Abstract
Introduction Cervicomedullary compression often requires an anterior approach to address the compressive vector. In certain cases an endoscopic endonasal approach (EEA) is ideal for decompression. It is essential that an adequate decompression be achieved and verified before the patient leaves the operating room. The purpose of this study was to evaluate the use intraoperative computed tomography (IO-CT) in assessing the adequacy of decompression.
Methods A retrospective chart review revealed 11 cases of EEA odontoid resection IO-CT verification of decompression. Operative reports and review of imaging was used to determine if further decompression was performed following the intraoperative scan.
Results Out of 11 EEA cases, 4 (36%) patients showed evidence of residual compression following an initial IO-CT. Further operative decompression was undertaken following the first scan in all cases. A second intraoperative scan was then used to confirm complete decompression. No patient left the operating room with residual compression.
Discussion IO-CT provided valuable utility in 36% of the cases after the initial resection was incomplete. The standard fluoroscopic guidance may not provide adequate resolution and enhanced utility like IO-CT.
Keywords: endoscopy, endonasal, odontoid, rheumatoid disease
Introduction
Ventral cervicomedullary compression may result from several pathologic entities, including congenital anomalies, degenerative pannus, and metastatic disease. Traditional management algorithms include in situ fixation and an attempt at closed reduction followed by posterior fusion.1 For cases with irreducible ventral compression, an anterior decompression may be required. A transoral approach (TOA) has been the traditional corridor for resection of lesions in this region,1,2 but growing enthusiasm for endonasal access (via an endoscopic endonasal approach [EEA]) to the craniocervical junction is changing this paradigm.3,4,5
Despite differences in operative technique, intraoperative imaging modalities have traditionally been similar for both approaches. Intraoperative fluoroscopy is often used in conjunction with radio-opaque contrast to confirm decompression.6,7 The introduction of advanced imaging modalities in the 1970s has allowed for diagnostic-quality, high-resolution images to guide intraoperative decision making.8,9 More specifically, intraoperative computed tomography (IO-CT) has proved useful for delineating the adequacy of surgical decompression at the cervicomedullary junction.10,11
Methods
Between February 2004 and August 2011, 31 patients underwent endoscopic endonasal surgery (EES) for craniocervical decompression at the authors' institution. Eleven of the surgeries were performed with the aid of IO-CT between August 2008 and March 2011. A 64-slice multidetector CT scanner (GE LightSpeed, GE Healthcare, Chalfont St. Giles, UK) was used to acquire the CT scans. Contiguous 0.625- or 1.25-mm images at 120 kVP and 240 mA were obtained. Image acquisition time was <1 minute in all cases. The goal of this study was to determine the utility of IO-CT for verifying adequate decompression and thus avoiding a return to the operating room.
All patients were placed in a radiolucent Mayfield head holder affixed to the IO-CT scanner bed. Image guidance (Stryker, Kalamazoo, MI, USA) was registered using a preoperative CT angiogram. A binarial approach to the posterior nasopharynx was undertaken in all cases as previously described5. Briefly, the nasopharyngeal fascia was dissected away from the inferior clivus, anterior arch of C1, and odontoid process. The arch of C1 was resected and the odontoid process was removed. In cases of degenerative or rheumatoid pannus, the soft tissue pannus was resected until visualization of pulsatile tectorial membrane or dura was achieved.
Following decompression, sterile drapes were placed over the operative field and the intraoperative CT scan was obtained. The patient was removed from the CT bore while the scan was assessed by the operating surgeons for extent of decompression (Fig. 1). In cases where the decompression was felt to be adequate, reconstruction of the defect was begun. In cases where residual compression was observed, further decompression was undertaken and finally confirmed with a repeat IO-CT scan (Fig. 2).
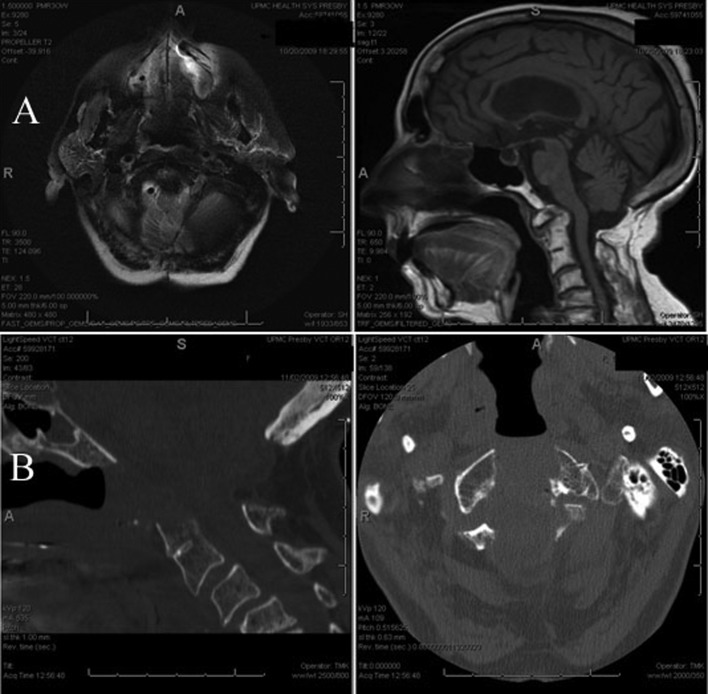
Fig. 1.
