Summary
A fundamental rule of cell division is that daughter cells inherit half the DNA complement and an appropriate proportion of cellular organelles. The highly asymmetric cell divisions of female meiosis present a different challenge because one of the daughters, the polar body, is destined to degenerate, putting at risk essential maternally inherited organelles such as mitochondria. We have therefore investigated mitochondrial inheritance during the meiotic divisions of the mouse oocyte. We find that mitochondria are aggregated around the spindle by a dynein-mediated mechanism during meiosis I, and migrate together with the spindle towards the oocyte cortex. However, at cell division they are not equally segregated and move instead towards the oocyte-directed spindle pole and are excluded from the polar body. We show that this asymmetrical inheritance in favour of the oocyte is not caused by bias in the spindle itself but is dependent on an intact actin cytoskeleton, spindle–cortex proximity, and cell cycle progression. Thus, oocyte-biased inheritance of mitochondria is a variation on rules that normally govern organelle segregation at cell division, and ensures that essential maternally inherited mitochondria are retained to provide ATP for early mammalian development.
Key words: Mitochondria, Oocyte, Asymmetry
Introduction
Most cell divisions result in the production of two equally sized daughter cells each of which inherit half of the DNA and an equal proportion of cellular organelles. In order to facilitate segregation, organelles such as mitochondria fragment and disperse throughout the cytoplasm in a cell cycle dependent manner before being distributed to the two daughter cells at cytokinesis (Taguchi et al., 2007). Variations to this pattern exist in some asymmetrically dividing cells, where polarisation of the mother cell and differential segregation of fate determinants and organelles at cytokinesis results in daughter cells of different sizes and with differing fates (Gönczy, 2008; Ouellet and Barral, 2012). In contrast asymmetric division in the budding yeast Saccharomyces cerevisiae involves extraordinary measures to ensure equal inheritance of organelles to the emerging bud. If trafficking of mitochondria into the bud fails, a mitochondrial inheritance cell cycle checkpoint blocks cytokinesis (Peraza-Reyes et al., 2010).
Extrusion of the polar body during the meiotic divisions of the oocyte is another highly asymmetrical cell division (Brunet and Verlhac, 2011) but little is known about how cytoplasmic organelles are organised and distributed during this event. While the division of DNA during cytokinesis must be equal, the loss of important cellular organelles to the polar body could be detrimental to oocyte health. This may be particularly relevant in the case of mitochondria, which provide the primary source of ATP during oocyte maturation and early embryogenesis due to a block in glycolytic activity that lasts until the blastocyst stage (Biggers et al., 1967; Dumollard et al., 2007; Leese, 1995). Furthermore, mitochondrial replication is suppressed in the fully grown oocyte and does not resume until after implantation (Pikó and Taylor, 1987), such that the mitochondrial complement contained within the oocyte must be capable of sustaining the early stages of mammalian development.
In mammalian oocytes, aggregation of mitochondria around the first meiotic spindle (Van Blerkom and Runner, 1984; Yu et al., 2010) renders these organelles particularly vulnerable to being partitioned into the polar body where they would be destined to degenerate. We have therefore investigated mitochondrial inheritance during the meiotic divisions of the mouse oocyte and find that meiosis I-specific mechanisms exist to bias mitochondrial segregation in favour of the oocyte.
Results
Mitochondria redistribute during oocyte maturation
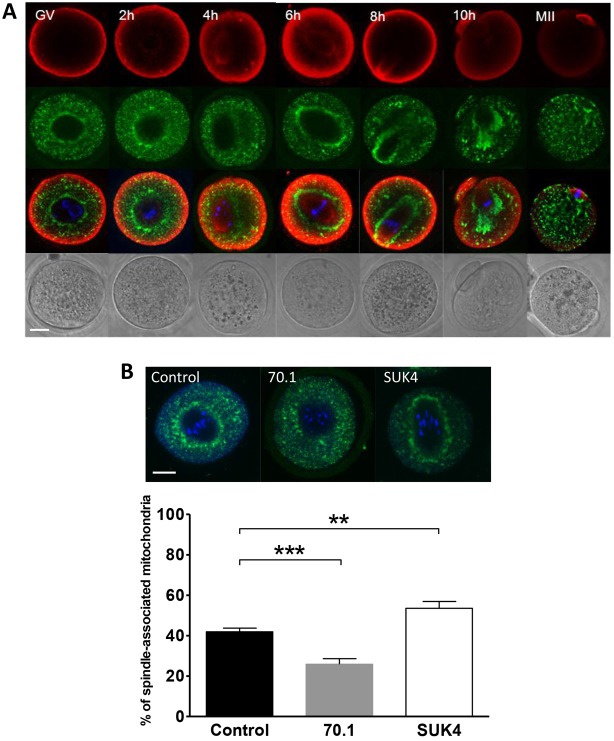
To begin investigating mitochondrial dynamics and inheritance in the mouse oocyte, we examined the distribution of mitochondria during oocyte maturation at two-hourly intervals, from germinal vesicle (GV) stage to arrest at metaphase of meiosis II (MII) (Fig. 1A). In agreement with previous findings, mitochondria accumulated around the developing meiotic spindle (Van Blerkom and Runner, 1984; Yu et al., 2010) and furthermore remained associated with the spindle during its migration to the cortex. Analysis of the proportion of spindle-associated mitochondria revealed that just over 40% of the oocyte mitochondria aggregated around the spindle (see Fig. 1B). Interestingly, we observed very few mitochondria in the polar body and by the time of MII arrest mitochondria had largely distributed homogenously throughout the oocyte with occasional minor enrichment in the spindle region. The spindle aggregation of mitochondria was linked to meiotic progression because, in accordance with previous studies (Van Blerkom and Runner, 1984), mitochondria remained distributed throughout the cytoplasm in oocytes arrested at the GV stage. To verify that the aggregation was not an artefact of in vitro conditions we administered hCG and recovered oocytes 6 hours later. Mitochondria in the in vivo stimulated oocytes were distributed in a manner similar to in vitro cultured oocytes, with a prominent spindle aggregation.
Fig. 1.
Reorganisation of mitochondria during oocyte maturation. (A) Oocytes were fixed at 2 hour intervals from GV stage to MII arrest (n≥7 oocytes at each stage), stained for tubulin (red), mitochondria (green) and DNA (blue) and imaged by confocal microscopy. Mitochondria accumulate around the spindle and follow the path of its migration to the cortex during MI but are distributed throughout the oocyte at MII. (B) Oocytes arrested at GV stage were microinjected with 70.1 to disrupt dynein function (n = 22), SUK4 to inhibit kinesin (KIF5) function (n = 22), or a vehicle control (n = 33). Microinjected oocytes were released from meiotic arrest, fixed at 6 hours, stained for mitochondria (green) and DNA (blue) and imaged by confocal microscopy. The percentage of spindle-associated mitochondria was calculated and was found to be reduced by inhibition of dynein activity (***P<0.001) and enhanced by inhibition of kinesin activity (**P<0.01). Scale bars: 20 µm.
Mitochondrial association with the spindle is regulated by a balance between dynein and kinesin activity
In most eukaryotic cell types mitochondrial transport occurs along microtubule tracks and these are thought to be responsible for the organisation of mitochondria around the first meiotic spindle (Van Blerkom, 1991; Yu et al., 2010). We confirmed this by disrupting the microtubule and microfilament networks from GV stage using small molecule inhibitors. Disruption of the microtubule network with nocodazole (noc) prevented the aggregation of mitochondria normally seen around the spindle 6 hours after release from meiotic arrest (supplementary material Fig. S1). Conversely, disruption of actin filaments with latrunculin A (LatA) did not affect accumulation of mitochondria around the spindle (supplementary material Fig. S1).
