Abstract
Clinical decision support (CDS) has been shown to improve clinical processes, promote patient safety, and reduce costs in healthcare settings, and it is now a requirement for clinicians as part of the Meaningful Use Regulation. However, most evidence for CDS has been evaluated primarily in internal medicine care settings, and colon and rectal surgery (CRS) has unique needs with CDS that are not frequently described in the literature. The authors reviewed published literature in informatics and medical journals, combined with expert opinion to define CDS, describe the evidence for CDS, outline the implementation process for CDS, and present applications of CDS in CRS.CDS functionalities such as order sets, documentation templates, and order facilitation aids are most often described in the literature and most likely to be beneficial in CRS. Further research is necessary to identify and better evaluate additional CDS systems in the setting of CRS.
Keywords: clinical decision support systems, colorectal surgery, electronic health records, patient safety
Objectives: On completion of this article, the reader should be able to define and provide examples of clinical decision support for colon and rectal surgery.
Following the two seminal and influential Institute of Medicine reports, “To Err Is Human”1 and “Crossing the Quality Chasm,”2 clinicians and healthcare organizations have been implementing electronic health records (EHRs) in an attempt to achieve the sought-after transformational breakthroughs to improve quality and service while simultaneously reducing costs. Further, early studies of computerized provider order entry (CPOE) with embedded clinical decision support (CDS) suggest that such systems could significantly reduce the rate of medication errors by as much as 81%.3,4,5,6,7,8,9 However, these transformations have been much harder to achieve than anyone expected.10,11 More recently the American Recovery and Reinvestment Act (ARRA) stimulus with its Health Information Technology for Economic and Clinical Health (HITECH) Act has significantly increased the pressure on healthcare providers to implement state of the art EHRs with at least a minimal amount of CDS with the Meaningful Use Regulation, led by the Centers for Medicare and Medicaid Services and the Department of Health and Human Services.12,13
Historically, the majority of CDS for patient care has been developed for internal medicine. The literature on CDS therefore is primarily focused on applications, interventions, and outcomes outside of the surgical subspecialty domain. Colon and rectal surgery (CRS) practice, however, has unique needs for health information technology (HIT) and CDS that may differ from what is traditionally described.14 Although many of the clinical examples described in the literature are not directly applicable to CRS, we nevertheless believe that CDS can be beneficial in CRS settings. In this article, we define CDS, describe the evidence for CDS, outline the implementation process for CDS, and present some potential applications of CDS in CRS.
Definition of Clinical Decision Support
Several definitions of CDS have been described in various manuscripts, textbooks, and regulations.15 One broad definition for CDS is “a process for enhancing health-related decisions and actions with pertinent, organized clinical knowledge and patient information to improve health and healthcare delivery.”16 That said, CDS is often best defined by examples. Some of the most familiar examples of CDS are drug-drug interaction alerts, computerized dosing reminders, and guideline-based order sets. These are tools, generally embedded in an EHR, to help clinicians make decisions or remind them of data or facts they may have forgotten or overlooked.
Several approaches for providing and classifying CDS exist. In particular, one recent report describes a taxonomy for CDS, providing definitions and examples of each CDS type.17 The major categories within the taxonomy include medication dosing support, order facilitators, point of care alerts or reminders, relevant information display, expert systems, and workflow support. We describe these categories in detail with several examples relevant to CRS below.
Evidence for Clinical Decision Support
Extensive literature has described the effect of several different types of CDS on patient, process, and cost outcomes.18,19,20,21,22 Most studies report benefits of CDS implementations on process outcomes; examples of these include appropriate ordering of medications, preventive therapies, and laboratory test result monitoring.20,23 A recent systematic review determined that the random-effects combined odds ratios for studies reporting adherence to recommendations for preventive services, ordering clinical studies, and prescribing appropriate treatments was 1.42, 1.72, and 1.575, respectively, confirming a positive effect of CDS on process outcomes.23
In addition to improved process outcomes, CDS has also resulted in significant cost savings.23 One study reported a net savings of $16.7 million and net operating budget savings of $9.5 million over 10 years, citing renal dosing guidance, nursing time utilization, specific drug guidance, and adverse drug event prevention as items resulting in the greatest cumulative savings.22 However, initial costs of implementing HIT to deliver CDS are extremely high, often limiting implementations in smaller or less well-funded organizations from realizing these savings.24 With the release of the Meaningful Use Regulation, which includes incentive payments for those attesting prior to 2015,and penalties beginning afterward for those who have not attested, in the coming several years, implementation of CDS will continue to increase.12