Preoperative sagittal and axial computed tomography (CT) scan showing degenerative C1–2 changes causing cervicomedullary compression (A). Intraoperative CT verifying decompression (B).
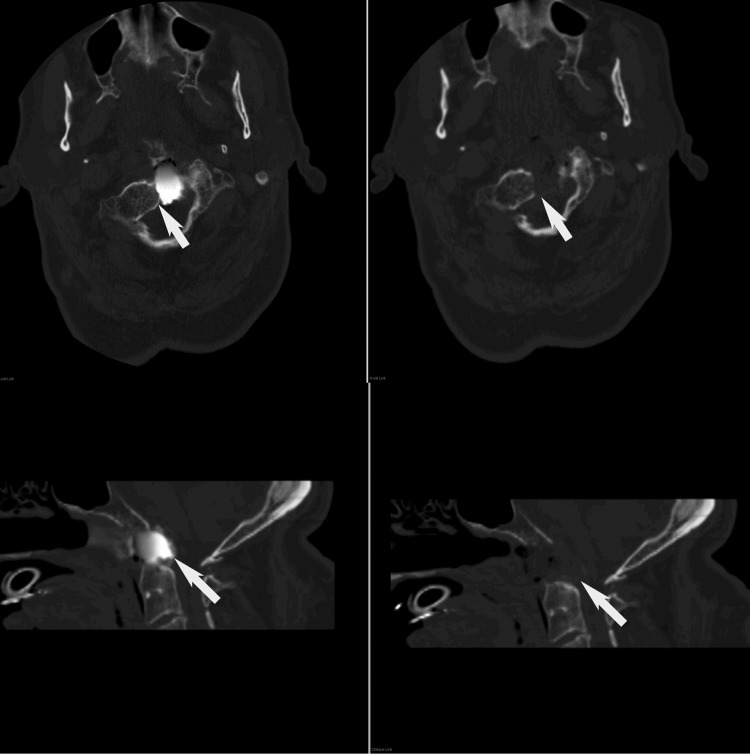
Fig. 2.
Left, initial intraoperative scan showing residual compression (arrow). Radio-opaque dye was used to provide contrast during this scan. Right, second intraoperative scan showing removal of the residual compression.
Results
Between 2008 and 2011, 11 patients (8 female and 3 male) with a mean age of 44 years (range 9 to 79) underwent a fully endoscopic endonasal odontoid resection utilizing an IO-CT scan. Out of the 11 surgeries, 4 cases showed residual compression on the intraoperative scan. All 4 patients had residual compression resected during the same anesthetic. Decompression in these cases was verified with a second intraoperative scan. This translates to a utility rate of 36% for IO-CT imaging in EES (Table 1).
Table 1. Patients and pathologies addressed by endoscopic endonasal approach with intraoperative computed tomography (IO-CT).
