Abstract
Objective
Treatment-naïve rheumatoid arthritis (RA) patients suffer from defective early B cell tolerance checkpoints and fail to remove developing autoreactive B cells. However, it is unclear if these B cell tolerance checkpoint defects are primary to the disease or the result of ongoing inflammatory processes in these patients. The aim of this study is to assess the impact of standard immunosuppressive treatments, methotrexate and anti-TNFα agents, on early B cell tolerance checkpoints in RA patients.
Methods
We tested the reactivity of recombinant antibodies cloned from single B cells from RA patients pre- and post-therapy with methotrexate and/or anti-TNFα agents. We were able thereby to determine the evolution of the frequency of autoreactive clones in the mature naïve B cell compartment of RA patients before and after treatment.
Results
We found that post-treatment frequencies of autoreactive mature naïve B cells in the blood of RA patients were elevated and remained similar to those of the same patients pre-treatment.
Conclusion
Despite patients’ improvement, methotrexate and anti-TNFα agents do not correct the accumulation of peripheral autoreactive mature naïve B cells in RA patients, suggesting that inflammation is not responsible for defective early B cell tolerance checkpoints in RA.
The efficacy of B cell depletion by anti-CD20 therapy in the treatment of rheumatoid arthritis (RA) suggests a critical role for B cells in the pathology of RA (1), although the pathogenic mechanisms are unknown. We previously described abnormalities in early B cell tolerance checkpoints in treatment- naïve active RA, which deviated from the evolution of autoreactive B cell frequencies during B cell development in healthy donors (2). Random V(D)J recombination generates a large number of autoreactive B cells that are counterselected in healthy donors at two major checkpoints. A first checkpoint occurs in the bone marrow between the early immature and immature B cell stages and silences most polyreactive and anti-nuclear antibody expressing B cells (3, 4). A second counterselection step takes place in the periphery and further removes autoreactive B cells that recently emigrated from the bone marrow before they enter the long-lived mature naïve B cell pool (3, 4). In clinically active RA, we found that both central and peripheral B cell tolerance checkpoints were defective resulting in the accumulation of a large number of autoreactive B cells in the mature naïve B cell compartment of these patients (2).
It remains to be determined if defective B cell tolerance checkpoints resulting in altered antibody reactivity selection is primary to the disease or merely represent a byproduct of the disease induced by cytokine imbalances. Methotrexate and anti-TNFα agents remain the standard of care in RA (5), although little is known about their effect on the loss of B cell tolerance and the persistence of autoreactive B cells. By analyzing RA patients who clinically improved after either methotrexate or anti-TNFα therapy, we demonstrate that B cell tolerance checkpoints remain defective post-therapy as evidenced by the increased frequency of self-reactive mature naïve B cells. This suggests that the early B cell tolerance defects in RA do not result from the production of proinflammatory cytokines, but rather are more likely due to intrinsic genetic predisposition.
PATIENTS AND METHODS
Patients
Patients either treated with methotrexate (RA01, RA02, RA03) or anti-TNFα agents (RA05, RA11, RA24) were enrolled at Hospital for Special Surgery, New York (Supplemental Table 1). RA01 and RA03 patients were further studied after anti-TNFα was either added or substituted to their initial regimen several years later (Supplemental Table 1). All patients were off of corticosteroids for at least three months. All except RA11, RA24 and HD15 who is a 53 year old Hispanic female had been studied before any treatment (2). RA patients were compared to 6 adult healthy donors including HD01 (GO); HD02 (JB); HD03 (JH); HD10; HD11 (3, 6–8) and HD15. All blood samples were collected after individuals signed informed consent in accordance with IRB-reviewed protocols.
