Abstract
Histopathological results are critical for the diagnosis and surgical decision regarding gastric cancer. However, opposite opinions from radiology and pathology can sometimes affect clinical decisions. The two cases reported in this article were both highly suspected as gastric cancer by clinical manifestations and radiologic findings, although both showed negative results in the first biopsy examination. One was confirmed as gastric cancer by the time of the 6th biopsy, while the other was still negative even after 8 biopsies. With a definite pathologic result and the agreement of the patient for the latter case, both of them finally received surgery. Postoperative pathological examination revealed findings that were the same as Borrmann type IV gastric cancer. We believed that duplicate biopsies under radiologic guidance were necessary for highly suspected gastric cancer cases in the absence of a definite pathology result, and patients should be under close follow-up. We propose that, if gastric cancer is highly suspected when typical radiology changes of widely diffuse gastric parietal lesions suffice to exclude lymphoma and other similar situations, and even in absence of a positive biopsy result, a diagnostic laparotomy under laparoscopy and even radical gastrectomy may be reasonably performed by an experienced gastric cancer center with the agreement of the patient after being decided by a multidisciplinary discussion team.
Keywords: Gastric cancer, Pathology, Diagnosis, Borrmann type
Core tip: Histopathological diagnosis is the diagnostic gold standard of gastric cancer required by medical ethics and practice guideline. However, cases with repeated suspected false negative pathological results always concern medical practitioners a great deal. This article might illustrate a possible standard process for these cases. We propose that, if gastric cancer is highly suspected when typical radiology changes of widely diffuse gastric parietal lesions suffice to exclude lymphoma and other similar situations, and even in absence of a positive biopsy result, surgery could be perform with the agreement of the patient after being decided by a multidisciplinary discussion team.
INTRODUCTION
Gastric cancer is one of the most common cancers of the digestive system, and shows the highest morbidity and mortality among all digestive malignancies in China. Early detection, diagnosis, and treatment are of significant importance in improving patient cure rate and 5-year survival. Gastroscopic biopsy remains one of the primary means for the screening of the disease, and at the same time the gold standard of diagnosis[1]. However, biopsy accuracy relies on samples obtained from gastrofiberscopy so much that doctors would be confused when a pathologic examination showed a negative result inconsistent with radiologic findings and other results. Thus, treatment is often delayed due to medical ethics considerations, which require a definite pathological diagnosis. Both cases we are reporting in this article were negative in repeated biopsies but suggested as gastric cancer by radiologic and gastrofiberscopy examination. We intend to demonstrate the clinical decisions for similar patients.
CASE REPORT
Case 1
A 50-year-old male presented on May 15, 2009, and was admitted due to recurrent upper abdominal discomfort associated with acid reflux, hiccups, and weight loss for 3 mo. Two months previously, it was suggested that he have an abdominal computed tomography (CT) scan in another hospital, and an obviously thickened antral wall was discovered. The CT report considered a differential diagnosis of gastric cancer and primary gastric lymphoma. The patient then had his first gastroscopy examination. Gastroduodenoscopy did not find any apparent ulcer of the stomach, but did show stenosis of the antrum and pyloric. The biopsy result was antral mucosal inflammation. Finally, the