Abstract
AIM: To evaluate and compare detection of lymphatic and blood vessel invasion (LVI and BVI) by hematoxylin-eosin (HE) and immunohistochemistry (IHC) in gastric cancer specimens, and to correlate with lymph node status.
METHODS: IHC using D2-40 (a lymphatic endothelial marker) and CD34 (a pan-endothelial marker) was performed to study LVI and BVI in surgical specimens from a consecutive series of 95 primary gastric cancer cases. The results of the IHC study were compared with the detection by HE using McNemar test and kappa index. The morphologic features of the tumors and the presence of LVI and BVI were related to the presence of lymph node metastasis. A χ2 test was performed to obtain associations between LVI and BVI and other prognostic factors for gastric cancer.
RESULTS: The detection rate of LVI was considerably higher than that of BVI. The IHC study identified eight false-positive cases and 13 false-negative cases for LVI, and 24 false-positive cases and 10 false-negative cases for BVI. The average Kappa value determined was moderate for LVI (κ = 0.50) and low for BVI (κ = 0.20). Both LVI and BVI were statistically associated with the presence of lymph node metastasis (HE: P = 0.001, P = 0.013, and IHC: P = 0.001, P = 0.019). The morphologic features associated with LVI were location of the tumor in the distal third of the stomach (P = 0.039), Borrmann’s macroscopic type (P = 0.001), organ invasion (P = 0.03) and the depth of tumor invasion (P = 0.001). The presence of BVI was related only to the depth of tumor invasion (P = 0.003).
CONCLUSION: The immunohistochemical identification of lymphatic and blood vessels is useful for increasing the accuracy of the diagnosis of vessel invasion and for predicting lymph node metastasis.
Keywords: Gastric cancer, Tumour-node-metastesis staging, Lymph node metastasis, Predictive factor, Lymphatic vessel invasion, Blood vessel invasion, Immunohistochemistry, CD34, D2-40
Core tip: The presence of lymphatic vessel invasion in gastric cancer is the strongest risk factor for lymph node metastasis and is known as an independent prognostic factor. The subjective evaluation of vessel invasion performed with conventional hematoxylin-eosin staining can lead to inaccurate false-positive and false-negative results. This study shows that the immunohistochemical identification of lymphatic and blood vessels is useful for increasing the accuracy of the diagnosis of lymphatic and blood vessel invasion and for predicting lymph node metastasis in gastric cancer.
INTRODUCTION
Gastric cancer (GC) is the fourth most common cancer and the second most common cause of cancer deaths in the world[1]. A steady decline in the incidence and mortality rates of gastric carcinoma has been observed worldwide over the past several decades, but there is a significant variation in incidence between the populations at the greatest and least risk[2]. In areas without endoscopic screening for GC, especially in developing countries, GC presents as an advanced disease and has a high frequency of nodal involvement[3]. Surgery is the only effective intervention for a cure or for long-term survival and nodal status is one of the most important independent predictors of patient survival[4].
The depth of invasion is an independent prognostic factor for gastric carcinoma and is associated with patient survival[5,6]. Early GC is limited to the mucosa and submucosa and is associated with a better prognosis. In Japan, where asymptomatic patients are screened, there is a high incidence of early diagnosis, ranging from 30% to 50%, in contrast with the smaller fraction of 16%-24% in Western countries[2]. Minimizing the number of invasive procedures used in cancer treatment is critical for improving the patient’s quality of life. Minimally invasive treatments, such as endoscopic mucosal resection, may be possible only in highly selective cases of early GC[7-9].
Lymph node metastasis is one of the most important prognostic factors in patients with GC[2,10]. Studies have estimated that the lymph nodes will be involved in 3%-5% of cases of gastric adenocarcinoma limited to the mucosa, in 11%-25% of cases limited to the submucosa, in 50% of T2 tumors and in 83% of T3 tumors[11]. Hence the accurate assessment of potential lymph node metastasis is an important issue for the appropriate treatment of early GC.
The histologic identification of lymphatic vessel invasion (LVI) by tumor cells has long been recognized as a potential prognostic indicator and a predictor of patient outcomes in various malignancies[12-18]. One of the earliest steps in the metastatic cascade is (lympho)vascular invasion, i.e., the penetration of tumor cells into lymph and/or blood vessels in and around the primary tumor[19-21]. Therefore, tumor cell emboli in the lymph and blood vessels are considered to be the morphological correlates of metastases to loco-regional lymph nodes and to distant hematogenous sites, respectively. Consistent with the distribution of lymphatic vessels in the gastric wall, LVI is most frequently observed in the muscularis mucosa layer and in the superficial submucosa[22,23].
Usually, LVI and blood vessel invasion (BVI) are identified based on conventional hematoxylin-eosin (HE) staining, and the diagnosis is made based on the presence of tumor emboli within the vascular channels lined by a single layer of endothelial cells, with or without red blood cells[14,19,20]. However, if the cancer cells completely obliterate the lumen, it is not possible to diagnose vascular invasion. Additionally, retraction artifacts that isolate tumor aggregates via tissue shrinkage during fixation are sometimes confused with true tumor emboli in lymphatic vessels. Besides, using that criterion, vascular invasion detected on HE sections does not always allow for a distinction between BVI and LVI[14].