Single cell sorting
Peripheral B cells were purified from the blood of RA patients by positive selection with anti-human CD20 magnetic microbeads (Miltenyi Biotec). CD20-enriched peripheral B cells from RA patients were stained with anti-human CD27-FITC, anti-human CD10-PE, anti-human IgM-Biotin, and allophycocyanin (APC) anti-human CD19 (Pharmingen, Becton Dickinson). Biotinylated antibodies were revealed using PE-cy7 conjugated streptavidin (Pharmingen, Becton Dickinson). Single CD19+CD10−IgM+CD27−mature naïve B cells were sorted on a FACSVantage (Becton Dickinson) into 96-well PCR plates containing 4μl lysis solution (0.5X PBS containing 10 mM DTT, 8U RNAsin (Promega), 0.4U 5′-3′ RNAse Inhibitor (Eppendorf), and immediately frozen on dry ice. All samples were stored at −70°C. Additional flow cytometry analyses for regulatory T cells were performed using anti-CD62L-FITC, CD25-PE, CD4-PE-cy7, CD127-APC.
cDNA, RT-PCR, antibody production and purification
RNA from single cells was reverse-transcribed in the original 96 well plate in 12.5μl reactions containing 100U of Superscript II RT (Gibco BRL) for 45 minutes at 42°C. RT-PCR reactions, primer sequences, cloning strategy, expression vectors, antibody expression, and purification were as described (3). Ig sequences were analyzed by Ig BLAST comparison with GenBank. Heavy chain CDR3 was defined as the interval between the conserved cysteine at position 92 in the VH framework 3 and the conserved tryptophan at position 103 in JH segments.
ELISAs and immunofluorescence assays
Antibody concentration and reactivity against specific antigens were as described (3). A high (polyreactive ED38) HEp-2 reactive antibody was used as positive control in HEp-2 reactivity and polyreactivity ELISAs. This control antibody establishes that recombinant antibodies with absorbance values above 0.5 could be considered reactive with the coated antigen. Antibodies were polyreactive when they recognized 4 antigens that include single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), insulin and LPS.
For detection of anti-nuclear antibodies (ANAs), HEp-2 coated slides (Bion Enterprises) were incubated with purified recombinant antibodies at 50–100 μg/ml. FITC conjugated goat anti-human IgG was used as detection reagent.
RESULTS
Impacts of methotrexate and anti-TNFα agents on peripheral B cell subpopulations
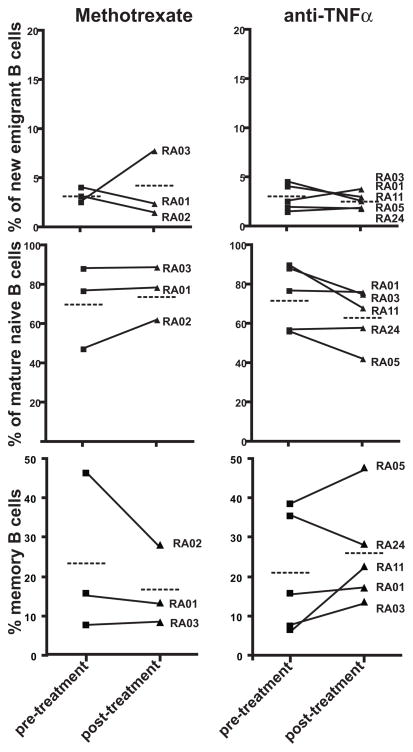
Methotrexate and anti-TNFα agents are the standard of care in RA (5). Recent reports analyzing B cell subpopulation changes in RA patients after anti-TNFα treatment provided conflicting results (9, 10). Hence, we assessed by flow cytometry the frequency of different B cell subpopulations in the blood of the same RA patients before and after therapy with methotrexate and/or anti-TNFα agents. We found that the frequency of CD19+CD10+IgMhiCD27− new emigrant/transitional B cells was not affected by any treatment and represented 1.5–8% of total B cells in all RA patients (Figure 1, top panel). The frequencies of CD19+CD10−IgM+CD27− mature naïve B cells and CD19+CD10−CD27+ memory B cells were not significantly altered by either methotrexate or anti-TNFα treatment (Figure 1, middle and lower panel). However, 3 out of 5 patients on anti-TNFα blockade showed decreased frequencies of naïve B cells and increased proportion of CD27+ circulating memory B cells as previously reported by others (10) (Figure 1, middle and lower panel).