Cargoes are transported along microtubules by the activity of the motor proteins dynein and kinesin, which move towards the minus and plus ends of microtubules respectively (Hirokawa et al., 1998). We therefore addressed the mechanisms governing the microtubule dependent migration of mitochondria to the spindle region by disrupting the activities of dynein and kinesin-1. In oocytes where dynein activity had been inhibited from GV stage using a function-blocking antibody 70.1 (Heald et al., 1997; Varadi et al., 2004), mitochondrial accumulation in the spindle region at 6 hours was reduced compared to control oocytes (25.8±2.8% versus 41.9±1.8% P<0.001), indicating a role for dynein activity in mediating mitochondrial redistribution during this period of oocyte maturation (Fig. 1B). The effect of dynein inhibition on mitochondrial transport to the spindle was confirmed by overexpression of p50, the dynamitin subunit of the dynactin complex (Burkhardt et al., 1997) (supplementary material Fig. S2). In contrast, inhibition of kinesin-1 activity using the function-blocking antibody SUK4 (Ingold et al., 1988) resulted in an increase of mitochondrial accumulation around the spindle to 53.5±3.4% (P<0.005), suggesting a role for kinesin-1 in regulating the extent of mitochondrial spindle association. The formation of the mitochondria–spindle superstructure is therefore regulated by the activity of both motors, with dynein activity out-competing kinesin-dependent trafficking during the period of spindle formation.
Mitochondria associate with migrating microtubule-organising centres
The mouse oocyte lacks centrioles (Szollosi et al., 1972) and the assembly of the acentriolar spindle is achieved instead by multiple microtubule-organising centres (MTOCs) that cluster to form the two poles of the spindle (Messinger and Albertini, 1991; Schuh and Ellenberg, 2007). It has been suggested that distinct populations of MTOCs may exist in the oocyte, with only perinuclear MTOCs able to participate in spindle formation whilst cytoplasmic MTOCs remain dispersed in the cytoplasm (Calarco, 2000; Combelles and Albertini, 2001; Messinger and Albertini, 1991). However, other data, including a live-cell imaging study, indicate that cytoplasmic MTOCs move towards the central region of the oocyte and are involved in spindle formation (Schuh and Ellenberg, 2007; Van Blerkom, 1991). During the period of mitochondrial redistribution to the spindle we observed several small clusters of mitochondria in the cytoplasm. Interestingly these cytoplasmic mitochondrial clusters were associated with microtubule asters, also known as microtubule organising centres (MTOCs) (Fig. 2A). We therefore postulated that the association of mitochondria with migrating cytoplasmic MTOCs might contribute to the recruitment of mitochondria to the spindle. In order to establish if this was the case, we undertook live-cell time-lapse imaging of mitochondria in oocytes during spindle formation and observed cytoplasmic clusters of mitochondria moving towards and joining the developing mitochondrial ring (Fig. 2B; supplementary material Movie 1). Thus it is likely that mitochondrial redistribution to the spindle region occurs at least in part through transport of MTOC-associated mitochondria towards the forming spindle. Indeed, dynein function has been demonstrated to be necessary for the inward movement of MTOCs and microtubule bundles at prophase/prometaphase during mitosis (Courtois et al., 2012; Rusan et al., 2002) which is consistent with the timing and direction of movement of MTOC-associated mitochondria during the initial phases of oocyte maturation, and in line with the impact of dynein inhibition on the percentage of mitochondria which become associated with the spindle.
Fig. 2.
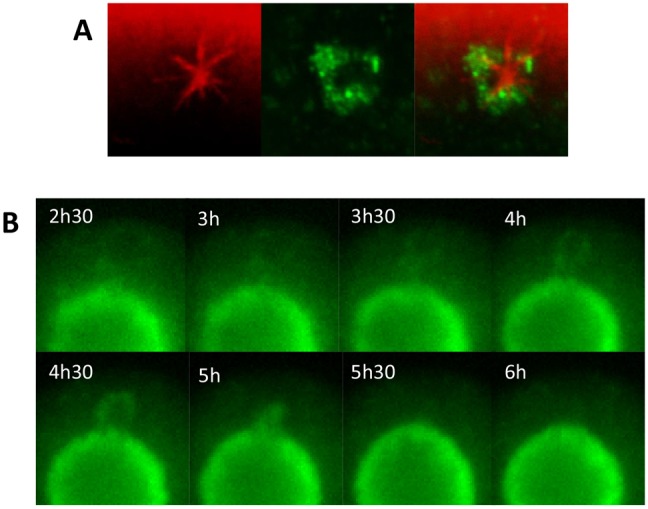
Mitochondria associate with cytoplasmic MTOCs. (A) Oocytes were fixed during spindle formation and stained for tubulin (red) and mitochondria (green). Mitochondria were found associated with cytoplasmic MTOCs. (B) Oocytes microinjected with mito-GFP to visualise mitochondria were imaged by live-cell time-lapse microscopy. Cytoplasmic clusters of mitochondria, presumed to be encircling MTOCs, were observed to join the spindle-associated mitochondrial ring. Times shown are hours after release.
Mitochondria are inherited asymmetrically in the first meiotic division
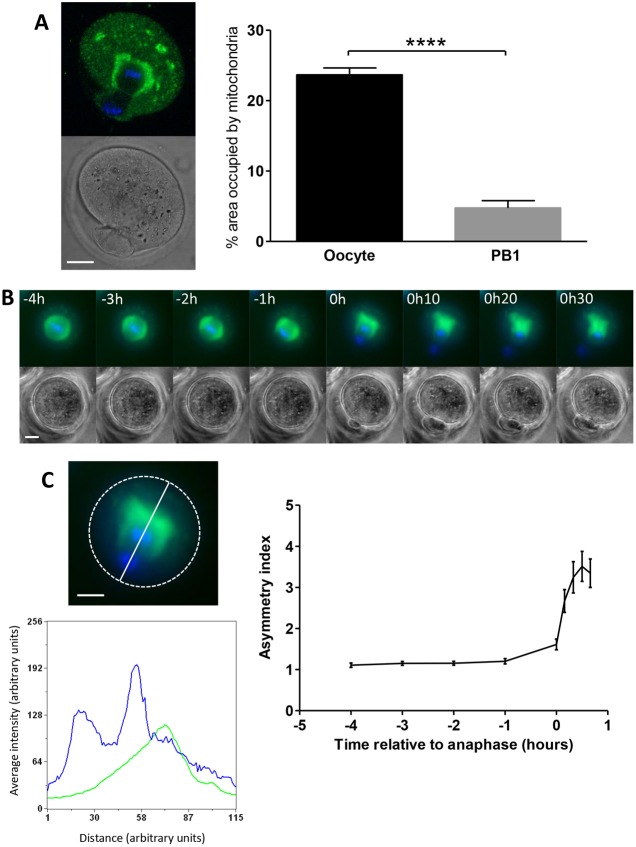
The organisation of mitochondria around the spindle just prior to cell division might suggest that about half of these spindle-associated mitochondria would be inherited by the polar body when the chromatin is divided equally at cytokinesis. This would equate to a loss of around 20% of the ATP producing capacity of the oocyte and may present problems for continuing development as mitochondrial replication is blocked until after implantation (Pikó and Taylor, 1987). We therefore set out to investigate how mitochondria are inherited during the asymmetric divisions of meiosis. Oocytes were fixed during polar body extrusion and stained for mitochondria and DNA. Using confocal microscopy we observed that in oocytes undergoing anaphase, mitochondria remained highly enriched around the oocyte-directed pole of the spindle but were largely absent from the spindle pole directed towards the emerging polar body (Fig. 3A). Analysis revealed that the percentage area occupied by mitochondria in the oocyte was significantly higher than in the polar body (23.7±1% versus 4.8±1%, respectively, P<0.0001). This suggests the existence of a mechanism for retaining mitochondria, thereby ensuring preferential inheritance of these organelles to the oocyte.
Fig. 3.
Mitochondria are retained in the oocyte during polar body extrusion. (A) Oocytes were fixed at the time of first polar body extrusion, stained for mitochondria (green) and DNA (blue) and imaged by confocal microscopy. The percentage area occupied by mitochondria in both the oocyte and polar body (PB) was calculated and a significant difference was found (n = 15, ****P<0.0001). (B) Mitochondria (green) and DNA (blue) were imaged using live-cell time-lapse microscopy during polar body extrusion. Mitochondria distribute asymmetrically during polar body extrusion, with the reorganisation commencing around the time of anaphase (time = 0). (C) Mitochondrial asymmetry was quantified by calculating the average mitochondrial fluorescence either side of the spindle mid-zone from a linescan through the plane of the dividing chromosomes (solid line on upper left panel). The dotted line indicates the oocyte cortex. The lower panel shows the graph derived from the linescan. Asymmetry begins to develop in the hour preceding anaphase and increases rapidly thereafter (n = 19). Scale bars: 20 µm.