Recently, interest in vascular invasion has increased because of the development of specific markers for the lymphatic endothelium used in immunohistochemistry (IHC), such as Prox-1, which is a transcription factor; Lyve-1, which is a hyaluronan receptor; podoplanin, which is a glomerular podocyte membrane protein and D2-40[21]. It has been demonstrated that D2-40 is the best marker for the lymphatic endothelium[24]. Used in combination with panendothelial markers such as CD34 or CD31, D2-40 permits the differentiation between BVI and LVI and the study of both processes in GC metastasis[25].
There have been numerous studies regarding LVI and BVI in GC. However, most of them have not defined the criteria used to determine the presence or absence of lymphatic and vascular invasion. Additionally, many large retrospective series of GC cases have extracted the reporting of (lympho)vascular invasion from the patients’ medical records, without histological reviews by central pathologists for consistency and without immunohistochemical studies[6,9,15,26]. Uncertain criteria for the diagnosis of (lympho)vascular invasion may affect the clinical assessment of prognosis and may change the course of therapy for the patients[27-30].
The aim of this study was to evaluate, in a consecutive series of patients with GC, a technique that uses a combined immunohistochemical expression profile to detect LVI and BVI and compare this technique to routine HE assessment. In addition, we analyzed the relationship between lymph node metastasis and clinicopathological findings, especially those of LVI and BVI re-evaluated by IHC staining.
MATERIALS AND METHODS
This study was reviewed and approved by the university’s research ethics committee (COEP-UFMG). Ninety-five consecutive cases of GC, diagnosed and treated between 2000 and 2006 and identified from the pathology archives, were selected for study. All patients underwent curative gastrectomy with standard lymphadenectomy at the Clinical Hospital of the Federal University of Minas Gerais. None of the patients had received preoperative radiation therapy or chemotherapy. In total, 57 patients underwent distal gastrectomy, 33 had total gastrectomy and five had partial gastrectomy.
All surgical specimens of the primary tumors and regional lymph nodes had been processed and examined histologically by routine HE staining, according to the institutional protocol[31]. The definitions of stages and the criteria for histological classification followed the World Health Organization classification[2] and the Japanese classification for GC[32]. The resected primary tumors and regional lymph nodes were reviewed histologically by two pathologists using HE staining.
IHC for CD34 and D2-40 was then performed on the corresponding paraffin blocks. Serial 4-µm-thick sections were deparaffinized in xylene and rehydrated. A hydrogen peroxide quench, using 3% H2O2 with methyl alcohol, was performed for 20 min. A 15-min incubation with a serum-free protein blocking agent was then performed. Antigen retrieval was performed using EDTA buffer at pH 9.0 (CD34), a citrate buffer at pH 6.0 (D2-40) and a steam cooker for 20 min, with a bench cool down period of 15 min. A 30-min incubation in the primary antibodies (Biogenex CD34 monoclonal mouse antihuman, clone QBEND/10, at 1:10 dilution; Dako D2-40 monoclonal mouse antihuman, clone D2-40, at 1:30 dilution) was performed at room temperature. Secondary detection consisted of a 30-min incubation using Labeled Streptavidin-Biotin link (Dako LSAB®+ System), and the staining was visualized by a 5-min incubation with diaminobenzidine tetrahydrochloride. The slides were counterstained with Harris’ hematoxylin and cover-slipped. A normal stomach was used for the control slide for each immunohistochemical stain. For the negative control, all of the reagents except for the primary antibody were used.
All slides were assessed simultaneously by two investigators (Gresta LT and Cabral MMDA) on a double-observation microscope, without knowledge of the clinicopathological data. The slides (HE, CD34 and D2-40) were screened for (lympho)vascular invasion using strict criteria[14]. With HE, invasion was considered only if the tumor cells were within an endothelium-lined, vessel-like structure. With IHC, vessels with endothelial cells stained by CD34, but not by D2-40, were recognized as blood vessels, and vessels with endothelial cells stained by both CD34 and D2-40 were recognized as lymphatic vessels. Every vessel with tumor cell invasion on one of the three consecutive sections was identified in the other slides and was classified as a blood vessel or lymphatic invasion, based on the immunohistochemical staining profile.
Statistical analysis
The statistical calculations were performed using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, United States). The McNemar test was used to determine the significance of intergroup differences. P ≤ 0.05 was considered to be statistically significant. The estimation of the agreement rate between the two methods was obtained using the Kappa statistic (κ). A χ2 test was applied for the analysis of associations between categorical variables.
RESULTS
The clinical and pathological characteristics of the 95 patients with GC are summarized in Table 1. Lymphatic vessels were identified by immunostaining as D2-40-positive and CD34-positive (Figure 1A-C). Blood vessels were identified by immunostaining as D2-40-negative and CD34-positive (Figure 1D-F).
Table 1.
