. GA: Glycyrrhizic acid; CCl4: Carbon tetrachloride.
We also found that the cytoplasmic fraction in the control group contained a negligible amount of Smac. However, Smac accumulated in the cytoplasm of livers in rats exposed to CCl4. GA treatment significantly inhibited the release of Smac induced by CCl4 (Figure 4E). The expression level of c-IAP1 corresponded to the decreased expression of Smac in the GA-treated group compared with the CCl4 group (Figure 4F), and the consequence was in accordance with the view that Smac has an antergic effect on c-IAP1 activity which can inhibit the activity of caspases[16-18]. These results indicated that GA could prevent CCl4-induced apoptosis by suppressing the activation of upstream caspase-3. GA treatment ameliorated CCl4-induced hepatic injury, and indicated the involvement of the p53 pathway in CCl4-induced hepatocyte apoptosis.
DISCUSSION
Liver fibrosis is a common outcome in many chronic liver diseases. Liver fibrosis and cirrhosis, as shown in recent studies, are reversible processes[40,41]. However, there have been few effective therapies for the treatment of hepatic fibrosis in recent years[42]. There is an urgent need to investigate the effect of innocuous anti-fibrotic agents[43]. CCl4-induced liver injury is one of the best-characterized models of hepatotoxicity, and can be used in the clinic to examine anti-hepatotoxic and/or hepatoprotective drugs[44].
GA, used in the treatment and control of chronic viral hepatitis, is now routinely used in Japan, due to its well-recognized transaminase-lowering effect in clinical applications[25,45,46]. Neominophagen C is a Japanese preparation containing 0.2% glycyrrhizin, 0.1% cysteine, and 2% glyceine, and mainly acts as an anti-inflammatory or cytoprotective drug rather than an antiviral. It can improve mortality in patients with subacute liver failure and ameliorate liver function in patients with subacute hepatic failure, chronic hepatitis, and cirrhosis[47].
Apoptosis is one of the events involved in the process of liver fibrosis. Thus, factors that affect apoptosis may be used to modulate liver fibrosis[33]. A line of evidence has shown that loss of p53 function is a common and considerable occurrence in the development of many human malignancies. In unstressed cells, expression of p53 is regulated and maintained at a low level through the ubiquitin/proteasome pathway[48]. Endogenous p53 activation in hepatocytes causes spontaneous liver fibrosis in double minute 2-knockout mice[3]. It also appears to modulate ethanol-induced hepatocyte apoptosis, since it was completely abrogated in mice with a p53 null background[49]. Mitochondria react to different cytotoxic stimuli, are central death regulators and play a vital role in p53-dependent death, in other words, the p53-dependent signal induces cell death through the mitochondrial pathway[50,51]. When the death signal is conducted to the mitochondria, the cell membrane permeability is increased and apoptosis-related proteins are released[52].
Many reports have demonstrated that drugs can ameliorate CCl4-mediated hepatic apoptosis in rats, such as branched-chain amino acids[32] and the water-soluble extract of Salvia miltiorrhiza[33]. GA has an anti-apoptotic effect through the inhibition of hepatic apoptosis[26,27]. It significantly inhibited hepatocyte apoptosis by down-regulating the expression of caspase-3 and inhibiting the release of cytochrome C from mitochondria into the cytoplasm[28]. GA can alter Kaposi sarcoma-associated herpesvirus latency by triggering p53-mediated apoptosis[53]. Here we demonstrated that intervention with GA from the early stage of chronic liver disease effectively attenuated p53-dependent hepatocyte apoptosis and liver fibrosis, thus retarding disease progression in rats.
Apoptosis and necrosis contribute to the process of liver fibrosis[29,33]. Whether necrotic liver injury or apoptosis is dominant in CCl4-induced liver injury models remains controversial. A previous study showed that CCl4 can induce acute hepatocellular damage which is characterized by necrotic cell death[54], while another study indicated that a substantial number of hepatocytes undergo apoptosis in the acute stage after CCl4 administration[29]. In the present study, we found both apoptosis and necrosis occurred in the CCl4-induced chronic liver injury model. These results were consistent with other reports[32,33]. Discrepancies may be attributed to the time points of observation.
