Abstract
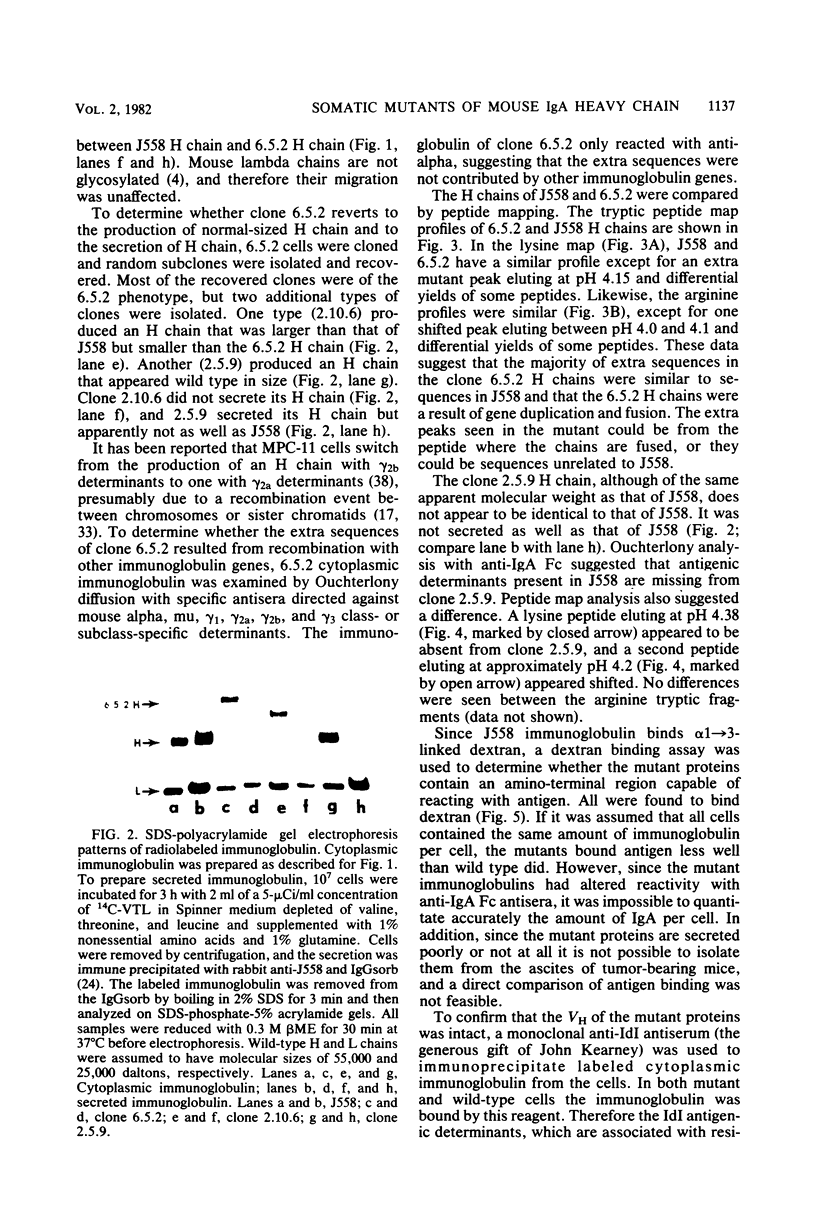
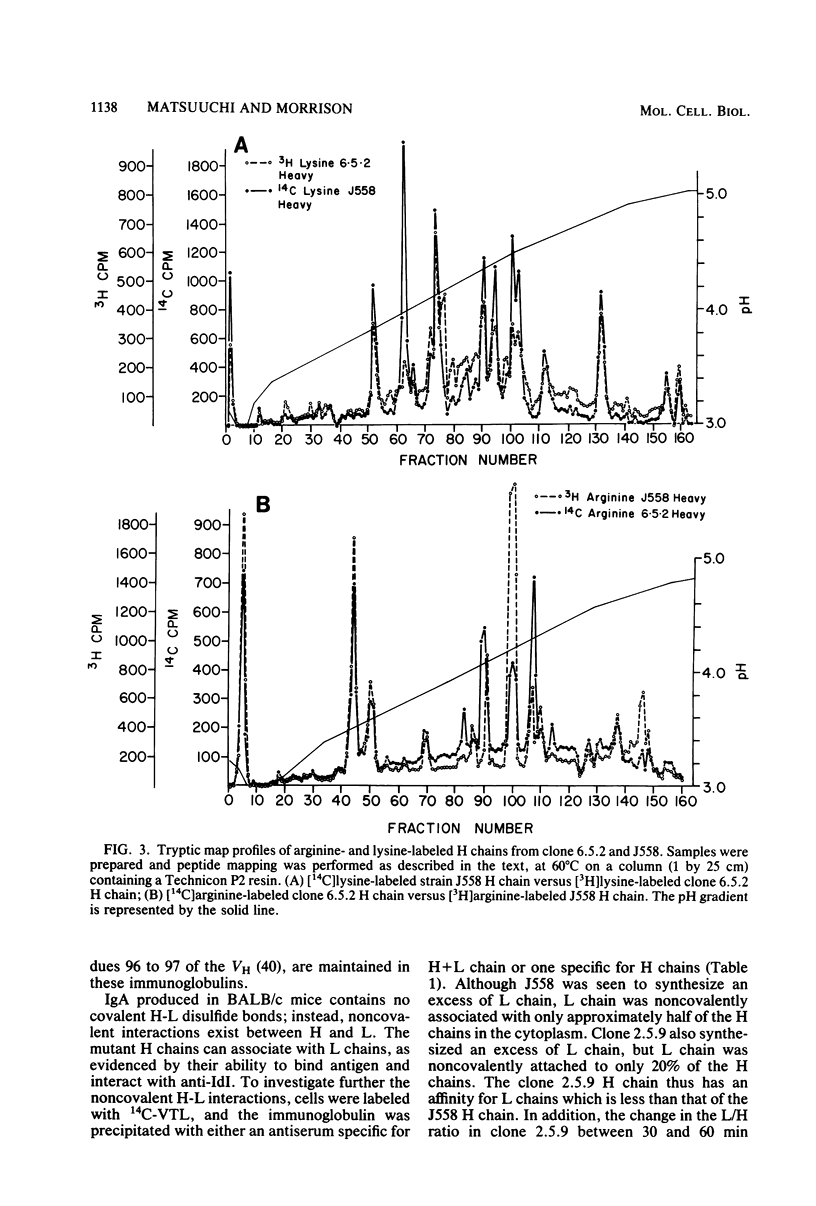
A mutant has been isolated from the J558 (immunoglobulin A, lambda, anti-alpha 1 leads to 3 dextran) cell line which synthesizes a heavy-chain immunoglobulin twice the size of normal heavy chain. Secondary variants that synthesized heavy chains either 1.5 times as large as wild type or the same size as wild type were identified. All mutants were serologically immunoglobulin continued to bind antigen, and retained the individual idiotype of the parent. Northern blot analysis and in vitro synthesis studies showed that the large heavy chains were primary synthetic products and not the consequence of abnormal covalent bonds. Cleavage of genomic DNA with restriction endonucleases and molecular hybridization studies showed new fragments in the 2 X and 1.5 X mutants which disappeared in the 1 X revertant. These data cannot easily be reconciled with the mutants arising either by unequal recombination or gene conversion. Further molecular characterization of these mutants should give additional insight into immunoglobulin gene evolution.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel C. A., Grey H. M. Studies on the structure of mouse gamma-A myeloma proteins. Biochemistry. 1968 Jul;7(7):2682–2688. doi: 10.1021/bi00847a035. [DOI] [PubMed] [Google Scholar]
- Adetugbo K., Milstein C., Secher D. S. Molecular analysis of spontaneous somatic mutants. Nature. 1977 Jan 27;265(5592):299–304. doi: 10.1038/265299a0. [DOI] [PubMed] [Google Scholar]
- Adetugbo K. Spontaneous somatic mutations. Structural studies on mutant immunoglobulins. J Biol Chem. 1978 Sep 10;253(17):6076–6080. [PubMed] [Google Scholar]
- Appella E. Amino acid sequences of two mouse immunoglobulin lambda chains. Proc Natl Acad Sci U S A. 1971 Mar;68(3):590–594. doi: 10.1073/pnas.68.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Birshtein B. K., Preud'homme J. L., Scharff M. D. Variants of mouse myeloma cells that produce short immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3478–3482. doi: 10.1073/pnas.71.9.3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Coffino P., Scharff M. D. Rate of somatic mutation in immunoglobulin production by mouse myeloma cells. Proc Natl Acad Sci U S A. 1971 Jan;68(1):219–223. doi: 10.1073/pnas.68.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech H. J., Preston R. K., Peck R. M., O'Connell A. P. Antitumor and mutagenic properties of a variety of heterocyclic nitrogen and sulfur mustards. J Med Chem. 1972 Jul;15(7):739–746. doi: 10.1021/jm00277a011. [DOI] [PubMed] [Google Scholar]