Clinicopathological characteristics of 95 patients with gastric cancer n (%)
| Clinicopathological data | |
| Sex | |
| Male | 62 (65.3) |
| Female | 33 (34.7) |
| Curvature | |
| Small curvature | 54 (56.8) |
| Large curvature | 10 (10.5) |
| Small and large | 14 (14.7) |
| Not evaluated | 17 (17.9) |
| Primary tumor | |
| pT1a | 9 (9.5) |
| pT1b | 12 (12.6) |
| pT2a | 12 (12.6) |
| pT2b | 8 (8.4) |
| pT3 | 51 (53.7) |
| pT4 | 3 (3.2) |
| Regional lymph nodes | |
| pN0 | 28 (29.5) |
| pN1 | 36 (37.9) |
| pN2 | 13 (13.7) |
| pN3 | 12 (12.6) |
| pNx | 6 (6.3) |
| Distant metastasis | |
| pMx | 87 (91.6) |
| pM1 | 8 (8.4) |
| Laurén classification | |
| Intestinal | 45 (47.4) |
| Diffuse | 25 (26.3) |
| Mixed/indeterminate | 25 (26.3) |
| WHO classification | |
| Adenocarcinoma NOS | 48 (50.5) |
| Tubular | 5 (5.3) |
| Papillary | 5 (5.3) |
| Mucinous | 4 (4.2) |
| Signet-ring cell | 22 (23.2) |
| Undifferentiated | 9 (9.5) |
| Others | 2 (2.2) |
| Ming classification | |
| Expansive | 40 (42.1) |
| Infiltrative | 40 (42.1) |
| Not evaluated | 15 (15.8) |
NOS: Not otherwise specified; WHO: World Health Organization; pT: Primary tumor; pN: Regional lymph nodes; pM: Distant metastasis.
Figure 1.
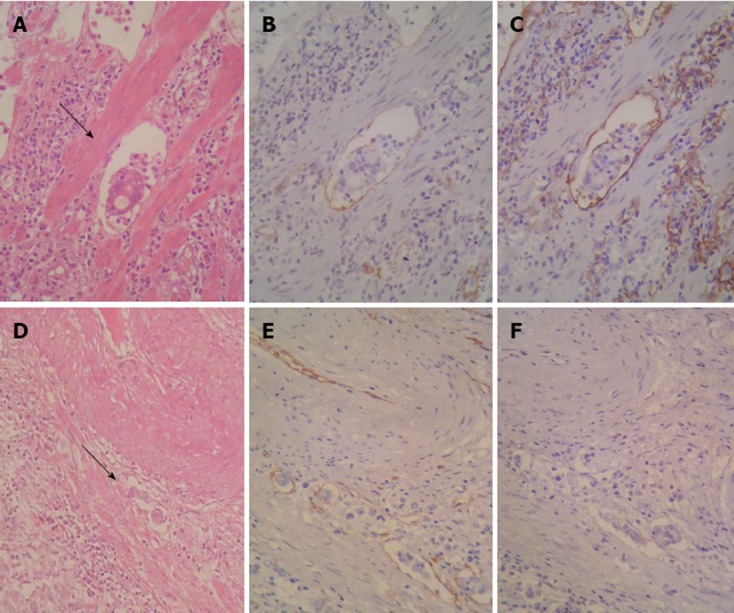
Sequential sections stained with hematoxylin and eosin and Immunohistochemistry showing neoplastic cell emboli within a space surrounded by the endothelial lining (arrows). A: Lymphatic vessel invasion (LVI)-hematoxylin and eosin (HE, × 400); B: LVI CD34 (× 400); C: LVI D2-40 (× 400); D: Blood vessel invasion (BVI) (HE, × 100); E: BVI CD34 (× 200); F: BVI D2-40 (× 200).
Lymphatic and BVI (HE and IHC)
Histological HE staining revealed LVI from the primary tumor in 61 of the 95 patients (64.2%). In 53 of those cases, LVI detected by HE staining was confirmed with D2-40 staining. In contrast, LVI was newly detected in 13 of 34 patients who had been diagnosed as free of LVI by HE staining. Figure 2 shows examples of false-positive and false-negative for LVI.
Figure 2.
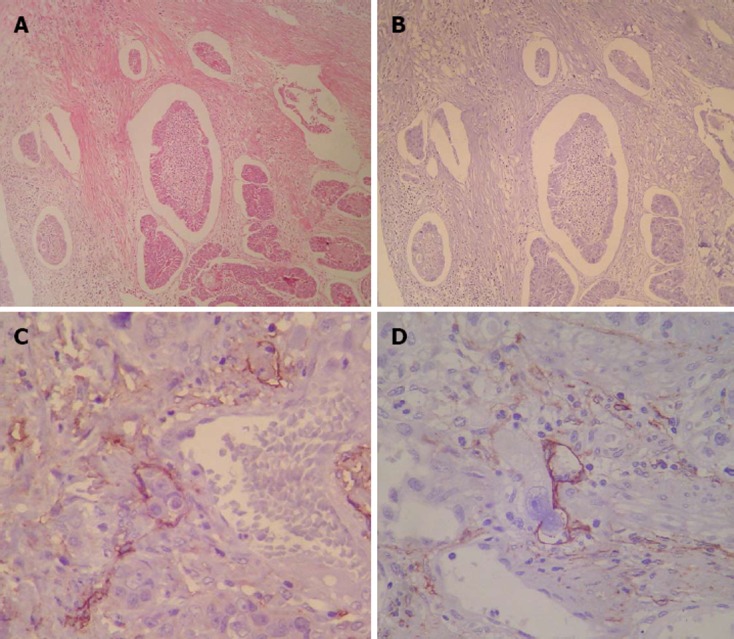
Example of a patient diagnosed for lymphatic vessel invasion by routine histological examination. A: Example of a patient diagnosed as positive for lymphatic vessel invasion (LVI) by routine histological examination; B: As false-positive for lymphatic vessel invasion by D2-40 (× 100); C, D: Examples of patients diagnosed as free of LVI by routine histological examination. False-negatives for LVI detected by D2-40 (× 400).