Steatosis and ballooning of hepatocytes are the earliest, most frequent, and most striking pathological changes observed in CCl4-induced liver injury[29,55,56], and we found this pathological change using H and E staining. According to immunohistochemical staining, p53 expression level was significantly increased in the CCl4 group compared with the GA group. Western blot analysis showed that p53 was sharply up-regulated in the CCl4 group compared to the GA group. This indicated that p53 was activated after CCl4 administration, however, GA reduced the expression level of p53.
To date, TUNEL assay[27], cleaved caspase-3 immunohistochemical staining[57] and serum CK18 fragment[58] have been identified as the markers of apoptosis. In the study we first detected DNA fragmentation of hepatocytes using the TUNEL assay. TUNEL-positive cells in the CCl4 group were significantly increased compared with the GA group. GA reduced the number of TUNEL-labeled cells[27]. However, the TUNEL assay is not a specific marker of apoptosis, thus we performed cleaved caspase-3 immunohistochemical staining. The results coincided with those from the TUNEL assay. Apoptosis, a form of cell death, is principally caused by activation of the caspase family of cysteine proteases[4]. In accordance with Western blotting analysis, accompanied by the reduction in p53, the expression level of Bcl-2 was sharply decreased and the expression level of Bax was obviously increased in the mitochondrial fraction of the CCl4 group, and the Bax/Bcl-2 ratio was elevated, while this tendency was reversed in the GA-treated group. Our results demonstrated that GA suppressed p53 activity, resulting in an increase in Bcl-2 and a decrease in Bax. In addition, GA inhibited the release of cytochrome C into the cytoplasm from mitochondria, and then inactivated caspase-9 and caspase-3. GA also reduced the expression of Smac, which was released from mitochondria, and bound to and antagonized c-IAP1, subsequently increased the inhibitory effect of c-IAP1 on caspase-3 and finally suppressed hepatocyte apoptosis. The degree of hepatic injury was associated with a substantial number of hepatocytes undergoing apoptosis[27]. The results also demonstrated that hepatic injury in the CCl4 group was more serious than that in the GA group on the basis of histological observation, Sirius red staining assay, serum transaminase and TUNEL analyses. To our knowledge, these findings were to report that the effects of GA on p53-mediated activity in hepatocyte apoptosis in the liver of CCl4-treated rats. Whether other mechanisms or pathways are involved in liver fibrosis requires further exploration.
In summary, our findings showed that GA exerted anti-apoptotic effects via a p53-dependent mitochondrial pathway (Figure 5). GA protected against CCl4-induced hepatocyte apoptosis by regulating the Bcl-2 family of proteins, expression of Smac and caspase cleavage. These anti-apoptotic effects were related to decreases in the expression of pro-apoptotic proteins in the cytoplasm and the inhibition of proteins associated with apoptosis in the mitochondria. These findings suggest that GA can attenuate CCl4-induced hepatocyte apoptosis via a p53-mediated mitochondrial pathway and can retard the progression of liver fibrosis induced by CCl4 in rats.
Figure 5.