Despite these findings and the initial promise of CDS success at improving patient outcomes, only a few centers have rigorously demonstrated significant, positive findings with CDS.18,23 Further, many of these studies focused on custom-built CDS implemented within locally developed health information technology systems in large, academic medical centers, and further research is necessary to better quantify the benefits of CDS across all healthcare settings and to demonstrate the scalability of CDS for subspecialty settings.25
The Clinical Decision Support Implementation Process
Creating a CDS program for any organization or practice is a difficult undertaking. It is important to build a shared vision among all stakeholders within the organization or practice, including how the CDS interventions will enhance clinical, operational, and financial performance.26 The vision should be informed by both internal and external factors. Currently, external regulatory pressures such as Meaningful Use, combined with internal pressures to develop and/or participate in an Accountable Care Organization (ACO),27 are driving these decisions in many healthcare organizations. Once the goals of a particular CDS program have been identified, the organization can begin to identify specific clinical objectives to address.The organization should attempt to broadly integrate these new CDS-focused objectives into existing clinical governance, planning, and operational committees.28
The next major hurdle is identifying the key personnel who will be responsible for the actual development and implementation of the CDS interventions. Key roles to be filled include clinical champions responsible for encouraging and teaching other clinicians regarding reasons for and use of the CDS interventions; technical resources responsible for implementing the CDS logic within the EHR; and administrative supporters who must ensure that there will be adequate funding for new parts of the technical architecture, have access to external consultants and vendor personnel that may be required to help implement the CDS, and test the new interventions.29 The organization must also investigate what is feasible, given the technical limitations of their particular EHR and other HIT systems coupled with the availability, accuracy, and timeliness of the data required to drive the CDS.30
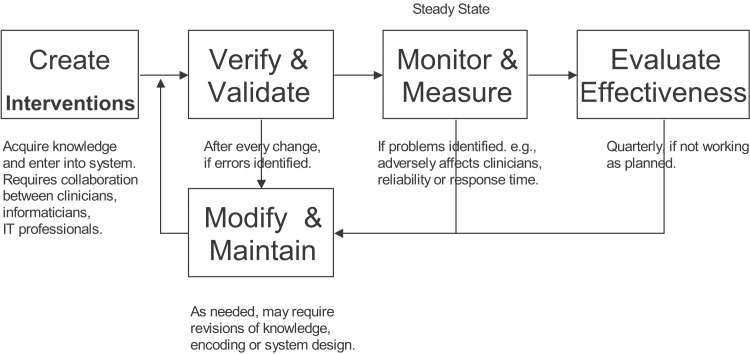
The organization should also consider how they will measure and monitor the effect of each CDS intervention because a critical component of any CDS intervention implementation is proper testing.31 These tests must be done at many different levels. For example, a technical person needs to ensure that the intervention performs as expected (e.g., storage of specific data values leads to the appropriate alert appearing in a pop-up window, or entry of a specific problem onto the patient's problem list creates a link to an appropriate order set). Similarly, each intervention must be tested by clinicians to ensure that it makes sense within their normal clinical workflow. Finally, following go-live, the intervention needs to be monitored closely to ensure that it is working as expected (e.g., the alert is firing when expected, but not more often than necessary, or the order set is appearing for patients with the appropriate conditions and the correct default items within the order set are selected).The organization should make sure that there is a mechanism to allow clinicians to provide feedback to the HIT staff regarding any potential issues with the CDS.32 These complaints should be quickly investigated and fixed if necessary. Finally, the organization should carefully monitor the intervention to ascertain whether it has positively affected the originally defined clinical objective.33 Often, the intervention will need to be optimized in some manner to get the desired outcome.34,35 Fig. 1 provides a diagram that illustrates the iterative nature of the CDS implementation process. It is important to note that it is not uncommon for CDS interventions to have to be modified in some way following implementation to get the desired effect.
Fig. 1.
An overview of the clinical decision support design, development, implementation, and evaluation lifecycle. Adapted from Osheroff et al.16
Applications of Clinical Decision Support for Colon and Rectal Surgery
Table 1,36,37,38,39,40,41,42,43,44,45,46 depicts the previously described CDS taxonomy17 and provides several examples of CDS relevant to CRS, with references when applicable. Medication dosing support is the first category of CDS and includes medication dose adjustment, formulary checking, dose checking, default doses, and indication-based ordering. This category of CDS has been demonstrated to be highly effective in both improving patient safety and reducing associated costs.22,47 Although few examples specific to CRS have been described in the literature, several examples exist that may be relevant, including dosing advisors for antibiotics based on renal function.47
Table 1. Types of clinical decision support and examples relevant to colon and rectal surgery.