The specimens examined using HE staining showed a false-negative BVI rate of 12.6% (12/95) and a false-positive rate of 25.2% (24/95). The positive rate of BVI determined by HE staining was 40% (38/95); however, BVI was confirmed by CD34 in only 27.4% of the cases (26/95).
Figure 3 shows the prevalence of LVI and BVI with conventional HE staining and IHC in 95 primary tumors. Table 2 shows the average kappa values for both methods determined separately for LVI and BVI. The agreement was fair for BVI (κ = 0.20) and medium for LVI (κ = 0.50).
Figure 3.
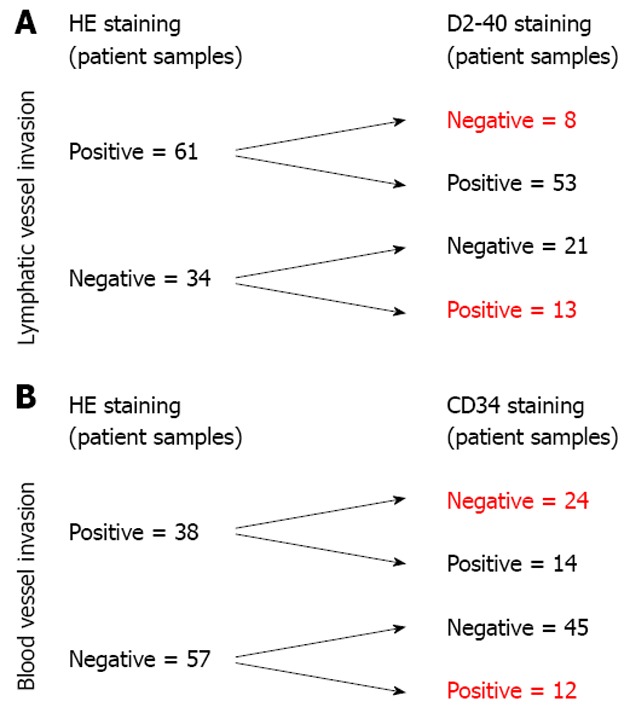
Diagnostic comparison in 95 patients with gastric cancer. A: Diagnostic comparison of lymphatic vessel invasion; B: Diagnostic comparison of blood vessel invasion. HE: Hematoxylin and eosin.
Table 2.
Diagnostic agreement between methods of detection for lymphatic and blood vessel invasion (n = 95)
| HE | IHC | P value | κ | ||
| LVI | Positive | 61 | 66 | 0.38 | 0.50 |
| Negative | 34 | 29 | |||
| BVI | Positive | 38 | 26 | 0.02 | 0.20 |
| Negative | 57 | 69 |
BVI: Blood vessel invasion; LVI: Lymphatic vessel invasion; HE: Hematoxylin and eosin; IHC: Immunohistochemistry.
Correlation of LVI and BVI with other prognostic factors
The LVI and BVI diagnosed by both HE and IHC were significantly correlated with lymph node metastasis, as shown in Table 3.
Table 3.
Correlation between lymphatic and blood vessel invasion and lymph node status (n = 89) n (%)
| Vascular invasion |
Lymph node metastasis |
P value | ||
| Negative | Positive | |||
| LVI–HE | Negative | 23 (71.8) | 9 (28.2) | |
| Positive | 5 (8.8) | 52 (91.2) | 0.001 | |
| LVI–IHC | Negative | 20 (74.0) | 7 (26.0) | |
| Positive | 8 (13.0) | 54 (87.0) | 0.001 | |
| BVI–HE | Negative | 22 (41.5) | 31 (58.5) | |
| Positive | 6 (16.6) | 30 (83.4) | 0.013 | |
| BVI–IHC | Negative | 25 (38.5) | 40 (61.5) | |
| Positive | 3 (12.5) | 21 (87.5) | 0.019 | |
IHC: Immunohistochemistry; HE: Hematoxylin and eosin; BVI: Blood vessel invasion; LVI: Lymphatic vessel invasion.
Table 4 shows other clinical-pathologic variables that were significantly correlated with LVI and BVI when detected by IHC.
Table 4.