Since we observed that mitochondria had adopted an asymmetric distribution around the spindle during anaphase, we next examined the relationship of asymmetric mitochondrial inheritance and meiotic cell cycle progression in more detail. We undertook live-cell time-lapse imaging of Hoechst-labelled oocytes expressing mito-GFP in the period before and during polar body extrusion. This revealed that mitochondria began to reorganise around the oocyte-directed spindle pole close to the time of anaphase (Fig. 3B; supplementary material Movie 2). This was analysed using an asymmetry index (see Fig. 3C and methods for explanation) which revealed that the change in mitochondrial symmetry began in the hour preceding anaphase. After anaphase the asymmetry index increased rapidly and reached a peak within 30 minutes (Fig. 3C).
The endoplasmic reticulum is inherited asymmetrically in the first meiotic division
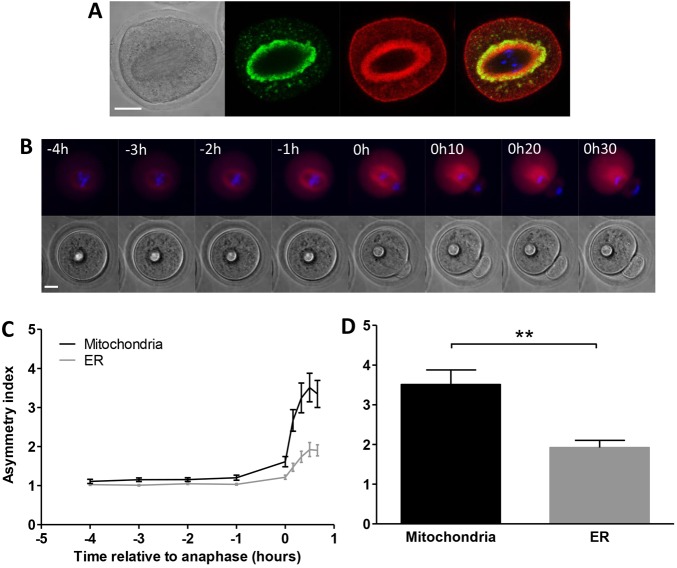
The endoplasmic reticulum (ER) redistributes to the spindle in a similar manner to mitochondria during oocyte maturation (FitzHarris et al., 2007) but it is not known how the ER segregates at polar body formation. In order to examine the spatial relationship of mitochondria and ER around the spindle, oocytes were co-labelled for ER and mitochondria and imaged by confocal microscopy. The ER was located closest to the spindle with the mitochondria tightly packed against it. Some mitochondria could be seen inter-digitating into the outer layers of ER (Fig. 4A). To examine if the ER was retained in the oocyte together with mitochondria we next undertook live-cell time-lapse imaging of oocytes in which the ER had been labelled with DiI (FitzHarris et al., 2007; Mehlmann et al., 1995). As with mitochondria, the spindle-associated ring of ER became asymmetrically distributed during polar body extrusion (Fig. 4B; supplementary material Movie 3). The timing of ER reorganisation was strikingly similar to that of the mitochondria, with the shift to asymmetry commencing shortly before anaphase and increasing progressively during formation of the polar body (Fig. 4C). The extent of asymmetry was less than that observed with mitochondria (1.92±0.18 versus 3.51±0.37 at 30 minutes post-anaphase, P<0.01; Fig. 4C,D), which may reflect tighter control over mitochondrial segregation, possibly facilitated by the discrete nature of mitochondria in the oocyte, compared to the tubular network of the ER.
Fig. 4.
Mitochondria and ER are similarly distributed at the spindle and during polar body extrusion. (A) Oocytes were microinjected with mito-GFP to visualise mitochondria (green) and dsRed2ER to visualize ER (red), and fixed 6 hours after release from meiotic arrest. Both ER and mitochondria were localised around the first meiotic spindle with the ER closest to the spindle. DNA is shown in blue. (B) Oocytes were microinjected with DiI to visualize the ER (red) and stained with Hoechst to visualize DNA (blue) and imaged by live-cell time-lapse microscopy. Similarly to mitochondria, the ER distributes asymmetrically during polar body extrusion. Time = 0 indicates anaphase. (C) Analysis of the extent of ER asymmetry shows that reorganisation of the ER commences in the hour preceding anaphase and increases progressively during polar body extrusion (n = 14), mirroring the temporal dynamics of mitochondrial asymmetry but at a reduced level. (D) Asymmetry of ER distribution was compared to that of mitochondria at 30 minutes post-anaphase and found to be significantly lower (1.92±0.18 versus 3.51±0.37; P<0.01). Scale bars: 20 µm.
Asymmetrical mitochondrial inheritance requires spindle-cortex proximity
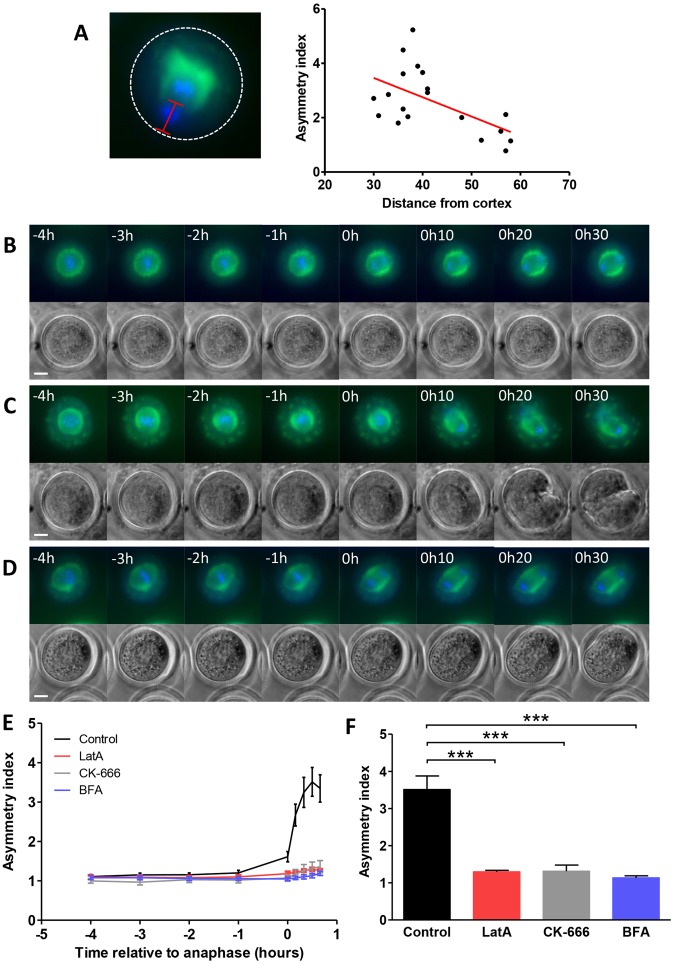
Two major factors are at play in the oocyte as organelle asymmetry is initiated; first, the spindle migrates to the cortex (Maro and Verlhac, 2002) and second, cyclin B1 is degraded thereby inactivating Cdk1 and triggering exit from metaphase (Ledan et al., 2001). To test whether the proximity of the spindle to the cortex is able to influence the level of mitochondrial asymmetry we examined the relationship between the mitochondrial asymmetry index and the spindle-cortical distance at a defined time point (10 minutes post-anaphase). Analysis revealed an inverse relationship (Fig. 5A) suggesting that mitochondrial behaviour is influenced by spindle-cortical interactions.
Fig. 5.
Asymmetrical mitochondrial inheritance requires spindle–cortex proximity. (A) The mid-zone to cortex distance at a defined time point (10 minutes post-anaphase) was measured (red line in left panel; dotted line indicates the oocyte cortex) and plotted against the asymmetry score. The red line on the graph is a line of best fit, r2 = 0.318. (B–D) Live-cell time-lapse imaging of oocytes that had been microinjected with mito-GFP to visualise mitochondria (green) and stained with Hoechst to visualise DNA (blue) showed that mitochondrial asymmetry does not develop in the presence of (B) 6 µM LatA, (C) 100 µM CK-666 or (D) 5 µM BFA to prevent spindle migration. Time = 0 indicates anaphase. (E) Mitochondrial asymmetry was quantified and no asymmetry was found when spindle migration was prevented by LatA, CK-666 or BFA. (F) The extent of mitochondrial asymmetry at 30 minutes post-anaphase was compared and control oocytes were found to contain mitochondria that were significantly more asymmetrically arranged than those in LatA-treated oocytes (n = 13, P<0.001), CK-666-treated oocytes (n = 6, P<0.001) and BFA-treated oocytes (n = 22, P<0.001). Scale bars: 20 µm.