- Dackowski W., Morrison S. L. Two alpha heavy chain disease proteins with different genomic deletions demonstrate that nonexpressed alpha heavy chain genes contain methylated bases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7091–7095. doi: 10.1073/pnas.78.11.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. R., Padlan E. A., Segal D. M. Immunoglobulin structures at high resolution. Contemp Top Mol Immunol. 1975;4:127–153. doi: 10.1007/978-1-4615-8930-3_5. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Early P. W., Davis M. M., Kaback D. B., Davidson N., Hood L. Immunoglobulin heavy chain gene organization in mice: analysis of a myeloma genomic clone containing variable and alpha constant regions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):857–861. doi: 10.1073/pnas.76.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmundson A. B., Ely K. R., Girling R. L., Abola E. E., Schiffer M., Westholm F. A., Fausch M. D., Deutsch H. F. Binding of 2,4-dinitrophenyl compounds and other small molecules to a crystalline lambda-type Bence-Jones dimer. Biochemistry. 1974 Aug 27;13(18):3816–3827. doi: 10.1021/bi00715a031. [DOI] [PubMed] [Google Scholar]
- Francus T., Dharmgrongartama B., Campbell R., Scharff M. D., Birshtein B. K. IgG2a-producing variants of an IgG2b-producing mouse myeloma cell line. J Exp Med. 1978 Jun 1;147(6):1535–1550. doi: 10.1084/jem.147.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey H. M., Sher A., Shalitin N. The subunit structure of mouse IgA. J Immunol. 1970 Jul;105(1):75–84. [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Fellows R. E., Lebovitz H. E. The evolutionary origins of the immunoglobulins. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1762–1769. doi: 10.1073/pnas.56.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Campbell J. H., Elgin S. C. The organization, expression, and evolution of antibody genes and other multigene families. Annu Rev Genet. 1975;9:305–353. doi: 10.1146/annurev.ge.09.120175.001513. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Klenk H. D., Scholtissek C., Rott R. Inhibition of glycoprotein biosynthesis of influenza virus by D-glucosamine and 2-deoxy-D-glucose. Virology. 1972 Sep;49(3):723–734. doi: 10.1016/0042-6822(72)90529-6. [DOI] [PubMed] [Google Scholar]
- Koshland M. E. Structure and function of the J chain. Adv Immunol. 1975;20:41–69. doi: 10.1016/s0065-2776(08)60206-0. [DOI] [PubMed] [Google Scholar]
- Koskimies S., Birshtein B. K. Primary and secondary variants in immunoglobulin heavy chain production. Nature. 1976 Dec 2;264(5585):480–482. doi: 10.1038/264480a0. [DOI] [PubMed] [Google Scholar]
- Lundblad A., Steller R., Kabat E. A., Hirst J. W., Weigert M. G., Cohn M. Immunochemical studies on mouse myeloma proteins with specificity for dextran or for levan. Immunochemistry. 1972 May;9(5):535–544. doi: 10.1016/0019-2791(72)90063-8. [DOI] [PubMed] [Google Scholar]
- Matsuuchi L., Morrison S. L. Estimation of antibodies specific for dextran. J Immunol. 1978 Sep;121(3):962–965. [PubMed] [Google Scholar]
