Abstract
The aim of this study was to evaluate the potential of microemulsions as a drug vehicle for transdermal delivery of citalopram. A computerized statistical technique of response surface methodology with mixture design was used to investigate and optimize the influence of the formulation compositions including a mixture of Brij 30/Brij 35 surfactants (at a ratio of 4:1, 20%–30%), isopropyl alcohol (20%–30%), and distilled water (40%–50%) on the properties of the drug-loaded microemulsions, including permeation rate (flux) and lag time. When microemulsions were used as a vehicle, the drug permeation rate increased significantly and the lag time shortened significantly when compared with the aqueous control of 40% isopropyl alcohol solution containing 3% citalopram, demonstrating that microemulsions are a promising vehicle for transdermal application. With regard to the pharmacokinetic parameters of citalopram, the flux required for the transdermal delivery system was about 1280 μg per hour. The microemulsions loaded with citalopram 3% and 10% showed respective flux rates of 179.6 μg/cm2 and 513.8 μg/cm2 per hour, indicating that the study formulation could provide effective therapeutic concentrations over a practical application area. The animal study showed that the optimized formulation (F15) containing 3% citalopram with an application area of 3.46 cm2 is able to reach a minimum effective therapeutic concentration with no erythematous reaction.
Keywords: citalopram, microemulsion, transdermal delivery systems
Introduction
Citalopram (1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydro-5-isobenzofurancar bonitrile, C20H21FN2O) is a selective serotonin re-uptake inhibitor with a molecular weight of 324.40 g/mol and a pKa of 9.59. It was approved by the US Food and Drug Administration in 1998 to treat the symptoms of major depression.1–3 In the clinical setting, citalopram is used alone or in combination with other antidepressant and/or antipsychotic drugs to treat a variety of psychiatric disorders.4 Citalopram is available as 10 mg, 20 mg, and 40 mg tablets. The recommended starting dose is 20 mg/day, with a maximum dose of 40 mg/day. After oral administration, the most common gastrointestinal side effects are nausea (21%) and xerostomia (20%). The nausea is caused when the 5HT3 receptors are activated by serotonin, when the receptor is exhibited in the digestive tract. 5HT3 receptors also stimulate vomiting, and the dose administered must be adjusted when this occurs.5,6 There has been a recent clinical report of subacute cutaneous lupus erythematosus caused by oral citalopram, highlighting that the indication for and dose of citalopram must be monitored carefully.7
There has been little research to date on transdermal antidepressants. However, several reports suggest the potential application and advantages of antidepressants administered via the transdermal route.8–10 A transdermal drug delivery system able to bypass the gastrointestinal tract may be a suitable administration route for citalopram to avoid gastrointestinal side effects. Moreover, citalopram has a low molecular weight and the daily dose needed is small, which makes it a promising candidate for transdermal delivery. The stratum corneum is a major obstacle in the transport of a foreign compound in a transdermal delivery system. In the past decade, numerous strategies have been investigated for their ability to increase drug permeation capacity through the skin, including vehicles, chemical penetration enhancers, iontophoresis, electroporation, ultrasound, and microneedle technologies, used alone or in combination.11–15 In recent years, ethosomes, microemulsions, liposomes, and polymeric nanoparticles have shown potential as drug vehicles in modifying the properties of therapeutic agents and in improving their transport through the skin.16–21
Microemulsions are optically isotropic and thermodynamically stable colloidal systems. Droplet size is typically in the range of 10–100 nm.22–24 They are typically composed of an aqueous phase, oil phase, surfactant, and cosurfactant. Microemulsions can be prepared by spontaneous emulsification, which offers several advantages over other drug vehicles, such as liposome and polymeric nanoparticles, including lower preparation costs, high drug loading, no need for an organic solvent, and a long shelf-life for both hydrophilic and lipophilic therapeutic agents.16–25 Numerous studies have demonstrated that microemulsions can improve drug transport through the skin compared with conventional topical preparations, such as gels, creams, and ointments.26–29 Hence, microemulsions were used as the vehicle for transdermal delivery of citalopram in the present study.
Obtaining an optimal transdermal formulation with the appropriate properties of a high permeation rate, a shortened lag time, and minimal requirement for experimental investigation is an important issue in the development of a pharmaceutical formulation. A statistical optimization tool based on response surface methodology and experimental formulations, such as central composite, Box-Behnken, factorial, and mixture designs,16,30–36 has been used widely, because it can evaluate the main effects and interaction of all variables simultaneously. Hence, response surface methodology with a constrained mixture design was used to develop an optimal citalopram-loaded microemulsion for topical application, and the effect of composition of the microemulsion on the ability of citalopram to permeate rat skin was investigated.
Materials and methods
Materials
Citalopram hydrobromide was purchased from Arch Pharmalabs Ltd (Mumbai, India). Verapamil chloride was purchased from Sigma-Aldrich (St Louis, MO, USA). Polyoxyl 23 lauryl ether (Brij 35) and polyoxyl 4 lauryl ether (Brij 30) were sourced from Acros Organics (Fair Lawn, NJ, USA). Isopropyl myristate and isopropyl alcohol were purchased from Merck Chemicals (Darmstadt, Germany). All other chemicals and solvents were of analytical reagent grade.
Construction of phase diagrams
Phase diagrams were constructed using the water titration method to obtain the level range of each component for the existence range of microemulsions. A surfactant mixture of Brij 30/Brij 35 at a ratio of 4:1, isopropyl myristate, and aqueous solution containing 40% cosurfactant including isopropyl alcohol, propylene glycol, and poly(ethylene glycol) (PEG) 400 were used. Mixtures of oil phase and the mixed surfactants were diluted at defined weight ratios (1/9, 2/8, 3/7, 4/6, 5/5, 6/4, 7/3, 8/2, 9/1) with aqueous phase dropwise under agitation until the mixture became clear. The amounts of components used were recorded to complete the phase diagrams.