Figure 1.
Effects of methotrexate and anti-TNFα treatment on peripheral B cell subpopulations. Comparative frequencies of CD19+CD10+IgMhiCD27− new emigrant B cell, CD19+CD10−IgM+CD27− mature naïve B cells and CD19+CD10−CD27+ memory B cells from RA patients before and after methotrexate and anti-TNFα therapies. Each symbol represents an individual and the average is shown with a dashed bar.
Expansion of CD62L− regulatory T cell population is induced by infliximab but not by other anti-TNFα blockade
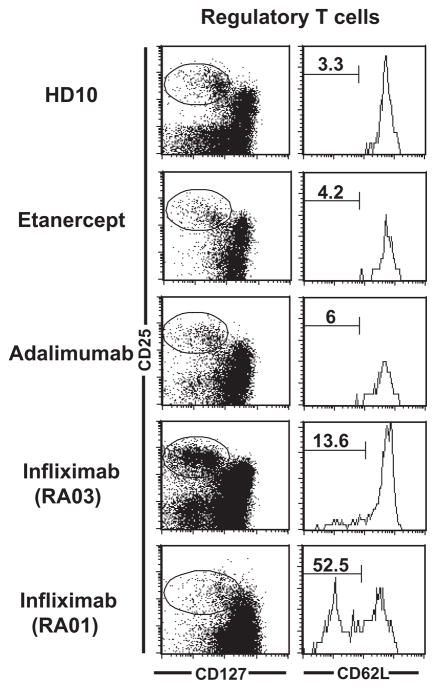
It has been reported that regulatory T (Treg) cell functions may be defective in RA patients and treatment with infliximab induces the expansion of potentially functional CD62L− Treg cells (11, 12). In agreement with these reports, we found that 4 and 2 month-long infliximab treatment induced the expansion of CD4+CD25hiCD127−/loCD62L−Treg cells in RA01 and RA03, respectively (Figure 2) (13, 14). In contrast, we found that treatment with other anti-TNFα agents, etanercept and adalimumab, did not generate CD62L− Treg cells. Indeed, their frequency remained very low and similar to control healthy donors and untreated RA patients (Figure 2). Hence, TNFα blockade in RA is not responsible for the induction of CD62L− Treg cells whose expansion seems to be specifically dependent on infliximab.
Figure 2.
Anti-TNFα agents do not all induce newly differentiated CD62L− Treg cells. Histograms show CD62L expression (right) on gated CD4+CD25hiCD127−/lo Treg cells (left). Newly differentiated CD62L− Treg cells are only induced by infliximab but not by etanercept and adalimumab anti-TNFα agents.
Methotrexate and anti-TNFα agent therapy do not correct the defective peripheral B cell tolerance checkpoint in RA
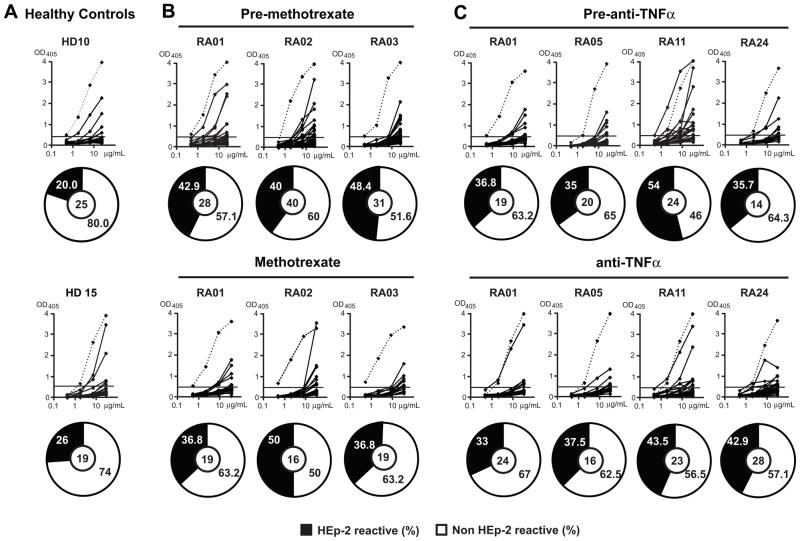
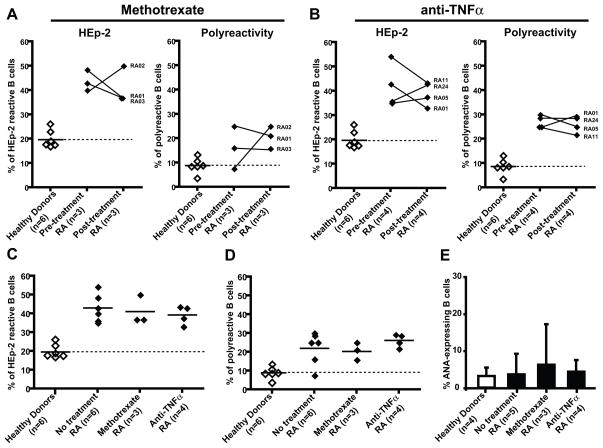
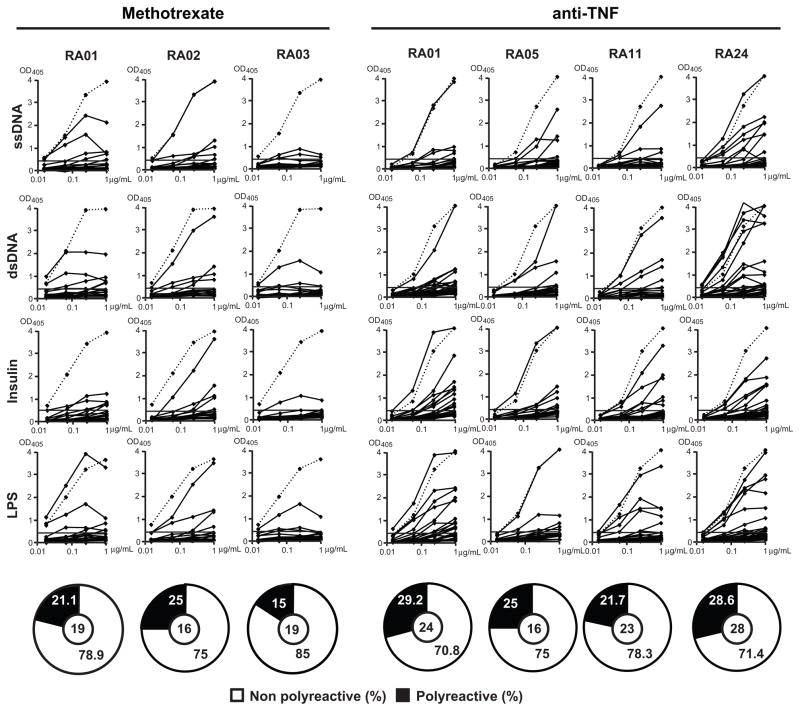