This observation predicts that retaining the spindle in a central location, preventing spindle-cortical interactions and allowing an equal cell division, would lead to a symmetrical distribution of mitochondria to the daughter cells. To test this prediction spindle migration was inhibited by disrupting the actin cytoskeleton (Azoury et al., 2008; Longo and Chen, 1985; Schuh and Ellenberg, 2008). We first inhibited the microfilament network using latrunculin A (LatA) which caused the spindle to remain in a central position and prevented cytokinesis. Anaphase proceeded normally in 91% of oocytes and in these oocytes there was no switch to an asymmetrical distribution of mitochondria either during or after anaphase (Fig. 5B; supplementary material Movie 4). Arp2/3, a branched actin filament nucleator, is also known to be required for spindle migration (Sun et al., 2011). In oocytes treated with the Arp2/3 inhibitor CK-666 (Nolen et al., 2009), anaphase occurred in 69% of oocytes. Thirty-two percent of these oocytes extruded a polar body of approximately normal size, indicating that spindle migration had not been inhibited and these oocytes were excluded from our analysis of mitochondrial asymmetry. The remaining 68% of oocytes underwent a cleavage division that yielded two cells of similar size indicating that spindle migration had been effectively prevented. This is in line with data from others studies showing that inhibition of Arp2/3 results a similar proportion of oocytes failing to undergo spindle migration (Sun et al., 2011; Yi et al., 2013) while the remainder are able to move the spindle to the cortex after a delay (Yi et al., 2013). In those oocytes where spindle migration was prevented, mitochondria remained in a symmetrical distribution around the spindle and were segregated to both daughter cells (Fig. 5C; supplementary material Movie 5). Finally, we used brefeldin A (BFA), which has been shown to prevent spindle migration (Wang et al., 2008) possibly through effects on ARF1 (Wang et al., 2009b). In BFA-treated oocytes, spindle migration was inhibited but anaphase occurred in 88% of oocytes. After undergoing anaphase, oocytes either attempted but failed to complete a symmetrical cytokinesis (57%), or divided symmetrically (43%). In both cases, mitochondria remained in a symmetrical distribution and were segregated to both daughter cells if cytokinesis occurred (Fig. 5D; supplementary material Movie 6). Analysis of mitochondrial asymmetry over time confirmed that in the absence of actin-dependent spindle migration mitochondria remained symmetrically distributed in all cases (Fig. 5E,F). The development of asymmetry is therefore not governed by the meiotic spindle itself but requires spindle-cortical interactions that ultimately lead to the mitochondria being prevented from entering the polar body.
Asymmetrical mitochondrial inheritance requires cell cycle progression
To determine whether spindle-cortical proximity is sufficient to trigger mitochondrial redistribution, conditions were established that allowed the spindle to migrate and remain close to the cortex, while preventing progress of the cell cycle. This was achieved by treating oocytes with the proteasome inhibitor MG132, which does not affect spindle migration (Fig. 6A; supplementary material Movie 7) but does inhibit cyclin B and securin destruction leading to a stable metaphase I arrest. Imaging of mitochondria for prolonged periods during metaphase I arrest with the spindle at the cortex revealed that the mitochondria remained symmetrically distributed around the spindle and there was no evidence of the mitochondria moving away from the cortical spindle pole. (Fig. 6A,C; supplementary material Movie 7). This suggests that progression out of metaphase is necessary to develop asymmetrical distribution of mitochondria. This was confirmed by reversing the metaphase arrest by washing out MG132 and observing the development of asymmetry accompanying progression into anaphase and subsequent polar body formation (Fig. 6B,C; supplementary material Movie 8). Taken together, these data show that asymmetrical mitochondrial inheritance requires both a cortically located spindle and progression through metaphase.
Fig. 6.
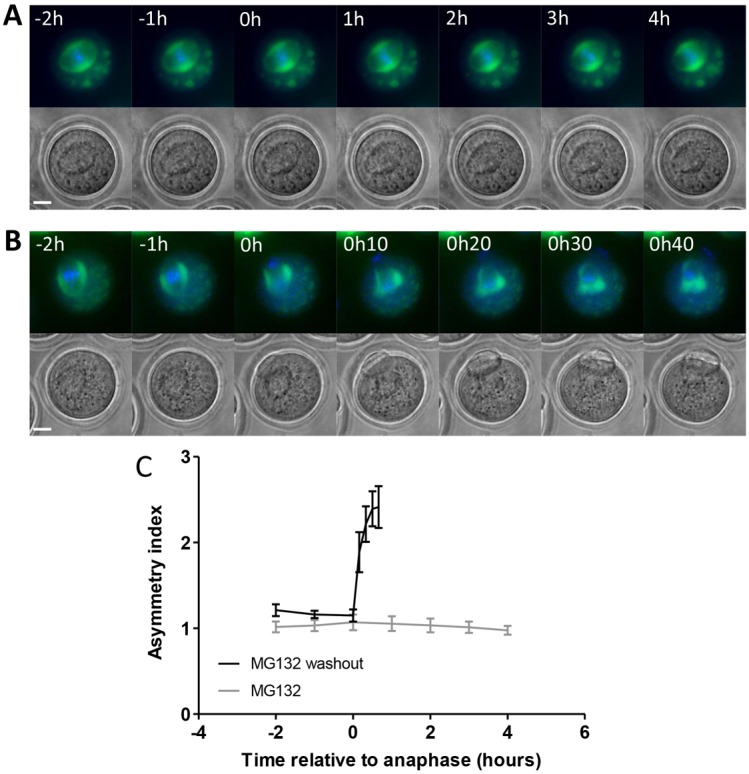
Asymmetrical mitochondrial inheritance requires cell cycle progression. (A) Live-cell time-lapse imaging of oocytes that had been microinjected with mito-GFP to visualise mitochondria (green) and stained with Hoechst to visualise DNA (blue) showed that mitochondrial asymmetry does not develop when the cell cycle is arrested at metaphase using 50 µM MG132 (n = 12). (B) MG132 was subsequently washed out, allowing the cell cycle to proceed and a polar body to be extruded, and mitochondrial asymmetry developed. (C) Mitochondrial asymmetry was quantified and no asymmetry was found in the presence of MG132 even over an extended time period. Time = 0 indicates anaphase for MG132 washout experiments. Owing to the absence of anaphase in MG132-treated oocytes, time = 0 for these oocytes was defined as 11 hour 30 minutes after release from meiotic arrest, similar to the average time of anaphase in control oocytes (11 hours 17 minutes ± 10 minutes, n = 48) and in MG132 washout oocytes (11 hour 43 minutes ± 11 minutes, n = 11). Asymmetry developed during polar body extrusion with similar dynamics to that in control oocytes when MG132 was washed out. Scale bars: 20 µm.
Mitochondria are not inherited asymmetrically in the second meiotic division
The second meiotic spindle is not surrounded by sheath of mitochondria (see Fig. 1A). However, it is not known whether mitochondria associate with the MII spindle during its formation or if mitochondria in the vicinity of the spindle are excluded from the second polar body.
We therefore examined the distribution of mitochondria during formation of the second meiotic spindle using live-cell time-lapse imaging in oocytes expressing mito-GFP. We observed that following extrusion of the first polar body the mitochondria which had been retained in the oocyte moved towards the cortex and formed a ring around the developing second meiotic spindle (Fig. 7A; supplementary material Movie 9). The aggregation of mitochondria around both the first and second meiotic spindle as they develop suggests that mitochondria are required locally for spindle formation in the mammalian oocyte.
Fig. 7.