Preparation of citalopram-loaded microemulsions
A constrained mixture design37 consisting of three independent variables was used in this study to prepare model citalopram-loaded microemulsions. The oil phase was fixed at 10% because citalopram is a hydrophilic compound. The effect of the amount of oil on drug solubility in the microemulsion was negligible. The amounts of mixed surfactants, isopropyl alcohol, and double-distilled water were used as independent variables. The range of independent variables was selected according to the phase diagrams. The total amount of the varying excipients was maintained at 90% of the total amount of the formulation. Based on the constrained mixture model (Design-Expert® software, Stat-Ease Corporation, Minneapolis, MN, USA), 14 citalopram-loaded model microemulsions were arranged randomly. The compositions of these citalopram-loaded microemulsions are listed in Table 1. The mixed Brij 30/Brij 35 surfactant was prepared in advance at a ratio of 4:1. The isopropyl myristate and aqueous phases containing cosurfactant were incorporated into the mixed surfactant in order, and shaken for one minute at each step. All microemulsions became transparent. Citalopram 3%–10% was dissolved in the final microemulsion formulations. No precipitation was observed.
Table 1.
Composition, physiochemical properties, and permeability parameters of citalopram-loaded model microemulsions
| X1 | X2 | X3 | Size (nm) | PI | Viscosity (cps × 103) | Flux(μg/cm2/h) | LT (h) | |
|---|---|---|---|---|---|---|---|---|
| F01 | 23.3 | 23.3 | 43.3 | 13.9 ± 0.7 | 0.39 ± 0.18 | 10.23 ± 0.06 | 163.8 ± 4.5 | 1.3 ± 0.6 |
| F02 | 21.7 | 26.7 | 41.7 | 16.0 ± 2.0 | 0.38 ± 0.13 | 8.9 ± 0.07 | 161.5 ± 6.8 | 1.0 ± 0.0 |
| F03 | 20.0 | 30.0 | 40.0 | 16.5 ± 0.1 | 0.47 ± 0.04 | 7.53 ± 0.06 | 165.0 ± 20.7 | 0.8 ± 0.3 |
| F04 | 25.0 | 25.0 | 40.0 | 38.0 ± 10.5 | 0.43 ± 0.10 | 9.80 ± 0.10 | 172.1 ± 14.1 | 2.0 ± 0.0 |
| F05 | 20.0 | 20.0 | 50.0 | 19.9 ± 3.7 | 0.30 ± 0.15 | 10.73 ± 0.12 | 153.8 ± 16.1 | 1.2 ± 0.8 |
| F06 | 20.0 | 30.0 | 40.0 | 17.3 ± 2.4 | 0.43 ± 0.12 | 7.08 ± 0.07 | 179.8 ± 11.8 | 1.5 ± 0.9 |
| F07 | 20.0 | 20.0 | 50.0 | 17.7 ± 3.2 | 0.33 ± 0.18 | 10.17 ± 0.06 | 179.8 ± 18.1 | 1.7 ± 0.6 |
| F08 | 25.0 | 25.0 | 40.0 | 20.8 ± 6.4 | 0.36 ± 0.09 | 9.22 ± 0.04 | 176.8 ± 15.9 | 1.7 ± 0.6 |
| F09 | 30.0 | 20.0 | 40.0 | 18.5 ± 10.7 | 0.44 ± 0.10 | 13.07 ± 0.06 | 191.8 ± 14.6 | 2.7 ± 0.6 |
| F10 | 21.7 | 21.7 | 46.7 | 14.5 ± 0.5 | 0.33 ± 0.01 | 10.23 ± 0.06 | 188.6 ± 13.4 | 2.3 ± 0.6 |
| F11 | 30.0 | 20.0 | 40.0 | 32.1 ± 5.5 | 0.34 ± 0.05 | 12.70 ± 0.00 | 216.1 ± 11.0 | 2.7 ± 0.6 |
| F12 | 25.0 | 20.0 | 45.0 | 13.5 ± 0.2 | 0.45 ± 0.09 | 12.00 ± 0.10 | 211.0 ± 8.1 | 3.0 ± 0.0 |
| F13 | 26.7 | 21.7 | 41.7 | 13.2 ± 0.1 | 0.45 ± 0.09 | 11.30 ± 0.10 | 221.5 ± 8.9 | 2.7 ± 0.6 |
| F14 | 20.0 | 25.0 | 45.0 | 13.2 ± 0.1 | 0.36 ± 0.14 | 9.52 ± 0.10 | 245.6 ± 14.0 | 2.7 ± 0.6 |
Notes: Amount of citalopram and isopropyl myristate was fixed at 300 mg and 1.0 g, respectively; total amount of three variables of X1 (Brij 30/Brij 35 = 4/1, 20%–30%), X2 (isopropyl alcohol 20%–30%), and X3 (distilled water, 40%–50%) was 9.0 g X1 + X2 + X3 = 1.
Abbreviations: LT, lag time; PI, polydispersity index.
Characterization of microemulsions
A cone-plate viscometer (Model LVDV-II, Brookfield Engineering Laboratories, Middleboro, MA, USA) was used to determine the viscosity of the citalopram-loaded microemulsions. Five milliliters of sample was loaded into the plate, and the temperature was maintained at 37°C for 3 minutes using a thermostatic water pump. The rotation rate was set at 120 rpm. Readings were taken 20 seconds after measurement was started. Each datum point represents the average of three determinations.
A photocorrelation spectroscope equipped with laser light scattering (Zetasizer 3000HSA, Malvern Instruments, Malvern, UK) was used to measure the droplet sizes in the citalopram-loaded microemulsions. The helium-neon laser was set at 633 nm. A 50 μL sample was diluted in distilled water to 3 mL, loaded into a cuvette, and placed in the scattering chamber at room temperature. Light scattering was set at a fixed angle of 90°.
Skin permeation study
The ability of citalopram in the various microemulsions to permeate skin was determined using a modified Franz diffusion cell. Abdominal skin excised from a Wistar rat (275–300 g) was mounted between the receptor cell and donor cell with the stratum corneum facing upwards. The effective diffusion area of the cell was 3.46 cm2. The donor cell was loaded with 1 mL samples of the citalopram microemulsions and occluded by Parafilm® (Pechiney Plastic Packaging Company Chicago, IL, USA). The receptor cell was filled with 20 mL of phosphate buffer (0.05 M, pH 7.4), and its temperature was maintained at 37°C ± 0.5°C during the experiment. At specific intervals, 500 μL of receptor medium was withdrawn and analyzed using high-pressure liquid chromatography38 All experiments were repeated three times and averaged. The skin permeation protocol was approved by the Institutional Animal Care and Use Committee of Kaohsiung Medical University. The committee confirmed that the permeation experiment followed the guidelines set out by the Guide for Laboratory Fact Lines and Care.