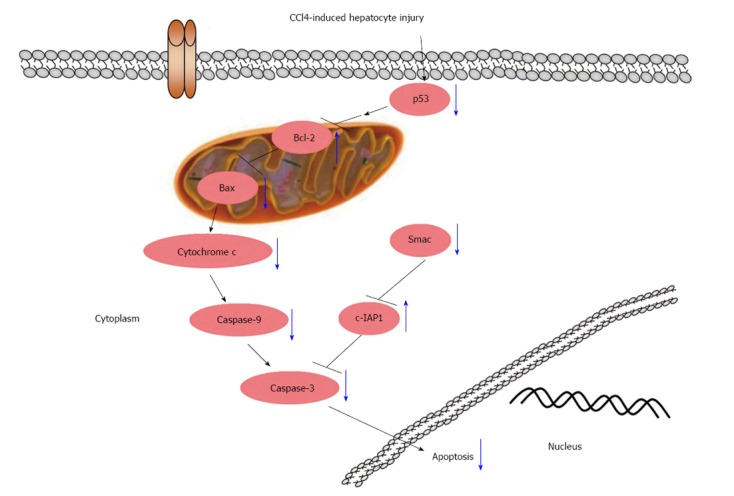
Schematic diagram of the effect of glycyrrhizic acid on the interruption of p53 signaling in carbon tetrachloride-induced hepatocyte apoptosis (blue arrows). Glycyrrhizic acid (GA) suppressed the activation of p53, decreased the expression level of Bax and increased the expression level of Bcl-2, which resulted in reduced cytochrome C release from the mitochondria into the cytoplasm, and inactivated caspase-9 and -3; GA also significantly inhibited Smac release from mitochondria into the cytoplasm and elevated the expression level of c-IAP1, resulting in inhibition of caspase-3 activity. Ultimately, GA suppressed the apoptosis of hepatocytes.
COMMENTS
Background
Liver fibrosis, induced by various pathological factors, is a common outcome in many chronic liver diseases, and is a serious threat to human health. However, there have been few effective therapies for the treatment of hepatic fibrosis in recent years. The authors investigated whether glycyrrhizic acid (GA) could attenuate hepatocyte apoptosis via a p53-mediated mitochondrial pathway and retard the progression of liver fibrosis induced by CCl4 in rats.
Research frontiers
In this study, the authors found that GA attenuated hepatocyte apoptosis via a p53-mediated mitochondrial pathway and retarded the progression of liver fibrosis induced by carbon tetrachloride (CCl4) in rats, which may be a potential alternative treatment approach in patients with liver injury.
Innovations and breakthroughs
This study sought to investigate the effects of GA on p53-dependent apoptosis in CCl4-induced hepatic injury. The study data showed that GA protected against CCl4-induced hepatocyte apoptosis by regulating the Bcl-2 family of proteins, expression of Smac and caspase cleavage.
Applications
This study provides valuable experimental evidence for future anti-liver fibrosis drug studies, and may provide an effective therapy for retarding the process of liver fibrosis.
Terminology
Liver fibrosis, induced by various pathological factors, is a common outcome in many chronic liver diseases, and eventually leads to liver cirrhosis. Apoptosis is gene-controlled and auto-programmed cell death in order to maintain homeostasis. Apoptosis is different from necrosis, as it is an initiative process rather than a passive process and involves gene activation, expression and regulation.
Peer review
This is a good study in which the authors presented experimental evidence that GA exerts anti-apoptotic effects via a p53-dependent mitochondrial pathway in CCl4-induced hepatocyte apoptosis in rats. The results are interesting and suggest that GA could protect against CCl4-induced hepatocyte apoptosis by regulating Bcl-2 family of proteins, expression of Smac and caspases cleavage.