Correlation of lymphatic and blood vessel invasion detected by immunohistochemistry with other prognostic factors (n = 95) n (%)
| Data |
LVI |
BVI |
||||
| Negative | Positive | P value | Negative | Positive | P value | |
| Tumor location | ||||||
| Distal third | 15 (27.3) | 40 (72.7) | 0.0391 | 42 (76.3) | 13 (23.7) | 0.281 |
| Other locations | 14 (35.0) | 26 (65.0) | 29 (72.5) | 11 (27.5) | ||
| Curvature | ||||||
| Small curvature | 19 (35.2) | 35 (64.8) | 0.122 | 41 (76.0) | 13 (24.0) | 0.131 |
| Large curvature | 3 (30.0) | 7 (70.0) | 8 (80.0) | 2 (20.0) | ||
| Small and large | 1 (7.2) | 13 (92.8) | 7 (50.0) | 7 (50.0) | ||
| Macroscopy | ||||||
| Borrmann I | 5 (71.4) | 2 (28.6) | 0.0011 | 7 (100.0) | 0 (0.0) | 0.24 |
| Borrmann II | 4 (13.3) | 26 (86.7) | 21 (70.0) | 9 (30.0) | ||
| Borrmann III | 5 (18.5) | 22 (81.5) | 17 (62.9) | 10 (37.1) | ||
| Borrmann IV | 0 (0.0) | 12 (100.0) | 7 (58.3) | 5 (41.7) | ||
| Organ invasion | ||||||
| Negative | 26 (45.6) | 31 (54.4) | 0.0031 | 46 (80.7) | 11 (19.3) | 0.297 |
| Duodenum | 1 (4.3) | 22 (95.7) | 15 (65.2) | 8 (34.8) | ||
| Esophagus | 1 (12.5) | 7 (87.5) | 6 (75.0) | 2 (25.0) | ||
| Both E + D | 0 (0.0) | 3 (100.0) | 1 (33.3) | 2 (66.7) | ||
| Other | 1 (25.0) | 3 (75.0) | 3 (75.0) | 1 (25.0) | ||
| Tumor depth | ||||||
| Early | 17 (80.9) | 4 (19.1) | 0.0011 | 21 (100.0) | 0 (0.0) | 0.0031 |
| Advanced | 12 (16.2) | 62 (83.8) | 50 (67.5) | 24 (32.5) | ||
| Laurén histology | ||||||
| Intestinal | 12 (26.6) | 33 (73.3) | 0.228 | 36 (80.0) | 9 (20.0) | 0.332 |
| Diffuse | 11 (44.0) | 14 (56.0) | 19 (76.0) | 6 (24.0) | ||
| Mixed/not classified | 6 (24.0) | 19 (76.0) | 16 (64.0) | 9 (36.0) | ||
BVI: Blood vessel invasion; LVI: Lymphatic vessel invasion; E + D: Esophagus + duodenum.
DISCUSSION
To improve the detection of vascular invasion in GC, which is normally performed by routine HE staining, and to distinguish LVI from BVI, we introduced an IHC method using the combination of two markers: one specific to the lymphatic vessel endothelium (D2-40) and the other pan-endothelial (CD34). In addition to the effective detection of LVI and BVI, this method also enabled the correct evaluation of the predictive value of vascular invasion in GC for the occurrence of lymph node metastasis. As we expected, the identification of LVI and BVI was also correlated with other prognostic factors important for GC, such as tumor depth and organ invasion, which have been detected in other studies.
In this study, the group of 95 patients generally reflected the profile of GC described in the literature with regard to age, gender, location of tumor and Laurén histological type. The patients with diffuse-type carcinoma were significantly younger than those with the intestinal-type (P = 0.04). This peculiar feature of diffuse-type neoplasms is well established and reflects differences in pathogenesis that are generally linked to genetic factors, whereas intestinal-type neoplasms are more influenced by environmental factors, such as diet and infection with Helicobacter pylori.
Data from the literature indicate that most diagnoses in developing countries occur late, when the disease is already in the advanced stage[31]. In our study, 53.7% of the GCs were in-depth stage pT3 tumors. Additionally, more than half of our sample (64.2%) had lymph node metastasis at the time of diagnosis.
The prevalence of LVI and BVI in GC has been determined in various studies to vary from 7.2% to 86%[9,15,28,33-35]. This wide variation in results could be explained by the different methods used to evaluate vascular invasion, i.e., HE only or usage of IHC staining with endothelium-specific markers. Three consistent instances of this variation include the studies of Arigami et al[36], of Sako et al[22] and of Yonemura et al[23], who reported higher rates of detection of LVI with the IHC method when compared to routine staining with HE. All three studies strengthened the role of IHC in the analysis of vascular invasion in GC[36].
We observed that the difference between HE and IHC when detecting LVI was not statistically significant (P = 0.38). However, there were 8 cases of false-positive and 13 cases of false-negative that were isolated only after IHC. Thus, the Kappa coefficient was considered to be only moderate for LVI (κ = 0.50). The evaluation of LVI by only HE is subject to these misconceptions because of the inability to distinguish retraction artifacts around glands or cell groups from true vascular invasion. Occasionally, neoplastic cells occupy the vascular lumen completely, which makes their identification impossible without specific marking of the lymphatic endothelium. Additionally, false-positive results can occur when BVI is misinterpreted as LVI with HE staining only[36].
The correlation between LVI and the presence of metastasis was statistically significant when assessed by both methods (P = 0.001). This finding agrees with published data, in which LVI of the primary tumor was found to be crucial for the occurrence of lymph node metastasis[37]. Therefore, it is possible to infer that LVI is more widely found in patients with lymph node metastases than in those in which the examined lymph nodes are negative.
We found 24 cases of false-positives for BVI with HE and 10 false-negatives identified by IHC. Therefore, the detection of BVI was more accurate and significantly less frequent with IHC than with HE (P = 0.02). This result produced a very low Kappa coefficient (κ = 0.20) because of the identification of a large number of cases as false-positives. The false-positive results obtained could be explained as cases of LVI that were inadvertently interpreted as BVI, as it is not possible to distinguish between blood vessels and lymphatic vessels in all cases using only HE[14].
Our results show that BVI evaluated by both methods is positively correlated with the presence of lymph node metastasis, in contrast to what has been demonstrated by some previous studies[7]. It is interesting to note that although the previous studies have examined large numbers of cases, they performed retrospective review studies that included only cases of early GC, which explains the low occurrence of lymph node metastasis and BVI. Our study, in contrast, analyzed BVI and lymph node metastasis not only in early GC but also in advanced cases of GC, which resulted in the statistical significance described in Table 4.