| Type | Example |
|---|---|
| Medication dosing support | |
| Medication dose adjustment | Dosing advisor for antibiotics based on renal function.47 |
| Formulary checking | Suggest nifedipine ointment as a more cost-effective alternative to diltiazem ointment in the treatment of anal fissure.36 |
| Single-dose range checking | Alert on a single dose of acetaminophen 2 g for postoperative analgesia. |
| Maximum daily dose checking | Alert on a total daily dose of acetaminophen 7 g for postoperative analgesia. |
| Maximum lifetime dose checking | Alert if the total cumulative amount of radiation therapy exceeds the recommended maximum amount in the setting of neoadjuvant chemoradiotherapy for rectal cancer.37 |
| Default doses/pick lists | Provide a list of 100 mg, 200 mg, 300 mg, 400 mg, 600 mg, and 800 mg doses for ibuprofen with a default of 400 mg for postoperative analgesia in adults following an anorectal procedure. |
| Indication-based dosing | Order 2% diltiazem ointment topically for anal fissure, but 60 mg three times daily by mouth for hypertension. |
| Order facilitators | |
| Medication order sentences | Allow the provider to order “Heparin 5000 Units Subcutaneously t.i.d.” as a single unit for perioperative thromboembolic disease prophylactic prevention. |
| Subsequent or corollary orders | Order hemoglobin test when mesalamine is ordered. |
| Indication-based ordering | Suggest perioperative intravenous antibiotics at the time of starting an abdominal surgery.38,39 |
| Service-specific order sets | Colorectal surgery inpatient admission order set49 |
| Condition-specific order sets | Abdominal pain order set |
| Procedure-specific order sets | Postoperative colectomy order set48 |
| Condition-specific treatment protocol | Inflammatory bowel disease treatment protocol |
| Transfer order set | Transfer to surgery step-down unit order set |
| Nonmedication order sentences | Allow the provider to order “Call HO for temperature > 101, SBP > 180, DBP < 90, HR > 120, HR < 50, RR > 30, RR < 10, O2 saturation < 92%” as a single unit. |
| Point of care alerts/reminders | |
| Drug/condition interaction checking | Alert when a provider orders metronidazole in the setting of Clostridium difficile colitis for a female patient of childbearing age. |
| Drug/drug interaction checking | Alert about the possibility of thrombosis when a provider orders omeprazole in a patient receiving clopidogrel.40 |
| Drug/allergy interaction checking | Alert when a provider orders cefotetan for perioperative antibiotics in a patient with a documented penicillin allergy. |
| Plan of care alerts | Reminders to reassess the need for restraints and reorder if necessary at least every 24 h. |
| Critical laboratory value checking | Notify providers about positive FOBT results.54 |
| Duplicate order checking | Alert when a provider orders metoprolol in a surgical patient with active order for atenolol or when it is already on the medication list. |
| Care reminders | Remind providers to order a yearly FOBT for patients between 45 and 85.52,53 |
| Look-alike/sound-alike medication warnings | Warn providers ordering prednisone or prednisolone to ensure that they have chosen the drug they intended. |
| Ticklers | Alert a provider when colonoscopy has been ordered but not scheduled or performed within three months. |
| Problem list management | When ordering mesalamine, ask the provider if he/she would like to add inflammatory bowel disease to the problem list if not already documented.41 |
| Radiology ordering support | Order a CT scan of the abdomen and pelvis (rather than only the abdomen) in a patient with abdominal pain to ensure full visualization of the peritoneal cavity. |
| IV/PO conversion | Convert patient from IV metronidazole to PO metronidazole when patient is no longer NPO. |
| High-risk state monitoring | Alert the provider to order contact precautions for patients with known methicillin-resistant Staphylococcus aureus colonization. |
| Polypharmacy alerts | Alert the provider that a patient is on >8 medications and suggest pharmacy consult.42 |
| Relevant information display | |
| Context-sensitive information retrieval | Allow the provider to link directly to prescribing information for a medication at the time of ordering.19 |
| Patient-specific relevant data displays | Display recent creatinine levels when ordering Fleets Phospho-Soda with a bowel preparation. |
| Medication/test cost display | Indicate that a CBC costs $66 at the time of ordering. |
| Tallman lettering | Show prednisone and prednisolone as predniSONE and prednisoLONE in a pick list.43 |
| Context-sensitive user interface | Provide a special interface for chemotherapy order entry, which might include relevant data display, special facilities for ordering complex or time-based protocols and reference information. |
| Expert systems | |
| Antibiotic ordering support | Suggest metronidazole for empiric antibiotic therapy for patients with suspected Clostridium difficile. |
| Ventilator support | Unless the FiO2 is already at 1.0, suggest increasing the FiO2 by 0.1 if the PaO2 is > 50 but < 60 mmHg in patients with acute respiratory distress syndrome.44 |
| Diagnostic support | Provide a tool to help providers distinguish between types of inflammatory bowel disease and related conditions. |
| Risk assessment tools | Estimate 10-year diverticulitis recurrence risk for a patient with complicated diverticulitis.55 |
| Prognostic tools | Estimate survival for colorectal cancer patients based on pathologic tumor grade and stage.45 |
| Transfusion support | Suggest fresh frozen plasma for patients with a high INR and taking warfarin. |
| Nutrition ordering tools | Suggest increased protein in TPN for patients with active infection. |
| Laboratory test interpretation | Based on microbiology sensitivity testing, report that a patient with an intraabdominal abscess has vancomycin-resistant enterococcus and needs to be placed on contact precautions. |
| Treatment planning | A computerized system to facilitate management of surgical sepsis.56 |
| Triage tools | Computer-based recommendations for patients with acute abdominal pain.46 |
| Syndromic surveillance | City-wide reporting and monitoring of emergency department chief complaints to detect E. coli O157:H7 outbreaks. |
| Workflow support | |
| Order routing | Route order for stoma marking to wound ostomy continence nursing. |
| Registry functions | Send a letter to eligible patients to recommend FOBT.57,58,59 |
| Medication reconciliation | Upon postprocedure admission, automatically generate a preadmission medication list based on outpatient medication orders and pharmacy dispensing data. |
| Automatic order termination | Automatically terminate antibiotic orders after the conclusion of the order duration. |
| Order approvals | Send all colonoscopy orders to CRS for approval. |
| Free-text order parsing | Allow the user to enter the text “amox 500 mg QID 10d” and translate that to a complete, structured amoxicillin order that can be automatically processed by the pharmacy system. |
| Documentation aids | Structured documentation template for a CRS visit that has common findings. |
Abbreviations: t.i.d., three times daily; HO, house officer; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; RR, respiratory rate; FOBT, fecal occult blood test; CT, computed tomography; IV, intravenous; PO, by mouth; NPO, nothing by mouth; CBC, complete blood count; INR, international normalized ratio; TPN, total parenteral nutrition; CRS, colon and rectal surgery; QID, four times daily.
