Abstract
Patients with prosthetic heart valves require chronic oral anticoagulation. In this clinical scenario, physicians must be mindful of the thromboembolic and bleeding risks related to chronic anticoagulant therapy. Currently, only vitamin K antagonists are approved for this indication. This paper reviews the main heart valve guidelines focusing on the use of oral anticoagulation in these patients.
Keywords: Heart valves, Anticoagulation, Vitamin K antagonist, Warfarin, Guidelines
Valvular heart disease continues to have a significant societal impact [1]. Every year in North America, approximately 100,000 patients undergo valve replacement procedures [1], with rheumatic and degenerative valve disease representing the most common indications for intervention. Excluding homografts, there are currently three types of commercially available valvular prostheses: mechanical valves, bioprosthetic valves, and transcatheter valves which were recently approved for endovascular implantation (e.g., Edwards Sapien and Medtronic CoreValve). The estimated risk of a thromboembolic event depends on the type of prosthesis and its anatomical position (aortic, mitral, or tricuspid). Patients with mechanical valves are at high risk for thromboembolic events, necessitating the use of vitamin K antagonists (VKAs). Patients with bioprosthetic valves are often treated with systemic anticoagulation for a shorter period of time following implantation. For valves placed endovascularly, the current major society guidelines do not consider these devices because they were not approved for clinical use at the time the guidelines were developed. However, recently published data from the PARTNER trial, an evaluation of transcatheter valves in patients at high operative risk for traditional aortic valve replacement, suggest that dual antiplatelet therapy (aspirin and clopidogrel) for 6 months after the procedure may be sufficient for prevention of thromboembolic events [2].
VKAs are the principal category of oral anticoagulants. Their clinical use dates back to the early 1940s [3]. In North America, warfarin is the sole representative of this class of drugs. Phenprocoumon, fluindione, and acenocoumarol are widely used in South America and Europe. The anticoagulant effects of VKAs are due to inhibition of vitamin K-dependent gamma-carboxylation of coagulation factors II, VII, IX, and X. The main difference among these compounds is their half-life, which is shortest with acenocoumarol, intermediate with warfarin and fluindione, and longest with phenprocoumon. There are no large trials comparing the efficacy of these drugs; however, small studies suggest that warfarin may be better than acenocoumarol in avoiding excessive anticoagulation and its sequelae [4]. Phenprocoumon seems to confer better international normalized ratio (INR) stability during patient follow-up when compared with warfarin or acenocoumarol [5, 6]. In addition to better stability, phenprocoumon has also recently been associated with fewer bleeding episodes than warfarin [7].
There are no universal dosage recommendations for VKAs, but rather each patient should have their therapy tailored via serial INR monitoring. The prospective, randomized SPORTIF II (Stroke Prevention Using Oral Thrombin Inhibitor in Atrial Fibrillation) trial showed that atrial fibrillation patients had INRs that were in the therapeutic range only 44% of the time during the first 12 weeks of therapy [8]. Recent pharmacogenetic studies have shed some light on the often marked individual response variability associated with VKAs [9–11]. VKAs are also known to have large and variable interactions with food and other drugs. Thus, the management of oral anticoagulation remains a challenge for both the clinician and patient.
In this paper, we review the current evidence and guidelines for antithrombotic therapy in patients with prosthetic heart valves.
Estimating individual bleeding risk
Before the implantation of an artificial heart valve, it is crucial to estimate the individual risk of bleeding. Mechanical heart valves are highly thrombogenic, and require a lifelong commitment to anticoagulant therapy. Several risk scores have been developed to help predict bleeding events [12, 13]; however, most of these scoring systems were created to assess bleeding risk in patients with atrial fibrillation and have not been validated in subjects with valvular heart disease alone. Age is by far the strongest predictor of hemorrhage [14, 15]. The risk of bleeding appears to steadily increase once a patient is >75 years old. Pharmacogenetic factors also seem to influence the risk of bleeding during anticoagulant therapy; in particular, polymorphisms of the cytochrome P450 CYP2C9 enzyme seem to confer greater risk [10]. The greater likelihood of bleeding is associated with the CYP2C9*2 and CYP2C9*3 alleles, which require lower mean daily warfarin dosing. Also, variation in the genes coding for vitamin K epoxide reductase (VKORC1) have been associated with hemorrhagic risk [9, 11]. Other comorbidities such as chronic kidney disease, previous gastrointestinal bleeding, anemia, and previous stroke or myocardial infarction have all been independently associated with major bleeding events [16] and led to the development of the Outpatient Bleeding Risk Index, a risk-scoring system. The Outpatient Bleeding Risk Index has been prospectively validated and is often a useful tool for helping to guide clinician decision making (Table 1). In general, the incidence of major bleeding in patients with mechanical valves treated with warfarin, or its derivatives, is approximately 1.4 per 100 patient-years [17, 18].
Table 1.
