Abstract
Background
Near-infrared has been shown to penetrate deeper than optical light sources independent of skin color, allowing safer treatment for the Asian skin type. Many studies have indicated the efficacy of various types of devices, but have not included a sufficiently objective evaluation. In this study, we used three-dimensional imaging for objective evaluation of facial skin tightening using a water-filtered near-infrared device.
Methods
Twenty Japanese patients were treated with the water-filtered near-infrared (1,000–1,800 nm) device using a contact-cooling and nonfreezing gel stored in a freezer. Three-dimensional imaging was performed, and quantitative volume measurements were taken to evaluate the change in post-treatment volume. The patients then provided their subjective assessments.
Results
Objective assessments of the treated cheek volume evaluated by a three-dimensional color schematic representation with quantitative volume measurements showed significant improvement 3 months after treatment. The mean volume reduction at the last post-treatment visit was 2.554 ± 0.999 mL. The post-treatment volume was significantly reduced compared with the pretreatment volume in all patients (P < 0.0001). Eighty-five percent of patients reported satisfaction with the improvement of skin laxity, and 80% of patients reported satisfaction with improvement of rhytids, such as the nasolabial folds. Side effects, such as epidermal burns and scar formation, were not observed throughout the study.
Conclusion
The advantages of this water-filtered near-infrared treatment are its high efficacy for skin tightening, associated with a minimal level of discomfort and minimal side effects. Together, these characteristics facilitate our ability to administer repeated treatments and provide alternative or adjunctive treatment for patients, with improved results. This study provides a qualitative and quantitative volumetric assessment, establishing the ability of this technology to reduce volume through noninvasive skin tightening.
Keywords: objective evaluation, quantitative volume measurement, skin laxity, three-dimensional imaging, rhytids
Introduction
Nonablative photorejuvenation has become an integral procedure in the emerging discipline of laser dermatologic surgery.1,2 Rhytids and laxity are caused by a reduction in the quantity and quality of collagen and elastin in the dermis and hypodermis,3 as well as a combination of intrinsic and extrinsic factors, such as photoaging.
Compared with ultraviolet and visible light radiation, which are both attenuated by melanin,4 near-infrared is able to penetrate deep into human tissue where it causes photochemical changes.5 Whereas nonablative skin rejuvenation that uses intense pulse light heats up the superficial dermis, near-infrared or radiofrequency has been introduced for nonablative tissue tightening by volumetric heating of the deep tissues.6 Because near-infrared is primarily absorbed by water in the skin, near-infrared irradiation can effectively heat the dermis.7 Dermal heating induces the release of inflammatory chemical mediators that stimulate the collagen healing process, and initiates a cascade of inflammatory events that includes fibroblastic proliferation and upregulation of collagen expression.8,9 One of the effects of dermal heating is an immediate change in collagen structure, followed by long-term stimulation of neocollagenesis.10 Deep tissue heating stimulates formation of new collagen, which can achieve skin tightening.11 These thermal effects improve rhytids, laxity, and contours on both the face and body.
We previously described the tightening effects of near-infrared objectively and histologically, and reported that near-infrared can achieve skin tightening12–15 and muscle thinning,16,17 as well as nonthermally induce various responses in the skin and subcutaneous tissues.18–27 In addition to the tightening effects, near-infrared nonthermally relaxes and weakens dystonic or hypertrophic muscles, which is effective in reducing rhytids16,17 and improving enlarged masseter muscles.
In this study, we used a water-filtered near-infrared device with a contact cooling and nonfreezing gel stored in a freezer. The near-infrared device emits a near-infrared spectrum at 1,000–1,800 nm, with water filtering to remove the wavelengths at 1,400–1,500 nm that are strongly absorbed by water and hemoglobin, which prevents blood vessel dilation, and enables near-infrared to be delivered to the deeper tissues without pain or epidermal burns. Further, the contact-cooling and freezer-stored gel reduces the temperature, perspiration, and blood vessel dilation at the skin surface, enabling safe delivery of near-infrared energy into the deeper tissues, including muscle.17,23
Several studies have suggested the efficacy of the near-infrared device but these studies did not include a sufficiently objective evaluation of post-treatment volumetric tissue changes. Conventional evaluations using photographs have been widely used, but do not provide an accurate objective assessment.28 Therefore, in this study, a three-dimensional photographic system was used to evaluate the amount of post-treatment volume change. The aim was to assess and provide definitive clinical evidence of skin tightening using the water-filtered near-infrared device with the contact-cooling and freezer-stored gel and including both subjective and objective evaluations.
Materials and methods
Japanese patients
Twenty Japanese patients (18 women, two men) of mean age 49.40 ± 12.51 (32–71) years with Fitzpatrick skin type III–V were enrolled in this study. All patients had visited the Clinica Tanaka Anti-Aging Center seeking treatment for facial skin laxity and reduction of rhytids.
None of the patients had a history of any type of skin disease or cosmetic procedure that affected the treatment area within the previous 3 years. Patients did not use any specific skin care products or follow any specific diet. No patients reported weight loss during the study period. No topical pretreatment medication was used, and the post-treatment skin care regimen consisted of a gentle cleanser and sun block. All patients gave written informed consent for participation in the study after reading the experimental protocol and being advised about the risks of treatments.