| Patient | Sex | Age | Surgery date | Pathology | Evidence of residual compression on initial IO-CT scan |
|---|---|---|---|---|---|
| 1 | F | 11 | 08/2008 | Basilar invagination with retroflexed odontoid process | No |
| 2 | M | 74 | 09/2008 | Odontoid pannus | Yes |
| 3 | F | 18 | 10/2008 | Basilar invagination with chiari | No |
| 4 | F | 71 | 10/2008 | Odontoid pannus with synovial cyst | No |
| 5 | F | 10 | 02/2009 | Basilar invagination | Yes |
| 6 | F | 41 | 03/2009 | Os odontoideum with C1–2 subluxation | No |
| 7 | M | 31 | 08/2009 | Cranial settling s/p chiari decompression and previous transoral resection | No |
| 8 | F | 9 | 09/2009 | Basilar invagination | No |
| 9 | F | 79 | 11/2009 | Basilar invagination s/p chiari decompression | Yes |
| 10 | M | 79 | 11/2009 | Basilar invagination | Yes |
| 11 | F | 61 | 03/2011 | Basilar invagination | No |
Discussion
Since the introduction of CT in the early 1970s,12 it has been well known that this imaging modality held great potential for diagnostic purposes. However, the adaptation of IO-CT in neurosurgery has been relatively slow compared with its near ubiquitous use in perioperative settings. This underuse is likely attributable to the cost and operating room (OR) space required to implement such a system. Since the 1980s there has been an effort to promote IO-CT integration for various neurosurgical diagnoses ranging from skull base pathologies to intracranial metastases.8,13 However, early limitations in CT imaging pertained to image quality, high radiation exposure, and lack of reconstructive software.14
Modern systems provide high-resolution images with coronal, sagittal and three-dimensional reconstructions.15 Regarding the issue of practicality in the ORs, Uhl et al have found that IO-CT only interrupted surgery for 9 to 15 minutes in a total of 230 cases (136 intracranial, 94 spinal); in their study, it changed the course of 16 (7%) cases by helping identify nonideal pedicle screw placements.14
In addition to superior image quality and practicality, modern CT scanners minimize radiation exposure to the OR staff compared with fluoroscopic imaging systems.16 By increasing the number of detectors, current CT configurations require fewer x-ray tube rotations for a given volume and dramatically shorten the data acquisition period compared with fluoroscopy or conventional tomography.17,18 Newer mobile CT scanners minimize exposure to patients by implementing online tube current modulation. In this technique, x-ray dose is dynamically reduced when patient diameter is smaller without compromising image quality.19 Clearly, IO-CT holds the potential to deliver superior levels of accuracy while minimizing radiation exposure and improving resection extent in a timely manner.
Among the 11 cases of endonasal odontoid resection with IO-CT performed over the last 3 years at our institution, the added imaging technique improved the detection of residual compression after initial resection in 36% of cases. By using IO-CT, four patients had an improved radiographic decompression under the same anesthetic. Especially given the learning curve associated with endoscopic surgery, this may provide significant value in maintaining quality of care during the acquisition of this learning curve. Although the sample size is relatively small, it must be noted that the incidence of odontoid pannus is considerably lower than that of other cervical spine pathologies in the general population,20 and the need for anterior decompression is rare.
The rationale for using IO-CT in bony craniocervical junction decompression surgery is important to understand. Intraoperative CT-based navigation provides real-time feedback to the surgeon, which is invaluable for the rapid confirmation of adequacy of decompression. Due to its enhanced accuracy, CT-based image guidance can account for subtle changes in patient positioning and spinal alignment. The first IO-CT scan in our protocol is used to gauge the extent of decompression, and, if residual compression is observed, subsequently resect it. When performed, this second resection is followed by another IO-CT scan to confirm the complete removal of all compressive elements. Although the clinical significance of this additional decompression is unknown, it can only be beneficial to ensure an ideal radiographic result.
Traditional fluoroscopic techniques are efficient, have low maintenance costs, and are commonly used in many surgical suites. However, their inferior image quality relative to CT scans, as demonstrated by previous studies, is a cause for concern.21,22 Another disadvantage of the standard C-arm fluoroscopy guidance is the lack of three-dimensional view including coronal and sagittal reconstructions, which can be essential to a surgeon's understanding of complex craniocervical anatomy. With EES, the inferior lateral margins are often more challenging to completely access, and it is impossible to ensure the adequacy of bone removal in this location with fluoroscopy. In another study of IO-CT use for improving placement of spinal instrumentation performed at our institution, three patients in the fluoroscopy group required repeat surgery as opposed to none in the IO-CT group.18 Similarly, in our series of endonasal odontoid resections, repeat surgery was potentially avoided in 36% of the cases after residual compression was detected with the aid of IO-CT. An alternative to standard fluoroscopy is three-dimensional C-arm fluoroscopy. Although this technology can improve resolution and provide coronal/sagittal reconstructions, it is not up to par with the quality of modern IO-CT.7,18,23 More importantly, the three-dimensional fluoroscopy system cannot be used for intracranial pathologies due to the poor resolution of soft tissues and blood vessels.24 Comparison of preoperative CT with any other intraoperative imaging technique will add yet another degree of uncertainty and inaccuracy. Lastly, other modalities such as intraoperative MRI are costly and inefficient, requiring compatible instruments and anesthesia equipment.25,26 In addition, almost all patients who are appropriate candidates for ventral decompression have largely bony compression, which is far better evaluated with CT than MRI. In the rare cases where a significant soft tissue component exists, CT is able to evaluate this as well (Fig. 3).