Outpatient bleeding risk index
| 1. Bleeding risk factors | Points assigned |
| Age ≥65 years | 1 |
| History of stroke | 1 |
| History of gastrointestinal bleeding | 1 |
| Recent MI or Hct< 30% or SCr> 1.5 mg/dl or Diabetes mellitus | 1 point maximum if any is checked |
| 2. Bleeding risk group | Total points assigned |
| Low | 0 = 3%/year |
| Intermediate | 1–2 = 12%/year |
| High | 3–4 = 48%/year |
MI myocardial infarction, Hct hematocrit, SCr serum creatinine Adapted from Am J Med 1998;105:91–99 [15]
Risk of thromboembolism
It is important to remember that each type of prosthetic heart valve has its own thrombogenicity profile. Mechanical heart valves have a significantly greater risk of thromboembolic phenomena compared with bioprosthetic valves. There are three basic types of mechanical valves: caged-ball, tilting-disk, and bileaflet valves. Their individual thrombogenicity is directly related to the materials and engineering characteristics of each design [19]. The caged-ball valve is the most thrombogenic, while bileaflet implants have lower thrombotic risk.
The risk of thrombosis is also directly related to valve position. Valves placed in the mitral position have an increased risk of thromboembolic complications as compared with those implanted in the aortic position [17]. This likely occurs due to higher blood flow velocity at the aortic valve orifice.
Bioprosthetic valves are heterografts made primarily of porcine or bovine tissue, each with comparable risks of thromboembolism. Homografts are either preserved human aortic valves or pulmonary autografts, usually implanted in the aortic position. It is generally believed that homografts and autografts have very low thrombogenicity and do not require systemic anticoagulation [20].
The incidence of major embolism (defined as causing death, residual neurologic deficit, or peripheral ischemia requiring surgery) in the absence of antithrombotic therapy for patients with mechanical valves has been suggested to be approximately 4 per 100 patient-years [17]. Initial studies suggested that patients with mechanical valves may have an even higher rate of major thromboembolic complications in the absence of systemic anticoagulation (23 per 100 patient-years) [21, 22]. The effect of different antithrombotic agents and combinations of agents on ischemic events is illustrated in Table 2. This risk decreases to 2.2 per 100 patient-years with only antiplatelet therapy, and is further reduced to 1 per 100 patient-years with warfarin, including valves in both the mitral and aortic positions [17].
Table 2.
Estimated incidence rates of valve thrombosis and major and total embolisms: effect of antithrombotic treatment
| Anticoagulation | Incidence rates per 100 patient-years
|
||
|---|---|---|---|
| Valve thrombosis | Major embolism | Total embolisma | |
| None | 1.8 | 4.0 | 8.6 |
| Antiplatelet | 1.6 | 2.2 | 8.2 |
| Dipyridamole | 4.1 | 5.4 | 11.2 |
| Aspirinb | 1.0 | 1.4 | 7.5 |
| Warfarin | 0.2 | 1.0 | 1.8 |
| Warfarin and antiplatelet | 0.1 | 1.7 | 3.2 |
Total embolism includes all reported incidences of valve thrombosis, major embolism, and minor embolism.
Aspirin alone or in combination with dipyridamole or pentoxifylline. Adapted from Circulation 1994;89:635–641 [17]
Anticoagulation intensity
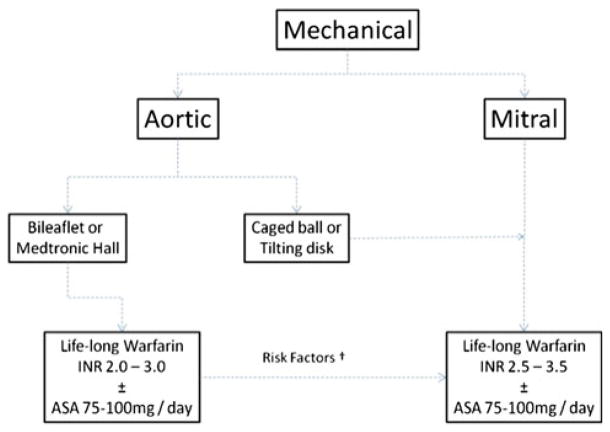
A Dutch study, reported in 1995 in the New England Journal of Medicine, sought to determine an appropriate “intensity goal” for anticoagulant therapy in patients with prosthetic heart valves [18]. The investigators found that the INR value necessary to achieve an optimal risk–benefit ratio between bleeding and thrombosis was between 2.5 and 4.9. To achieve this level of anticoagulation, the authors recommended targeting an INR of 3.0–4.0 postoperatively. In a more recent analysis published in 2009, the same investigators prospectively followed more than 4,000 patients and found that the optimal intensity of anticoagulation for those with mechanical heart valves was an INR of 2.5–2.9 [23]. This newly recommended target level of anticoagulation is lower than the previous level probably due to the smaller number of patients with older valve types. There were fewer patients with caged-ball and single-disk designs in this second cohort, and the prosthesis materials used today are less thrombogenic than those available in the past. Table 3 shows the risk of events according to INR levels in patients with mechanical heart valves [23]. The AREVA study compared moderate anticoagulation (INR of 2.0–3.0) versus the usual regimen (INR of 3.0–4.5) after single-valve replacement with a mechanical prosthesis. Investigators demonstrated that the rates of thromboembolic events were similar with each anticoagulation strategy. In addition, the incidence of hemorrhagic events was significantly lower in those patients with INR of 2.0–3.0 [24]. The German Experience With Low Intensity Anticoagulation (GELIA study) [25] also reported similar thromboembolic and bleeding outcomes among three different INR strata (3.0–4.5; 2.5–4.0; and the lower intensity 2.0–3.5). Summarizing the evidence, guidelines recommend that patients with mechanical valves in the aortic position should have an INR of 2.0–3.0 and an INR of 2.5–3.5 with valves in the mitral position. The same recommendation is made for caged-ball valves in the aortic position (Fig. 1).