Near-infrared treatment
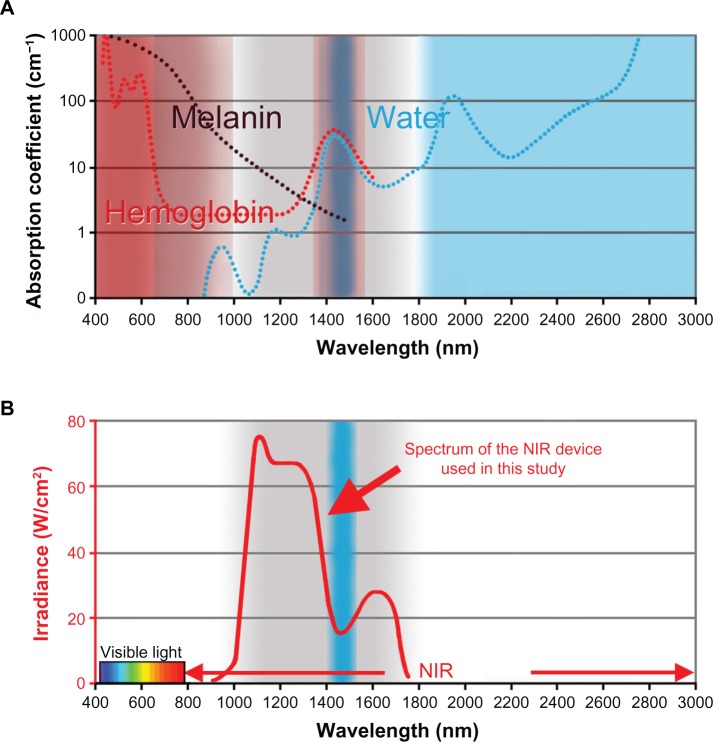
A near-infrared device (Titan®, Cutera Inc, Brisbane, CA, USA) with water-filtered near-infrared for targeting via selective photothermolysis (excluding melanin and hemoglobin) with contact-cooling and freezer-stored gel was used. The near-infrared device emits a near-infrared spectrum at 1,000–1,800 nm, with water filtering to remove wavelengths mainly at 1,400–1,500 nm, that are strongly absorbed by water and hemoglobin (Figure 1). Water filtering of these highly absorbable wavelengths prevents dilation of blood vessels, and enables near-infrared to be delivered to the deeper tissues without pain or epidermal burns.
Figure 1.
(A) Absorption coefficients of melanin (black), hemoglobin (red), and water (blue). (B) The NIR device used in the present study emits a spectrum of near-infrared from 1,000 nm to 1,800 nm (bold red), with filtering of wavelengths between 1,400 nm and 1,500 nm (blue belt), which are strongly absorbed by water and hemoglobin. Cited and revised from Figure 1 in Tanaka et al. Non-thermal cytocidal effect of NIR irradiation on cultured cancer cells using specialized device. Cancer Sci. 2010;101:1396–1402.20
Abbreviation: NIR, near-infrared.
To reduce temperature, perspiration, and blood vessel dilation at the skin surface, the sapphire contact-cooling tip was set to a fixed temperature of 20°C to provide contact cooling. The sapphire block was cooled with fluids using thermoelectric coolers. Cooling fluids were circulated by a pump and a cooling system. Further, nonfreezing gel stored in a freezer was applied to the skin. Pre and parallel irradiation cooling of the superficial layers was accomplished using this temperature-controlled sapphire window and freezer-stored gel, which prevented excessive superficial heating and enabled near-infrared to be delivered to the deeper tissues. These specific wavelengths and the cooling system enable near-infrared to achieve skin tightening and muscle relaxation, which is demonstrated by the ability to treat without anesthesia, complications, or side effects.12–14,16,17
Before treatment, the patient’s face was wiped with an alcohol pad or washed to remove any makeup. No topical anesthetics or oral analgesics were administered before, during, or after treatment. Before each treatment, freezer-stored gel was applied to the skin. The gel was used to keep approximately 2 mm of gel-filled space between the hand piece and the skin surface, avoiding direct or bad contact. The freezer-stored gel was very cold (approximately −10°C to 10°C) at the beginning of near-infrared treatment, which enabled near-infrared to reach the deeper tissues without absorption of near-infrared energy by superficial tissues and to achieve improvement in wrinkles and muscle thinning, as shown in our prior research17 (Figure 2A). During treatment, as the gel and superficial layers of the skin gradually became warmer (approximately 10°C–30°C), near-infrared energy was absorbed in the superficial layers of the skin, which was effective for skin tightening (Figure 2B).
Figure 2.
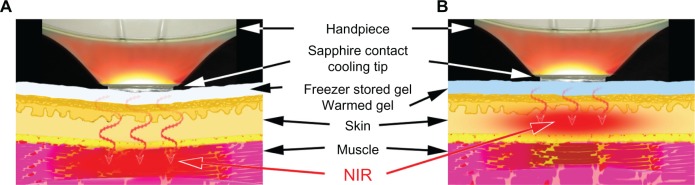
Schematic of our NIR treatment for skin tightening. (A) NIR treatment targeting superficial muscles with the freezer-stored gel (approximately −10°C to 10°C). (B) NIR, treatment targeting dermis with the gel (approximately 10°C–30°C).