patient was treated as gastritis for 2 mo, but no alleviation of discomfort was achieved. Thus, he came to our hospital for further treatment and was suggested to have further examinations after admission. General blood tests that included alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), tumor marker-CA125 (CA125), tumor marker-CA19-9 (CA19-9), and squamous cell carcinoma antigen revealed nothing abnormal. A second abdominal CT scan in our hospital found circular thickening of the antrum stomach wall and retention of gastric contents (Figure 1). Possible gastric cancer was considered. But after a consultation of biopsy slides of the previous scan, we had the same comments. Therefore, a second gastroscope biopsy was conducted in our hospital, only to find chronic inflammation of antral mucosa and local fibrous tissue proliferation, rather than any malignant cells. Since the patient was highly suspected for Borrmann type IV gastric cancer by our multidisciplinary discussion team (MDT), a third biopsy was performed with endoscopic ultrasound as guidance. We sampled 15 tissue cores by electric biopsy forceps through holing in the thickening antrum stomach wall. Endoscopic ultrasonography also supported that the thickening antral wall was a typical change of malignant lesions. However, pathological diagnosis reported chronic inflammation of the antral mucosa without any atypical cells (Figure 1). The patient was discharged for another month of continuous gastritis treatment, which failed to bring about any improvements. One month later, the patient came back for a fourth gastroscope biopsy, but with immunohistochemical examination this time. As before, the report showed chronic inflammation of the antral mucosa and local fibrous tissue proliferation. Immunohistochemistry results were: suspicious atypical cells with M-CEA (±), S-100 (-), actin (-), Syn (-), CD117 (-), CD56 (-), glandular epithelium CK (-), small lymph L26 (+), CD79a (+), the LCA (+), CD3 (+), the tissue cells CD68 (+) and plasma cells CD38 (+). Although there was no definite pathologic result of gastric cancer, Borrmann type IV gastric cancer was strongly suspected. Therefore a further examination of positron emission tomography-CT (PET-CT) was performed and reported. It showed uneven diffuse thickening of the antrum stomach wall, with antrum metabolic imaging not supporting this malignant change and no abnormal metabolic lesions or standard uptake value (SUV) occurring in the other tissues. Conventional medical management seemed to be ineffective and the patient gradually felt worse and worse. On July 29th, he underwent alimentary tract barium meal examination, and the result indicated it was most probably primary gastric lymphoma or antrum cancer with submucosal infiltration, besides eosinophilic gastritis. The patient was discharged from hospital again because we failed to get a positive biopsy result supporting the diagnosis of cancer. On August 30th, one month after discharge, he was asked to have an abdominal CT and gastroscopy examination, which was the suggestion from the MDT last time. The sixth gastroscopy biopsy result confirmed it was gastric antrum signet-ring cell (Figure 1) carcinoma, accompanied with immunohistochemical stain results: signet ring-like cells CK (+), CEA (+). Abdominal CT examination reported gastric antrum cancer with perigastric lymph nodes enlargement. A surgery decision was made soon after the confirmation.
Figure 1.
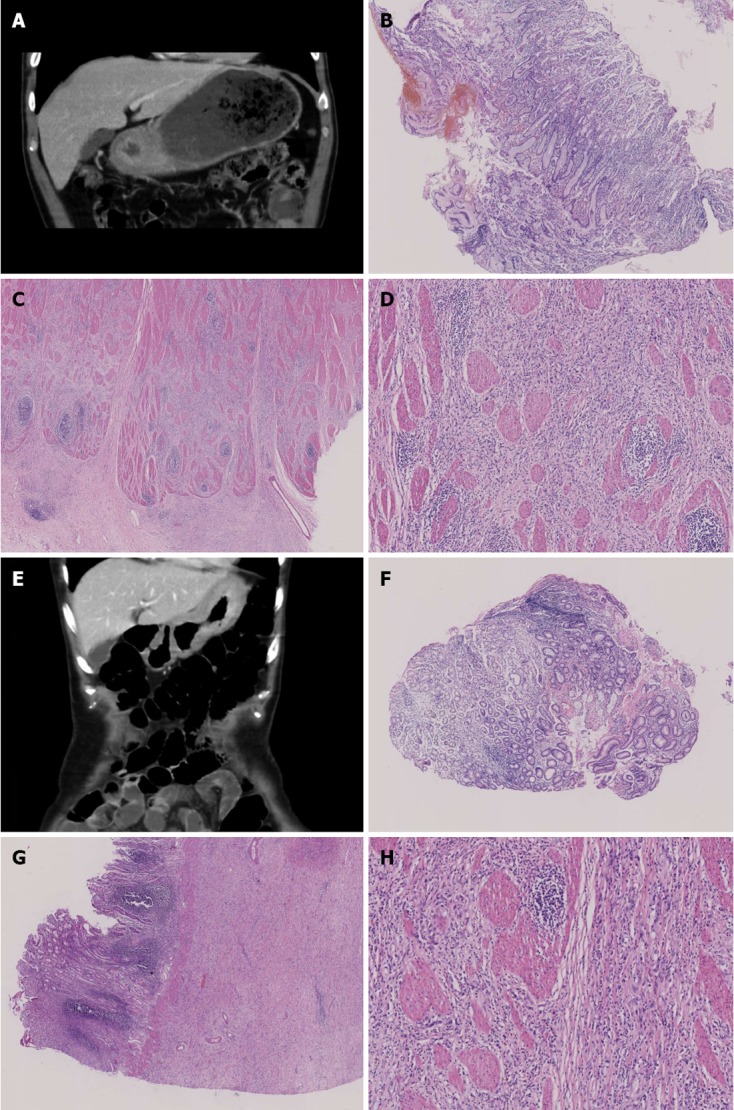