Table 3.
Incidence rates of untoward events according to INR in patients with mechanical heart valves
| INR | All events, incidencea (95% CI) | Life-threatening and fatal events, incidencea (95% CI) |
|---|---|---|
| < 2 | 31.9 (5.7, 79.4) | 10.6 (0.0, 42.6) |
| 2.0–2.4 | 6.7 (0.6, 19.4) | 3.3 (0.0, 13.3) |
| 2.5–2.9 | 2.0 (0.2, 5.7) | 1.0 (0.1, 4.4) |
| 3.0–3.9 | 2.5 (1.3, 4.0) | 1.1 (0.4, 2.1) |
| 4.0–4.9 | 3.3 (1.6, 5.5) | 1.8 (0.6, 3.5) |
| ≥5.0 | 24.7 (13.3, 39.7) | 5.3 (0.9, 13.2) |
CI indicates confidence interval, INR international normalized ratio.
Per 100 patient-years. Adapted from Arch Intern Med 2009;169: 1203–1209 [22]
Fig. 1.
†Atrial fibrillation, previous thromboembolism, left ventricular EF ≤ 35%, hypercoagulable condition. Adapted from Lancet 2009;374:565–76 [55]. Simplified decision tree for choice of antithrombotic therapy in patients with mechanical heart valves
Antiplatelet therapies for thromboembolic prophylaxis
The addition of antiplatelet agents to VKAs has been shown to reduce the occurrence of embolic complications and even death [26–28]. Patients deemed to be at high risk for embolic events, including those with concomitant atrial fibrillation, previous thromboembolism, and mitral pros-theses, may derive a greater benefit from concomitant antiplatelet therapies [26]. However, the additional bleeding risk must not be overlooked. The use of aspirin has been associated with an increase in the likelihood of minor bleeding, but not in major bleeding [26, 28]. Using low-dose aspirin (100 mg) may minimize the risk for hemorrhagic complications without compromising the antithrombotic effects. A systematic review by the Cochrane collaboration also found that the addition of antiplatelet agents to the antithrombotic regimen in patients with prosthetic heart valves reduces the risk of thrombo-embolic events and also mortality [29]. The most common agents used in this metanalysis were aspirin and dipyridamole. One should keep in mind that this review also reported an increase in bleeding complications associated with the addition of aspirin, however, there appears to be a net clinical benefit with a decrease in thromboembolic risk offsetting the bleeding risk.
At present, there are no compelling data to support the addition of clopidogrel for patients with prosthetic heart valves. Several studies suggest that “triple” therapy (clopidogrel + aspirin + warfarin) may be associated with a significant increase in hemorrhagic complications [28, 30, 31]. Given the risk of bleeding observed among patients taking a VKA and clopidogrel, great care must be taken to avoid hemorrhagic complications among patients with prosthetic heart valves who have an additional requirement for an antiplatelet agent. Triple therapy should only be used for a short period of time (e.g., after stent implantation). The guidelines recommend stopping clopidogrel as soon as possible to decrease the risk of serious hemorrhage [32, 33]. Studies have already shown an increase in the risk of bleeding with this drug combination [30, 31]. If the concomitant use of these drugs is necessary, care should be taken during patient follow-up to ensure safety regarding bleeding risk. A randomized trial is underway to determine how we should proceed with anti-platelet therapy in patients already on chronic VKA therapy who receive a drug-eluting stent [34]; this may help further inform the safety of triple therapy.
The concept of using dual therapy with only aspirin and clopidogrel has been considered an undesirable strategy due to the high risk of embolic events seen in observational studies of patients with mechanical heart valves. Although several animal studies suggest that this combination can be a safe and acceptable substitute for VKA therapy, further data are required before advocating such a strategy for mechanical valve patients [35, 36]. The PROACT trial (ClinicalTrials.gov identifier NCT00291525) is currently randomizing low-risk patients undergoing aortic valve replacement with the On-X mechanical valve (On-X Life Technologies, Inc, Austin, TX) to receive either a combination of aspirin and clopidogrel or VKA therapy for thromboembolic prophylaxis. The On-X valve is composed of pure pyrolytic carbon which is believed to reduce its thrombogenicity relative to other mechanical valves [37]. Completion of the PROACT trial is anticipated in 2015.
Bioprosthetic valve anticoagulation
In terms of anticoagulation, current American Heart Association (AHA)/American College of Cardiology (ACC) and European Society of Cardiology (ESC) guidelines for bioprosthetic valves recommend anticoagulation during the first 90 postoperative days [29, 33]. The current American College of Chest Physicians (ACCP) guidelines recommend anticoagulation for bioprosthetic valves when in the mitral position [28]. However, these recommendations are generally weak and based on dated reports. In fact, in the absence of other thromboembolic risk factors, practicing clinicians commonly treat bioprosthetic valve patients with only antiplatelet therapy following implantation, a strategy supported by prospective observational data [38–40]. Recent clinical studies have supported anti-platelet therapy as sufficient for antithrombotic protection for bioprosthetic valves in the aortic position [34]. Thus, in the setting of a bioprosthetic valve, whether or not anticoagulation is needed in the early postoperative period remains a point of debate among clinicians and a point of uncertainty in the guidelines [34].