Abbreviation: NIR, near-infrared.
One or two treatments with a one-month interval between treatments were performed on the cheeks for 3–4 passes at a 34–43 J/cm2 output (80–120 shots), which enabled an effective procedure without severe burning sensation. Fluences were varied, depending on mild pink red erythema and sensation of heat by the patient. If the patients reported a strong sensation of heat, the hand piece was moved slightly away from the point of heat sensation. Although higher efficacy can be obtained with a higher fluence, the energy was lowered if the patients reported sensation of heat.
Objective assessments
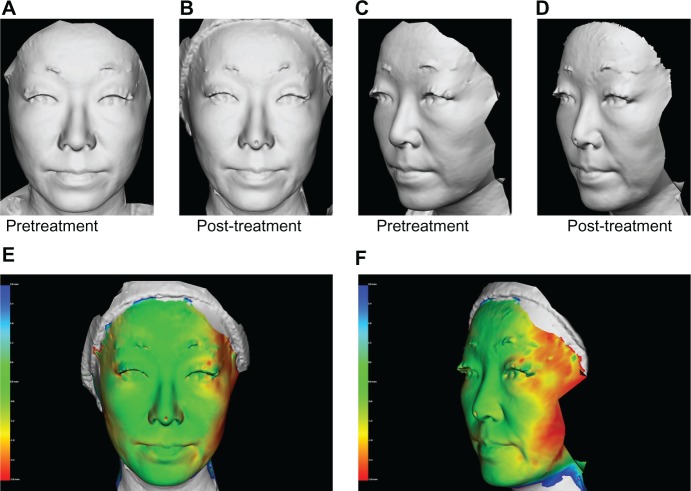
Digital photographs and three-dimensional imaging with quantitative volume measurements were performed as objective assessments using a Vectra camera and software (Canfield Scientific Inc, Fairfield, NJ, USA). This system is designed to capture the surface shape accurately as well as two-dimensional color information for the human body. The capture sequence of the Vectra was set to less than 3 msec in order to capture the shape accurately even if the subject was not perfectly still. A three-dimensional color schematic representation indicates the volume changes between pretreatment and post-treatment images in the face, and shows the varying degrees of tightening achieved in colors ranging from yellow to red. Green areas indicate no changes to the face. In this study, volume change was calculated using software, and recorded in milliliters. Volume reduction was regarded as achievement of skin tightening and improvement of an enlarged masseter muscle. Care was taken to ensure similar nonsmiling facial tone in both pretreatment and post-treatment photographs.
Subjective assessments
Subjective volunteer assessments were performed using questionnaires in which the patients were asked to rate their degree of satisfaction in terms of skin laxity and rhytids based on a five-point scale ranging from 0 to 4 (0 = worse, 1 = little satisfaction or not satisfied, 2 = fairly satisfied, 3 = satisfied, and 4 = very satisfied). Questionnaires were completed 3 months after the final treatment.
Statistical analyses
The differences were examined for statistical significance using the Wilcoxon signed-rank test. A P < 0.05 was set as the cutoff for statistical significance. Data are represented as the mean ± standard deviation.
Results
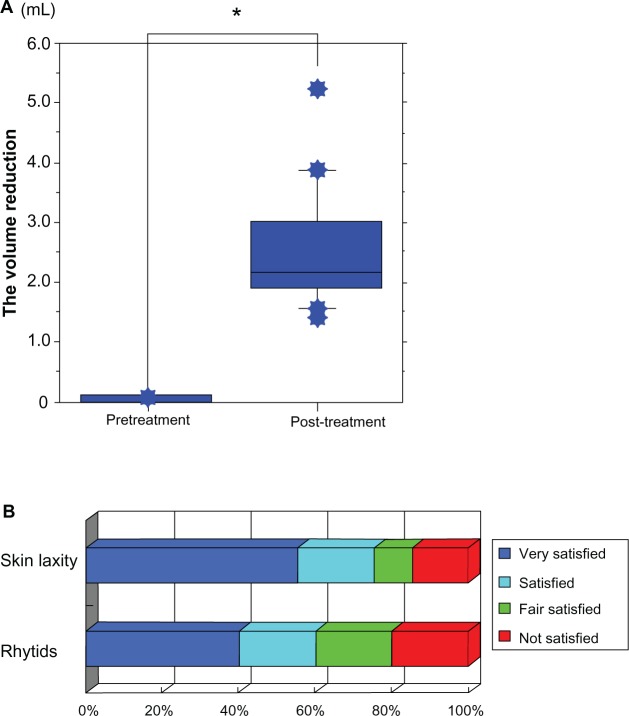
Objective assessments evaluated by three-dimensional color schematic representation with quantitative volume measurements showed significant improvement in skin laxity and the enlarged masseter muscle after treatment (Figures 3–5). The mean volume reduction measured at the last post-treatment visit was 2.554 ± 0.999 mL. The post-treatment volume was significantly reduced compared with the pretreatment volume (P < 0.0001, Figure 6A). Eighty-five percent of patients reported satisfaction with improvement in skin laxity, and 80% reported satisfaction with improvement of rhytids, such as the nasolabial folds (Figure 6B). The mean degree of satisfaction in terms of skin laxity and rhytids based on a five-point scale from 0 to 4 was 3.15 ± 1.137 and 2.80 ± 1.196, respectively. Complications were minor and transient, with slight burning sensation reported by five patients and mild erythema by one patient. Patients did not report severe pain during near-infrared treatment, even though it was performed without anesthesia and contact cooling. Side effects, such as epidermal burns, atrophy of adipose tissue, and contraction, were not observed during the study.