Methotrexate is one of the most commonly prescribed medications in RA and many RA patients respond positively to this inexpensive agent. In addition, anti-TNFα agents have shown remarkable efficacy in the treatment of RA (15). Moreover, anti-B cell therapy has demonstrated the important role for B cells in RA (1). We aimed to characterize the effects of methotrexate or anti-TNFα agents on the early defective B cell tolerance checkpoints in RA patients and determine whether methotrexate and anti-TNFα agents restore the removal of autoreactive B cells. We cloned and expressed in vitro 159 antibodies from single mature naïve B cells from the blood of RA patients before and after treatment with either methotrexate and/or anti-TNFα agents. Antibody reactivity was tested using ELISA and immunofluorescence assays (IFA) as previously described (3) (Supplemental tables 2–11). We found that the frequencies of HEp-2 reactive B cells from RA patients under methotrexate and/or anti-TNFα therapy represented 36.8–50 % (average: 41%) and 33–43.5% (average: 39%) respectively, and were not significantly different from an average frequency of 42% found in the same RA patients before treatment (Figure 3B, C, Figure 5C). In contrast, the frequencies of HEp-2 reactive clones in healthy donors only averaged 20% (Figure 5). Recombinant antibodies were also tested by ELISA for polyreactivity against four different antigens as previously reported (3). We found that after treatment with methotrexate and/or anti-TNFα polyreactive clones represented 20%–26% of mature naïve B cells. These frequencies were also similar to those of polyreactive clones in RA patients before treatment (22%, Figure 4 and Figure 5D). In addition, the frequencies of ANA-expressing B cells in treated RA patients were similar to those in untreated patients (3.8–5%) and were comparable to the frequencies of ANA-expressing mature naïve B cells in healthy donors (3.1%), suggesting that despite the global increase in autoreactive B cells in RA patients, ANA-expressing B cells are counterselected properly in these individuals (Figure 5E).