Footnotes
Supported by Leading Academic Discipline Project of State Administration of Traditional Chinese Medicine of China
P- Reviewers Di Costanzo GG, Germanidis G, Liedtke AC, Yagi K S- Editor Wen LL L- Editor A E- Editor Ma S
References
- 1.Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- 2.Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 3.Kodama T, Takehara T, Hikita H, Shimizu S, Shigekawa M, Tsunematsu H, Li W, Miyagi T, Hosui A, Tatsumi T, et al. Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. J Clin Invest. 2011;121:3343–3356. doi: 10.1172/JCI44957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue S, Browne G, Melino G, Cohen GM. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ. 2009;16:1053–1061. doi: 10.1038/cdd.2009.29. [DOI] [PubMed] [Google Scholar]
- 5.Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:6198–6202. doi: 10.3748/wjg.v12.i38.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attallah AM, Shiha GE, Ismail H, Mansy SE, El-Sherbiny R, El-Dosoky I. Expression of p53 protein in liver and sera of patients with liver fibrosis, liver cirrhosis or hepatocellular carcinoma associated with chronic HCV infection. Clin Biochem. 2009;42:455–461. doi: 10.1016/j.clinbiochem.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Papakyriakou P, Tzardi M, Valatas V, Kanavaros P, Karydi E, Notas G, Xidakis C, Kouroumalis E. Apoptosis and apoptosis related proteins in chronic viral liver disease. Apoptosis. 2002;7:133–141. doi: 10.1023/a:1014472430976. [DOI] [PubMed] [Google Scholar]
- 8.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 9.Chen LH, Hsu CY, Weng CF. Involvement of P53 and Bax/Bad triggering apoptosis in thioacetamide-induced hepatic epithelial cells. World J Gastroenterol. 2006;12:5175–5181. doi: 10.3748/wjg.v12.i32.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhi H, Guicciardi ME, Gores GJ. Hepatocyte death: a clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jürgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X, Wang X. Cytochrome c promotes caspase-9 activation by inducing nucleotide binding to Apaf-1. J Biol Chem. 2000;275:31199–31203. doi: 10.1074/jbc.C000405200. [DOI] [PubMed] [Google Scholar]
- 13.Kappler M, Köhler T, Kampf C, Diestelkötter P, Würl P, Schmitz M, Bartel F, Lautenschläger C, Rieber EP, Schmidt H, et al. Increased survivin transcript levels: an independent negative predictor of survival in soft tissue sarcoma patients. Int J Cancer. 2001;95:360–363. doi: 10.1002/1097-0215(20011120)95:6<360::aid-ijc1063>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, Altieri DC. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396:580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- 15.Fraser AG, James C, Evan GI, Hengartner MO. Caenorhabditis elegans inhibitor of apoptosis protein (IAP) homologue BIR-1 plays a conserved role in cytokinesis. Curr Biol. 1999;9:292–301. doi: 10.1016/s0960-9822(99)80137-7. [DOI] [PubMed] [Google Scholar]
- 16.Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 17.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 18.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 19.Carter BZ, Mak DH, Schober WD, Koller E, Pinilla C, Vassilev LT, Reed JC, Andreeff M. Simultaneous activation of p53 and inhibition of XIAP enhance the activation of apoptosis signaling pathways in AML. Blood. 2010;115:306–314. doi: 10.1182/blood-2009-03-212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu JP, McIntosh H, Lin H. Chinese medicinal herbs for chronic hepatitis B. Cochrane Database Syst Rev. 2001;(1):CD001940. doi: 10.1002/14651858.CD001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang SS, Zhang X, Jia XL, Cheng YA, Song P, Liu EQ, He Q, Li ZF. Protective effects of emodin and astragalus polysaccharides on chronic hepatic injury in rats. Chin Med J (Engl) 2008;121:1010–1014. [PubMed] [Google Scholar]
- 22.Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura M, Watanabe H, Abo T. Selective activation of extrathymic T cells in the liver by glycyrrhizin. Biotherapy. 1992;5:167–176. doi: 10.1007/BF02171049. [DOI] [PubMed] [Google Scholar]