Order facilitators are the next category of CDS, including order sentences, subsequent or corollary orders, indication-based ordering, and order sets. For busy surgeons, order facilitators can reduce the amount of time spent entering orders and leave more time for providing patient care. Order sets, collections of orders that are grouped by a specific clinical purpose, are frequently used by hospitals to ensure adherence to guidelines or protocols.48 In surgical settings, order set examples include those for admission to the CRS service or for postoperative care of patients.48,49
Point-of-care alerts and reminders comprise the third category of CDS, representing one of the CDS types most frequently evaluated in the literature. This type of CDS prompts clinicians about drug-condition, drug-drug, and drug-allergy interactions; reminds clinicians to assess specific care items; and notifies clinicians about critical laboratory values or high-risk states. These reminders can be passive alerts that display additional text, change existing text colors, or show images, without interrupting the workflow, and can also be interruptive alerts, which require that providers acknowledge or respond to the alert before resuming order entry.50,51 For colon and rectal diseases, alerts often prompt primary care providers to order or remind patients about cancer-screening tests or to follow up with patients after abnormal screening test results.52,53,54 Alerts can also be beneficial directly to CRS practice, given the protocoled nature of many CRS conditions and the high number of potential interactions with antibiotics and other medications frequently ordered.
The fourth type of CDS is relevant information display. This type ensures that clinicians have up-to-date and necessary patient data to make decisions in providing care to the patients, such as showing recent laboratory test values during medication ordering. Specific examples for CRS have not been extensively described in the literature, although knowledge about laboratory trends or cost of care is an important consideration for clinicians across all care settings.
Expert systems are the fifth type of CDS, and these apply advanced logic or computational methods to assist clinicians in ordering, diagnosing, treating, and interpreting elements within the EHR. One example specific to CRS includes a study that applied statistical methods to predict outcomes for patients with diverticulitis.55 Another study evaluated a treatment planning system that suggests volume resuscitation and medication therapy, such as use of antibiotics and vasopressors, for surgical patients identified as having sepsis, and found that use of the CDS improved mortality in the observed patients.56
The final type of CDS is workflow support, including order routing, registry functions, medication reconciliation, automatic order termination, order approvals, free-text order parsing, and documentation aids. Registry functions are especially important for CRS, where appropriate patient follow-up is imperative for providing optimal care. For example, several studies have described reminders sent to patients, through mailed letters or telephone calls, to remind patients about completion of fecal occult blood tests.57,58,59
Challenges for Clinical Decision Support
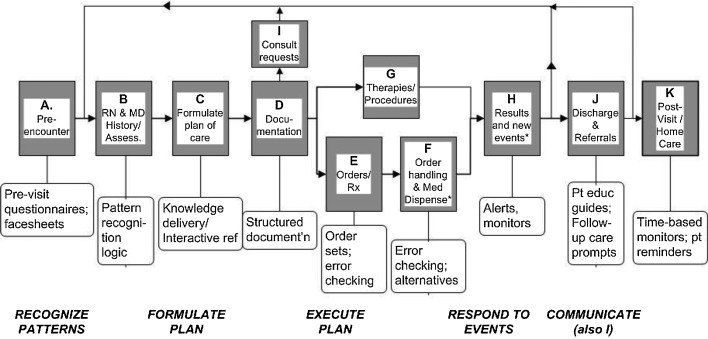
Despite the reported benefits CDS can provide, several barriers and challenges exist that frequently prevent patient safety and other healthcare improvements. One challenge is that CDS is often not implemented in a manner that allows it to be as effective as possible in practice. First, CDS must be presented in a manner that fits into the optimal workflow for clinicians and is intuitive for clinicians to view and respond.60,61 Fig. 2 depicts the basic workflow for clinical encounters, indicating core actions that are associated with each step in the workflow and CDS interventions that are appropriate for each core action.16
Fig. 2.