Figure 3.
High frequency of HEp-2 reactive mature naïve B cells from RA patients after methotrexate and anti-TNFα treatment. Antibodies from mature naïve B cells from healthy donors (A) and RA patients before and after treatment with methotrexate (B), and before and after anti-TNFα therapy (C) were tested by ELISA for anti-HEp-2 cell reactivity. Dotted lines show ED38 positive control. Horizontal lines show cut-off OD405 for positive reactivity. The frequency of HEp-2-reactive and non HEp-2-reactive clones is summarized in pie charts with the number of antibodies tested indicated in the centers.
Figure 5.
Methotrexate and anti-TNFα treatments do not correct the defective peripheral B cell tolerance checkpoint. Comparative frequencies of HEp-2 reactive and polyreactive clones before and after methotrexate or anti-TNFα treatment is depicted in (A) and (B) respectively. The frequencies of HEp-2 reactive (C) and polyreactive (D) B cells in healthy donors and RA patients before and after methotrexate and anti-TNFα treatment are shown. Each diamond is an individual and the average is represented with a bar. E, The percentages of ANA-expressing clones in mature naïve B cells from healthy donors, untreated RA patients and patients after either treatment are shown. Standard deviations are indicated.
Figure 4.
Mature naïve B cells from RA patients after methotrexate or anti-TNFα treatments express polyreactive antibodies. Antibodies from mature naïve B cells from RA patients after methotrexate and anti-TNFα treatment were tested by ELISA for reactivity with single-stranded DNA (ss-DNA), double-stranded DNA (ds-DNA), insulin and lipopolysaccharide (LPS). Dotted lines show ED38 positive control. Horizontal lines shoe cut-off OD405 for positive reactivity. For each individual, the frequency of polyreactive (in black) and non polyreactive (in white) clones is summarized in pie-charts with the number of antibodies tested indicated in the centers.
In conclusion, the proportion of autoreactive B cells in RA patients remained elevated after either methotrexate or anti-TNFα blockade, demonstrating that these anti-inflammatory agents do not correct the defective peripheral B cell tolerance checkpoint in RA.
DISCUSSION
A pathogenic role for B cells in autoimmune diseases has been clearly demonstrated in several mouse models and likely relies on antibody-independent B cell functions such as autoreactive T cell priming through antigen-presenting cell functions and/or cytokine production (16, 17). In humans, the success of B cell depleting anti-CD20 treatment in RA, type 1 diabetes and multiple sclerosis also underlines an important role for B cells in the development of these autoimmune diseases (1, 18, 19). We previously reported that active untreated patients with RA display abnormal early B cell tolerance checkpoints resulting in the accumulation of large numbers of autoreactive B cells in the periphery. However, it remained to be determined whether defects in early B cell tolerance checkpoints in RA were primary or secondary to the disease. We report herein that methotrexate and the three longest-available anti-TNFα therapies, which are widely used to inhibit inflammatory disorders, do not restore early B cell tolerance in RA patients, suggesting that B cell tolerance defects do not result from an imbalance of proinflammatory cytokines in these patients. Similarly, defects in early B cell tolerance checkpoints observed in active systemic lupus erythematosus (SLE) (20) persist in patients in clinical remission, suggesting that disease activity does not correlate with impaired autoreactive B cell removal (21).