In vivo animal study
Male Wistar rats weighing 250–275 g were anesthetized throughout the study using urethane 25% (Sigma-Aldrich) given as an intraperitoneal injection (0.33 μL per mg of rat body weight before the study). Glass cylinders, each with an available area of 3.46 cm2, were glued onto rat abdominal skin. Next, 1 mL of each microemulsion formulation was added to a cylinder. Blood samples (0.5 mL) were drawn from the jugular vein at each sampling time. The methods used to prepare the samples were modified from those used in a previous study,39 ie, a 150 μL plasma sample was added to 50 μL of verapamil as the internal standard and vortexed for 2 minutes, then added to 600 μL of methanol and vortexed for a further 2 minutes. The mixtures were centrifuged at 2500 g for 5 minutes to spin down the protein aggregates. The supernatants were then analyzed by high-pressure liquid chromatography.
Skin erythema study
Glass cylinders with an available area of 2.42 cm2 were glued onto rat abdominal skin, and a 1 mL sample of each microemulsion formulations was added to each cylinder. The formulation were moved at the sampling point and the rat skins were examined using a chroma meter (MiniScan® XE Plus, HunterLab, Reston, VA, USA). The color reflectance of the rat skin was recorded as Commission Internationale de l’Eclairage (CIE) in three-dimensions (L* for a*, b*). A defined area near the treated site was designated as the control.40
Chromatographic conditions
An L-7100 series high-pressure liquid chromatography system (Hitachi, Tokyo, Japan) and a Supelco Discovery® Bio Wide Pore C5 column (150 × 4.6 mm ID, particle size 5 μm, Sigma-Aldrich) were used for analysis of the citalopram concentration and modified from a previously reported method.3 A mixture of 0.025 M potassium dihydrogen phosphate and acetonitrile at a ratio of 60:40 (v/v) was used as the mobile phase. The flow rate was set at 1 mL per minute. The detection wavelength was set at 237 nm. Verapamil 300 μg/mL was used as the internal standard. The citalopram concentration ranged from 5 μg/mL to 1000 μg/mL with linearity (r2 = 0.9993). The limit of quantitation was 1 μg/mL. For the in vivo animal study, the plasma concentration was measured by fluorescence detection modified from a previous method.39 An L-2480 series fluorescence detector (Hitachi) and a Supelco Discovery Bio Wide Pore C5 column (Sigma-Aldrich) were used. The excitation wavelength was 249 nm, and the emission wavelength was 302 nm. The in vivo concentration of citalopram ranged from 5 ng/mL to 500 ng/mL with linearity (r2 = 0.9999). The limit of quantitation was 1 ng/mL.
Data analysis
The cumulative amount of citalopram transported across rat skin was plotted as a function of time. The slopes of the resulting linear plots in the steady state were calculated and the permeation rate (flux) was determined from the slope. Time to first detection of the drug was selected as the lag time. The independent variables (X1, X2, and X3) and responses (flux and lag time) of all model citalopram-loaded microemulsions were analyzed using Design-Expert® software (Stat-Ease Corporation). Polynomial linear, quadratic, and cubic equations were used to show the relationship between the independent variables and responses. Statistical parameters, including the multiple correlation coefficient (R2), adjusted multiple correlation coefficient (adjusted R2), coefficient of variation, P value for the model (<0.05), and P value for lack of fit (>0.05) validated by Design-Expert software (Stat-Ease Corporation) were used to select the model equation with best fit.
Results and discussion
Pseudoternary phase diagrams
The excipients used to prepare the microemulsions were pharmaceutically accepted ingredients. Isopropyl myristate is used widely as a nongreasy emollient and emulsifying agent in topical cosmetic and pharmaceutical products.41 Moreover, it shows a permeation enhancement effect,42 so it was used as the oil phase. Brij 30 and Brij 35 are nonionic surfactants with reliable biological compatibility, and are also widely used in topical pharmaceutical and cosmetic products.41 The hydrophilic-lipophilic balance required for isopropyl myristate was about 11.1. Therefore, the mixed Brij 30/Brij 35 surfactant was used at a ratio of 4:1 to give a blended hydrophilic-lipophilic balance of about 11.1. Previous studies have reported that a short-chain alkanol cosurfactant can be incorporated into the interfacial layer, resulting in reduced interfacial energy and tension. Cosurfactants can also modify the hydrophilic-lipophilic balance of the surfactant to an appropriate value suitable for formation of a microemulsion. Addition of a cosurfactant can decrease the amount of surfactant needed in a microemulsion.42,43 The effect of a cosurfactant depends on its chain length; only appropriate chain lengths are suitable for good formation of a microemulsion.44 Therefore, three kinds of alkanol, ie, isopropyl alcohol, propylene glycol, and PEG 400, were used in this study to evaluate the effect of cosurfactants on the formation of microemulsions. Pseudoternary phase diagrams were constructed to determine the appropriate compositions of the microemulsions and to evaluate the effect of the cosurfactants. As shown in Figure 1, larger microemulsion regions were obtained when the microemulsion was prepared with isopropyl alcohol. A large microemulsion region gives the investigator the flexibility to vary the ingredients to obtain an appropriate formulation with low surfactant and cosurfactant concentrations and a higher permeation rate. Hence, isopropyl alcohol was used subsequently.
Figure 1.