Figure 3.
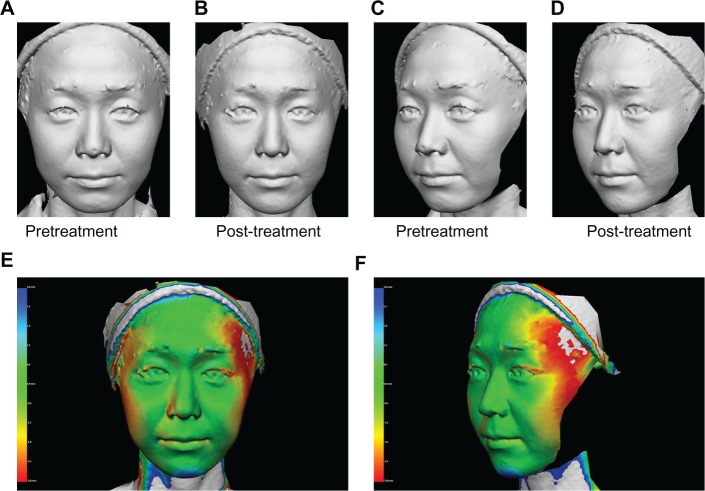
A 36-year-old Japanese woman (A and C) pretreatment and (B and D) 60 days post-treatment, and (E and F) three-dimensional color schematic representation. The varying degrees of tightening achieved are shown in colors yellow to red. Green areas indicate areas that remained unchanged. Two treatments with a one-month interval between treatments were performed on the cheeks with four passes at 42 J/cm2 (100 shots). Significant improvements were noted in the gray image and three-dimensional color schematic representation. Volume reduction was 3.140 mL.
Figure 5.
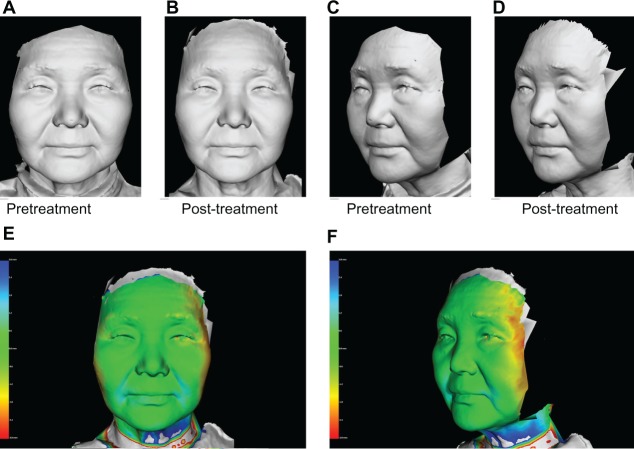
A 65-year-old Japanese woman (A and C) pretreatment and (B and D) 60 days post-treatment, and (E and F) three-dimensional color schematic representation. Two treatments with a one-month interval between treatments were performed with four passes at 50 J/cm2 (100 shots). Significant improvements were noted in the gray image and three-dimensional color schematic representation. Volume reduction was 2.097 mL.
Figure 6.
(A) Mean volume reduction measured by comparison of volume at the first pretreatment visit and the last post-treatment visit. The post-treatment volume was significantly reduced compared with the pretreatment volume in all patients (P < 0.0001). Data are presented as the mean ± standard deviation. *Indicates a significant difference. (B) Subjective volunteer assessments were performed using questionnaires. The patients were asked to rate their degree of satisfaction regarding improvement in skin laxity and rhytids. Subjective volunteer assessments are shown as follows: very satisfied (blue), satisfied (light blue), fairly satisfied (green), and not satisfied (red).