Patients with concomitant atrial fibrillation
Valvular atrial fibrillation is always considered a high-risk factor for arterial embolization. The occurrence of this arrhythmia in patients with a bioprosthetic mitral valve is a Class I indication for chronic VKA use. In patients with a mechanical heart valve and the presence of an additional risk factor, adding aspirin seems to confer a great clinical benefit. This strategy is supported by all of the major society guidelines and is based on randomized clinical data. The addition of aspirin also seems to be important for patients with bioprosthetic valves in the mitral position who develop atrial fibrillation [28, 29]. This strategy has been shown to reduce adverse clinical outcomes including stroke and death in a randomized, placebo-controlled clinical trial [28].
Time to start anticoagulation
There are no randomized data that address this important issue, however, all major guidelines [28, 29, 35] recommend starting VKA therapy in the first 24–48 h after the surgical procedure. This is also true for patients with bioprosthetic valves during the first 3 months after implantation. The recommended target INR in this situation is 2.5. However, in clinical practice, there is widespread use of aspirin (low dose: 75–100 mg) as an alternative to VKA therapy. The randomized TRAC study [41] included 193 patients and compared the use of VKA with antiplatelet agents in the first 3 months after valve implantation. There was no difference regarding the incidence of thromboembolic events, but the rate of hemorrhagic complications was significantly higher with the VKA strategy. An important limitation of this small trial is that the VKA used was acenocoumarol, a short-acting agent that is known to have a less stable anticoagulation profile when compared with longer acting agents [6]. In addition, the antiplatelet used in this study was not aspirin but triflusal, a derivate of salicylic acid.
Bridging therapy
Often, patients with mechanical valves require temporary interruption of VKA therapy for clinical or surgical reasons and will usually require some temporary anticoagulation during this time. This is referred to as a bridge therapy. The risk of thromboembolism during short-term interruption of anticoagulation is not well known. Mathematical modeling estimates suggest that the daily risk of thrombosis in patients with mechanical (mitral or aortic) heart valves is approximately 0.046% per day in the absence of VKA therapy [42]. Whether or not this represents an acceptable risk for short-term interruption of systemic anticoagulation is an important consideration for the treating clinician.
Risk stratification for thromboembolism is essential when evaluating the need for heparin or low molecular weight heparin bridging in patients with prosthetic heart valves. According to the ACCP guidelines [32], patients considered to be at the highest risk for thromboembolism (>10% risk per year) include those with a mitral valve prostheses, those with an older generation aortic valve prostheses (caged-ball, single-disk), or those with a history of stroke or transient ischemic attack (TIA) within the previous 6 months. Patients considered to be at moderate risk (4–10% per year) include those with a bileaflet aortic valve and any of the following: atrial fibrillation, prior stroke or TIA, or other risk factors for stroke (hypertension, diabetes, congestive heart failure, or age >75). Patients at the lowest risk (<4% per year) include those with bileaflet aortic valves and no other risk factors for stroke.
The ESC guidelines for the management of patients with prosthetic heart valves consider patients with mechanical valves in the mitral position as having an increased risk for thromboembolic events, regardless of valve type [33]. They also consider the presence of 1 of the following factors to be high risk comorbidities: previous thrombo-embolism, atrial fibrillation, left atrial diameter >50 mm, left atrial dense spontaneous echo contrast, mitral stenosis of any degree, left ventricular ejection fraction <35%, and any documented hypercoagulable state. In the ACC/AHA guidelines, risk factors for a thromboembolic event include atrial fibrillation, previous thromboembolism, left ventricular systolic dysfunction (<35%), and any hypercoagulable condition [33, 43, 44].
Management options for bridging therapy differ among the various guidelines. We believe that the use of bridging therapy is reasonable to consider in patients with a high risk for thromboembolic events. Low molecular weight heparin should be used in this patient category when there is a low perceived risk of bleeding. In patients with a higher bleeding risk (e.g., women, low body weight, end stage renal failure), hospital admission and the use of unfractionated heparin is encouraged. Tables 4 and 5 highlight the available guideline recommendations that should assist clinicians in managing anticoagulation for their patients with prosthetic heart valves.
Table 4.