Computerized tomography and pathological findings. A: Diffuse thickened antral detected by computerized tomography (CT) scan; B: Repeated false negative slides without cancer cells detected for the previous 5 biopsies [hematoxylin-eosin (HE) stain, × 40]; C: Positive slide with cancer cells observed by the 6th biopsy (HE stain, × 100); D: Postoperative slide with cancer cells observed, many signet ring-like cells can be observed in the upper-right quadrant of the slide (HE stain, × 200); E: Diffuse thickened fundic and gastric body detected by CT scan; F: Repeated false negative slides without cancer cells detected for 8 biopsies (HE stain, × 40); G: Postoperative slide with cancer cells observed and the whole gastric wall infiltrated (HE stain, × 100); H: Fibrosis stomach wall, scattered or small focal distributed signet ring-like cells could be observed (HE stain, × 200).
During laparotomy, we found the antropylorus where lesions located was thickened, and the perigastric lymph nodes, especially those of the lesser curvature, were obviously enlarged from the 0.5 to 2.5 cm. In addition, multiple metastatic nodules were detected on the omentum. There were no findings of metastasis to other organs within the abdominal cavity and no dissemination of the peritoneum was observed. Radical distal gastrectomy (D2 lymphadenectomy) was therefore performed. Postoperative pathological examination showed that gastric poorly differentiated adenocarcinoma infiltrated the whole gastric wall, and with myenteric nerve invasion (Figure 1) and lymph nodes metastasis (12/27). The patient was finally diagnosed as having antrum signet ring cell carcinoma (T3N2M0, IIIB period, Borrmann type IV). Eight courses of postoperative adjuvant chemotherapy treatment followed by the XELOX plan were initiated since the fourth week after surgery. Our follow-up data show that the patient is still alive.
Case 2
A 52-year-old female had been suffering from abdominal dull pain with intermittent melena and weight loss for 11 mo, and her medical history showed nothing special. Before she came to us, she had been in a local hospital three times for the same complain, and received five gastrofiberscopy examinations with biopsies, since the doctors strongly suspected she had gastric cancer. Unfortunately, every biopsy result remained negative, as did the upper gastrointestinal barium meal examination. The barium meal examination regarded it as gastritis due to the imaging findings: a thick mess of the gastric body mucosa folds was detected but no signs of niche or filling defect, gastric motility and tension were well, and barium could go through the pyloric canal smoothly. However, views of the gastrofiberscopy reports were unified: hypertrophy of gastric body mucosal folds; gastric cancer, and duodenal ulcers was considered. Due to the previous ineffective medical management and being unqualified for any further examination or treatment at the local hospital, the patient was referred to our hospital. General blood tests, including tumor markers AFP, CEA, CA125, CA19-9 and squamous cell carcinoma antigen, were performed after admission, which all showed results within the normal ranges. Gastric endoscopic ultrasonography reported a differential diagnosis of adenoma and lymphoma due to the thickened stomach wall of the fundus and gastric body. The patient was suggested to have a sixth biopsy. Under a gastroduodenoscopy, this time in our hospital, we found the gastric mucosa had obvious congestion, edema, was stiff, and the gastric folds were thicker and harder than normal. A biopsy was then performed. Pathological findings showed that no adenocarcinoma was found with hematoxylin-eosin stain, a small amount of abnormal glands were noticed under cytokeratin immunohistochemical stain, M-CEA (-), and a particularly high positive rate of Ki-67 was not seen in the samples. Pathology failed to diagnose the cancer. An abdominal CT examination revealed a diffusely thickened and stiff stomach wall of the gastric fundus and body, a fixed stomach shape, obvious changes to the mucosa of the thickened stomach wall during the contrast enhanced phase, and a number of enlarged lymph nodes in the lesser curvature and retroperitoneal area (Figure 1). The CT report stated possible lymphoma. However, gastric