Discussion
Regardless of skin type, skin laxity is one of the most common complaints reported by aging patients. Although invasive or ablative procedures, such as facelifts or laser resurfacing, are effective in skin tightening, the downtime and potential adverse effects are not well accepted.29 Noninvasive skin tightening procedures can be particularly applicable to skin of color, such as in Asians, because such procedures are independent of skin type.29 Further, the skin aging process in Asians differs from that in Caucasians, with mid-face aging, such as sagging of the malar fat pads, being a common manifestation.30 Therefore, skin tightening is an important aspect in the management of skin aging in patients of color.29 Although radiofrequency treatment is effective in the improvement of skin laxity, the pain and cost associated with this procedure suggest the need for alternative treatment options.29
Recently, a water-filtered near-infrared device with contact cooling was shown to be effective in the treatment of skin laxity.29 Various kinds of near-infrared devices have been used in multiple medical fields. Near-infrared has been used as an option for the treatment of wound healing disorders31–33 and malignant tumors.34–37 Photobiomodulation with near-infrared irradiation increases mitochondrial metabolism,5,38–40 facilitates wound healing, and promotes angiogenesis in the skin.41 While near-infrared irradiation appears to damage tumor tissue, it has also been shown to reduce cellular protein damage produced by biological oxidants in normal cells.42 Near-infrared also induces nonthermal DNA damage of mitotic cells due to the absence of nuclear lamin protection,22,23,27 and suppresses the proliferation of various kinds of malignant cells.20–22
Near-infrared devices that emit wide wavelengths of near-infrared without a water filter or contact cooling were used in previous in vivo studies.31,43,44 Because near-infrared is primarily absorbed by water and hemoglobin, a substantial amount of energy was absorbed in the superficial layers of skin, and only limited near-infrared energy could be delivered to deeper tissues.22,23
Wavelengths below 1,000 nm are preferentially absorbed by melanin in the superficial layers of the skin. Wavelengths of 1,400–1,500 nm and those above 1,850 nm are absorbed heavily by water in the superficial layers of the skin, which results in heating and can lead to painful sensations and burns.35 Filtering out wavelengths below 1,000 nm, wavelengths around 1,450 nm and above 1,850 nm enable delivery of near-infrared to deeper tissues.45 These specific wavelengths allow a penetration depth ideal for targeting the reticular dermis.6 The depth of near-infrared penetration is estimated to be 1–2 mm,7 with some heat reaching as deep as 2–4 mm6 and even 5 mm.46 This specific near-infrared induces dermal heating thermally and nonthermally induces stimulation of collagen, elastin, and water-binding protein, resulting in skin tightening.13,22,23 Collagens are triple helices of polypeptide chains held together by many hydrogen bonds. Near-infrared induces breakage of hydrogen bonds and conversion from a crystalline to an amorphous state,47 and promotes generation of new collagen.7,48 This results in thickening and shortening of collagen fibrils, and increases tissue tension as well as, ultimately, tissue tightening.48–50 Near-infrared induces high collagen density in the dermis, resulting in long-term epidermal smoothness without scar formation, which provides safe, consistent, and long-term skin rejuvenation effects.14
In practice, near-infrared increases the surface temperature and has thermal effects, so contact cooling is needed to reduce temperature, perspiration, and dilation of blood vessels on the skin surface. Contact cooling and chilled gel are sufficient and effective for skin tightening. However, our near-infrared report regarding reduction of rhytids and myalgia suggested that cooler contact cooling with an ice pack enabled near-infrared to be delivered to the deeper tissues.17 Therefore, in this study, we used freezer-stored gel that appeared to be effective for reducing rhytids and improving enlarged masseter muscles. During treatment, as the gel and the superficial layers of the skin gradually became warm, the energy of near-infrared was absorbed in the superficial layers of the skin, which was effective for skin tightening. These specific wavelengths, the contact cooling, and the freezer-stored gel enabled near-infrared to be delivered to the deeper tissues without pain or epidermal burns, and allowed for tightening effects in both the superficial and deep tissues.
Near-infrared is an electromagnetic wave that simultaneously exhibits both wave and particle properties, and is strongly absorbed by water, hemoglobin, and myoglobin.22 The near-infrared spectrum of biological materials is a result of the overtones and a combination of the stretching vibrations of bonds in O–H, C–H, and N–H groups.51 Water is a polar molecule possessing hydrogen bonds and an electrical dipole moment. A water molecule will be resonated by near-infrared and absorb near-infrared due to the intramolecular O–H hydrogen bonds and electrical dipole moment.52 Our collagen, elastin, and cancer studies suggest that near-infrared may mainly resonate hydrogen bonds, helical structures, alpha helices, and DNA.22 Alpha helices are thought to be resonated by near-infrared and have strong amide bands in the infrared spectrum, with characteristic frequencies and intensities.53
Appropriate near-infrared irradiation also nonthermally relaxes and weakens dystonic or hypertrophic muscles to reduce rhytids and myalgia.17 Because muscles contain hemoglobin and myoglobin that strongly absorb near-infrared, muscles are easily damaged by near-infrared.54 Our previous histologic studies show that near-infrared nonthermally induces degeneration of myoglobin, resulting in apoptosis of vascular smooth muscle cells, long-lasting vasodilation,18 and long-lasting muscle thinning.16,17 Hemoglobin and myoglobin are oxygen-carrying proteins with many alpha helices. It is possible that near-infrared induces resonance of hydrogen bonds and helical structures and degrades proteins containing hydrogen bonds and helical structures, resulting in damage to the storage and transport of oxygen. This could be one of the mechanisms of near-infrared-induced apoptosis in muscle.22,23
This simple technique of using near-infrared may offer an alternative method to relax and weaken dystonic or hypertrophic muscles, which is effective in reducing rhytids16,17 and improving enlarged masseter muscles. Further studies are needed to investigate the range and mechanisms of the biological effects of near-infrared in humans.
One of the major issues in all clinical studies of skin tightening is the lack of an accepted standard for accurate assessment of the degree of skin tightening.29 Many studies have suggested the efficacy of various esthetic devices, but these studies have not included sufficiently objective evaluation. Conventional evaluations using photographs have been widely used, but do not provide accurate objective assessment.28 A three-dimensional imaging approach with quantitative volume measurements is an effective visual communication tool for skin tightening.28 Standardized lighting and optically guided object positioning are essential for appropriate pretreatment and post-treatment evaluation. Photographs are taken from two directions and a three-dimensional quantitative analysis is systemically performed. Even if significant improvement cannot be observed by two-dimensional photographic images or gray images, three-dimensional images can demonstrate the improvement. Thus, three-dimensional quantitative analysis can evaluate and demonstrate the effectiveness and duration of results objectively, as well as show to patients results that are not demonstrable with standard, two-dimensional photography.