- 24.Crance JM, Lévêque F, Biziagos E, van Cuyck-Gandré H, Jouan A, Deloince R. Studies on mechanism of action of glycyrrhizin against hepatitis A virus replication in vitro. Antiviral Res. 1994;23:63–76. doi: 10.1016/0166-3542(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 25.Sato H, Goto W, Yamamura J, Kurokawa M, Kageyama S, Takahara T, Watanabe A, Shiraki K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Res. 1996;30:171–177. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- 26.Gwak GY, Moon TG, Lee DH, Yoo BC. Glycyrrhizin attenuates HMGB1-induced hepatocyte apoptosis by inhibiting the p38-dependent mitochondrial pathway. World J Gastroenterol. 2012;18:679–684. doi: 10.3748/wjg.v18.i7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikeda T, Abe K, Kuroda N, Kida Y, Inoue H, Wake K, Morito M, Sato T. The inhibition of apoptosis by glycyrrhizin in hepatic injury induced by injection of lipopolysaccharide / D-galactosamine in mice. Arch Histol Cytol. 2008;71:163–178. doi: 10.1679/aohc.71.163. [DOI] [PubMed] [Google Scholar]
- 28.Tang B, Qiao H, Meng F, Sun X. Glycyrrhizin attenuates endotoxin- induced acute liver injury after partial hepatectomy in rats. Braz J Med Biol Res. 2007;40:1637–1646. doi: 10.1590/s0100-879x2006005000173. [DOI] [PubMed] [Google Scholar]
- 29.Shi J, Aisaki K, Ikawa Y, Wake K. Evidence of hepatocyte apoptosis in rat liver after the administration of carbon tetrachloride. Am J Pathol. 1998;153:515–525. doi: 10.1016/S0002-9440(10)65594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G137–G144. doi: 10.1152/ajpgi.00080.2005. [DOI] [PubMed] [Google Scholar]
- 31.Aram G, Potter JJ, Liu X, Wang L, Torbenson MS, Mezey E. Deficiency of nicotinamide adenine dinucleotide phosphate, reduced form oxidase enhances hepatocellular injury but attenuates fibrosis after chronic carbon tetrachloride administration. Hepatology. 2009;49:911–919. doi: 10.1002/hep.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuwahata M, Kubota H, Kanouchi H, Ito S, Ogawa A, Kobayashi Y, Kido Y. Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr Res. 2012;32:522–529. doi: 10.1016/j.nutres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Lee TY, Chang HH, Wang GJ, Chiu JH, Yang YY, Lin HC. Water-soluble extract of Salvia miltiorrhiza ameliorates carbon tetrachloride-mediated hepatic apoptosis in rats. J Pharm Pharmacol. 2006;58:659–665. doi: 10.1211/jpp.58.5.0011. [DOI] [PubMed] [Google Scholar]
- 34.Weiler-Normann C, Herkel J, Lohse AW. Mouse models of liver fibrosis. Z Gastroenterol. 2007;45:43–50. doi: 10.1055/s-2006-927387. [DOI] [PubMed] [Google Scholar]
- 35.Cai Y, Shen XZ, Wang JY. Effects of glycyrrhizin on genes expression during the process of liver fibrosis. Zhonghua Yixue Zazhi. 2003;83:1122–1125. [PubMed] [Google Scholar]
- 36.Wang JY, Zhang QS, Guo JS, Hu MY. Effects of glycyrrhetinic acid on collagen metabolism of hepatic stellate cells at different stages of liver fibrosis in rats. World J Gastroenterol. 2001;7:115–119. doi: 10.3748/wjg.v7.i1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Consensus on evaluation of the diagnosis and efficacy of hepatic fibrosis. Zhonghua Ganzangbing Zazhi. 2002;10:327–328. [PubMed] [Google Scholar]
- 38.Giannone FA, Baldassarre M, Domenicali M, Zaccherini G, Trevisani F, Bernardi M, Caraceni P. Reversal of liver fibrosis by the antagonism of endocannabinoid CB1 receptor in a rat model of CCl(4)-induced advanced cirrhosis. Lab Invest. 2012;92:384–395. doi: 10.1038/labinvest.2011.191. [DOI] [PubMed] [Google Scholar]
- 39.Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis. 2006;27:2018–2027. doi: 10.1093/carcin/bgl043. [DOI] [PubMed] [Google Scholar]
- 40.Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- 41.Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82–S88. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 42.Hidvegi T, Ewing M, Hale P, Dippold C, Beckett C, Kemp C, Maurice N, Mukherjee A, Goldbach C, Watkins S, et al. An autophagy-enhancing drug promotes degradation of mutant alpha1-antitrypsin Z and reduces hepatic fibrosis. Science. 2010;329:229–232. doi: 10.1126/science.1190354. [DOI] [PubMed] [Google Scholar]