cancer was highly suspected according to our experience and the patient’s clinical manifestation. A seventh deep layer biopsy was therefore carried out, but only to disappointingly show a negative result again. After deliberation by the MDT, including experts from departments of imaging, ultrasound diagnosis, pathology, surgery, and internal medicine, we insisted it to be Borrmann type IV stomach adenocarcinoma that showed hypertrophic gastric lesions, and that conventional biopsy methods would always display a low positive rate. We decided to take endoscopic ultrasound guided fine-needle aspiration for the eighth biopsy. We made two punctures deep into the submucosa and muscularis at the stomach wall of the lower segment of the gastric body for samples where the endoscopic ultrasound showed disappearing gastric structure. Unfortunately, our pathologist told us that cancer cells were still not detected in the samples, only normal gastric mucosa epithelium and some lamina propria glands. A liquid-based cytology test was also negative (Figure 1). However, according to our experience, Borrmann type IV stomach cancer was strongly suspected and surgery was recommended after an MDT discussion. Naturally, the patient refused to have surgery without a definite histological diagnosis. So a PET-CT examination was followed. It reported that the stomach wall of the gastric fundus and body were obvious thickened to a maximum of 1.5 cm. Abnormal 18F-fluorodeoxyglucose (18F-FDG) uptake was also observed, with a maximum SUV of approximately 3.1. The thickened gastric wall and slightly active metabolic imaging indicated the possibility of poorly differentiated adenocarcinoma. Considering that the patient’s clinical symptoms were aggravating, surgery was again recommended, and she agreed to undergo surgery and a laparotomy was performed.
During the laparotomy, we found the stomach wall was thickened overall and the tumor, as large as 14 cm × 9 cm, had invaded the serosa, accompanied by the enlargement of the perigastric lymph nodes. The center of the transverse colon, omentum, the spleen, and partial left adrenal were all invaded. Enlarged retroperitoneal lymph nodes were palpable. Adhesion or ascites was not detected, and there were no findings of metastasis to the rest organs in the abdominal cavity. So we decided to perform gastric cancer extended radical mastectomy (radical total gastrectomy, splenectomy, partial resection of the left adrenal, D2 lymphadenectomy, and Roux-en-Y esophagojejunostomy). Postoperative pathological examination showed that the stomach wall tissue was fibrotic with small focal distributed signet ring-like cells (Figure 1), CK (+), M-CEA (+), CK20 multifocal (+), poorly differentiated adenocarcinoma infiltrated the whole stomach wall, no cancer cells were found in the Splenic tissue, transverse colon, surgical margins or omentum, lymph nodes metastasis (0/32). She was diagnosed with gastric signet ring cell carcinoma (T4aN0M0, IIB stage, Borrmann type IV). As with the former case, the patient was suggested to receive postoperative adjuvant chemotherapy treatment, and is still alive.
DISCUSSION
Histopathological diagnosis is the diagnostic gold standard, as well as the treatment basis of gastric cancer due to its high specificity and negative predictive value[2,3]. Since a definite preoperative pathological result is required by medical ethics and practice guidelines, we could not carelessly make clinical decisions only according to our experience. But it is definite that there no test methods that can be 100% confirmed for a diagnosis. Thus, when the biopsy results didn’t agree with the patient’s
clinical manifestations and other examination results (such as CT, endoscopic ultrasound, PET-CT, etc., in our article), both patients were suggested to have repeated gastroscopic biopsies to create a proper medical plan for MDT discussion.