Although volume measurement was performed 3 months after treatment, the post-treatment volume was found to be significantly reduced compared with the pretreatment volume in all patients. Given that the effects of this treatment are observed clinically for at least several months after treatment, further studies involving volume measurements with a longer follow-up time are needed. Most of the patients were satisfied with their improvement in skin laxity and rhytids, even though the results of the volume measurements were not significant in some patients.
However, more rounds of treatments or higher output may enhance the effects of this treatment strategy. Only a few patients were dissatisfied with their individual results, and most were patients with thicker skin. Therefore, additional rounds of treatment or higher output may be needed for these patients.
Side effects, such as epidermal burns, adipose tissue atrophy, and contraction, were not observed during the study. Further studies are necessary to determine if a higher output, increased frequency of treatments, or longer periods of treatment may be even more effective for skin tightening.
It should be noted that this was a preliminary study based on a fairly small number of patients. We cannot exclude the possibility that intrinsic and extrinsic factors in everyday life may have contributed to the changes demonstrated in this study. Therefore, further studies in this area are warranted in a larger number of patients and with longer post-treatment periods to evaluate variations in treatment parameters and correlations with environmental factors in patients.
Conclusion
This study examined an Asian population for volumetric changes and subjective effects after water-filtered near-infrared treatment with contact cooling. The statistically significant volume reduction measured in treated areas proves a long-lasting skin tightening effect, with a good subjective patient satisfaction rate. In our view, this study highlights the advantage of water-filtered near-infrared treatment in achieving higher efficacy and reduced discomfort, as well as low-cost, noninvasive skin tightening. The results indicate that water-filtered near-infrared irradiation with contact cooling provides safe and effective treatment of facial skin laxity and reduction of rhytids.
Figure 4.
A 49-year-old Japanese woman (A and C) pretreatment and (B and D) 60 days post-treatment, and (E and F) three-dimensional color schematic representation. Only one treatment was performed with four passes at 42 J/cm2 (80 shots). Significant improvements were noted in the gray image and three-dimensional color schematic representation. Volume reduction was 5.248 mL.
Acknowledgments
We thank Scott Davenport and Chris West from Cutera Inc for technical information related to the near-infrared device and helpful comments, and Yohei Nagaoka for performing the three-dimensional imaging in this work.
Footnotes
Disclosure
This study was conducted without financial support from any third party. The authors report no conflicts of interest in this work.
References
- 1.Nelson JS, Majaron B, Kelly KM. What is nonablative photorejuvenation of human skin? Semin Cutan Med Surg. 2002;21:238–250. doi: 10.1053/sder.2002.36764. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia AC, Dover JS, Arndt KA, Stewart B, Alam M. Patient satisfaction and reported long-term therapeutic efficacy associated with 1,320 nm Nd: YAG laser treatment of acne scarring and photoaging. Dermatol Surg. 2006;32:346–352. doi: 10.1111/j.1524-4725.2006.32071.x. [DOI] [PubMed] [Google Scholar]
- 3.Elman M, Harth Y. Novel multi-source phase-controlled radiofrequency technology for non-ablative and micro-ablative treatment of rhytids, lax skin and acne scars. Laser Ther. 2011;20:139–144. doi: 10.5978/islsm.20.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–527. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 5.Karu T. Invited Review. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol Biol B. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 6.Dierickx CC. The role of deep heating for noninvasive skin rejuvenation. Lasers Surg Med. 2006;38:799–807. doi: 10.1002/lsm.20446. [DOI] [PubMed] [Google Scholar]
- 7.Zelickson B, Ross V, Kist D, Counters J, Davenport S, Spooner G. Ultrastructural effects of an infrared handpiece on forehead and abdominal skin. Dermatol Surg. 2006;32:897–901. doi: 10.1111/j.1524-4725.2006.32193.x. [DOI] [PubMed] [Google Scholar]
- 8.Alam M, Hsu TS, Dover JS, Wrone DA, Arndt KA. Nonablative laser and light treatments: histology and tissue effects – a review. Lasers Surg Med. 2003;33:30–39. doi: 10.1002/lsm.10195. [DOI] [PubMed] [Google Scholar]
- 9.Lipper GM, Perez M. Nonablative acne scar reduction after a series of treatments with a short-pulsed 1,064-nm neodymium: YAG laser. Dermatol Surg. 2006;32:998–1006. doi: 10.1111/j.1524-4725.2006.32222.x. [DOI] [PubMed] [Google Scholar]