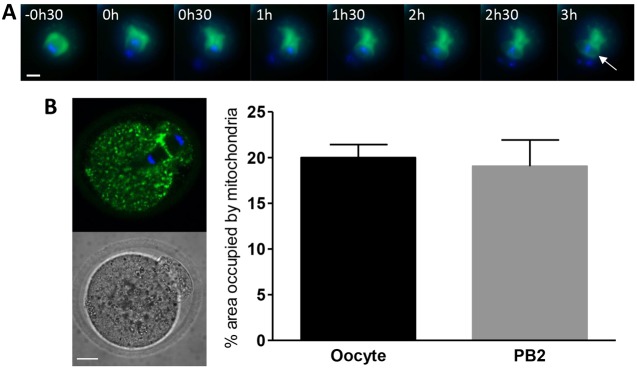
Mitochondrial dynamics during meiosis II. (A) Mitochondria (green) and DNA (blue) were imaged using live-cell time-lapse microscopy during extrusion of the first polar body and formation of the second meiotic spindle. Time is measured from the start of anaphase I. Mitochondria are seen to accumulate around the developing second meiotic spindle (white arrow). (B) Oocytes that had been parthenogenetically activated were fixed during second polar body extrusion, stained for mitochondria (green) and DNA (blue), and imaged by confocal microscopy. The percentage area occupied by mitochondria in both the oocyte and polar body (PB) was calculated and no consistent difference between the oocyte and polar body (PB) was found (n = 14). Scale bars: 20 µm.
By the time of MII arrest the spindle is no longer enclosed by a ring of mitochondria (see Fig. 1A). However they are found in the vicinity of the spindle and are often enriched in this area (Calarco, 1995; Cecconi et al., 2006; Dumollard et al., 2006). Given the exclusion of mitochondria from the first polar body we tested whether there was any asymmetry in the distribution of mitochondria during the second meiotic division. Eggs which had been parthenogenetically activated with ethanol were fixed during extrusion of the second polar body and stained for mitochondria and DNA. Analysis of confocal images of eggs undergoing anaphase showed that on average there was no difference in the percentage area occupied by mitochondria in the oocyte when compared to the polar body during meiosis II (20±1.4% versus 19±2.9%, respectively; Fig. 7B). Variability between individual oocytes was a feature of this experiment and would be predicted if the relatively small polar body captured different numbers of randomly dispersed clusters or aggregates of mitochondria. The retention of mitochondria in the oocyte is therefore meiosis I specific, and likely reflects the increased risk of mitochondrial loss caused by the aggregation of mitochondria around the first meiotic spindle.
Discussion
We find that mitochondrial inheritance during the highly asymmetric divisions of female meiosis represents a unique mode of distributing organelles between daughter cells. In previously described examples of asymmetric division, for example in the early embryo of the nematode worm C. elegans, in Drosophila neuroblasts and in stem cells (Gönczy, 2008) both of the resulting cells are viable, and contribute to the generation of diversity and to self-renewal. In contrast, the polar bodies which are extruded during female meiosis are destined to degenerate while the oocyte must retain sufficient maternal stores to sustain early development. The retention of mitochondria by the oocyte and exclusion from the polar body is entirely consistent with the ultimate fate of the daughter cells; the oocyte providing the functional female gamete and the polar body degenerating within a few hours. The basis of this pattern of inheritance is founded in mitochondrial dynamics during acentriolar spindle formation and in a previously undiscovered reorganization of mitochondria at the onset of anaphase in meiosis I.
Mitochondria are highly dynamic in the oocyte and it is the aggregation of mitochondria around the developing first meiotic spindle that sets up the need for reorganisation at polar body formation. The association of mitochondria with the spindle is driven by dynein-mediated transport, ultimately delivering more than 40% of the oocyte mitochondria to the spindle periphery. Numerous cytoplasmic MTOCs have been shown to progressively cluster in the centre of the oocyte as a result both of direct MTOC–MTOC interaction and of interactions between MTOCs and the nuclear envelope (Schuh and Ellenberg, 2007). We observed mitochondria associated with MTOCs in the oocyte cytoplasm, as well as cytoplasmic rings of mitochondria moving towards the spindle region. Dynein has been shown to be necessary for the recruitment of MTOCs during the first mitotic division in the mouse (Courtois et al., 2012) as well as for the inward movement of microtubule bundles at prophase/prometaphase in mitosis (Rusan et al., 2002). Therefore we postulate that the accumulation of mitochondria around the spindle may progress through two complementary dynein-dependent mechanisms. First, mitochondria may be trafficked by dynein to microtubule minus ends at cytoplasmic MTOCs, followed by dynein-dependent recruitment of these MTOCs to the spindle region. Second, microtubules emanating from MTOCs clustered in the centre of the oocyte (Schuh and Ellenberg, 2007) could also allow direct dynein-driven trafficking of mitochondria to the spindle region.
The formation of the mitochondria–spindle superstructure is however regulated by the opposing activity of two motor proteins, with dynein activity out-competing kinesin-dependent trafficking during the period of spindle formation. The action of kinesin motors could function to limit both the recruitment of mitochondria to MTOCs and the direct trafficking of mitochondria along microtubules to the spindle region. The co-existence of dynein and kinesin-dependent traffic for a single cargo ensures at least a proportion of oocyte mitochondria remain in the cytosol distinct from the spindle. This may be important for serving ATP supply at regions of high ATP demand such as the Na-K ATPase at the plasma membrane (Houghton et al., 2003; Kelly and McBride, 1990).
The risk inherent in this superstructure is that at cytokinesis the spindle architecture would normally lead to 50% of the spindle-associated mitochondria being inherited by the polar body when the chromatin is divided equally at cytokinesis. The fact that the structure develops in spite of this risk, and that it develops both in meiosis I and II suggests that it may play a functional role or act as a functional unit and confer an advantage on the oocyte. Mitochondria aggregate in regions of high cellular demand in systems such as the sperm axenome, active neuronal synapses and between muscle fibres (Hollenbeck and Saxton, 2005; MacAskill et al., 2010). The developing meiotic spindle may be another example of a cell function requiring local ATP production. The presence of ER in a similar distribution around the spindle during meiosis I may further contribute to it acting as a functional unit as this arrangement can facilitate Ca2+ cycling between the ER and mitochondria leading to an increase in mitochondrial activity (Cardenas et al., 2010; de Brito and Scorrano, 2010; Dumollard et al., 2003; Dumollard et al., 2004). Mitochondrial ATP appears to be important for the development and maintenance of the spindle because interventions expected to impact on mitochondrial function also lead to spindle abnormalities (Johnson et al., 2007; Wang et al., 2009a; Zhang et al., 2006). Direct evidence that spindle function is impacted by local ATP production will require an experimental manipulation that specifically targets dynein-mediated mitochondrial trafficking.
Preservation of mitochondrial numbers is particularly crucial in the mammalian oocyte; they are responsible for the vast majority of ATP production (Dumollard et al., 2007; Leese, 1995), there is little if any biogenesis until implantation (Pikó and Taylor, 1987), and the lack of a highly developed cristae structure (Sathananthan and Trounson, 2000) suggests the respiratory capacity of each individual mitochondrion is relatively limited. Interestingly, Tfam+/− oocytes can develop normally to blastocyst stage despite substantially reduced mtDNA copy number, however a critical threshold of 40,000–50,000 copies of mtDNA is required for successful post-implantation development (Wai et al., 2010). In the literature, a reduction in ATP content of around 20% upwards is associated with abnormalities in the mature oocyte or with failure to proceed normally through development (Johnson et al., 2007; Selesniemi et al., 2011; Thouas et al., 2004; Van Blerkom et al., 1995; Zeng et al., 2007). The loss of 20% of the oocyte mitochondrial complement due to the spindle-mitochondria superstructure at cytokinesis in the first meiotic division would equate to a similar decrease in ATP production or the requirement of the remaining mitochondria to increase ATP generation by 20%; a response that would lead to increased ROS generation with potential damage to mitochondria and other cellular processes (Valko et al., 2007).
The fidelity of meiotic divisions and the ability of the oocyte to develop after fertilisation are known to be exquisitely sensitive to perturbations in ATP and ROS (Igosheva et al., 2010; Liu et al., 2000; Thouas et al., 2004; Thouas et al., 2006; Zhang et al., 2006). More specifically, Ca2+ transients at fertilisation trigger a threefold increase in ATP levels in the oocyte, presumably to meet the energy demands of egg activation (Dumollard et al., 2004; Dumollard et al., 2008), and perturbing mitochondrial activity leads to a loss of control of basal Ca2+ levels (Dumollard et al., 2004). In light of the critical role mitochondria play in supplying ATP in oocytes, a mechanism for selectively retaining mitochondria at the time of polar body extrusion may be essential to maximise the ability of the oocyte to support early development.