The presence of lymph node metastasis is considered to be the most important prognostic factor in GC, and it is related to the presence of vascular invasion[37,38]. Retrospective studies have shown that the presence of LVI and BVI detected by the IHC method is related to tumor recurrence in patients with and without lymph node metastasis and is also related to a low survival rate[16,36,39]. In this regard, our study revealed the importance of LVI and BVI as predictive factors, even in the absence of lymph node involvement.
The early GC concept applies to those tumors with more superficial infiltration of the gastric wall. It is thought that cases of early GC are less likely to show invasion of blood and lymph vessels. Our data show that, compared with advanced GC, early GC exhibits less lymph node involvement (P = 0.001), less LVI (P = 0.001) and less BVI (P = 0.007) detected by IHC. However, studies of lymphatic network density in the normal gastric wall have found that the concentration of lymphatic vessels is considerably greater in the muscularis mucosa, which can be infiltrated in early GC[22].
The risk of lymph node metastasis in early GC is only 3.2% for the intramucosal and is approximately 19.2% when invasion reaches the submucosa[40]. Our results agree with these findings. We found two cases of early GC (11.2%) with invasion of the submucosa and lymph node metastasis. Conversely, in 59 cases of advanced GC (83.0%), the lymph nodes were positive for metastasis.
At present, non invasive imaging methods to properly evaluate the likelihood of lymph node metastasis in GC do not exist. Thus, lymph node staging in early GC still relies on the assessment of specific tumor characteristics that are related to increased lymph node metastasis, i.e., depth of tumor infiltration in the gastric wall, tumor size greater than 2.0 cm, Laurén histological classification and LVI. It is noteworthy that, among these factors, the presence of lymphatic vessel involvement is the most significant isolated predictive factor for the occurrence of lymph node metastasis[7]. Thus, it is essential to include LVI and BVI evaluation by IHC in routine pathologic protocols of GC surgical specimens.
Gastrectomy with lymphadenectomy is indicated in poorly differentiated intramucosal carcinomas, with dimensions larger than 20 mm or in submucosal carcinomas. However, these criteria are quite strict and may result in unnecessary surgery. Gotoda et al[41] proposed more expanded criteria for the endoscopic treatment of early GC that combined histological type, LVI and BVI, ulceration and tumor size, thereby enabling the expansion of the universe of patients with early GC who could potentially be eligible for endoscopic resection, even with submucosal invasion.
The meta-analysis published by Kwee et al[40] revealed several variables significantly associated with the presence of lymph node metastasis in early GC. Most of these predictive factors may be perfectly evaluated through preoperative exams, endoscopy with biopsy and non-invasive imaging methods, such as computed tomography and endoscopic ultrasound. However, the presence or absence of LVI and BVI can only be judged by a histopathological study after tumor resection.
LVI and BVI must be systematically analyzed as the histological parameters with the greatest prognostic significance and as decisive factors in the choice of complementary adjuvant therapy. Therefore, we suggest that more sensitive and more specific methods be incorporated into the routine protocols for histopathological examination of GC.
Our results show that the application of IHC using two combined markers (CD34 and D2-40) provides a more accurate detection of LVI and BVI when compared to routine staining with HE. These findings may be of great value in clinical practice, especially in cases in which it is not possible to determine the precise lymph node status because of an insufficient number of lymph nodes or because lymphadenectomy was not performed.
ACKNOWLEDGMENTS
The authors would like to pay tribute to professor Ana Margarida MF Nogueira in recognition of her great contribuition to our work and to gastrointestinal pathology.
COMMENTS
Background
Gastric cancer (GC) is the fourth most common cancer and the second most common cause of cancer deaths in the world. Lymph node metastasis is one of the most important prognostic factors in patients with GC. The presence of lymphatic vessel invasion in GC is the strongest risk factor for lymph node metastasis and is known as an independent prognostic factor.
Research frontiers
Usually, lymphatic and blood vessel invasion (BVI) are identified based on conventional hematoxylin-eosin staining, and the diagnosis is made based on the presence of tumor emboli within the vascular channels lined by a single layer of endothelial cells, with or without red blood cells. However, this subjective evaluation can lead to inaccurate false-positive and false-negative results.
Innovations and breakthroughs
Recently, interest in vascular invasion has increased because of the development of specific markers for the lymphatic endothelium used in immunohistochemistry (IHC), such as Prox-1, which is a transcription factor; Lyve-1, which is a hyaluronan receptor; podoplanin, which is a glomerular podocyte membrane protein and D2-40. It has been demonstrated that D2-40 is the best marker for the lymphatic endothelium. Used in combination with panendothelial markers such as CD34 or CD31, D2-40 permits the differentiation between lymphatic and BVI and the study of both processes in GC metastasis.
Applications
Uncertain criteria for the diagnosis of (lympho)vascular invasion may affect the clinical assessment of prognosis and may change the course of therapy for the patients. This study shows that the immunohistochemical identification of lymphatic and blood vessels is useful for increasing the accuracy of the diagnosis of lymphatic and BVI and for predicting lymph node metastasis in GC.
Terminology
Blood and lymphatic vessels show different functions and phenotypic expression. IHC is a procedure which identifies specific antigens expressed by the cells or tissues, making it possible to determine their origin. Vascular invasion is the presence of tumor cells within the lumen of blood or lymphatic vascular spaces, which can be transported into the bloodstream, producing metastasis.