- 10.Sadick N, Sorhaindo L. The radiofrequency frontier: a review of radiofrequency and combined radiofrequency pulsed light technology in aesthetic medicine. Facial Plastic Surg. 2005;21:131–138. doi: 10.1055/s-2005-872414. [DOI] [PubMed] [Google Scholar]
- 11.Kist D, Burns AJ, Sanner R, Counters J, Zelickson B. Ultrastructural evaluation of multiple pass low energy versus single pass high energy radio-frequency treatment. Lasers Surg Med. 2006;38:150–154. doi: 10.1002/lsm.20303. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka Y, Matsuo K, Yuzuriha S, Shinohara H. Differential long-term stimulation of type I versus type III collagen after infrared irradiation. Dermatol Surg. 2009;35:1099–1104. doi: 10.1111/j.1524-4725.2009.01194.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Matsuo K, Yuzuriha S. Long-term evaluation of collagen and elastin following infrared (1000 to 1800 nm) irradiation. J Drugs Dermatol. 2009;8:708–712. [PubMed] [Google Scholar]
- 14.Tanaka Y, Matsuo K, Yuzuriha S. Long-term histological comparison between near-infrared irradiated skin and scar tissues. Clin Cosmet Investig Dermatol. 2010;3:143–149. doi: 10.2147/CCID.S15729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Matsuo K, Yuzuriha S. Objective assessment of skin rejuvenation using near-infrared 1064-nm neodymium: YAG laser in Asians. Clin Cosmet Investig Dermatol. 2011;4:123–130. doi: 10.2147/CCID.S22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka Y, Matsuo K, Yuzuriha S. Long-lasting muscle thinning induced by infrared irradiation specialized with wavelength and contact cooling: a preliminary report. ePlasty. 2010;10:e40. [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka Y, Matsuo K, Yuzuriha S. Long-lasting relaxation of corrugator supercilii muscle contraction induced by near infrared irradiation. ePlasty. 2011;11:e6. [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka Y, Matsuo K, Yuzuriha S. Near-infrared irradiation non-thermally induces long-lasting vasodilation by causing apoptosis of vascular smooth muscle cells. ePlasty. 2011;11:e22. [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y, Matsuo K, Yuzuriha S. Near-infrared irradiation non-thermally affects subcutaneous adipocytes and bones. ePlasty. 2011;11:e12. [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, Matsuo K, Yuzuriha S, Yan H, Nakayama J. Non-thermal cytocidal effect of infrared irradiation on cultured cancer cells using specialized device. Cancer Sci. 2010;101:1396–1402. doi: 10.1111/j.1349-7006.2010.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka Y, Tatewaki N, Nishida H, Eitsuka T, Ikekawa N, Nakayama J. Non-thermal DNA damage of cancer cells using near-infrared irradiation. Cancer Sci. 2012;103:1467–1473. doi: 10.1111/j.1349-7006.2012.02310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka Y, Matsuo K. In: Non-Thermal Effects of Near-Infrared Irradiation on Melanoma. Breakthroughs in Melanoma Research. Tanaka Y, editor. Bratislava, Croatia: InTech; 2011. [Google Scholar]
- 23.Tanaka Y. The impact of near-infrared radiation in dermatology. Review. World J Dermatol. 2012;1:30–37. [Google Scholar]
- 24.Tanaka Y, Kawashima M. The biological effects of near-infrared. Aesthet Dermatol. 2012;22:100–109. Japanese. [Google Scholar]
- 25.Tanaka Y, Tsunemi Y, Kawashima M, Nishida H. The impact of near-infrared in plastic surgery. Plastic Surgery: An International Journal. 2013 Mar; in press. [Google Scholar]
- 26.Tanaka Y, Gale L. The effect of near-infrared between 1100–1800 nm together with a water-filter and a contact cooling. Anaplastology. 2013 May; in press. [Google Scholar]
- 27.Tanaka Y, Gale L. Beneficial applications and deleterious effects of near-infrared from biological and medical points of view. Optics and Photonics Journal. 2013 May; in press. [Google Scholar]
- 28.Tanaka Y.Objective assessment of skin tightening using multisource, phase-controlled radiofrequency in Asians J Cosmet Dermatol Sci Appl 201331110–116.Available from: http://www.scirp.org/journal/JCDSA/Accessed June 10, 2013 [Google Scholar]
- 29.Chan HH, Yu CS, Shek S, Yeung CK, Kono T, Wei WI. A prospective, split face, single-blinded study looking at the use of an infrared device with contact cooling in the treatment of skin laxity in Asians. Lasers Surg Med. 2008;40:146–152. doi: 10.1002/lsm.20586. [DOI] [PubMed] [Google Scholar]
- 30.Matory WE. Skin care. In: Matory WE, editor. Ethnic Considerations in Facial Aesthetic Surgery. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 31.Danno K, Mori N, Toda K, Kobayashi T, Utani A. Near-infrared irradiation stimulates cutaneous wound repair: laboratory experiments on possible mechanisms. Photodermatol Photoimmunol Photomed. 2001;17:261–265. doi: 10.1034/j.1600-0781.2001.170603.x. [DOI] [PubMed] [Google Scholar]
- 32.Horwitz LR, Burke TJ, Carnegie D. Augmentation of wound healing using monochromatic infrared energy. Exploration of a new technology for wound management. Adv Wound Care. 1999;12:35–40. [PubMed] [Google Scholar]