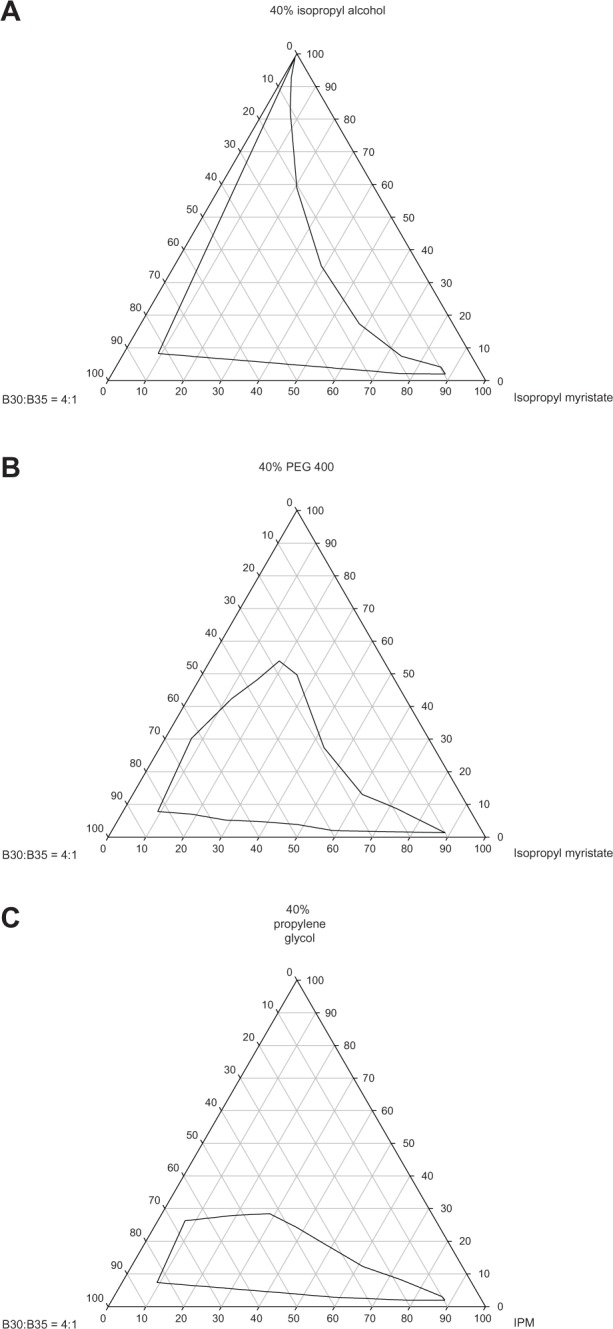
Pseudoternary phase of microemulsion composed of isopropyl myristate, mixed Brij 30/Brij 35 surfactant and aqueous solution containing 40% different cosurfactants. Isopropyl alcohol (A), poly(ethylene glycol) (PEG) 400 (B), and propylene glycol (C).
Abbreviation: IPM, isopropyl myristate.
Based on the pseudoternary phase diagrams, the quantitative measures of the independent variables, ie, X1, X2, and X3, were set at 25%–30%, 25%–30%, and 45%–60%, respectively. Various citalopram-loaded microemulsion formulations were prepared according to the constrained mixture design, and their physicochemical properties and permeation parameters were determined.
Physicochemical characteristics of microemulsions
Droplet size, polydispersity index, and viscosity are shown in Table 1. The droplet size of the model citalopram-loaded microemulsions ranged from 13.2 nm to 38.0 nm. The polydispersity index ranged from 0.30 to 0.47. All measurements below 0.5 indicated narrow deviation from the average size. The viscosity of the citalopram-loaded microemulsions ranged from 7.08 to 13.07 × 103 cps at 37°C. Our results demonstrate that the combinations in the formulation had a predominant effect on the physicochemical properties of the microemulsions. All the drug-loaded microemulsions remained clear during the in vitro experimental period and at room temperature for more than one year.
Skin permeation study
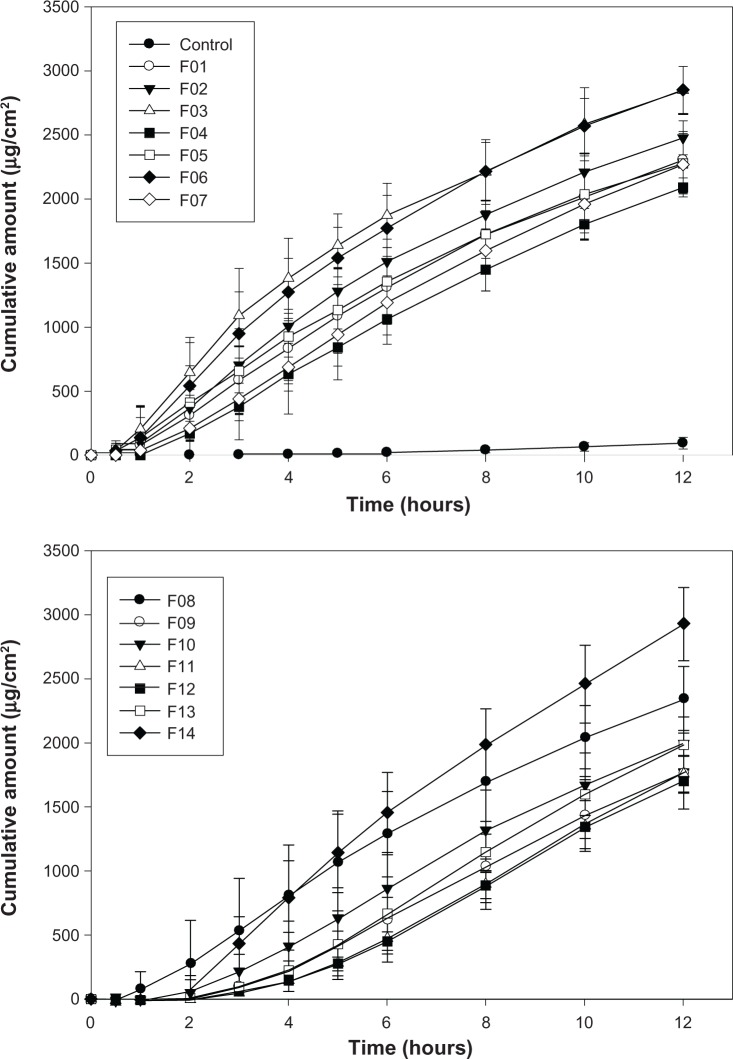
The influence of the ingredients in the microemulsions, including the surfactant, cosurfactant, and water mixture on drug penetration capacity was investigated by response surface methodology. Fourteen 3% citalopram-loaded model microemulsions were prepared and the permeation parameters were determined by in vitro permeation study. An aqueous solution of 40% isopropyl alcohol containing 3% citalopram was used as a control to evaluate the enhancement effect of the microemulsions.
The permeation profiles for the model microemulsions and the aqueous control are plotted in Figure 2. Zero-order release kinetics were suitable to fit the curves for all formulations (R2 > 0.9920). The flux (μg/cm2 per hour) was calculated. The permeation parameters for all formulations are summarized in Table 1. The flux of the microemulsions ranged from 153.77 μg/cm2 to 245.62 μg/cm2 per hour, while the lag time ranged from 0.83 hours to 3.0 hours. The wide deviations demonstrate that the permeability of citalopram from the microemulsions was significantly influenced by the composition, ie, proportions of surfactant, cosurfactant, and water in the mixture. The flux and lag time of the aqueous control were 24.2 ± 2.5 μg/cm2 per hour and 6.2 ± 0.8 hours, respectively.