Peer review
Authors demonstrated that the IHC of two different endothelial markers D2-40 and CD34 is useful for accurate diagnosis of vessel invasion and for predicting lymph node metastasis.
Footnotes
Supported by Grants from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior
P- Reviewers Langley RR, Pan WS, Shen LZ, Shibata MA S- Editor Gou SX L- Editor A E- Editor Xiong L
References
- 1.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol. 2009;472:467–477. doi: 10.1007/978-1-60327-492-0_23. [DOI] [PubMed] [Google Scholar]
- 2.Aaltonen LA, Hamilton SR, World Health Organization, International Agency for Research on Cancer. Pathology and genetics of tumours of the digestive system. Lyon. Oxford: IARC Press/Oxford University Press distributor; 2000. [Google Scholar]
- 3.Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–649. doi: 10.1016/j.bpg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Coburn NG. Lymph nodes and gastric cancer. J Surg Oncol. 2009;99:199–206. doi: 10.1002/jso.21224. [DOI] [PubMed] [Google Scholar]
- 5.Zheng HC, Li XH, Hara T, Masuda S, Yang XH, Guan YF, Takano Y. Mixed-type gastric carcinomas exhibit more aggressive features and indicate the histogenesis of carcinomas. Virchows Arch. 2008;452:525–534. doi: 10.1007/s00428-007-0572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talamonti MS, Kim SP, Yao KA, Wayne JD, Feinglass J, Bennett CL, Rao S. Surgical outcomes of patients with gastric carcinoma: the importance of primary tumor location and microvessel invasion. Surgery. 2003;134:720–777; discussion 727-729. doi: 10.1016/s0039-6060(03)00337-4. [DOI] [PubMed] [Google Scholar]
- 7.An JY, Baik YH, Choi MG, Noh JH, Sohn TS, Kim S. Predictive factors for lymph node metastasis in early gastric cancer with submucosal invasion: analysis of a single institutional experience. Ann Surg. 2007;246:749–753. doi: 10.1097/SLA.0b013e31811f3fb7. [DOI] [PubMed] [Google Scholar]
- 8.Hanaoka N, Tanabe S, Mikami T, Okayasu I, Saigenji K. Mixed-histologic-type submucosal invasive gastric cancer as a risk factor for lymph node metastasis: feasibility of endoscopic submucosal dissection. Endoscopy. 2009;41:427–432. doi: 10.1055/s-0029-1214495. [DOI] [PubMed] [Google Scholar]
- 9.Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol. 2004;85:181–15; discussion 186. doi: 10.1002/jso.20018. [DOI] [PubMed] [Google Scholar]
- 10.Greene FL, American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 11.de Gara CJ, Hanson J, Hamilton S. A population-based study of tumor-node relationship, resection margins, and surgeon volume on gastric cancer survival. Am J Surg. 2003;186:23–27. doi: 10.1016/s0002-9610(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 12.Arnaout-Alkarain A, Kahn HJ, Narod SA, Sun PA, Marks AN. Significance of lymph vessel invasion identified by the endothelial lymphatic marker D2-40 in node negative breast cancer. Mod Pathol. 2007;20:183–191. doi: 10.1038/modpathol.3800728. [DOI] [PubMed] [Google Scholar]
- 13.Niakosari F, Kahn HJ, Marks A, From L. Detection of lymphatic invasion in primary melanoma with monoclonal antibody D2-40: a new selective immunohistochemical marker of lymphatic endothelium. Arch Dermatol. 2005;141:440–444. doi: 10.1001/archderm.141.4.440. [DOI] [PubMed] [Google Scholar]
- 14.Van den Eynden GG, Van der Auwera I, Van Laere SJ, Colpaert CG, van Dam P, Dirix LY, Vermeulen PB, Van Marck EA. Distinguishing blood and lymph vessel invasion in breast cancer: a prospective immunohistochemical study. Br J Cancer. 2006;94:1643–1649. doi: 10.1038/sj.bjc.6603152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasu J, Nishina T, Hirasaki S, Moriwaki T, Hyodo I, Kurita A, Nishimura R. Predictive factors of lymph node metastasis in patients with undifferentiated early gastric cancers. J Clin Gastroenterol. 2006;40:412–415. doi: 10.1097/00004836-200605000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura Y, Yasuoka H, Tsujimoto M, Kurozumi K, Nakahara M, Nakao K, Kakudo K. Importance of lymph vessels in gastric cancer: a prognostic indicator in general and a predictor for lymph node metastasis in early stage cancer. J Clin Pathol. 2006;59:77–82. doi: 10.1136/jcp.2005.028779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii M, Ota M, Saito S, Kinugasa Y, Akamoto S, Ito I. Lymphatic vessel invasion detected by monoclonal antibody D2-40 as a predictor of lymph node metastasis in T1 colorectal cancer. Int J Colorectal Dis. 2009;24:1069–1074. doi: 10.1007/s00384-009-0699-x. [DOI] [PubMed] [Google Scholar]
- 18.Harris EI, Lewin DN, Wang HL, Lauwers GY, Srivastava A, Shyr Y, Shakhtour B, Revetta F, Washington MK. Lymphovascular invasion in colorectal cancer: an interobserver variability study. Am J Surg Pathol. 2008;32:1816–1821. doi: 10.1097/PAS.0b013e3181816083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weidner N. New paradigm for vessel intravasation by tumor cells. Am J Pathol. 2002;160:1937–1939. doi: 10.1016/S0002-9440(10)61141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shayan R, Achen MG, Stacker SA. Lymphatic vessels in cancer metastasis: bridging the gaps. Carcinogenesis. 2006;27:1729–1738. doi: 10.1093/carcin/bgl031. [DOI] [PubMed] [Google Scholar]