- 33.Schramm JM, Warner D, Hardesty RA, Oberg KC. A unique combination of infrared and microwave radiation accelerates wound healing. Plast Reconstr Surg. 2003;111:258–266. doi: 10.1097/01.PRS.0000033065.10876.2E. [DOI] [PubMed] [Google Scholar]
- 34.Bäumler W, Abels C, Karrer S, et al. Photo-oxidative killing of human colonic cancer cells using indocyanine green and infrared light. Br J Cancer. 1999;80:360–363. doi: 10.1038/sj.bjc.6690363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelleher DK, Thews O, Rzeznik J, Scherz A, Salomon Y, Vaupel P. Hot topic. Water-filtered infrared-A radiation: a novel technique for localized hyperthermia in combination with bacteriochlorophyll-based photodynamic therapy. Int J Hyperthermia. 1999;15:467–474. doi: 10.1080/026567399285468. [DOI] [PubMed] [Google Scholar]
- 36.Dees C, Harkins J, Petersen MG, Fisher WG, Wachter EA. Treatment of murine cutaneous melanoma with near infrared light. Photochem Photobiol. 2002;75:296–301. doi: 10.1562/0031-8655(2002)075<0296:tomcmw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Orenstein A, Kostenich G, Kopolovic Y, Babushkina T, Malik Z. Enhancement of ALA-PDT damage by IR-induced hyperthermia on a colon carcinoma model. Photochem Photobiol. 1999;69:703–707. [PubMed] [Google Scholar]
- 38.Passarella S, Casamassima E, Molinari S, et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett. 1984;175:95–99. doi: 10.1016/0014-5793(84)80577-3. [DOI] [PubMed] [Google Scholar]
- 39.Yu W, Naim JO, McGowan M, Ippolito K, Lanzafame RJ. Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria. Photochem Photobiol. 1997;66:866–871. doi: 10.1111/j.1751-1097.1997.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 40.Wilden L, Karthein R. Import of radiation phenomena of electrons and therapeutic low-level laser in regard to the mitochondrial energy transfer. J Clin Laser Med Surg. 1998;16:159–165. doi: 10.1089/clm.1998.16.159. [DOI] [PubMed] [Google Scholar]
- 41.Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation. J Clin Periodontol. 1996;23:492–496. doi: 10.1111/j.1600-051x.1996.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 42.Kujawa J, Zavodnik IB, Lapshina A, Labieniec M, Bryszewska M. Cell survival, DNA, and protein damage in B14 cells under low-intensity near-infrared (810 nm) laser irradiation. Photomed Laser Surg. 2004;22:504–508. doi: 10.1089/pho.2004.22.504. [DOI] [PubMed] [Google Scholar]
- 43.Kim HH, Lee MJ, Lee SR, et al. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice. Mech Aging Dev. 2005;126:1170–1177. doi: 10.1016/j.mad.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Kligman LH. Intensification of ultraviolet-induced dermal damage by infrared radiation. Arch Dermatol Res. 1982;272:229–238. doi: 10.1007/BF00509050. [DOI] [PubMed] [Google Scholar]
- 45.Davenport SA, Gollnick DA, Levernier M, Spooner GJR. Method and system for treatment of post-partum abdominal skin redundancy or laxity. 2006. United States Patent 20060052847.
- 46.Esparza JR. Near painless, nonablative, immediate skin contraction induced by low-fluence irradiation with new infrared device: a report of 25 patients. Dermatol Surg. 2006;32:601–610. doi: 10.1111/j.1524-4725.2006.32130.x. [DOI] [PubMed] [Google Scholar]
- 47.Lennox FG. Shrinkage of collagen. Biochim Biophys Acta. 1949;3:170–187. [Google Scholar]
- 48.Goldberg DJ, Hussain M, Fazeli A, Berlin AL. Treatment of skin laxity of the lower face and neck in older individuals with a broad spectrum infrared light device. J Cosmet Laser Ther. 2007;9:35–40. doi: 10.1080/14764170601186107. [DOI] [PubMed] [Google Scholar]
- 49.Ross EV, McKinlay JR, Anderson RR. Why does carbon dioxide resurfacing work? Arch Dermatol. 1999;135:444–454. doi: 10.1001/archderm.135.4.444. [DOI] [PubMed] [Google Scholar]
- 50.Ross EV, Sajben FP, Hsia J. Non-ablative skin remodeling: selective dermal heating with a mid-infrared laser and contact cooling combination. Lasers Surg Med. 2000;26:186–195. doi: 10.1002/(sici)1096-9101(2000)26:2<186::aid-lsm9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 51.Weyer LG.Near-infrared spectroscopy of organic substances Appl Spectrosc Rev 1985211–43.Available from: http://www.tandfonline.com//abs/10.1080/05704928508060427Accessed June 10, 2013 [Google Scholar]
- 52.Tsai CH, Chen JC, Wang WJ. Near-infrared absorption property of biological soft tissue constituents. J Med Biol Eng. 2001;21:7–14. [Google Scholar]
- 53.Nevskaya NA, Chirgadze YN. Infrared spectra and resonance interactions of amide-I and II vibrations of alpha-helix. Biopolymers. 1976;15:637–648. doi: 10.1002/bip.1976.360150404. [DOI] [PubMed] [Google Scholar]
- 54.Srinivasan S, Pogue BW, Jiang S, et al. Interpreting hemoglobin and water concentration, oxygen saturation, and scattering measured in vivo by near-infrared breast tomography. Proc Natl Acad Sci U S A. 2003;100:12349–12354. doi: 10.1073/pnas.2032822100. [DOI] [PMC free article] [PubMed] [Google Scholar]