Flowchart of clinical workflow with core actions and typical clinical decision support opportunities. Adapted from Osheroff et al.16
CDS must also be specific and relevant to both the clinician and patient receiving the CDS. For example, an alert about a medication contraindicated in pregnancy should be suppressed for a patient who is postmenopausal or has had a hysterectomy. However, it can be difficult to implement highly specific CDS systems, as these require that relevant patient information be computable in acoded instead of free-text format. When coded or structured data are not available, some computation methods may be applied to transform the free-text information into coded data, using techniques such as natural language processing.62 These methods have been applied to process surgical and pathology reports, which could improve CDS delivered to CRS clinicians.63,64,65
Further problems arise with ineffective CDS solutions, such as alert fatigue, which occurs when clinicians receive too many CDS alerts and subsequently ignore many alerts that are displayed by the system.66 Studies have found that 49–96% of alerts displayed to clinicians are overridden, most often due to frequent alerts that are irrelevant or not serious.67 To prevent alert fatigue, it is necessary that institutions effectively evaluate and refine the implementation of any CDS tool both prior to and following implementation.68 Computer-based pharmacy surveillance of patients with high-risk conditions or of alerts with high rates of overrides may also prevent errors when the CDS is insufficient.69,70
Another challenge to implementing effective CDS is the difficulty in managing and sharing CDS knowledge.71,72 Currently, most CDS systems are implemented locally, and replicating these systems at other institutions requires considerable effort by HIT staff and may be prone to errors. The development of methods for sharing CDS across institutions and EHRs could increase CDS adoption. One approach to sharing CDS utilizes a service-oriented architecture, where CDS interventions are hosted remotely and institutions can subscribe to receive CDS without having to develop and maintain the services locally.73 Another approach might involve CDS repositories, where an organization makes rules available, and the rules are downloaded locally, where they can be maintained, modified, and integrated into existing EHRs.71
Conclusion
CDS can be an effective method to improve clinical processes and patient safety. Given the episodic nature of CRS care, CDS such as order sets, documentation templates, and any variety of order facilitation aids is most likely to be beneficial in CRS, whereas other types of CDS (such as registry management tools) or reminders may be more useful for primary care providers. However, colon and rectal surgeons can still play an important role in developing CRS-related CDS for other providers by, for example, participating in the development of screening reminder systems that help primary care providers select appropriate screening tests and intervals. Even though the tools would primarily be used by primary care providers, colon and rectal surgeons have special expertise that could contribute to the process of development. Further research is necessary to identify and better evaluate additional CDS systems in the setting of CRS.
References
- 1.Committee on Quality of Health Care in America, Institute of Medicine . Washington, DC: National Academies Press; 2000. To Err Is Human: Building a Safer Health System. [PubMed] [Google Scholar]
- 2.Committee on Quality of Health Care in America, Institute of Medicine . Washington, DC: National Academies Press; 2001. Crossing the Quality Chasm: A New Health System for the 21st Century. [Google Scholar]
- 3.Bates D W, Cullen D J, Laird N. et al. ADE Prevention Study Group . Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA. 1995;274(1):29–34. [PubMed] [Google Scholar]
- 4.Lesar T S, Briceland L, Stein D S. Factors related to errors in medication prescribing. JAMA. 1997;277(4):312–317. [PubMed] [Google Scholar]
- 5.Bobb A, Gleason K, Husch M, Feinglass J, Yarnold P R, Noskin G A. The epidemiology of prescribing errors: the potential impact of computerized prescriber order entry. Arch Intern Med. 2004;164(7):785–792. doi: 10.1001/archinte.164.7.785. [DOI] [PubMed] [Google Scholar]
- 6.Reckmann M H, Westbrook J I, Koh Y, Lo C, Day R O. Does computerized provider order entry reduce prescribing errors for hospital inpatients? A systematic review. J Am Med Inform Assoc. 2009;16(5):613–623. doi: 10.1197/jamia.M3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ammenwerth E, Schnell-Inderst P, Machan C, Siebert U. The effect of electronic prescribing on medication errors and adverse drug events: a systematic review. J Am Med Inform Assoc. 2008;15(5):585–600. doi: 10.1197/jamia.M2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Rosse F, Maat B, Rademaker C MA, van Vught A J, Egberts A CG, Bollen C W. The effect of computerized physician order entry on medication prescription errors and clinical outcome in pediatric and intensive care: a systematic review. Pediatrics. 2009;123(4):1184–1190. doi: 10.1542/peds.2008-1494. [DOI] [PubMed] [Google Scholar]