Even as the only diagnostic gold standard for gastric cancer, gastroscopy biopsies still have a certain percentage of false negative (positive) rates. A prospective study on 1331 cases by Tatsuta et al[2] reported a false negative rate of 3.7% and a false-positive rate of 0.6% for gastroscopy biopsies. The data derived from a statistics of the patients’ postoperative pathology, autopsy and clinical follow-up etc. According to the study, early gastric cancer, Borrmann type IV gastric cancer, and leiomyosarcoma were the main causes of false negative cases, and the false positive situations were all caused by active ulcer. Therefore, if the biopsy outcomes were suspected to be false negative (positive), repeated examinations for confirmed diagnosis are necessary. That was why we were so hesitated to make surgery decisions without a pathological diagnosis for both cases. However, at the same time, we were very anxious that the increasing number of biopsies would naturally increase the risk of bleeding at the biopsy site, rather than improve accuracy. A study by Choi et al[3] thus recommended 3-4 biopsies from visible tissue through endoscopy to make a correct pathologic diagnosis. When 6 or more biopsy specimens were obtained, diagnostic accuracy would reach 100%. But they also admitted the special difficulty for Borrmann type IV gastric cancer and insisted that if the negative results were accompanied with a malignant impression under endoscopic examination, a re-biopsy should be performed with careful targeting, which were the measures we performed in the cases reported. Similarly, statistics from the database of our hospital, including 1747 gastric cancer patients (Table 1), revealed a general false negative rate of 2.1% for the initial pathological diagnosis, but a second biopsy followed by multiple and deep sampling could mostly bring a definite outcome. However, we found it difficult to have a positive result, even with careful targeting, if the second biopsy was still negative. Among the false negative cases, Borrmann type IV gastric cancer covered the highest false negative rate, and the cases we reported were the most notable two. Borrmann type IV gastric cancer often calls for several biopsies before a confirmatory diagnosis, due to its peculiar biological properties in which a submucosal spread of malignant cells is present without a mucosal lesion. Malignant cells could not be obtained by subsequent repeated endoscopic biopsy. However, repeated biopsies with the guide of CT and ultrasound might increase the accuracy[4-7]. Ahn et al[4] insisted that if cases were strongly suspected malignant prior to surgery, and surgery is being considered, endoscopic stomach mucosal resection should be recommended despite any development of stomach perforation or complications. In fact, the risk and uselessness of repeated biopsies were our inevitable concern. Thus, further noninvasive measures, though not the gold standard but of great importance, were under consideration for diagnosis. Upper gastrointestinal imaging, especially for the double contrast barium-air test, is one of the most commonly-used methods for the diagnosis of gastric cancer. Park et al[8] even pointed out that upper gastrointestinal imaging was superior to gastrofiberscopy for the diagnosis and localization of Borrmann type IV gastric cancer. Practically, alimentary tract barium meals were suggested in case 1 and the result supported this point. But most doctors would hardly be persuaded by this, especially when it is controversial in comparison to other, more credible, results (such as pathology and CT). In addition, PET-CT was suggested and performed in both patients. However, the use of PET-CT in the diagnosis of gastric cancer and evaluation of lymph nodes metastasis is still controversial[9]. The reported diagnosis accuracy varies from 60% to 95% in different studies[10-13]. For cases in which ordinary CT did not prompt a diagnosis of gastric cancer, we could not carelessly make a diagnosis of cancer simply according to the high gastrointestinal 18F-FDG uptake[14,15]. Since such situations could also happen in normal physiological conditions, gastritis as well as stomach ulcers could also show false positive results. Furthermore, being related to the rich mucin content, Borrmann type IV gastric cancer is often associated with a false negative result. It should be distinguished from mucinous adenocarcinoma, signet ring cell carcinoma, and poorly differentiated adenocarcinoma that are also low in FDG uptake[9,11]. The two cases we reported also suggest the limitations of PET-CT in the diagnosis of Borrmann type IV gastric cancer. Case 1 was with a false negative result, though the result of case 2 proved to be true postoperative. However, interfering by the CT report of possible lymphoma and the negative pathologic results, even the MDT felt the decision was tough.
Table 1.