The precise mechanism by which the mitochondria remain anchored in the oocyte is not yet established. Manipulating the position of the spindle in the oocyte has revealed that the asymmetry of mitochondrial inheritance is not an inherent property of the spindle and that spindle-cortex interactions are necessary but not sufficient. Also necessary is progression through meiosis I. A direct role for the decrease in Cdk1 activity on motors (Addinall et al., 2001; Niclas et al., 1996) or cytoskeletal organization (Field et al., 2011) is one possible mechanism for withdrawing mitochondria to the oocyte-directed pole of the spindle. Alternatively, forces associated with cortical contraction and associated cytoplasmic reorganization (Brunet and Verlhac, 2011) may repel mitochondria from the polarized cortex destined to become the polar body. It may be of interest to investigate mitochondrial distribution at polar body extrusion in oocytes of other species to understand whether the retention of mitochondria proceeds through an evolutionarily conserved mechanism.
Notably, we show here that mitochondria also associate with the second meiotic spindle during its formation suggesting that similar mechanisms may be working at both MI and MII to cause mitochondrial aggregation around the spindle. However mitochondria around the second meiotic spindle appear to disperse throughout the oocyte once spindle formation is completed. This suggests that the MII spindle is able to be maintained in the absence of the large aggregation of mitochondria present at MI, perhaps because of its smaller size and the fact that it has no requirement for migration. Additionally, dispersal of mitochondria throughout the oocyte at MII may well be important for their role in Ca2+ oscillations at fertilization (Dumollard et al., 2004).
The lack of a specific retention mechanism for mitochondria during the second meiotic division is therefore entirely consistent with the more random and lower risk distribution of mitochondria at MII arrest and may reflect inherent differences in the pattern of polar body extrusion in the first and second meiotic divisions. In MI, the spindle interacts with the cortex end on while at MII it is parallel with the cortex. This may lead to different cytoplasmic and actin dynamics (Yi et al., 2011; Yi et al., 2013) that result in organelles specifically being excluded from the first polar body but not the second. The variability seen in the percentage area occupied by mitochondria in the second polar body is likely explained by the random chance of the polar body inheriting small aggregates of mitochondria in the vicinity of the MII spindle.
To conclude, the highly choreographed mitochondrial dynamics during spindle formation and the first meiotic division serve to ensure a mechanism for providing the developing spindle with closely apposed mitochondria, and the oocyte with maximal numbers of mitochondria, thereby allowing mitochondrial activity to remain at the lowest possible set-point. Previous reports have found asymmetric distribution of some non-essential organelles such as P-granules and aggresomes at cell division (Ouellet and Barral, 2012) but to our knowledge this is the first report of asymmetrical mitochondrial inheritance and represents a deviation from previously described mechanisms for distributing these organelles at cytokinesis. This likely reflects the crucial role of these organelles in the oocyte and early embryo and ensures maintenance of the oocyte mitochondrial complement, maximising the capacity for generating ATP known to be necessary to support early development.
Materials and Methods
Oocyte collection and culture
Germinal vesicle stage oocytes were collected from 4–6-week-old female MF1 mice that had been administered 7 international units (IU) pregnant mare’s serum gonadotropin (PMSG; Intervet) by intraperitoneal injection 48 hours earlier. To recover mature (MII) oocytes, mice were injected intraperitoneally with 7.5 IU human chorionic gonadotropin (hCG; Intervet) 44–48 hours after PMSG injection. Oocytes were placed into M2 medium (Sigma-Aldrich) at a temperature of 37°C. GV arrest was maintained where necessary by the addition of 200 µM 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich) to the medium. Subsequent release from GV arrest was achieved by repeatedly washing oocytes in M2 medium without IBMX. For second polar body extrusion experiments, MII oocytes were parthenogenetically activated at 18–20 hours post-hCG by exposure to 7% ethanol in M2 medium for 7 minutes at 25°C. Oocytes were subsequently washed repeatedly in M2 medium without ethanol and maintained at 37°C.
All animal experiments were performed according to approved guidelines.
Microinjection
Oocytes were pressure injected using a micropipette and micromanipulators mounted on a Leica Axiovert 135 inverted microscope. A brief over compensation of negative capacitance was used to penetrate the plasma membrane and microinjection was performed using a fixed pressure pulse through a pico-pump (World Precision Instruments). Microinjection volume was estimated at 5% by cytoplasmic displacement.
Manipulation of the cytoskeleton and cell cycle
Oocytes were incubated with 10 µM nocodazole (Sigma-Aldrich) or 6 µM latrunculin A (Sigma-Aldrich) from GV stage to disrupt the microtubule and actin cytoskeletons respectively. Clone 70.1 (Sigma-Aldrich), used to disrupt cytoplasmic dynein activity, was microinjected into GV stage oocytes to an estimated final concentration of 1–2 mg/ml. SUK4 (gift from J. Kittler, UCL), a function-blocking antibody against Kinesin-1, was microinjected into GV stage oocytes to an estimated final concentration of 12–15 µg/ml. p50-EGFP or a vehicle control were microinjected into GV stage oocytes which were incubated overnight in M16 medium at 37°C in an atmosphere of 5% CO2 in air to allow expression of the protein. Oocytes were subsequently released from meiotic arrest by repeated washing in M16 without IBMX. Latrunculin A, CK-666 (Tocris Bioscience), BFA (Sigma-Aldrich) and MG132 (Calbiochem) were added at 100× concentrations during live-cell imaging, 6 hours after release from meiotic arrest. MG132 was subsequently washed out 9 hours after release from meiotic arrest in MG132 washout experiments.
Live-cell time-lapse imaging
Live-cell imaging of mitochondria was carried out using mitochondrially targeted GFP in a pRN3 vector (mito-GFP). mRNA was produced from linearised template DNA using the mMessage mMachine T3 kit (Applied Biosystems). ER was visualised by microinjection of DiI18 (Molecular Probes) as a saturated solution in soybean oil (Sigma-Aldrich). DNA was visualised by a 5 second incubation of oocytes in 10 µg/ml Hoechst 33342 (Invitrogen). Imaging was carried out at 20× magnification using a Zeiss Axiovert 200 microscope equipped with a CoolSnapHQ cooled CCD camera and an XBO 75 xenon short-arc lamp microscope illuminating system. MetaFluor software was used to control acquisition and oocytes were maintained at 37°C by the use of a heated stage.
Confocal microscopy
Oocytes were fixed for 50 minutes at room temperature in 4% paraformaldehyde in PBS, permeabilised for 10 minutes at room temperature using 0.025% Triton X-100 in PBS and blocked using 3 mg/ml BSA in PBS either for 1 hour at 37°C or overnight at 4°C. Mitochondria were stained using either an anti-cytochrome c antibody or an anti-TOM20 antibody (Santa Cruz Biotechnology), and an Alexa Fluor 488 secondary antibody (Molecular Probes). Tubulin was stained using an anti-β-tubulin antibody (Sigma-Aldrich) and an Alexa Fluor 546 secondary antibody (Molecular Probes). All antibody incubations were carried out at 37°C for 1 hour in PBS containing 1 mg/ml BSA. DNA was stained using Hoechst 33342 at a concentration of 10 µg/ml for 10 minutes at room temperature. For ER and mitochondria experiments, oocytes were microinjected with mRNA for dsRed2ER and mito-GFP and fixed 6 hours after release from meiotic arrest. Confocal imaging was carried out on a Zeiss LSM 510 confocal microscope using a 63× oil-immersion lens with a numerical aperture of 1.4.
Image analysis and statistics
Image analysis was carried out using MetaMorph software. Spindle-associated mitochondria were defined in a standardised manner on equatorial confocal sections. The area occupied by spindle-associated mitochondria was divided by the area occupied by mitochondria in the whole oocyte to give a measure of the percentage of mitochondria which were associated with the spindle.
In order to calculate the percentage area occupied by mitochondria in the oocyte or polar body, confocal sections where the chromosomes at opposing ends of the spindle had been aligned in the horizontal plane were used. The area occupied by mitochondria in the oocyte and polar body was calculated and values were expressed as a percentage of the total area of the oocyte or polar body.