- 9.Bates D W, Teich J M, Lee J. et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999;6(4):313–321. doi: 10.1136/jamia.1999.00660313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee on Quality of Health Care in America, Institute of Medicine . Washington, DC: The National Academies Press; 1997. The Computer-Based Patient Record: An Essential Technology for Health Care, Revised Edition. [PubMed] [Google Scholar]
- 11.Sittig D F, Stead W W. Computer-based physician order entry: the state of the art. J Am Med Inform Assoc. 1994;1(2):108–123. doi: 10.1136/jamia.1994.95236142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363(6):501–504. doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 13.Marcotte L, Seidman J, Trudel K. et al. Achieving meaningful use of health information technology: a guide for physicians to the EHR incentive programs. Arch Intern Med. 2012;172(9):731–736. doi: 10.1001/archinternmed.2012.872. [DOI] [PubMed] [Google Scholar]
- 14.Melton G B. Biomedical and health informatics for surgery. Adv Surg. 2010;44(1):117–130. doi: 10.1016/j.yasu.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Richardson J E, Ash J S, Sittig D F. et al. Multiple perspectives on the meaning of clinical decision support. AMIA Annu Symp Proc. 2010;2010:672–676. [PMC free article] [PubMed] [Google Scholar]
- 16.Osheroff J, Teich J, Levick D, Chicago, IL: Healthcare Information and Management Systems Society; 2012. Improving Outcomes with Clinical Decision Support: An Implementer's Guide. 2nd ed. [Google Scholar]
- 17.Wright A, Sittig D F, Ash J S. et al. Development and evaluation of a comprehensive clinical decision support taxonomy: comparison of front-end tools in commercial and internally developed electronic health record systems. J Am Med Inform Assoc. 2011;18(3):232–242. doi: 10.1136/amiajnl-2011-000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaushal R, Shojania K G, Bates D W. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163(12):1409–1416. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- 19.Kawamoto K, Houlihan C A, Balas E A, Lobach D F. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt D L, Haynes R B, Hanna S E, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: a systematic review. JAMA. 1998;280(15):1339–1346. doi: 10.1001/jama.280.15.1339. [DOI] [PubMed] [Google Scholar]
- 21.Kuperman G J, Bobb A, Payne T H. et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14(1):29–40. doi: 10.1197/jamia.M2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaushal R, Jha A K, Franz C. et al. Brigham and Women's Hospital CPOE Working Group . Return on investment for a computerized physician order entry system. J Am Med Inform Assoc. 2006;13(3):261–266. doi: 10.1197/jamia.M1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bright T J, Wong A, Dhurjati R. et al. Effect of clinical decision-support systems: a systematic review. Ann Intern Med. 2012;157(1):29–43. doi: 10.7326/0003-4819-157-1-201207030-00450. [DOI] [PubMed] [Google Scholar]
- 24.Kuperman G J, Gibson R F. Computer physician order entry: benefits, costs, and issues. Ann Intern Med. 2003;139(1):31–39. doi: 10.7326/0003-4819-139-1-200307010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Chaudhry B, Wang J, Wu S. et al. Systematic review: impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med. 2006;144(10):742–752. doi: 10.7326/0003-4819-144-10-200605160-00125. [DOI] [PubMed] [Google Scholar]
- 26.Sirajuddin A M, Osheroff J A, Sittig D F, Chuo J, Velasco F, Collins D A. Implementation pearls from a new guidebook on improving medication use and outcomes with clinical decision support. Effective CDS is essential for addressing healthcare performance improvement imperatives. J Healthc Inf Manag. 2009;23(4):38–45. [PMC free article] [PubMed] [Google Scholar]
- 27.Berwick D M. Making good on ACOs' promise—the final rule for the Medicare shared savings program. N Engl J Med. 2011;365(19):1753–1756. doi: 10.1056/NEJMp1111671. [DOI] [PubMed] [Google Scholar]
- 28.Wright A, Sittig D F, Ash J S. et al. Governance for clinical decision support: case studies and recommended practices from leading institutions. J Am Med Inform Assoc. 2011;18(2):187–194. doi: 10.1136/jamia.2009.002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ash J S, Stavri P Z, Dykstra R, Fournier L. Implementing computerized physician order entry: the importance of special people. Int J Med Inform. 2003;69(2–3):235–250. doi: 10.1016/s1386-5056(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 30.Sittig D F, Wright A, Meltzer S. et al. Comparison of clinical knowledge management capabilities of commercially-available and leading internally-developed electronic health records. BMC Med Inform Decis Mak. 2011;11:13. doi: 10.1186/1472-6947-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henriksen K Battles J B Marks E S Lewin D I, Eds. Advances in Patient Safety: From Research to Implementation (Volume 3: Implementation Issues) Rockville, MD: Agency for Healthcare Research and Quality (US)2005. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21250003. Accessed: May 29, 2012 [PubMed] [Google Scholar]