Gastroscope statistics from the database of the First Affiliated Hospital n (%)
| Items | Total | F (-) 1st | (+) 1st | F (-) 2nd | (+) 2nd | F (-) ≥ 3 | (+) ≥ 3 |
| Borrmann IV | 194 | 21 (10.8) | 173 (89.2) | 10 (5.2) | 11 (5.7) | 6 (3.1) | 4 (2.1) |
| Early type | 165 | 11 (6.7) | 154 (93.3) | 1 (0.6) | 10 (6.1) | 0 (0.0) | 1 (0.6) |
| Other types | 1388 | 4 (0.3) | 1384 (99.7) | 0 (0.0) | 4 (0.3) | 0 (0.0) | 0 (0.0) |
| Total | 1747 | 36 (2.1) | 1711 (97.9) | 11 (0.6) | 25 (1.4) | 6 (0.3) | 5 (0.3) |
F (-) 1st: False negative diagnosis of the 1st biopsy; (+) 1st: True positive diagnosis of the 1st biopsy; 2nd: The 2nd biopsy; ≥ 3: More than 3 times of biopsies.
Actually, reasonable treatments determined by a pathologic result were a real concern of doctors, and the reason for the repeated biopsies. Borrmann type IV gastric cancer usually shows an extensive diffuse thickened stomach wall through a CT scan, but so do stomach sarcoma, gastrointestinal stromal tumors, hiatal hernia, gastric lymphoma, benign gastric ulcer, benign tumor, and gastritis. Combined medical methods such as endoscopic ultrasound, and upper gastrointestinal contrast, would make it easier for the differentiation of most of the diseases mentioned, although gastric lymphoma was the one we mainly concerned about. Since gastric lymphoma also originated from the submucosa, it would firstly invade and grow with a wide lesion. Therefore, gastric lymphomas often have false negative biopsy results, and are also difficult to differentiate from gastric cancer through CT and endoscopic ultrasound. The differential diagnosis of gastric cancer from primary gastric lymphoma is especially important, since gastric cancer mainly depends on surgical treatment combined with radiotherapy and chemotherapy as a supplement at present. However, it is not the first choice for primary gastric lymphoma. Patients of mucosa associated lymphoid tissue lymphoma could mostly be cured simply by Helicobacter pylori (H. pylori) eradication, local radiotherapy, or chemotherapy[16]. For invasive gastric lymphomas, such as the diffuse large B-cell lymphoma patients, if they were at an early stage, chemotherapy combined with local radiotherapy would be suggested instead of surgical treatment. Patients’ life quality would be improved during the course of treatment[17]. Surgical therapy would be suggested only when meeting with the failure of gastric retention treatment, focal lesion, or uncontrollable complications such as serious perforation and bleeding[18]. Therefore, both patients of our reported cases, with imaging findings of diffuse gastric wall thickening, were suggested to have immunohistochemistry tests at the same time as the biopsy, so that we could make a conclusion according to the immunohistochemistry result and the specific lymphoma phenotype[19]. 18F-FDG PET-CT also played an important role in the differentiation and diagnosis of gastric lymphoma, but it is more valuable for a therapeutic evaluation[20], while CT and endoscopic ultrasonography were superior in evaluating gastric lesions and lymph node status[7,21] and promising a more accurate guide for the biopsy. Fan et al[22] concluded that gastric cancer lesions often covered less than 50% of the stomach wall under a CT scan, but usually more than 75% if it were gastric lymphoma. Furthermore, enlarged perigastric lymph nodes tend to be in one area, but for gastric lymphoma there would be multiple enlarged areas. Interesting, they pointed out that this might indicate a diagnosis of lymphoma if it were associated with the enlargement of the retroperitoneal lymph nodes inferior renal hilus[22]. However, it seemed unsuitable for our cases. Needless to say, a confirmed diagnosis of gastric lymphoma also depends on the biopsy results. But similar findings under gastrofiberscopy of superficial ulcers or protuberant submucosal mess in the stomach often lead to a misdiagnosis of adenocarcinoma or benign ulcers. According to a study by Guzicka-Kazimierczak et al[23], endoscopic ultrasound-guided biopsies, multiple biopsies, and H. pylori tests would contribute to a diagnosis. However, it seemed not helpful for our cases either.