Comparison among guidelines
| ACC/AHA 2006 guidelines/2008 focused update | ESC 2007 guidelines | ACCP 2008 guidelines | |
|---|---|---|---|
| Target INR according to valve type and location | Mechanical Aortica,b 2–3 Mitral 2.5–3.5 Aortic + Mitral 2.5–3.5 Multiple 2.5–3.2 |
See Table 5 | Mechanicalb Aorticc Mitral 2.5–3.5 Aortic + Mitral 2.5–3.5 Multiple 2.5–3.5 |
| Adding ASA to VKA | If high-risk factors,a add ASA (75–100 mg/day) May be reasonable to give clopidogrel (75 mg/day) if ASA C/I |
Add if concomitant CAD, PAD, or recurrent embolic event | Add ASA (50–100 mg/ day) for high-risk patients or previous embolic event |
| Bioprosthesis | ASA 75–100 mg/day Consider VKA if risk factorsa Risk factors with C/I to VKA consider a higher dose of ASA (75–325 mg/day) |
No evidence to support the long-term use of antiplatelet agents in patients who do not have an indication other than the presence of the bioprosthesis itself | ASA 50–100 mg/day Consider VKA if high risk conditiona |
| Bioprosthesis–VKA during first 3 months after implant in patients without risk factor for thromboembolism | Reasonable Target INR = 2.5a |
Yes (any position) Target INR = 2.5 |
Only in mitral valves Target INR = 2.5 |
| Anticoagulation during early postoperative period | Early use of UFH after prosthetic valve replacement—before warfarin achieves therapeutic levels—is controversial | IV UFH until INR is therapeutic Oral anticoagulation should be started during the first postoperative days |
IV UFH or subcutaneous LMWH until INR is therapeutic for 2 consecutive days |
| Bridge therapy | Should be considered in high-risk patients Preferably UFH Use of LMWH is not directly addressed |
Hospital admission in advance and bridge with IV UFH Recommend against LMWH as outpatient |
Unclear, though should be considered in high- risk patients Recommend considering use of LMWH as outpatient |
| Thromboembolic event during VKA | Add ASA and consider increasing INR target | Add ASA | Add ASA and consider increasing INR target |
Atrial fibrillation, previous thromboembolism, left ventricular dysfunction, and hypercoagulable condition (target INR, 3.0; range, 2.5–3.5).
Caged-ball or caged-disk valve, VKA therapy (target INR, 3.0; range, 2.5–3.5).
Additional risk factors for thromboembolism, such as atrial fibrillation, anterior-apical ST-segment elevation myocardial infarction, left atrial enlargement, hypercoagulable state, or low ejection fraction, we recommend VKA therapy (target INR, 3.0; range, 2.5–3.5). ACC indicates American College of Cardiology, ACCP American College of Chest Physicians, AHA American Heart Association, ASA aspirin, CAD coronary artery disease, C/I contraindicated, ESC European Society of Cardiology, INR international normalized ratio, IV intravenous, LMWH low molecular weight heparin, PAD peripheral artery disease, UFH unfractionated heparin, VKA vitamin K antagonist
Table 5.
ESC guide to target INR according to thrombosis risk
| Prosthesis thrombogenicityb | Patient-related risk factorsa
|
|
|---|---|---|
| No risk factor | ≥ 1 Risk factor | |
| Low | 2.5 | 3.0 |
| Medium | 3.0 | 3.5 |
| High | 3.5 | 4.0 |
Patient-related risk factors: mitral, tricuspid, or pulmonary valve replacement; previous thromboembolism; atrial fibrillation; left atrial diameter > 50 mm; left atrial dense spontaneous contrast; mitral stenosis of any degree; left ventricular ejection fraction < 35%; hypercoagulable state.
Prosthesis thrombogenicity: Low = Carbomedics (aortic position), Medtronic Hall, St Jude Medical (without Silzone); Medium = Bjork-Shiley, other bileaflet valves; High = Lillehei-Kaster, Omniscience, Starr-Edwards
Management of patients with an intracranial bleeding
The care of prosthetic heart valve patients during and after a catastrophic event, such as intracranial bleeding, is not entirely defined. Available data come from small patient series. Temporary discontinuation of warfarin for 1–2 weeks appears to be relatively safe [45, 46]. In the acute phase of bleeding, total reversal of the anticoagulation with vitamin K and fresh frozen plasma is required to avoid expansion of the bleeding that is directly related to mortality and neurological impairment. The decision to restart long-term VKA therapy in patients who have suffered a warfarin-related intracranial hemorrhage is often difficult [47]. After discussing risks with the patient and family, a reasonable approach is careful reintroduction of the VKA agent, often at a lower target INR level, as soon as the risk of a recurrent serious bleeding episode is judged to be lower than the risk of thromboembolism. Neurology or neurosurgical consultation may be helpful to assist in determining the risk of recurrent intracranial bleeding for a given patient.
Valve thrombosis
Valve thrombosis is a complication directly related to the type of valve and its position in the heart. The management of acute valve thrombosis is an urgent situation, which results in often life-threatening hemodynamic perturbations. There are no large clinical trials that address this condition. Whether to employ thrombolytic therapy or early surgical intervention remains a controversial subject. Surgical treatment is associated with high mortality. With lytic therapy there is great concern regarding the risk of cerebral embolization [48, 49] when treating left-sided valve thrombosis [50].