- 32.Sittig D F Singh H Rights and responsibilities of users of electronic health records CMAJ 2012184131479–1483.. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22331971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucher N, Koo S, Quiroz R. et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352(10):969–977. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 34.Stutman H R, Fineman R, Meyer K, Jones D. Optimizing the acceptance of medication-based alerts by physicians during CPOE implementation in a community hospital environment. AMIA Annu Symp Proc. 2007:701–705. [PMC free article] [PubMed] [Google Scholar]
- 35.Paterno M D, Maviglia S M, Gorman P N. et al. Tiering drug-drug interaction alerts by severity increases compliance rates. J Am Med Inform Assoc. 2009;16(1):40–46. doi: 10.1197/jamia.M2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haq Z, Rahman M, Chowdhury R A, Baten M A, Khatun M. Chemical sphincterotomy—first line of treatment for chronic anal fissure. Mymensingh Med J. 2005;14(1):88–90. [PubMed] [Google Scholar]
- 37.Chang M L, Hou J K. Cancer risk related to gastrointestinal diagnostic radiation exposure. Curr Gastroenterol Rep. 2011;13(5):449–457. doi: 10.1007/s11894-011-0214-8. [DOI] [PubMed] [Google Scholar]
- 38.Haynes K, Linkin D R, Fishman N O. et al. Effectiveness of an information technology intervention to improve prophylactic antibacterial use in the postoperative period. J Am Med Inform Assoc. 2011;18(2):164–168. doi: 10.1136/jamia.2009.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair B G, Newman S-F, Peterson G N, Wu W-Y, Schwid H A. Feedback mechanisms including real-time electronic alerts to achieve near 100% timely prophylactic antibiotic administration in surgical cases. Anesth Analg. 2010;111(5):1293–1300. doi: 10.1213/ANE.0b013e3181f46d89. [DOI] [PubMed] [Google Scholar]
- 40.Magid S K, Pancoast P E, Fields T, Bradley D G, Williams R B. Employing clinical decision support to attain our strategic goal: the safe care of the surgical patient. J Healthc Inf Manag. 2007;21(2):18–25. [PubMed] [Google Scholar]
- 41.Wright A, Chen E S, Maloney F L. An automated technique for identifying associations between medications, laboratory results and problems. J Biomed Inform. 2010;43(6):891–901. doi: 10.1016/j.jbi.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 42.Patterson S M, Hughes C, Kerse N, Cardwell C R, Bradley M C. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2012;5:CD008165. doi: 10.1002/14651858.CD008165.pub2. [DOI] [PubMed] [Google Scholar]
- 43.Filik R, Purdy K, Gale A, Gerrett D. Labeling of medicines and patient safety: evaluating methods of reducing drug name confusion. Hum Factors. 2006;48(1):39–47. doi: 10.1518/001872006776412199. [DOI] [PubMed] [Google Scholar]
- 44.Sittig D F, Pace N L, Gardner R M, Beck E, Morris A H. Implementation of a computerized patient advice system using the HELP clinical information system. Comput Biomed Res. 1989;22(5):474–487. doi: 10.1016/0010-4809(89)90040-2. [DOI] [PubMed] [Google Scholar]
- 45.Claret L, Girard P, Hoff P M. et al. Model-based prediction of phase III overall survival in colorectal cancer on the basis of phase II tumor dynamics. J Clin Oncol. 2009;27(25):4103–4108. doi: 10.1200/JCO.2008.21.0807. [DOI] [PubMed] [Google Scholar]
- 46.Farion K J, Michalowski W, Rubin S, Wilk S, Correll R, Gaboury I. Prospective evaluation of the MET-AP system providing triage plans for acute pediatric abdominal pain. Int J Med Inform. 2008;77(3):208–218. doi: 10.1016/j.ijmedinf.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Evans R S, Pestotnik S L, Classen D C. et al. A computer-assisted management program for antibiotics and other antiinfective agents. N Engl J Med. 1998;338(4):232–238. doi: 10.1056/NEJM199801223380406. [DOI] [PubMed] [Google Scholar]
- 48.Wright A, Feblowitz J C, Pang J E. et al. Use of order sets in inpatient computerized provider order entry systems: A comparative analysis of usage patterns at seven sites. Int J Med Inform. 2012;81(11):733–745. doi: 10.1016/j.ijmedinf.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayman A V, Chang E T, Molokie R E, Kahng L S, Prystowsky J B, Bentrem D J. Assessing compliance with national quality measures to improve colorectal cancer care at the VA. Am J Surg. 2010;200(5):572–576. doi: 10.1016/j.amjsurg.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 50.McCoy A B, Waitman L R, Gadd C S. et al. A computerized provider order entry intervention for medication safety during acute kidney injury: a quality improvement report. Am J Kidney Dis. 2010;56(5):832–841. doi: 10.1053/j.ajkd.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo H G, Matheny M E, Seger D L, Bates D W, Gandhi T K. Impact of non-interruptive medication laboratory monitoring alerts in ambulatory care. J Am Med Inform Assoc. 2009;16(1):66–71. doi: 10.1197/jamia.M2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McDonald C J, Hui S L, Smith D M. et al. Reminders to physicians from an introspective computer medical record. A two-year randomized trial. Ann Intern Med. 1984;100(1):130–138. doi: 10.7326/0003-4819-100-1-130. [DOI] [PubMed] [Google Scholar]