The two difficult cases we reported were both highly suspected as Borrmann type IV gastric cancer by the MDT preoperative, but cancer cells could not be detected in early biopsies. A confirmed diagnosis of cancer was mainly as a result of the deterioration of the clinical course. Since, for case 1, the patient was finally pathologically diagnosed as having cancer only in after the sixth biopsy after more than 6 mo from admittance, while the case 2 patient had to undergo surgery to find the truth, because her clinical symptoms were aggravating.
In conclusion, a definite preoperative pathological diagnosis of Borrmann type IV gastric cancer is difficult in many cases due to its characteristic submucosa originated development process. Repeated endoscopic biopsies and deep biopsy guided by CT and endoscopic ultrasonography that reaches the proper area of the stomach may be necessary. Compared with histopathology radiology (such as CT, PET-CT, upper gastrointestinal imaging), ultrasonography, and endoscopy are not the diagnostic gold standard for gastric cancer, and diagnostic value can not be ignored. Especially when cancer cells could not be detected despite repeated biopsies, the results of the methods mentioned above should be considered together with the clinical manifestations of patients. For those with radiology performance of widely diffuse lesions of the stomach wall, it may be highly characterized by Borrmann type IV gastric cancer. But surgery decisions should be carefully made in case of primary gastric lymphoma, which might benefit more from a non-surgical treatment method. We propose that, if gastric lymphoma could be excluded for such cases, even in the absence of any positive biopsy results supporting the diagnosis of gastric cancer, a diagnostic laparotomy and even radical gastrectomy may be reasonably performed by an experienced gastric cancer center with the agreement of the patient after being decided by a multidisciplinary discussion team.
ACKNOWLEDGMENTS
We thank Peng ZP and Chen WF from the pathology department of the First Affiliated Hospital of Sun Yat-sen University for pathological support and Xu CC from the Zhongshan Ophthalmic Center of Sun Yat-sen University for editorial assistance.
Footnotes
Supported by The Science and Technology Development Project of Guangdong Province, No. 2011B031800240 and No. 2012B031800389
P- Reviewers Guo JM, Rodriguez DC S- Editor Gou SX L- Editor Rutherford A E- Editor Li JY
References
- 1.Viudez-Berral A, Miranda-Murua C, Arias-de-la-Vega F, Hernández-García I, Artajona-Rosino A, Díaz-de-Liaño Á, Vera-García R. Current management of gastric cancer. Rev Esp Enferm Dig. 2012;104:134–141. doi: 10.4321/s1130-01082012000300006. [DOI] [PubMed] [Google Scholar]
- 2.Tatsuta M, Iishi H, Okuda S, Oshima A, Taniguchi H. Prospective evaluation of diagnostic accuracy of gastrofiberscopic biopsy in diagnosis of gastric cancer. Cancer. 1989;63:1415–1420. doi: 10.1002/1097-0142(19890401)63:7<1415::aid-cncr2820630731>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Choi Y, Choi HS, Jeon WK, Kim BI, Park DI, Cho YK, Kim HJ, Park JH, Sohn CI. Optimal number of endoscopic biopsies in diagnosis of advanced gastric and colorectal cancer. J Korean Med Sci. 2012;27:36–39. doi: 10.3346/jkms.2012.27.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn JB, Ha TK, Lee HR, Kwon SJ. An Insufficient Preoperative Diagnosis of Borrmann Type 4 Gastric Cancer in Spite of EMR. J Gastric Cancer. 2011;11:59–63. doi: 10.5230/jgc.2011.11.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim DY, Kim HR, Kim YJ, Kim S. Clinicopathological features of patients with Borrmann type IV gastric carcinoma. ANZ J Surg. 2002;72:739–742. doi: 10.1046/j.1445-2197.2002.02523.x. [DOI] [PubMed] [Google Scholar]