A small study comparing two protocols of streptokinase infusion (accelerated versus regular) found that the success rate of fibrinolytic therapy was low overall (59%), and particularly low in patients with New York Heart Association (NYHA) functional class III/IV symptoms (24%) [51]. In addition, the accelerated streptokinase infusion fared no better than the standard infusion. The ACC/AHA guidelines currently recommend that emergent surgery is most reasonable for patients with a thrombosed left-sided prosthetic valve and NYHA functional class III/ IV symptoms. Fibrinolytic therapy should be considered for patients in whom surgical intervention carries a prohibitively high risk or for those with absolute contraindications to surgery. In patients with a small clot burden who are NYHA functional class I or II, treatment with short-term intravenous unfractionated heparin therapy or continuous infusion of fibrinolytic therapy may be considered [43, 44]. The ACC/AHA guidelines also state that fibrinolytic therapy is reasonable for thrombosed right-sided prosthetic heart valves with NYHA functional class III/IV symptoms or a large clot burden.
The ESC guidelines [33] recommend urgent or emergent valve replacement as the treatment of choice for obstructive thrombosis in critically ill patients without serious comorbidity. Also recommended is implantation of a less thrombogenic prostheses in the place of the previously thrombosed one. Furthermore, fibrinolysis should be considered for the following situations: critically ill patients unlikely to survive surgery because of comorbidities or severely impaired cardiac function prior to developing valve thrombosis; cases in which surgery is not immediately available and the patient cannot be transferred; or thrombosis of tricuspid or pulmonary valve prostheses because of the higher success rate and lower incidence of embolism.
Ongoing and future clinical trials
PERIOP 2 (A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin) is a randomized trial that is currently enrolling patients to better evaluate the safety of bridging therapy [52]. Patients with both prosthetic heart valves and atrial fibrillation will be included; however, high-risk patients, such as those with caged-ball valves and prior stroke or TIA, are excluded.
The Randomized On-X Anticoagulation Trial [37] is currently testing the hypothesis that a specific design of prosthetic heart valve can be safely used in the mitral or aortic position with lower anticoagulation intensities. This is a longitudinal, randomized, multicenter study consisting of 20 centers in the United States enrolling no more than 1,200 patients (200 in each of 6 groups). The study consists of the following 3 arms and test therapies: (1) low-risk aortic valve replacement—aspirin plus clopidogrel; (2) high-risk aortic valve replacement—warfarin at INR of 1.5–2.0 plus aspirin; and (3) mitral valve replacement—warfarin at an INR of 2.0–2.5 plus aspirin. Each arm has an equivalent control and follow-up will continue through 5 years in each patient. The primary endpoints are the incidence of thromboembolic complications and bleeding events.
ESCAT III (Very Low Dose Oral Anticoagulation and Thromboembolic and Bleeding Complications) is another ongoing randomized trial in 1,800 patients with mechanical heart valve replacement [53]. The study aims to evaluate whether self-management of oral anticoagulation can reduce the risk of developing thromboembolic events and improve long-term survival compared with INR control by a general practitioner in mechanical heart valve recipients. During the first six postoperative months, low-dose INR self-management will be performed by all patients with INR measurement once a week. Thereafter, 600 patients will continue with this treatment regimen, whereas the other 1,200 patients will continue with very low-dose oral anticoagulation. Of these 1,200 patients, 600 will perform INR measurement once a week and 600 will perform INR measurement twice a week. Patients will be followed for 24 months. The main endpoints are thromboembolic events, bleeding events, and mortality.
The purpose of the EmbraceAC trial (Comparison of ATI-5923, a Novel Vitamin K Antagonist, With Warfarin in Patients Requiring Chronic Anticoagulation) [54] is to test an experimental drug, ATI-5923, against warfarin. The study is intended to demonstrate whether ATI-5923 is superior to warfarin for keeping INR values in the desired therapeutic range. Patients who require chronic anticoagulation with 1 or more of the following conditions are eligible for the study: atrial fibrillation or atrial flutter, prosthetic heart valve, venous thromboembolic disease, or history of myocardial infarction or cardiomyopathy.
Future directions
The future of patient management lies in ongoing advances in technology and pharmacology. Tissue engineering for the development of biological grafts with better long-term durability and less thrombogenic properties is one way to advance the care of patients with valvular heart disease. Additionally, the creation of new oral anticoagulant therapies with more predictable dose–response and safety profiles should be anticipated. Use of pharmacogenetics to better predict the response of an individual patient to anticoagulants may further guide therapeutic decisions in the future. In the meantime, careful appraisal of individual patients and their risk–benefit profile remains imperative for the management of this complex patient population.