- 53.McPhee S J, Bird J A, Jenkins C N, Fordham D. Promoting cancer screening. A randomized, controlled trial of three interventions. Arch Intern Med. 1989;149(8):1866–1872. doi: 10.1001/archinte.149.8.1866. [DOI] [PubMed] [Google Scholar]
- 54.Singh H, Kadiyala H, Bhagwath G. et al. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. Am J Gastroenterol. 2009;104(4):942–952. doi: 10.1038/ajg.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chapman J R Dozois E J Wolff B G Gullerud R E Larson D R Diverticulitis: a progressive disease? Do multiple recurrences predict less favorable outcomes? Ann Surg 20062436876–830., discussion 880–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore L J Turner K L Todd S R McKinley B Moore F A Computerized clinical decision support improves mortality in intra abdominal surgical sepsis Am J Surg 20102006839–843., discussion 843–844 [DOI] [PubMed] [Google Scholar]
- 57.Ornstein S M, Garr D R, Jenkins R G, Rust P F, Arnon A. Computer-generated physician and patient reminders. Tools to improve population adherence to selected preventive services. J Fam Pract. 1991;32(1):82–90. [PubMed] [Google Scholar]
- 58.Goldberg D, Schiff G D, McNutt R, Furumoto-Dawson A, Hammerman M, Hoffman A. Mailings timed to patients' appointments: a controlled trial of fecal occult blood test cards. Am J Prev Med. 2004;26(5):431–435. doi: 10.1016/j.amepre.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 59.Mosen D M, Feldstein A C, Perrin N. et al. Automated telephone calls improved completion of fecal occult blood testing. Med Care. 2010;48(7):604–610. doi: 10.1097/MLR.0b013e3181dbdce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller R A, Waitman L R, Chen S, Rosenbloom S T. The anatomy of decision support during inpatient care provider order entry (CPOE): empirical observations from a decade of CPOE experience at Vanderbilt. J Biomed Inform. 2005;38(6):469–485. doi: 10.1016/j.jbi.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koppel R, Metlay J P, Cohen A. et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–1203. doi: 10.1001/jama.293.10.1197. [DOI] [PubMed] [Google Scholar]
- 62.Hripcsak G, Friedman C, Alderson P O, DuMouchel W, Johnson S B, Clayton P D. Unlocking clinical data from narrative reports: a study of natural language processing. Ann Intern Med. 1995;122(9):681–688. doi: 10.7326/0003-4819-122-9-199505010-00007. [DOI] [PubMed] [Google Scholar]
- 63.Meng F, D'Avolio L W, Chen A A, Taira R K, Kangarloo H. Generating models of surgical procedures using UMLS concepts and multiple sequence alignment. AMIA Annu Symp Proc. 2005:520–524. [PMC free article] [PubMed] [Google Scholar]
- 64.Lamiell J M, Wojcik Z M, Isaacks J. Computer auditing of surgical operative reports written in English. Proc Annu Symp Comput Appl Med Care. 1993:269–273. [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner J C, Rogers J E, Baud R H, Scherrer J R. Natural language generation of surgical procedures. Int J Med Inform. 1999;53(2–3):175–192. doi: 10.1016/s1386-5056(98)00158-0. [DOI] [PubMed] [Google Scholar]
- 66.Murphy D R, Reis B, Sittig D F, Singh H. Notifications received by primary care practitioners in electronic health records: a taxonomy and time analysis. Am J Med. 2012;125(2):209.e 1–7. doi: 10.1016/j.amjmed.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 67.van der Sijs H, Aarts J, Vulto A, Berg M. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13(2):138–147. doi: 10.1197/jamia.M1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCoy A B, Waitman L R, Lewis J B. et al. A framework for evaluating the appropriateness of clinical decision support alerts and responses. J Am Med Inform Assoc. 2012;19(3):346–352. doi: 10.1136/amiajnl-2011-000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McCoy A B, Cox Z L, Neal E B. et al. The comparative effectiveness of real-time pharmacy surveillance and clinical decision support to reduce adverse drug events in acute kidney injury: a randomized, controlled trial. Appl Clin Inform. 2012;3(2):221–238. doi: 10.4338/ACI-2012-03-RA-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Waitman L R, Phillips I E, McCoy A B. et al. Adopting real-time surveillance dashboards as a component of an enterprisewide medication safety strategy. Jt Comm J Qual Patient Saf. 2011;37(7):326–332. doi: 10.1016/s1553-7250(11)37041-9. [DOI] [PubMed] [Google Scholar]
- 71.Sittig D F, Wright A, Osheroff J A. et al. Grand challenges in clinical decision support. J Biomed Inform. 2008;41(2):387–392. doi: 10.1016/j.jbi.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osheroff J A, Teich J M, Middleton B, Steen E B, Wright A, Detmer D E. A roadmap for national action on clinical decision support. J Am Med Inform Assoc. 2007;14(2):141–145. doi: 10.1197/jamia.M2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawamoto K, Lobach D F. Proposal for fulfilling strategic objectives of the U.S. roadmap for national action on clinical decision support through a service-oriented architecture leveraging HL7 services. J Am Med Inform Assoc. 2007;14(2):146–155. doi: 10.1197/jamia.M2298. [DOI] [PMC free article] [PubMed] [Google Scholar]