Figure 2.
In vitro penetration-time profile of model citalopram formulations and aqueous control of 40% isopropyl alcohol containing 3% citalopram through rat skin (n = 3).
When the microemulsion was used as a vehicle, the drug flux increased by 6.4–10.1-fold and the lag time shortened from 6.2 hours to 0.8–3.0 hours, as compared with the aqueous 40% isopropyl alcohol solution. These results were in agreement with previous studies45–50 reporting that microemulsion systems can increase transdermal drug transport. The mechanism might be attributed to the drug activity in the formulation being promoted by the combined effect of the hydrophilic and lipophilic components of the microemulsion. Further, microemulsions can decrease the interface tension between vehicle and skin, leading to faster permeation.29,51
To estimate the quantitative effects of the formulation factor on the permeation parameters of citalopram-loaded microemulsions and to obtain the appropriate formulation, the data collected for responses (flux and lag time) in the model microemulsions were analyzed statistically using response surface methodology and Design-Expert software. The (Stat-Ease Corporation) mathematical polynomial equation to describe the flux may be written as:
The P value for the model equation was less than 0.05, showing that the model is suitable for describing the relationship between the independent variables and the response. The P value for lack of ft was 0.2331, showing no indication of significance, which further confirmed the satisfactory fitness of the model. The coefficients for the X term conveyed the intensity of effect of the independent variables.
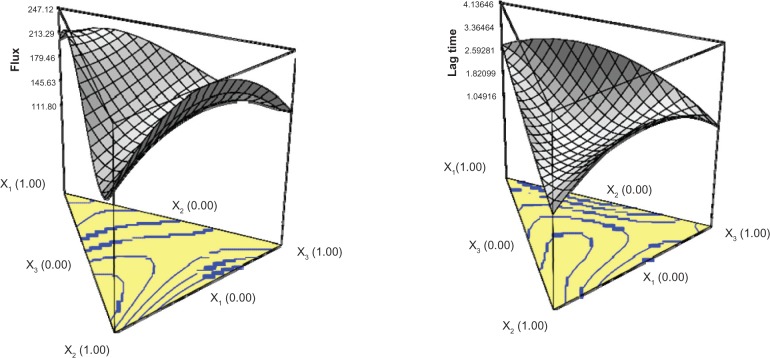
Coefficients with more than one factor term or higher order term in the regression model polynomial equation represent interaction or quadratic relationships, respectively. The interaction of the three factors showed the highest effect on drug permeation. In the main factor, the surfactant (X1) showed the greatest effect, followed by the cosurfactant (X2) and aqueous phase (X3). The response surface plot was constructed to illustrate the relationship between the independent and dependent variables used (Figure 3). Higher flux was observed in microemulsions with specific ratios of surfactant/cosurfactant/aqueous phase (eg, X1 at a low level, and X2 and X3 at a medium level). Surfactants can increase membrane fluidity, drug solubilization, and extraction of lipid from the stratum corneum, resulting in better permeation.52 With addition of excess surfactant, the thermodynamic activity of the drug in microemulsion may decrease, leading to decreased drug transport.53
Figure 3.
Three-dimensional response surface plots illustrating the effect of surfactant (X1), isopropyl alcohol (X2), and distilled water (X3) on the flux and lag time of citalopram from microemulsions.
The polynomial equation to depict lag time may be written as:
The P values of the model and lack of fit were <0.0001 and 0.1218, respectively, indicating that the selected equation can depict the relationship between formulation factors and lag time. The polynomial equation demonstrated that interaction of the three factors had the most significant effect. The surface response plot is shown in Figure 3. It was found that microemulsions with X1 and X3 at low levels and X2 at high levels had a shortened lag time.
Appropriate transdermal microemulsions should have higher flux and shortened lag time simultaneously. The response surface methodology predicted an appropriate formulation with flux and lag time values of 180.23 μg/cm2 per hour and 1.60 hours, respectively, when X1, X2, and X3 values were 0.24, 0.23, and 0.43, respectively. A new drug-loaded microemulsion (F15) was prepared according to these levels of formulation factors to obtain flux and lag time values of 179.63 ± 20.44 μg/cm2 per hour and 1.67 ± 0.58 hours, respectively. Observed and predicted values showed no significant difference, indicating that response surface methodology is a potential tool for designing citalopram-loaded microemulsions.
In order to increase flux of microemulsion (F15) further, the loading dose was increased from 3% to 10%. As shown in Figure 4, the flux increased with increasing drug concentration. The flux levels were 179.6 ± 20.4, 308.4 ± 29.1, 427.3 ± 38.1, and 513.8 ± 52.8 μg/cm2 per hour for 3%, 5%, 7%, and 10% citalopram-loaded microemulsions, respectively, showing a linear relationship over the loading dose range of 3%–10% (R2 > 0.9817).
Figure 4.
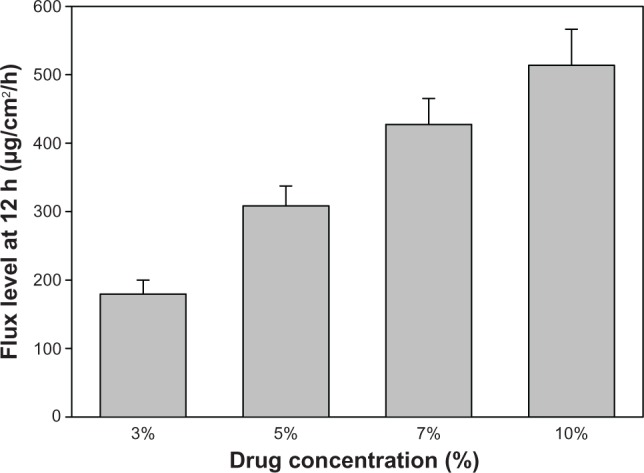
Various concentrations of citalopram in microemulsion (F15) on flux level per unit area (μg/cm2 per hour) from time zero to 12 hours (n = 3).
Previous studies1,2 reported that the Cmax was 34.42–46.2 ng/mL, and the total body clearance of citalopram was about 0.457 L/kg per hour after oral administration of a 40 mg dose. Thus, if the transdermal permeation of human skin for citalopram from the microemulsion (F15) is similar to that of rat skin, the flux required from the citalopram-loaded microemulsions is about 1280 μg per hour. The area of administration required for the citalopram-loaded microemulsion to obtain a minimum effective therapeutic concentration was 7.1, 4.2, 3.0, and 2.5 cm2, respectively, and these values are in the practical range for topical application.