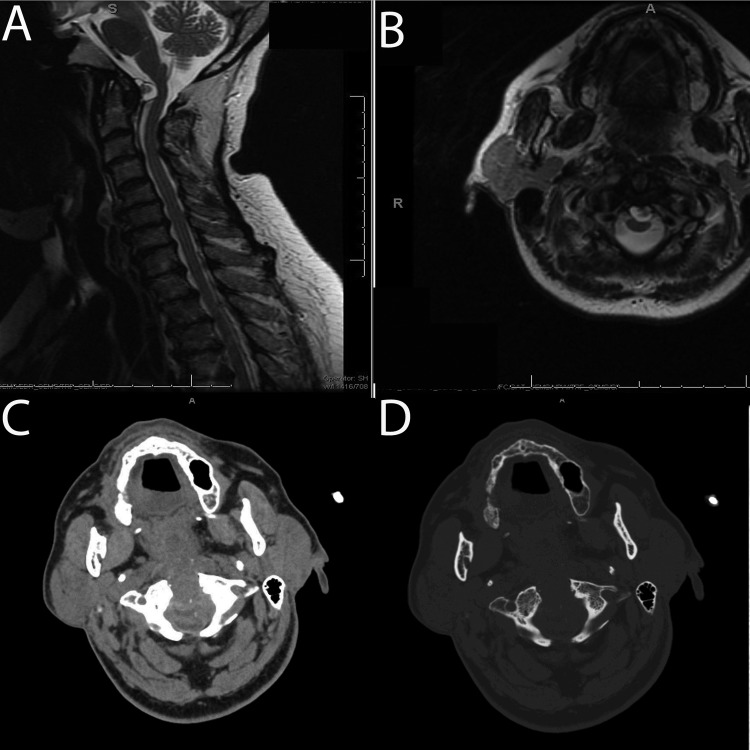
Fig. 3.
Preoperative sagittal (A) and axial (B) T2 magnetic resonance imaging showing cervical medullary compression caused primarily by a retro-odontoid synovial cyst. Intraoperative computed tomography scan with soft tissue (C) and bone algorithm (D) following decompression showing removal of the soft tissue compression.
Conclusions
Intraoperative CT provides useful information to rapidly confirm adequacy of decompression during endoscopic endonasal odontoid resection and cervicomedullary junction decompression. When available, this modality should be used to prevent the need for secondary surgeries due to inadequate initial decompression. This may hold increased value during the learning curve for new techniques such as EES.
References
- 1.Menezes A H, VanGilder J C. Transoral-transpharyngeal approach to the anterior craniocervical junction. Ten-year experience with 72 patients. J Neurosurg. 1988;69:895–903. doi: 10.3171/jns.1988.69.6.0895. [DOI] [PubMed] [Google Scholar]
- 2.Crockard H A. Transoral surgery: some lessons learned. Br J Neurosurg. 1995;9:283–293. doi: 10.1080/02688699550041304. [DOI] [PubMed] [Google Scholar]
- 3.Cavallo L M, de Divitiis O, Solari D. Transnasal and transoral approaches to the craniocervical junction: two routes for a variable destination. World Neurosurg. 2011;76:74–75. [Google Scholar]
- 4.Gempt J, Lehmberg J, Grams A E, Berends L, Meyer B, Stoffel M. Endoscopic transnasal resection of the odontoid: case series and clinical course. Eur Spine J. 2011;20:661–666. doi: 10.1007/s00586-010-1629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassam A B, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19:E6. [PubMed] [Google Scholar]
- 6.Holly L T, Foley K T. Intraoperative spinal navigation. Spine. 2003;28(15, Suppl):S54–S61. doi: 10.1097/01.BRS.0000076899.78522.D9. [DOI] [PubMed] [Google Scholar]
- 7.Lekovic G P, Potts E A, Karahalios D G, Hall G. A comparison of two techniques in image-guided thoracic pedicle screw placement: a retrospective study of 37 patients and 277 pedicle screws. J Neurosurg Spine. 2007;7:393–398. doi: 10.3171/SPI-07/10/393. [DOI] [PubMed] [Google Scholar]
- 8.Kondziolka D, Lunsford L D. Intraoperative navigation during resection of brain metastases. Neurosurg Clin N Am. 1996;7:267–277. [PubMed] [Google Scholar]
- 9.Shalit M N, Israeli Y, Matz S, Cohen M L. Intra-operative computerized axial tomography. Surg Neurol. 1979;11:382–384. [PubMed] [Google Scholar]
- 10.Hum B Feigenbaum F Cleary K Henderson F C Intraoperative computed tomography for complex craniocervical operations and spinal tumor resections Neurosurgery 200047374–380., discussion 380–381 [DOI] [PubMed] [Google Scholar]
- 11.Pillai P Baig M N Karas C S Ammirati M Endoscopic image-guided transoral approach to the craniovertebral junction: an anatomic study comparing surgical exposure and surgical freedom obtained with the endoscope and the operating microscope Neurosurgery 200964502437–442., discussion 442–444 [DOI] [PubMed] [Google Scholar]