TNFα plays a central role in the pathology of RA by recruiting leukocytes to the joints, inducing the secretion of pro-inflammatory cytokines such as IL-1 and participating in joint damage (15). TNFα may also impact early B cell development by modifying the frequency and absolute numbers of B cell subpopulations in the peripheral blood of RA patients (9, 10). However, conflicting results on B cell subpopulation alterations after anti-TNFα therapy have been reported by different groups (9, 10). These apparent discrepancies may result from the use of different anti-TNFα agents (9, 10). Nonetheless, studies following the same RA patients before and after anti-TNFα treatment tend to reach similar conclusions that suggest a decrease in mature naïve B cells in conjunction with an increase in memory B cells after anti-TNFα therapy (this report and (10)).
Anti-TNFα therapy has been reported to affect Treg cells, which may impact and suppress autoreactive B cells (22). Indeed, the anti-TNFα agent infliximab rescues the function of Treg and induces the emergence of a subset of CD62L−CD4+CD25hiFoxp3+ T cells (11, 23). In agreement with these reports, we also observed an increased frequency of CD62L− Treg in our patients treated with infliximab compared to their pre-treatment status. However, other anti-TNFα agents such as etanercept and adalimumab did not show any effects on Treg cell frequencies. The intrinsic nature of infliximab and adalimumab antibodies (chimeric vs. humanized) or delivery routes (intravenous vs. subcutaneous) may account for these differences. Nonetheless, anti-TNFα agents do not systematically impact Treg cell homeostasis and function, suggesting that these cells are not responsible for patients’ improvement after anti-TNFα therapy.
How do methotrexate and anti-TNFα therapies improve patients’ condition? We showed that anti-inflammatory effects of methotrexate and TNFα blockade do not restore the defective early B cell tolerance checkpoints in RA. However, methotrexate has been shown to reduce the release of pro-inflammatory cytokine such as IL-6 or TNFα, and to inhibit cell proliferation by interfering with DNA and RNA synthesis, potentially leading activated cells to apoptosis and cell death (24). In vitro, anti-TNFα agents showed cytotoxic effects on transmembrane TNFα-expressing cells (25). Anti-TNFα blockade can also induce the disruption of synovial lymphocyte aggregates and germinal centers as well as their follicular dendritic cell network, thereby blocking B cell activation (9, 26). Thus, patients’ improvement after methotrexate and anti-TNFα therapy is likely a result of blocking proinflammatory cytokines and inhibiting cell activation, downstream of early B cell tolerance checkpoints.
Thus, we demonstrate that the improvement of patients under methotrexate or anti-TNFα treatment is likely due to the anti-inflammatory effects of these drugs and not to a reestablishment of early B cell tolerance checkpoints, which may be controlled by intrinsic genetic factors. Indeed, we previously reported that BCR signaling was essential in regulating the central B cell tolerance checkpoint in humans (6). Little is known about BCR signaling defects in RA B cells, but abnormal BCR signaling has been reported in SLE B cells that also suffer from defective early B cell tolerance (20, 21, 27). More recently, the R620W missense polymorphism in the protein tyrosine phosphatase PTPN22 gene that segregates with RA, SLE and T1D has been shown to encode overactive PTPN22 phosphatases that alter BCR signaling (28–32). Hence, genetic alteration of BCR signaling threshold may result in defective early B cell tolerance checkpoints, which are likely to precede the onset of autoimmune diseases and favor the development of autoimmunity by producing large amounts of autoreactive B cells.
Supplementary Material
Acknowledgments
This work was supported by the Physician Scientist Development Award (American College of Rheumatology/Research and Education Foundation) to J. S. and by grants from the Department of Energy (DE-FG02-05ER64102) and AI061093 and AI071087 from NIH-NIAID to E. M.