The asymmetry score at polar body extrusion was calculated in oocytes where the chromosomes divided in the plane of imaging. A line scan was drawn either through the metaphase plate or through the plane of the dividing chromosomes across the whole oocyte, depending on the stage of the cell cycle of the oocyte. The metaphase plate or the central point between the dividing chromosomes was defined as the mid-point and the average fluorescence of mitochondria either side of this point on the line scan was measured and a ratio calculated.
Statistical analysis was carried out using either a t-test, paired t-test (for oocyte and polar body comparisons) or ANOVA with the Tukey–Kramer post-hoc test. P-values of <0.05 (*), <0.01 (**), <0.001 (***) and <0.0001 (****) are indicated. Error bars indicate standard error of the mean.
Supplementary Material
Acknowledgments
The authors are grateful to Greg FitzHarris, Jenny Bormann, Katie Howe, Gyorgy Szabadkai and Michael Duchen for support and useful discussions. They also thank Greg FitzHarris and Gyorgy Szabadkai for reading and providing comments on the manuscript.
Footnotes
Author contributions
J.C. raised the funding and conceived the project. C.D. developed the project and performed all of the experiments. C.D. and J.C. wrote the paper.
Funding
This work was supported by an MRC Programme Grant [grant number G1001705 to J.C.]. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.128744/-/DC1
References
- Addinall S. G., Mayr P. S., Doyle S., Sheehan J. K., Woodman P. G., Allan V. J. (2001). Phosphorylation by cdc2-CyclinB1 kinase releases cytoplasmic dynein from membranes. J. Biol. Chem. 276, 15939–15944 10.1074/jbc.M011628200 [DOI] [PubMed] [Google Scholar]
- Azoury J., Lee K. W., Georget V., Rassinier P., Leader B., Verlhac M. H. (2008). Spindle positioning in mouse oocytes relies on a dynamic meshwork of actin filaments. Curr. Biol. 18, 1514–1519 10.1016/j.cub.2008.08.044 [DOI] [PubMed] [Google Scholar]
- Biggers J. D., Whittingham D. G., Donahue R. P. (1967). The pattern of energy metabolism in the mouse oöcyte and zygote. Proc. Natl. Acad. Sci. USA 58, 560–567 10.1073/pnas.58.2.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S., Verlhac M. H. (2011). Positioning to get out of meiosis: the asymmetry of division. Hum. Reprod. Update 17, 68–75 10.1093/humupd/dmq044 [DOI] [PubMed] [Google Scholar]
- Burkhardt J. K., Echeverri C. J., Nilsson T., Vallee R. B. (1997). Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139, 469–484 10.1083/jcb.139.2.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarco P. G. (1995). Polarization of mitochondria in the unfertilized mouse oocyte. Dev. Genet. 16, 36–43 10.1002/dvg.1020160108 [DOI] [PubMed] [Google Scholar]
- Calarco P. G. (2000). Centrosome precursors in the acentriolar mouse oocyte. Microsc. Res. Tech. 49, 428–434 [DOI] [PubMed] [Google Scholar]
- Cardenas C., Miller R. A., Smith I., Bui T., Molgo J., Muller M., Vais H., Cheung K. H., Yang J., Parker I. et al. (2010). Essential regulation of cell bioenergetics by constitutive insp3 receptor ca2+ transfer to mitochondria. Cell 142, 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi S., Rossi G., Palmerini M. G. (2006). Mouse oocyte differentiation during antral follicle development. Microsc. Res. Tech. 69, 408–414 10.1002/jemt.20300 [DOI] [PubMed] [Google Scholar]
- Combelles C. M. H., Albertini D. F. (2001). Microtubule patterning during meiotic maturation in mouse oocytes is determined by cell cycle-specific sorting and redistribution of gamma-tubulin. Dev. Biol. 239, 281–294 10.1006/dbio.2001.0444 [DOI] [PubMed] [Google Scholar]
- Courtois A., Schuh M., Ellenberg J., Hiiragi T. (2012). The transition from meiotic to mitotic spindle assembly is gradual during early mammalian development. J. Cell Biol. 198, 357–370 10.1083/jcb.201202135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito O. M., Scorrano L. (2010). An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 29, 2715–2723 10.1038/emboj.2010.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumollard R., Hammar K., Porterfield M., Smith P. J., Cibert C., Rouvière C., Sardet C. (2003). Mitochondrial respiration and Ca2+ waves are linked during fertilization and meiosis completion. Development 130, 683–692 10.1242/dev.00296 [DOI] [PubMed] [Google Scholar]
- Dumollard R., Marangos P., Fitzharris G., Swann K., Duchen M., Carroll J. (2004). Sperm-triggered [Ca2+] oscillations and Ca2+ homeostasis in the mouse egg have an absolute requirement for mitochondrial ATP production. Development 131, 3057–3067 10.1242/dev.01181 [DOI] [PubMed] [Google Scholar]
- Dumollard R., Duchen M., Sardet C. (2006). Calcium signals and mitochondria at fertilisation. Semin. Cell Dev. Biol. 17, 314–323 10.1016/j.semcdb.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Dumollard R., Duchen M., Carroll J. (2007). The role of mitochondrial function in the oocyte and embryo. Current Topics in Developmental Biology: The Mitochondrion in the Germline and Early Development St. John J C, ed21–49San Diego, CA: Academic Press; [DOI] [PubMed] [Google Scholar]
- Dumollard R., Campbell K., Halet G., Carroll J., Swann K. (2008). Regulation of cytosolic and mitochondrial ATP levels in mouse eggs and zygotes. Dev. Biol. 316, 431–440 10.1016/j.ydbio.2008.02.004 [DOI] [PubMed] [Google Scholar]
- Field C. M., Wühr M., Anderson G. A., Kueh H. Y., Strickland D., Mitchison T. J. (2011). Actin behavior in bulk cytoplasm is cell cycle regulated in early vertebrate embryos. J. Cell Sci. 124, 2086–2095 10.1242/jcs.082263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzHarris G., Marangos P., Carroll J. (2007). Changes in endoplasmic reticulum structure during mouse oocyte maturation are controlled by the cytoskeleton and cytoplasmic dynein. Dev. Biol. 305, 133–144 10.1016/j.ydbio.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Gönczy P. (2008). Mechanisms of asymmetric cell division: flies and worms pave the way. Nat. Rev. Mol. Cell Biol. 9, 355–366 10.1038/nrm2388 [DOI] [PubMed] [Google Scholar]
- Heald R., Tournebize R., Habermann A., Karsenti E., Hyman A. (1997). Spindle assembly in Xenopus egg extracts: respective roles of centrosomes and microtubule self-organization. J. Cell Biol. 138, 615–628 10.1083/jcb.138.3.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Noda Y., Okada Y. (1998). Kinesin and dynein superfamily proteins in organelle transport and cell division. Curr. Opin. Cell Biol. 10, 60–73 10.1016/S0955-0674(98)80087-2 [DOI] [PubMed] [Google Scholar]
- Hollenbeck P. J., Saxton W. M. (2005). The axonal transport of mitochondria. J. Cell Sci. 118, 5411–5419 10.1242/jcs.02745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton F. D., Humpherson P. G., Hawkhead J. A., Hall C. J., Leese H. J. (2003). Na+, K+, ATPase activity in the human and bovine preimplantation embryo. Dev. Biol. 263, 360–366 10.1016/j.ydbio.2003.07.014 [DOI] [PubMed] [Google Scholar]
- Igosheva N., Abramov A. Y., Poston L., Eckert J. J., Fleming T. P., Duchen M. R., McConnell J. (2010). Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 5, e10074 10.1371/journal.pone.0010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingold A. L., Cohn S. A., Scholey J. M. (1988). Inhibition of kinesin-driven microtubule motility by monoclonal antibodies to kinesin heavy chains. J. Cell Biol. 107, 2657–2667 10.1083/jcb.107.6.2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. T., Freeman E. A., Gardner D. K., Hunt P. A. (2007). Oxidative metabolism of pyruvate is required for meiotic maturation of murine oocytes in vivo. Biol. Reprod. 77, 2–8 10.1095/biolreprod.106.059899 [DOI] [PubMed] [Google Scholar]
- Kelly J. M., McBride B. W. (1990). The sodium pump and other mechanisms of thermogenesis in selected tissues. Proc. Nutr. Soc. 49, 185–202 10.1079/PNS19900023 [DOI] [PubMed] [Google Scholar]