- 43.Lee TF, Lin YL, Huang YT. Studies on antiproliferative effects of phthalides from Ligusticum chuanxiong in hepatic stellate cells. Planta Med. 2007;73:527–534. doi: 10.1055/s-2007-981520. [DOI] [PubMed] [Google Scholar]
- 44.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 45.Arase Y, Ikeda K, Murashima N, Chayama K, Tsubota A, Koida I, Suzuki Y, Saitoh S, Kobayashi M, Kumada H. The long term efficacy of glycyrrhizin in chronic hepatitis C patients. Cancer. 1997;79:1494–1500. doi: 10.1002/(sici)1097-0142(19970415)79:8<1494::aid-cncr8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 46.van Rossum TG, Vulto AG, Hop WC, Brouwer JT, Niesters HG, Schalm SW. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double-blind, randomized, placebo-controlled phase I/II trial. J Gastroenterol Hepatol. 1999;14:1093–1099. doi: 10.1046/j.1440-1746.1999.02008.x. [DOI] [PubMed] [Google Scholar]
- 47.Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci. 2005;50:1807–1812. doi: 10.1007/s10620-005-2942-9. [DOI] [PubMed] [Google Scholar]
- 48.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pani G, Fusco S, Colavitti R, Borrello S, Maggiano N, Cravero AA, Farré SM, Galeotti T, Koch OR. Abrogation of hepatocyte apoptosis and early appearance of liver dysplasia in ethanol-fed p53-deficient mice. Biochem Biophys Res Commun. 2004;325:97–100. doi: 10.1016/j.bbrc.2004.09.213. [DOI] [PubMed] [Google Scholar]
- 50.Li W, Laskar A, Sultana N, Osman E, Ghosh M, Li Q, Yuan XM. Cell death induced by 7-oxysterols via lysosomal and mitochondrial pathways is p53-dependent. Free Radic Biol Med. 2012;53:2054–2061. doi: 10.1016/j.freeradbiomed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto Y, Kato S, Takahashi M, Okada Y, Yasuda K, Watanabe G, Imai H, Sato A, Ishioka C. Contribution of autophagic cell death to p53-dependent cell death in human glioblastoma cell line SF126. Cancer Sci. 2011;102:799–807. doi: 10.1111/j.1349-7006.2011.01857.x. [DOI] [PubMed] [Google Scholar]
- 52.Finkel E. The mitochondrion: is it central to apoptosis? Science. 2001;292:624–626. doi: 10.1126/science.292.5517.624. [DOI] [PubMed] [Google Scholar]
- 53.Curreli F, Friedman-Kien AE, Flore O. Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. J Clin Invest. 2005;115:642–652. doi: 10.1172/JCI23334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasuda M, Okabe T, Itoh J, Takekoshi S, Hasegawa H, Nagata H, Osamura RY, Watanabe K. Differentiation of necrotic cell death with or without lysosomal activation: application of acute liver injury models induced by carbon tetrachloride (CCL4) and dimethylnitrosamine (DMN) J Histochem Cytochem. 2000;48:1331–1339. doi: 10.1177/002215540004801004. [DOI] [PubMed] [Google Scholar]
- 55.Merino N, González R, González A, Remirez D. Histopathological evaluation on the effect of red propolis on liver damage induced by CCl4 in rats. Arch Med Res. 1996;27:285–289. [PubMed] [Google Scholar]
- 56.Takebe H, Sato I, Tajima S, Ikeda Y, Ito K, Nose T. [Effects of cianidanol (KB-53) on liver cirrhosis induced by CCl4 in rats: a pathological investigation] Nihon Yakurigaku Zasshi. 1983;81:585–591. [PubMed] [Google Scholar]
- 57.Boncompagni E, Gini E, Ferrigno A, Milanesi G, Gringeri E, Barni S, Cillo U, Vairetti M, Freitas I. Decreased apoptosis in fatty livers submitted to subnormothermic machine-perfusion respect to cold storage. Eur J Histochem. 2011;55:e40. doi: 10.4081/ejh.2011.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jazwinski AB, Thompson AJ, Clark PJ, Naggie S, Tillmann HL, Patel K. Elevated serum CK18 levels in chronic hepatitis C patients are associated with advanced fibrosis but not steatosis. J Viral Hepat. 2012;19:278–282. doi: 10.1111/j.1365-2893.2011.01546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