- 21.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 22.Sako A, Kitayama J, Ishikawa M, Yamashita H, Nagawa H. Impact of immunohistochemically identified lymphatic invasion on nodal metastasis in early gastric cancer. Gastric Cancer. 2006;9:295–302. doi: 10.1007/s10120-006-0396-1. [DOI] [PubMed] [Google Scholar]
- 23.Yonemura Y, Endou Y, Tabachi K, Kawamura T, Yun HY, Kameya T, Hayashi I, Bandou E, Sasaki T, Miura M. Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40. Hum Pathol. 2006;37:1193–1199. doi: 10.1016/j.humpath.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Kahn HJ, Marks A. A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest. 2002;82:1255–1257. doi: 10.1097/01.lab.0000028824.03032.ab. [DOI] [PubMed] [Google Scholar]
- 25.Ji RC. Lymphatic endothelial cells, tumor lymphangiogenesis and metastasis: New insights into intratumoral and peritumoral lymphatics. Cancer Metastasis Rev. 2006;25:677–694. doi: 10.1007/s10555-006-9026-y. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Zhang R, Lu Y, Li H, Lu P, Yao F, Jin F, Xu H, Wang S, Chen J. Prognostic role of lymphatic vessel invasion in early gastric cancer: a retrospective study of 188 cases. Surg Oncol. 2010;19:4–10. doi: 10.1016/j.suronc.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 28.Morita H, Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Nishimura C, Igarashi Y, Miki K, Ishii T. Histopathological predictor for regional lymph node metastasis in gastric cancer. Virchows Arch. 2009;454:143–151. doi: 10.1007/s00428-008-0717-3. [DOI] [PubMed] [Google Scholar]
- 29.Fujishiro M. Perspective on the practical indications of endoscopic submucosal dissection of gastrointestinal neoplasms. World J Gastroenterol. 2008;14:4289–4295. doi: 10.3748/wjg.14.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ichikawa D, Kubota T, Kikuchi S, Fujiwara H, Konishi H, Tsujiura M, Ikoma H, Nakanishi M, Okamoto K, Sakakura C, et al. Prognostic impact of lymphatic invasion in patients with node-negative gastric cancer. J Surg Oncol. 2009;100:111–114. doi: 10.1002/jso.21311. [DOI] [PubMed] [Google Scholar]
- 31.Lemes L, Neunschwander L, Matta L, Osório Filho J, Soares P, Cabral M, Nogueira A, Rodrigues M. Gastric carcinoma: analysis of 289 consecutive gastrectomy specimens in Belo Horizonte, Brazil. J Bras Patol Med Lab. 2003;39:9. [Google Scholar]
- 32.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 33.Maehara Y, Oshiro T, Baba H, Ohno S, Kohnoe S, Sugimachi K. Lymphatic invasion and potential for tumor growth and metastasis in patients with gastric cancer. Surgery. 1995;117:380–385. doi: 10.1016/s0039-6060(05)80056-x. [DOI] [PubMed] [Google Scholar]
- 34.Setälä LP, Kosma VM, Marin S, Lipponen PK, Eskelinen MJ, Syrjänen KJ, Alhava EM. Prognostic factors in gastric cancer: the value of vascular invasion, mitotic rate and lymphoplasmacytic infiltration. Br J Cancer. 1996;74:766–772. doi: 10.1038/bjc.1996.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamata I, Ishikawa Y, Akishima-Fukasawa Y, Ito K, Akasaka Y, Uzuki M, Fujimoto A, Morita H, Tamai S, Maehara T, et al. Significance of lymphatic invasion and cancer invasion-related proteins on lymph node metastasis in gastric cancer. J Gastroenterol Hepatol. 2009;24:1527–1533. doi: 10.1111/j.1440-1746.2009.05810.x. [DOI] [PubMed] [Google Scholar]
- 36.Arigami T, Natsugoe S, Uenosono Y, Arima H, Mataki Y, Ehi K, Yanagida S, Ishigami S, Hokita S, Aikou T. Lymphatic invasion using D2-40 monoclonal antibody and its relationship to lymph node micrometastasis in pN0 gastric cancer. Br J Cancer. 2005;93:688–693. doi: 10.1038/sj.bjc.6602739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4:448–456. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 38.Yokota T, Ishiyama S, Saito T, Teshima S, Narushima Y, Murata K, Iwamoto K, Yashima R, Yamauchi H, Kikuchi S. Lymph node metastasis as a significant prognostic factor in gastric cancer: a multiple logistic regression analysis. Scand J Gastroenterol. 2004;39:380–384. doi: 10.1080/00365520310008629. [DOI] [PubMed] [Google Scholar]
- 39.Kim JH, Park SS, Park SH, Kim SJ, Mok YJ, Kim CS, Lee JH, Kim YS. Clinical significance of immunohistochemically-identified lymphatic and/or blood vessel tumor invasion in gastric cancer. J Surg Res. 2010;162:177–183. doi: 10.1016/j.jss.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 40.Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer. 2008;11:134–148. doi: 10.1007/s10120-008-0476-5. [DOI] [PubMed] [Google Scholar]
- 41.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]