- 12.Petrik V Apok V Britton J A Bell B A Papadopoulos M C Godfrey Hounsfield and the dawn of computed tomography Neurosurgery 200658780–787., discussion 780–787 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Tamaki N, Tamura S. Image-guided navigational microsurgery for skull base and brainstem diseases. Neurosurg Q. 2002;12:100–107. [Google Scholar]
- 14.Uhl E Zausinger S Morhard D et al. Intraoperative computed tomography with integrated navigation system in a multidisciplinary operating suite Neurosurgery 200964502231–239., discussion 239–240 [DOI] [PubMed] [Google Scholar]
- 15.Kotecha R, Toledo-Pereyra L H. Advanced imaging technology in surgical innovation. J Invest Surg. 2011;24:243–249. doi: 10.3109/08941939.2011.624437. [DOI] [PubMed] [Google Scholar]
- 16.McCollough C H, Bruesewitz M R, Kofler J M Jr. CT dose reduction and dose management tools: overview of available options. Radiographics. 2006;26:503–512. doi: 10.1148/rg.262055138. [DOI] [PubMed] [Google Scholar]
- 17.Moon L, McHugh K. Advances in paediatric tumour imaging. Arch Dis Child. 2005;90:608–611. doi: 10.1136/adc.2004.051193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tormenti M J, Kostov D B, Gardner P A, Kanter A S, Spiro R M, Okonkwo D O. Intraoperative computed tomography image-guided navigation for posterior thoracolumbar spinal instrumentation in spinal deformity surgery. Neurosurg Focus. 2010;28:E11. doi: 10.3171/2010.1.FOCUS09275. [DOI] [PubMed] [Google Scholar]
- 19.Greess H, Lutze J, Nömayr A. et al. Dose reduction in subsecond multislice spiral CT examination of children by online tube current modulation. Eur Radiol. 2004;14:995–999. doi: 10.1007/s00330-004-2301-9. [DOI] [PubMed] [Google Scholar]
- 20.Naderi S. et al. Odontoid Fractures. Turk Neurosurg. 2006;16:81–84. [Google Scholar]
- 21.Mahnken A H, Wildberger J E, Gehbauer G. et al. Multidetector CT of the spine in multiple myeloma: comparison with MR imaging and radiography. AJR Am J Roentgenol. 2002;178:1429–1436. doi: 10.2214/ajr.178.6.1781429. [DOI] [PubMed] [Google Scholar]
- 22.Nuñez D B Jr Zuluaga A Fuentes-Bernardo D A Rivas L A Becerra J L Cervical spine trauma: how much more do we learn by routinely using helical CT? Radiographics 1996161307–1318., discussion 1318–1321 [DOI] [PubMed] [Google Scholar]
- 23.Hott J S, Papadopoulos S M, Theodore N, Dickman C A, Sonntag V K. Intraoperative Iso-C C-arm navigation in cervical spinal surgery: review of the first 52 cases. Spine. 2004;29:2856–2860. doi: 10.1097/01.brs.0000147742.20637.49. [DOI] [PubMed] [Google Scholar]
- 24.Pamir M N Seifert V Kiris T Intraoperative imaging. 2011 Available at: http://dx.doi.org/10.1007/978-3-211-99651-5 [PubMed]
- 25.Makary M, Chiocca E A, Erminy N. et al. Clinical and economic outcomes of low-field intraoperative MRI-guided tumor resection neurosurgery. J Magn Reson Imaging. 2011;34:1022–1030. doi: 10.1002/jmri.22739. [DOI] [PubMed] [Google Scholar]
- 26.Mushlin A I, Mooney C, Holloway R G, Detsky A S, Mattson D H, Phelps C E. The cost-effectiveness of magnetic resonance imaging for patients with equivocal neurological symptoms. Int J Technol Assess Health Care. 1997;13:21–34. doi: 10.1017/s0266462300010205. [DOI] [PubMed] [Google Scholar]