In vivo animal study
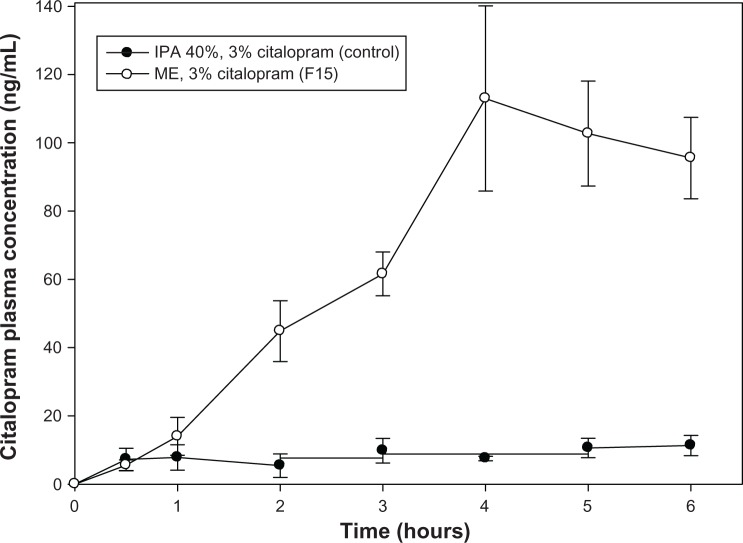
The in vivo transdermal study result is shown in Figure 5, indicating that the predicted optimized formulation (F15) provided an area under the concentration-time curve (AUC0–6) of about 402.59 ± 74.39 ng ⋅ hr/mL, which is 10-fold higher than for the controlled formulation (isopropyl alcohol 40%, 39.40 ± 10.10 ng ⋅ hr/mL). The Cmax for F15 is 112.97 ng/mL and the Tmax is at 4 hours. F15 reached the minimum effective therapeutic concentration (44.84 ± 15.45 ng/mL) in less than 2 hours after transdermal administration, indicating potentially adequate efficacy for the treatment of depression in the future.
Figure 5.
Plasma concentration-time curve for citalopram in rats after transdermal administration of 3% citalopram microemulsion (F15).
Note: Each sample point represents the mean of three experiments (mean ± standard error of the mean).
Abbreviations: IPA, isopropyl alcohol; ME, microemulsion.
Skin irritation study
The irritation test was estimated by the changing level in L* (white-black index) and a* (red-green index, Table 2). Both microemulsion formulations (containing 0% or 3% of citalopram) showed no significant difference in L* or a* at 6 hours, meaning that there is no erythematous reaction even after the formulation reaches the minimum effective therapeutic concentration for citalopram.40,54,55
Table 2.
Color difference in rat skin treated with microemulsions containing different amounts of citalopram at different time points as measured by colorimetry
| Control | 2 hours
|
6 hours
|
|||
|---|---|---|---|---|---|
| Citalopram 0% | Citalopram 3% | Citalopram 0% | Citalopram 3% | ||
| L* | 66.96 ± 2.32 | 62.95 ± 2.06 | 68.72 ± 0.95 | 62.40 ± 0.80 | 67.62 ± 0.94 |
| a* | 4.65 ± 0.88 | 7.87 ± 2.74 | 5.44 ± 0.85 | 8.56 ± 1.93 | 6.65 ± 1.92 |
| b* | 1.62 ± 1.53 | 2.31 ± 0.36 | −1.74 ± 1.03 | 1.35 ± 0.64 | −2.27 ± 0.56 |
Notes: Control, untreated rat skin site. L*, white-black index; a*, red-green index; b*, yellow-blue index. All four groups of formulations showed no significant difference in L* (white-black index) or a* (red-green index) compared with untreated skin near attachment site. All data represent the mean of three experiments.
Conclusion
The permeation rate of citalopram from microemulsions was conspicuously increased and the lag times were also shortened. The compositional proportions in the microemulsion are important in determining the efficacy of the microemulsion as a drug transdermal delivery system. Response surface methodology is a potential tool for investigating the influence of independent variables on dependent variables (responses) and for obtaining an appropriate formulation. The flux of experimental citalopram-loaded microemulsions is expected to provide adequate therapeutic efficacy in a workable application area. Throughout the in vivo study, the formulation was able to reach therapeutic plasma levels, and is worthy of investigation as a transdermal antidepressant drug delivery system.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Gutierrez MM, Abramowitz W. Pharmacokinetic comparison of oral solution and tablet formulations of citalopram: a single-dose, randomized, crossover study. Clin Ther. 2000;22(12):1525–1532. doi: 10.1016/s0149-2918(00)83050-7. [DOI] [PubMed] [Google Scholar]
- 2.Mendoza L, Hajduch M, Kekulova H, Svobodova X, Mihal V, Svoboda M. Bioequivalence of two brands of citalopram 40 mg tablets after single oral administration to healthy volunteers. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149(1):169–172. [PubMed] [Google Scholar]
- 3.Suresh PS, Giri S, Husain R, Mullangi R. A highly sensitive LC-MS/MS method for the determination of S-citalopram in rat plasma: application to a pharmacokinetic study in rats. Biomed Chromatogr. 2010;24(10):1052–1058. doi: 10.1002/bmc.1405. [DOI] [PubMed] [Google Scholar]
- 4.Milne RJ, Goa KL. Citalopram. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in depressive illness. Drugs. 1991;41(3):450–477. doi: 10.2165/00003495-199141030-00008. [DOI] [PubMed] [Google Scholar]
- 5.Rang HP. Pharmacology. Edinburgh, UK: Churchill Livingstone; 2003. [Google Scholar]
- 6.Uptodate. 2012. http://www.uptodate.com/contents/citalopram-drug-information
- 7.Rohrs S, Geiser F, Conrad R. Citalopram-induced subacute cutaneous lupus erythematosus – first case and review concerning photosensitivity in selective serotonin reuptake inhibitors. Gen Hosp Psychiatry. 2012;34(5):541–545. doi: 10.1016/j.genhosppsych.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Vandenberg CM. MAOIs and transdermal delivery. J Clin Psychiatry. 2012;73(9):e28. doi: 10.4088/JCP.11096tx6c. [DOI] [PubMed] [Google Scholar]
- 9.VanDenBerg CM. The transdermal delivery system of monoamine oxidase inhibitors. J Clin Psychiatry. 2012;73(Suppl 1):25–30. doi: 10.4088/JCP.11096su1c.04. [DOI] [PubMed] [Google Scholar]
- 10.Patkar AA, Pae CU, Zarzar M. Transdermal selegiline. Drugs Today (Barc) 2007;43(6):361–377. doi: 10.1358/dot.2006.43.6.1050794. [DOI] [PubMed] [Google Scholar]
- 11.Kim HM, Lim YY, An JH, Kim MN, Kim BJ. Transdermal drug delivery using disk microneedle rollers in a hairless rat model. Int J Dermatol. 2012;51(7):859–863. doi: 10.1111/j.1365-4632.2011.05343.x. [DOI] [PubMed] [Google Scholar]