- Miyata T., Yasunaga T., Yamawaki-Kataoka Y., Obata M., Honjo T. Nucleotide sequence divergence of mouse immunoglobulin gamma 1 and gamma 2b chain genes and the hypothesis of intervening sequence-mediated domain transfer. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2143–2147. doi: 10.1073/pnas.77.4.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S. L. Murine heavy chain disease. Eur J Immunol. 1978 Mar;8(3):194–199. doi: 10.1002/eji.1830080311. [DOI] [PubMed] [Google Scholar]
- Morrison S. L., Scharff M. D. Heavy chain-producing variants of a mouse myeloma cell line. J Immunol. 1975 Feb;114(2 Pt 1):655–659. [PubMed] [Google Scholar]
- Morrison S. L. Sequentially derived mutants of the constant region of the heavy chain of murine immunoglobulins. J Immunol. 1979 Aug;123(2):793–800. [PubMed] [Google Scholar]
- Padlan E. A., Segal D. M., Spande T. F., Davies D. R., Rudikoff S., Potter M. Structure at 4.5 A resolution of a phosphorylcholine-binding fab. Nat New Biol. 1973 Oct 10;245(145):165–167. doi: 10.1038/newbio245165a0. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Chen B. L., Phizackerley R. P., Saul F. Three-dimensional structure of the Fab' fragment of a human immunoglobulin at 2,8-A resolution. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3305–3310. doi: 10.1073/pnas.70.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preud'Homme J. L., Birshtein B. K., Scharff M. D. Variants of a mouse myeloma cell line that synthesize immunoglobulin heavy chains having an altered serotype. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1427–1430. doi: 10.1073/pnas.72.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Schilling J., Clevinger B., Davie J. M., Hood L. Amino acid sequence of homogeneous antibodies to dextran and DNA rearrangements in heavy chain V-region gene segments. Nature. 1980 Jan 3;283(5742):35–40. doi: 10.1038/283035a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. The arrangement and rearrangement of antibody genes. Nature. 1978 Dec 21;276(5690):790–795. doi: 10.1038/276790a0. [DOI] [PubMed] [Google Scholar]
- Seki T., Appella E., Itano H. A. Chain models of 6.6S and 3.9S mouse myeloma gamma A immunoglobulin molecules. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1071–1078. doi: 10.1073/pnas.61.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Doolittle R. F. Antibody active sites and immunoglobulin molecules. Science. 1966 Jul 1;153(3731):13–25. doi: 10.1126/science.153.3731.13. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Unequal mitotic sister chromatin exchange as the mechanism of ribosomal RNA gene magnification. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1272–1276. doi: 10.1073/pnas.71.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Wims L. A., Morrison S. L. ICR-191 and ethyl methanesulfonate induced mutagenesis at the immunoglobulin locus in the Y5606 cultured myeloma cell line. Mutat Res. 1981 Apr;81(2):215–228. doi: 10.1016/0027-5107(81)90036-1. [DOI] [PubMed] [Google Scholar]