Contributor Information
Tiago L. L. Leiria, Instituto de Cardiologia do Rio Grande do Sul/Fundação Universitária de Cardiologia, Porto Alegre, Brazil
Renato D. Lopes, Duke Clinical Research Institute, Division of Cardiology, Department of Medicine, Duke University Medical Center, Box 3850, Durham, NC 27710, UK
Judson B. Williams, Duke Clinical Research Institute, Division of Cardiovascular and Thoracic Surgery, Department of Surgery, Duke University Medical Center, Durham, NC, UK
Jason N. Katz, Division of Cardiology & Division of Pulmonary/Critical Care Medicine, University of North Carolina Center for Heart and Vascular Care, Chapel Hill, NC, UK
Renato A. K. Kalil, Instituto de Cardiologia do Rio Grande do Sul/Fundação Universitária de Cardiologia, Porto Alegre, Brazil. Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre, Brazil
John H. Alexander, Email: John.H.Alexander@duke.edu, Duke Clinical Research Institute, Division of Cardiology, Department of Medicine, Duke University Medical Center, Box 3850, Durham, NC 27710, UK
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni A. The anticoagulants heparin and the dicoumarin 3,3′-methylene-bis-(4-hydroxycoumarin) Bull N Y Acad Med. 1942;18:433–458. [PMC free article] [PubMed] [Google Scholar]
- 4.Oliva Berini E, Galan Alvarez P, Pacheco Onrubia AM. Comparison of quality and hemorragic risk of oral anticoagulant therapy using acenocoumarol versus warfarin. Med Clin (Barc) 2008;131:96–97. doi: 10.1157/13124012. [DOI] [PubMed] [Google Scholar]
- 5.Jensen CF, Christensen TD, Maegaard M, Hasenkam JM. Quality of oral anticoagulant therapy in patients who perform self management: warfarin versus phenprocoumon. J Thromb Thrombolysis. 2009;28:276–281. doi: 10.1007/s11239-008-0274-2. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Gad isseur AA, Pasterkamp E, et al. Comparison of control and stability of oral anticoagulant therapy using acenocoumarol versus phenprocoumon. Thromb Haemost. 2003;90:260–266. doi: 10.1160/TH02-10-0179. [DOI] [PubMed] [Google Scholar]
- 7.Leiria TL, Pellanda L, Miglioranza MH, et al. Warfarin and phenprocoumon: experience of an outpatient anticoagulation clinic. Arq Bras Cardiol. 2010;94:41–45. doi: 10.1590/s0066-782x2010000100008. [DOI] [PubMed] [Google Scholar]
- 8.Petersen P, Grind M, Adler J. Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. SPORTIF II: a dose-guiding, tolerability, and safety study. J Am Coll Cardiol. 2003;41:1445–1451. doi: 10.1016/s0735-1097(03)00255-9. [DOI] [PubMed] [Google Scholar]
- 9.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz UI, Ritchie MD, Bradford Y, et al. Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med. 2008;358:999–1008. doi: 10.1056/NEJMoa0708078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadamuro J, Dieplinger B, Felder T, et al. Genetic determinants of acenocoumarol and phenprocoumon maintenance dose requirements. Eur J Clin Pharmacol. 2010;66:253–260. doi: 10.1007/s00228-009-0768-7. [DOI] [PubMed] [Google Scholar]
- 12.Kakar P, Lane D, Lip GY. Bleeding risk stratification models in deciding on anticoagulation in patients with atrial fibrillation: a useful complement to stroke risk stratification schema. Chest. 2006;130:1296–1299. doi: 10.1378/chest.130.5.1296. [DOI] [PubMed] [Google Scholar]
- 13.Kuijer PM, Hutten BA, Prins MN, Buller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159:457–460. doi: 10.1001/archinte.159.5.457. [DOI] [PubMed] [Google Scholar]
- 14.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996;348:423–428. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 15.Fang MC, Chang Y, Hylek EM, et al. Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med. 2004;141:745–752. doi: 10.7326/0003-4819-141-10-200411160-00005. [DOI] [PubMed] [Google Scholar]
- 16.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998;105:91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 17.Cannegieter SC, Rosendaal FR, Briet E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation. 1994;89:635–641. doi: 10.1161/01.cir.89.2.635. [DOI] [PubMed] [Google Scholar]
- 18.Cannegieter SC, Rosendaal FR, Wintzen AR, et al. Optimal oral anticoagulant therapy in patients with mechanical heart valves. N Engl J Med. 1995;333:11–17. doi: 10.1056/NEJM199507063330103. [DOI] [PubMed] [Google Scholar]
- 19.Dasi LP, Simon HA, Sucosky P, Yoganathan AP. Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol. 2009;36:225–237. doi: 10.1111/j.1440-1681.2008.05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ElBardissi AW, DeBardino DJ, Chen FY, Yamashita MH, Cohn LH. Is early antithrombotic therapy necessary in patients with bioprosthetic aortic valves in normal sinus rhythm? J Thorac Cardiovasc Surg. 2010;139:1137–1145. doi: 10.1016/j.jtcvs.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 21.Baudet EM, Puel V, McBride JT, et al. Long-term results of valve replacement with the St. Jude Medical prosthesis. J Thorac Cardiovasc Surg. 1995;109:858–870. doi: 10.1016/S0022-5223(95)70309-8. [DOI] [PubMed] [Google Scholar]