- Ledan E., Polanski Z., Terret M. E., Maro B. (2001). Meiotic maturation of the mouse oocyte requires an equilibrium between cyclin B synthesis and degradation. Dev. Biol. 232, 400–413 10.1006/dbio.2001.0188 [DOI] [PubMed] [Google Scholar]
- Leese H. J. (1995). Metabolic control during preimplantation mammalian development. Hum. Reprod. Update 1, 63–72 10.1093/humupd/1.1.63 [DOI] [PubMed] [Google Scholar]
- Liu L., Trimarchi J. R., Keefe D. L. (2000). Involvement of mitochondria in oxidative stress-induced cell death in mouse zygotes. Biol. Reprod. 62, 1745–1753 10.1095/biolreprod62.6.1745 [DOI] [PubMed] [Google Scholar]
- Longo F. J., Chen D. Y. (1985). Development of cortical polarity in mouse eggs: involvement of the meiotic apparatus. Dev. Biol. 107, 382–394 10.1016/0012-1606(85)90320-3 [DOI] [PubMed] [Google Scholar]
- MacAskill A. F., Atkin T. A., Kittler J. T. (2010). Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur. J. Neurosci. 32, 231–240 10.1111/j.1460-9568.2010.07345.x [DOI] [PubMed] [Google Scholar]
- Maro B., Verlhac M. H. (2002). Polar body formation: new rules for asymmetric divisions. Nat. Cell Biol. 4, E281–E283 10.1038/ncb1202-e281 [DOI] [PubMed] [Google Scholar]
- Mehlmann L. M., Terasaki M., Jaffe L. A., Kline D. (1995). Reorganization of the endoplasmic reticulum during meiotic maturation of the mouse oocyte. Dev. Biol. 170, 607–615 10.1006/dbio.1995.1240 [DOI] [PubMed] [Google Scholar]
- Messinger S. M., Albertini D. F. (1991). Centrosome and microtubule dynamics during meiotic progression in the mouse oocyte. J. Cell Sci. 100, 289–298 [DOI] [PubMed] [Google Scholar]
- Niclas J., Allan V. J., Vale R. D. (1996). Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J. Cell Biol. 133, 585–593 10.1083/jcb.133.3.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen B. J., Tomasevic N., Russell A., Pierce D. W., Jia Z., McCormick C. D., Hartman J., Sakowicz R., Pollard T. D. (2009). Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature 460, 1031–1034 10.1038/nature08231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet J., Barral Y. (2012). Organelle segregation during mitosis: lessons from asymmetrically dividing cells. J. Cell Biol. 196, 305–313 10.1083/jcb.201102078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza-Reyes L., Crider D. G., Pon L. A. (2010). Mitochondrial manoeuvres: latest insights and hypotheses on mitochondrial partitioning during mitosis in Saccharomyces cerevisiae. Bioessays 32, 1040–1049 10.1002/bies.201000083 [DOI] [PubMed] [Google Scholar]
- Pikó L., Taylor K. D. (1987). Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev. Biol. 123, 364–374 10.1016/0012-1606(87)90395-2 [DOI] [PubMed] [Google Scholar]
- Rusan N. M., Tulu U. S., Fagerstrom C., Wadsworth P. (2002). Reorganization of the microtubule array in prophase/prometaphase requires cytoplasmic dynein-dependent microtubule transport. J. Cell Biol. 158, 997–1003 10.1083/jcb.200204109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathananthan A. H., Trounson A. O. (2000). Mitochondrial morphology during preimplantational human embryogenesis. Hum. Reprod. 15, Suppl. 2148–159 10.1093/humrep/15.suppl_2.148 [DOI] [PubMed] [Google Scholar]
- Schuh M., Ellenberg J. (2007). Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 130, 484–498 10.1016/j.cell.2007.06.025 [DOI] [PubMed] [Google Scholar]
- Schuh M., Ellenberg J. (2008). A new model for asymmetric spindle positioning in mouse oocytes. Curr. Biol. 18, 1986–1992 10.1016/j.cub.2008.11.022 [DOI] [PubMed] [Google Scholar]
- Selesniemi K., Lee H. J., Muhlhauser A., Tilly J. L. (2011). Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc. Natl. Acad. Sci. USA 108, 12319–12324 10.1073/pnas.1018793108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. C., Wang Z. B., Xu Y. N., Lee S. E., Cui X. S., Kim N. H. (2011). Arp2/3 complex regulates asymmetric division and cytokinesis in mouse oocytes. PLoS ONE 6, e18392 10.1371/journal.pone.0018392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szollosi D., Calarco P., Donahue R. P. (1972). Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J. Cell Sci. 11, 521–541 [DOI] [PubMed] [Google Scholar]
- Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. (2007). Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 282, 11521–11529 10.1074/jbc.M607279200 [DOI] [PubMed] [Google Scholar]
- Thouas G. A., Trounson A. O., Wolvetang E. J., Jones G. M. (2004). Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro. Biol. Reprod. 71, 1936–1942 10.1095/biolreprod.104.033589 [DOI] [PubMed] [Google Scholar]
- Thouas G. A., Trounson A. O., Jones G. M. (2006). Developmental effects of sublethal mitochondrial injury in mouse oocytes. Biol. Reprod. 74, 969–977 10.1095/biolreprod.105.048611 [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M. T. D., Mazur M., Telser J. (2007). Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 39, 44–84 10.1016/j.biocel.2006.07.001 [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. (1991). Microtubule mediation of cytoplasmic and nuclear maturation during the early stages of resumed meiosis in cultured mouse oocytes. Proc. Natl. Acad. Sci. USA 88, 5031–5035 10.1073/pnas.88.11.5031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Blerkom J., Runner M. N. (1984). Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am. J. Anat. 171, 335–355 10.1002/aja.1001710309 [DOI] [PubMed] [Google Scholar]
- Van Blerkom J., Davis P. W., Lee J. (1995). ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 10, 415–424 [DOI] [PubMed] [Google Scholar]
- Varadi A., Johnson-Cadwell L. I., Cirulli V., Yoon Y., Allan V. J., Rutter G. A. (2004). Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J. Cell Sci. 117, 4389–4400 10.1242/jcs.01299 [DOI] [PubMed] [Google Scholar]
- Wai T., Ao A., Zhang X., Cyr D., Dufort D., Shoubridge E. A. (2010). The role of mitochondrial DNA copy number in mammalian fertility. Biol. Reprod. 83, 52–62 10.1095/biolreprod.109.080887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Z. B., Zhang X., FitzHarris G., Baltz J. M., Sun Q. Y., Liu X. J. (2008). Brefeldin A disrupts asymmetric spindle positioning in mouse oocytes. Dev. Biol. 313, 155–166 10.1016/j.ydbio.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Wang Q., Ratchford A. M., Chi M. M. Y., Schoeller E., Frolova A., Schedl T., Moley K. H. (2009a). Maternal diabetes causes mitochondrial dysfunction and meiotic defects in murine oocytes. Mol. Endocrinol. 23, 1603–1612 10.1210/me.2009-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Hu J., Guo X., Liu J. X., Gao S. (2009b). ADP-ribosylation factor 1 regulates asymmetric cell division in female meiosis in the mouse. Biol. Reprod. 80, 555–562 10.1095/biolreprod.108.073197 [DOI] [PubMed] [Google Scholar]
- Yi K., Unruh J. R., Deng M., Slaughter B. D., Rubinstein B., Li R. (2011). Dynamic maintenance of asymmetric meiotic spindle position through Arp2/3-complex-driven cytoplasmic streaming in mouse oocytes. Nat. Cell Biol. 13, 1252–1258 10.1038/ncb2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K., Rubinstein B., Unruh J. R., Guo F., Slaughter B. D., Li R. (2013). Sequential actin-based pushing forces drive meiosis I chromosome migration and symmetry breaking in oocytes. J. Cell Biol. 200, 567–576 10.1083/jcb.201211068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Dumollard R., Rossbach A., Lai F. A., Swann K. (2010). Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J. Cell. Physiol. 224, 672–680 10.1002/jcp.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H. t., Ren Z., Yeung W. S. B., Shu Y. m., Xu Y. w., Zhuang G. l., Liang X. y. (2007). Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum. Reprod. 22, 1681–1686 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wu X. Q., Lu S., Guo Y. L., Ma X. (2006). Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 16, 841–850 10.1038/sj.cr.7310095 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.