- 12.Nair A, Vyas H, Shah J, Kumar A. Effect of permeation enhancers on the iontophoretic transport of metoprolol tartrate and the drug retention in skin. Drug Deliv. 2011;18(1):19–25. doi: 10.3109/10717544.2010.509361. [DOI] [PubMed] [Google Scholar]
- 13.Ryan E, Garland MJ, Singh TR, et al. Microneedle-mediated transdermal bacteriophage delivery. Eur J Pharm Sci. 2012;47(2):297–304. doi: 10.1016/j.ejps.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai YH, Chang JT, Huang CT, Chang JS, Huang YB, Wu PC. Electrically-assisted skin delivery of buspirone submicron emulsions. J Food Drug Anal. 2012;20:22–26. [Google Scholar]
- 15.Vaghani SS, Gurjar M, Singh S, et al. Effect of iontophoresis and permeation enhancers on the permeation of an acyclovir gel. Curr Drug Deliv. 2010;7(4):329–333. doi: 10.2174/156720110793360603. [DOI] [PubMed] [Google Scholar]
- 16.Azeem A, Ahmad FJ, Khar RK, Talegaonkar S. Nanocarrier for the transdermal delivery of an antiparkinsonian drug. AAPS PharmSciTech. 2009;10(4):1093–1103. doi: 10.1208/s12249-009-9306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Wu Q, Zhang Z, Yuan L, Liu X, Zhou L. Preparation of curcumin-loaded liposomes and evaluation of their skin permeation and pharmacodynamics. Molecules. 2012;17(5):5972–5987. doi: 10.3390/molecules17055972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang JY, Leu YL, Chang CC, Lin CH, Tsai YH. Lipid nano/submicron emulsions as vehicles for topical flurbiprofen delivery. Drug Deliv. 2004;11(2):97–105. doi: 10.1080/10717540490280697. [DOI] [PubMed] [Google Scholar]
- 19.Fang YP, Huang YB, Wu PC, Tsai YH. Topical delivery of 5-aminolevulinic acid-encapsulated ethosomes in a hyperproliferative skin animal model using the CLSM technique to evaluate the penetration behavior. Eur J Pharm Biopharm. 2009;73(3):391–398. doi: 10.1016/j.ejpb.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Shi J, Ma F, Wang X, Wang F, Liao H. Formulation of liposomes gels of paeonol for transdermal drug delivery by Box-Behnken statistical design. J Liposome Res. 2012;22(4):270–278. doi: 10.3109/08982104.2012.690159. [DOI] [PubMed] [Google Scholar]
- 21.Zhu W, Yu A, Wang W, Dong R, Wu J, Zhai G. Formulation design of microemulsion for dermal delivery of penciclovir. Int J Pharm. 2008;360(1–2):184–190. doi: 10.1016/j.ijpharm.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Kim BS, Won M, Lee KM, Kim CS. In vitro permeation studies of nanoemulsions containing ketoprofen as a model drug. Drug Deliv. 2008;15(7):465–469. doi: 10.1080/10717540802328599. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Dong J, Chen J, Eastoe J, Li X. Design and optimization of a new self nanoemulsifying drug delivery system. J Colloid Interface Sci. 2009;330(2):443–448. doi: 10.1016/j.jcis.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 24.Wu H, Ramachandran C, Weiner ND, Roessler BJ. Topical transport of hydrophilic compounds using water-in-oil nanoemulsions. Int J Pharm. 2001;220(1–2):63–75. doi: 10.1016/s0378-5173(01)00671-8. [DOI] [PubMed] [Google Scholar]
- 25.Heuschkel S, Goebel A, Neubert RH. Microemulsions – modern colloidal carrier for dermal and transdermal drug delivery. J Pharm Sci. 2008;97(2):603–631. doi: 10.1002/jps.20995. [DOI] [PubMed] [Google Scholar]
- 26.Abramovic Z, Sustarsic U, Teskac K, Sentjurc M, Kristl J. Influence of nanosized delivery systems with benzyl nicotinate and penetration enhancers on skin oxygenation. Int J Pharm. 2008;359(1–2):220–227. doi: 10.1016/j.ijpharm.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Bolzinger MA, Briancon S, Pelletier J, Fessi H, Chevalier Y. Percutaneous release of caffeine from microemulsion, emulsion and gel dosage forms. Eur J Pharm Biopharm. 2008;68(2):446–451. doi: 10.1016/j.ejpb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Fini A, Bergamante V, Ceschel GC, Ronchi C, De Moraes CA. Control of transdermal permeation of hydrocortisone acetate from hydrophilic and lipophilic formulations. AAPS PharmSciTech. 2008;9(3):762–768. doi: 10.1208/s12249-008-9107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teichmann A, Heuschkel S, Jacobi U, et al. Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur J Pharm Biopharm. 2007;67(3):699–706. doi: 10.1016/j.ejpb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Aktas E, Eroglu H, Kockan U, Oner L. Systematic development of pH-independent controlled release tablets of carvedilol using central composite design and artificial neural networks. Drug Dev Ind Pharm. 2012 Jul 18; doi: 10.3109/03639045.2012.705291. Epub 2012 July 18. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhary H, Kohli K, Amin S, Rathee P, Kumar V. Optimization and formulation design of gels of diclofenac and curcumin for transdermal drug delivery by Box-Behnken statistical design. J Pharm Sci. 2011;100(2):580–593. doi: 10.1002/jps.22292. [DOI] [PubMed] [Google Scholar]
- 32.Gannu R, Palem CR, Yamsani SK, Yamsani VV, Yamsani MR. Enhanced bioavailability of buspirone from reservoir-based transdermal therapeutic system, optimization of formulation employing Box-Behnken statistical design. AAPS PharmSciTech. 2010;11(2):976–985. doi: 10.1208/s12249-010-9451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao J, Wang F, Wang X, et al. Development and optimization of baicalin-loaded solid lipid nanoparticles prepared by coacervation method using central composite design. Eur J Pharm Sci. 2012 Jul 20; doi: 10.1016/j.ejps.2012.07.006. Epub 2012 July 20. [DOI] [PubMed] [Google Scholar]