- 6.Hamashima C, Shibuya D, Yamazaki H, Inoue K, Fukao A, Saito H, Sobue T. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259–267. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- 7.Park JM, Ahn CW, Yi X, Hur H, Lee KM, Cho YK, Han SU. Efficacy of endoscopic ultrasonography for prediction of tumor depth in gastric cancer. J Gastric Cancer. 2011;11:109–115. doi: 10.5230/jgc.2011.11.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park MS, Ha HK, Choi BS, Kim KW, Myung SJ, Kim AY, Kim TK, Kim PN, Lee NJ, Lee JK, et al. Scirrhous gastric carcinoma: endoscopy versus upper gastrointestinal radiography. Radiology. 2004;231:421–426. doi: 10.1148/radiol.2312030248. [DOI] [PubMed] [Google Scholar]
- 9.Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, Lee JH, Ryu KW, Kim YW, Bae JM. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148–155. doi: 10.1007/s00259-005-1887-8. [DOI] [PubMed] [Google Scholar]
- 10.Yeung HW, Macapinlac H, Karpeh M, Finn RD, Larson SM. Accuracy of FDG-PET in Gastric Cancer. Preliminary Experience. Clin Positron Imaging. 1998;1:213–221. doi: 10.1016/s1095-0397(98)00018-1. [DOI] [PubMed] [Google Scholar]
- 11.Stahl A, Ott K, Weber WA, Becker K, Link T, Siewert JR, Schwaiger M, Fink U. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288–295. doi: 10.1007/s00259-002-1029-5. [DOI] [PubMed] [Google Scholar]
- 12.Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, Cho A, Lee JD. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582–1588. [PubMed] [Google Scholar]
- 13.Park MJ, Lee WJ, Lim HK, Park KW, Choi JY, Kim BT. Detecting recurrence of gastric cancer: the value of FDG PET/CT. Abdom Imaging. 2009;34:441–447. doi: 10.1007/s00261-008-9424-4. [DOI] [PubMed] [Google Scholar]
- 14.Heusner TA, Hahn S, Hamami ME, Kim UH, Baumeister R, Forsting M, Stahl A, Bockisch A, Antoch G. Gastrointestinal 18F-FDG accumulation on PET without a corresponding CT abnormality is not an early indicator of cancer development. Eur Radiol. 2009;19:2171–2179. doi: 10.1007/s00330-009-1405-7. [DOI] [PubMed] [Google Scholar]
- 15.Kim EY, Lee WJ, Choi D, Lee SJ, Choi JY, Kim BT, Kim HS. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol. 2011;79:183–188. doi: 10.1016/j.ejrad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Zucca E, Bertoni F, Roggero E, Cavalli F. The gastric marginal zone B-cell lymphoma of MALT type. Blood. 2000;96:410–419. [PubMed] [Google Scholar]
- 17.Taal BG, Burgers JM, van Heerde P, Hart AA, Somers R. The clinical spectrum and treatment of primary non-Hodgkin’s lymphoma of the stomach. Ann Oncol. 1993;4:839–846. doi: 10.1093/oxfordjournals.annonc.a058390. [DOI] [PubMed] [Google Scholar]
- 18.Chang MC, Huang MJ, Su YW, Chang YF, Lin J, Hsieh RK. Clinical outcome of primary gastric lymphoma treated with chemotherapy alone or surgery followed by chemotherapy. J Formos Med Assoc. 2006;105:194–202. doi: 10.1016/S0929-6646(09)60305-3. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S, Akazawa K, Yao T, Tsuneyoshi M. A clinicopathologic study of 233 cases with special reference to evaluation with the MIB-1 index. Cancer. 1995;76:1313–1324. doi: 10.1002/1097-0142(19951015)76:8<1313::aid-cncr2820760804>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Paes FM, Kalkanis DG, Sideras PA, Serafini AN. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. Radiographics. 2010;30:269–291. doi: 10.1148/rg.301095088. [DOI] [PubMed] [Google Scholar]
- 21.Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer. 2009;12:6–22. doi: 10.1007/s10120-008-0492-5. [DOI] [PubMed] [Google Scholar]
- 22.Fan WJ, Lu YC, Liu LZ, Shen JX, Xie CM, Li X, Zhang L. Comparison of CT findings between gastric cancer and gastric lymphoma. Ai Zheng. 2008;27:539–543. [PubMed] [Google Scholar]
- 23.Guzicka-Kazimierczak R, Zdziarska B, Kazimierczak A, Sledz M. Gastric non-Hodgkin’s lymphoma--clinical symptoms and diagnostic problems. Wiad Lek. 2011;64:3–8. [PubMed] [Google Scholar]