We thank Dr. S. Rudchenko for assistance with the single cell sorter and Drs. D. Goldenberg and D. Orange for providing samples.
Footnotes
Disclosures
The authors declare no competing financial interests.
References
- 1.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–81. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 2.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. J Exp Med. 2005;201(10):1659–67. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 4.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Curr Opin Immunol. 2008;20(6):632–8. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 5.van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol. 2009;5(10):531–41. doi: 10.1038/nrrheum.2009.182. [DOI] [PubMed] [Google Scholar]
- 6.Ng YS, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase is essential for human B cell tolerance. J Exp Med. 2004;200(7):927–34. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29(5):746–57. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, et al. Complement receptor 2/CD21-negative human naïve B cells mostly contain autoreactive unresponsive clones. Blood. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anolik JH, Ravikumar R, Barnard J, Owen T, Almudevar A, Milner EC, et al. Cutting edge: anti-tumor necrosis factor therapy in rheumatoid arthritis inhibits memory B lymphocytes via effects on lymphoid germinal centers and follicular dendritic cell networks. J Immunol. 2008;180(2):688–92. doi: 10.4049/jimmunol.180.2.688. [DOI] [PubMed] [Google Scholar]
- 10.Souto-Carneiro MM, Mahadevan V, Takada K, Fritsch-Stork R, Nanki T, Brown M, et al. Alterations in peripheral blood memory B cells in patients with active rheumatoid arthritis are dependent on the action of tumour necrosis factor. Arthritis Res Ther. 2009;11(3):R84. doi: 10.1186/ar2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204(1):33–9. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108(1):253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2(5):364–71. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 16.Martin F, Chan AC. B cell immunobiology in disease: evolving concepts from the clinic. Annu Rev Immunol. 2006;24:467–96. doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 17.Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM. T cell activation in rheumatoid synovium is B cell dependent. J Immunol. 2001;167(8):4710–8. doi: 10.4049/jimmunol.167.8.4710. [DOI] [PubMed] [Google Scholar]
- 18.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361(22):2143–52. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 20.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–11. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yurasov S, Tiller T, Tsuiji M, Velinzon K, Pascual V, Wardemann H, et al. Persistent expression of autoantibodies in SLE patients in remission. J Exp Med. 2006;203(10):2255–61. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2(12):1126–32. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 23.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200(3):277–85. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47(3):249–55. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- 25.Mitoma H, Horiuchi T, Tsukamoto H, Tamimoto Y, Kimoto Y, Uchino A, et al. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum. 2008;58(5):1248–57. doi: 10.1002/art.23447. [DOI] [PubMed] [Google Scholar]
- 26.Klaasen R, Thurlings RM, Wijbrandts CA, van Kuijk AW, Baeten D, Gerlag DM, et al. The relationship between synovial lymphocyte aggregates and the clinical response to infliximab in rheumatoid arthritis: a prospective study. Arthritis Rheum. 2009;60(11):3217–24. doi: 10.1002/art.24913. [DOI] [PubMed] [Google Scholar]
- 27.Liossis SN, Kovacs B, Dennis G, Kammer GM, Tsokos GC. B cells from patients with systemic lupus erythematosus display abnormal antigen receptor-mediated early signal transduction events. J Clin Invest. 1996;98(11):2549–57. doi: 10.1172/JCI119073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75(2):330–7. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36(4):337–8. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 30.Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75(3):504–7. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michou L, Lasbleiz S, Rat AC, Migliorini P, Balsa A, Westhovens R, et al. Linkage proof for PTPN22, a rheumatoid arthritis susceptibility gene and a human autoimmunity gene. Proc Natl Acad Sci U S A. 2007;104(5):1649–54. doi: 10.1073/pnas.0610250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arechiga AF, Habib T, He Y, Zhang X, Zhang ZY, Funk A, et al. Cutting edge: the PTPN22 allelic variant associated with autoimmunity impairs B cell signaling. J Immunol. 2009;182(6):3343–7. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.