- 34.Makraduli L, Crcarevska MS, Geskovski N, Dodov MG, Goracinova K. Factorial design analysis and optimisation of alginate-Ca-chitosan microspheres. J Microencapsul. 2013;30(1):81–92. doi: 10.3109/02652048.2012.700957. [DOI] [PubMed] [Google Scholar]
- 35.Malzert-Freon A, Hennequin D, Rault S. Partial least squares analysis and mixture design for the study of the influence of composition variables on lipidic nanoparticle characteristics. J Pharm Sci. 2010;99(11):4603–4615. doi: 10.1002/jps.22177. [DOI] [PubMed] [Google Scholar]
- 36.Pabari RM, Ramtoola Z. Application of face centred central composite design to optimise compression force and tablet diameter for the formulation of mechanically strong and fast disintegrating orodispersible tablets. Int J Pharm. 2012;430(1–2):18–25. doi: 10.1016/j.ijpharm.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Lewis GA, Mathieu D, Phan-Tan-Luu R. Pharmaceutical Experimental Design. New York, NY: Dekker; 1999. [Google Scholar]
- 38.Macek J, Ptacek P, Klima J. Rapid determination of citalopram in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2001;755(1–2):279–285. doi: 10.1016/s0378-4347(01)00121-9. [DOI] [PubMed] [Google Scholar]
- 39.Mendoza L, Hajduch M, Kekulova H, Svobodova X, Mihal V, Svoboda M. Bioequivalence of two brands of citalopram 40 mg tablets after single oral administration to healthy volunteers. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005;149(1):169–172. [PubMed] [Google Scholar]
- 40.Fang JY, Tsai MJ, Huang YB, Wu PC, Tsai YH. Percutaneous absorption and skin erythema: quantification of capsaicin and its synthetic derivatives from gels incorporated with benzalkonium chloride by using non-invasive bioengineering methods. Drug Dev Res. 1997;40:56–67. [Google Scholar]
- 41.Kibbe AH. Handbook of Pharmaceutical Excipients. 3rd ed. London, UK: Pharmaceutical Press; 2000. [Google Scholar]
- 42.Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003;254(2):99–107. doi: 10.1016/s0378-5173(02)00632-4. [DOI] [PubMed] [Google Scholar]
- 43.El Maghraby GM. Transdermal delivery of hydrocortisone from eucalyptus oil microemulsion: effects of cosurfactants. Int J Pharm. 2008;355(1–2):285–292. doi: 10.1016/j.ijpharm.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Ho HO, Hsiao CC, Sheu MT. Preparation of microemulsions using polyglycerol fatty acid esters as surfactant for the delivery of protein drugs. J Pharm Sci. 1996;85(2):138–143. doi: 10.1021/js950352h. [DOI] [PubMed] [Google Scholar]
- 45.Bouchemal K, Briancon S, Perrier E, Fessi H. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280(1–2):241–251. doi: 10.1016/j.ijpharm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Lee PJ, Langer R, Shastri VP. Novel microemulsion enhancer formulation for simultaneous transdermal delivery of hydrophilic and hydrophobic drugs. Pharm Res. 2003;20(2):264–269. doi: 10.1023/a:1022283423116. [DOI] [PubMed] [Google Scholar]
- 47.Paolino D, Ventura CA, Nistico S, Puglisi G, Fresta M. Lecithin microemulsions for the topical administration of ketoprofen: percutaneous adsorption through human skin and in vivo human skin tolerability. Int J Pharm. 2002;244(1–2):21–31. doi: 10.1016/s0378-5173(02)00295-8. [DOI] [PubMed] [Google Scholar]
- 48.Tsai YH, Lee KF, Huang YB, Huang CT, Wu PC. In vitro permeation and in vivo whitening effect of topical hesperetin microemulsion delivery system. Int J Pharm. 2010;388(1–2):257–262. doi: 10.1016/j.ijpharm.2009.12.051. [DOI] [PubMed] [Google Scholar]
- 49.Wu H, Ramachandran C, Bielinska AU, et al. Topical transfection using plasmid DNA in a water-in-oil nanoemulsion. Int J Pharm. 2001;221(1–2):23–34. doi: 10.1016/s0378-5173(01)00672-x. [DOI] [PubMed] [Google Scholar]
- 50.Yuan Y, Li SM, Mo FK, Zhong DF. Investigation of microemulsion system for transdermal delivery of meloxicam. Int J Pharm. 2006;321(1–2):117–123. doi: 10.1016/j.ijpharm.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Schmalfuß U, Neubert R, Wohlrab W. Modification of drug penetration into human skin using microemulsions. J Control Release. 1997;46:279–285. [Google Scholar]
- 52.Rege BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci. 2002;16(4–5):237–246. doi: 10.1016/s0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 53.Rhee YS, Choi JG, Park ES, Chi SC. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 2001;228(1–2):161–170. doi: 10.1016/s0378-5173(01)00827-4. [DOI] [PubMed] [Google Scholar]
- 54.Hoffmann K, Auer T, Stucker M, Dirschka T, el-Gammal S, Altmeyer P. Evaluation of the efficacy of H1 blockers by noninvasive measurement techniques. Dermatology. 1994;189(2):146–151. doi: 10.1159/000246819. [DOI] [PubMed] [Google Scholar]
- 55.Serup J, Agner T. Colorimetric quantification of erythema – a comparison of two colorimeters (Lange Micro Color and Minolta Chroma Meter CR-200) with a clinical scoring scheme and laser-Doppler flowmetry. Clin Exp Dermatol. 1990;15(4):267–272. doi: 10.1111/j.1365-2230.1990.tb02087.x. [DOI] [PubMed] [Google Scholar]