- 22.Bjork VO, Henze A. Management of thrombo-embolism after aortic valve replacement with the Bjork-Shiley tilting disc valve. Medicamental prevention with dicumarol in comparison with dipyridamole—acetylsalicylic acid. Surgical treatment of prosthetic thrombosis. Scand J Thorac Cardiovasc Surg. 1975;9:183–191. doi: 10.3109/14017437509138637. [DOI] [PubMed] [Google Scholar]
- 23.Torn M, Cannegieter SC, Bollen WL, et al. Optimal level of oral anticoagulant therapy for the prevention of arterial thrombosis in patients with mechanical heart valve prostheses, atrial fibrillation, or myocardial infarction: a prospective study of 4202 patients. Arch Intern Med. 2009;169:1203–1209. doi: 10.1001/archinternmed.2009.176. [DOI] [PubMed] [Google Scholar]
- 24.Acar J, Iung B, Boissel JP, et al. AREVA: multicenter randomized comparison of low-dose versus standard-dose anticoagulation in patients with mechanical prosthetic heart valves. Circulation. 1996;94:2107–2112. doi: 10.1161/01.cir.94.9.2107. [DOI] [PubMed] [Google Scholar]
- 25.Hering D, Piper C, Bergemann R, et al. Thromboembolic and bleeding complications following St. Jude medical valve replacement: results of the German experience with low-intensity anticoagulation study. Chest. 2005;127:53–59. doi: 10.1378/chest.127.1.53. [DOI] [PubMed] [Google Scholar]
- 26.Massel D, Little SH. Risks and benefits of adding anti-platelet therapy to warfarin among patients with prosthetic heart valves: a meta-analysis. J Am Coll Cardiol. 2001;37:569–578. doi: 10.1016/s0735-1097(00)01135-9. [DOI] [PubMed] [Google Scholar]
- 27.Larson RJ, Fisher ES. Should aspirin be continued in patients started on warfarin? J Gen Intern Med. 2004;19:879–886. doi: 10.1111/j.1525-1497.2004.30419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turpie AG, Gent M, Laupacis A, et al. A comparison of aspirin with placebo in patients treated with warfarin after heart-valve replacement. N Engl J Med. 1993;329:524–529. doi: 10.1056/NEJM199308193290802. [DOI] [PubMed] [Google Scholar]
- 29.Little SH, Massel DR. Antiplatelet and anticoagulation for patients with prosthetic heart valves. Cochrane Database Syst Rev. 2003;4:CD003464. doi: 10.1002/14651858.CD003464.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manzano-Fernandez S, Marin F, Pastor-Perez FJ, et al. Increased major bleeding complications related to triple anti-thrombotic therapy usage in patients with atrial fibrillation undergoing percutaneous coronary artery stenting. Chest. 2008;134:559–567. doi: 10.1378/chest.08-0350. [DOI] [PubMed] [Google Scholar]
- 31.Rogacka R, Chieffo A, Michev I, et al. Dual antiplatelet therapy after percutaneous coronary intervention with stent implantation in patients taking chronic oral anticoagulation. JACC Cardiovasc Interv. 2008;1:56–61. doi: 10.1016/j.jcin.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Salem DN, O’Gara PT, Madias C, Pauker SG. Valvular and structural heart disease: American college of chest physicians evidence-based clinical practice guidelines (8th edn) Chest. 2008;133:593S–629S. doi: 10.1378/chest.08-0724. [DOI] [PubMed] [Google Scholar]
- 33.Vahanian A, Baumgartner H, Bax J, et al. Guidelines on the management of valvular heart disease: the task force on the management of valvular heart disease of the European society of cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 34.ClinicalTrials. gov Identifier: NCT00776633. [Accessed 14 Jan 2011]. Triple Therapy in Patients on Oral Anticoagulation after Drug Eluting Stent Implantation (ISAR-TRIPLE) [Google Scholar]
- 35.Schlitt A, Hauroeder B, Buerke M, et al. Effects of combined therapy of clopidogrel and aspirin in preventing thrombus formation on mechanical heart valves in an ex vivo rabbit model. Thromb Res. 2002;107:39–43. doi: 10.1016/s0049-3848(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 36.McKellar SH, Thompson JL, III, Garcia-Rinaldi RF, Macdonald RJ, Sundt TM, III, Schaff HV. Short- and long-term efficacy of aspirin and clopidogrel for thromboprophylaxis for mechanical heart valves: an in vivo study in swine. J Thorac Cardiovasc Surg. 2008;136:908–914. doi: 10.1016/j.jtcvs.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 37.Butany J, Ahluwalia MS, Munroe C, et al. Mechanical heart valve prostheses: identification and evaluation. Cardiovasc Pathol. 2003;12:1–22. doi: 10.1016/s1054-8807(02)00128-x. [DOI] [PubMed] [Google Scholar]
- 38.Gherli T, Colli A, Fragnito C, et al. Comparing warfarin with aspirin after biological aortic valve replacement: a prospective study. Circulation. 2004;110:496–500. doi: 10.1161/01.cir.0000137122.95108.52. [DOI] [PubMed] [Google Scholar]
- 39.Colli A, Verhoye JP, Heijmen R, et al. Antithrombotic therapy after bioprosthetic aortic valve replacement: ACTION registry survey results. Eur J Cardiothorac Surg. 2008;33:531–536. doi: 10.1016/j.ejcts.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Colli A, D’Amico R, Mestres CA, et al. Is early anti-thrombotic therapy necessary after tissue mitral valve replacement? J Heart Valve Dis. 2010;19:405–411. [PubMed] [Google Scholar]
- 41.Aramendi JI, Mestres CA, Martinez-Leon J, Campos V, Munoz G, Navas C. Triflusal versus oral anticoagulation for primary prevention of thromboembolism after bioprosthetic valve replacement (trac): prospective, randomized, co-operative trial. Eur J Cardiothorac Surg. 2005;27:854–860. doi: 10.1016/j.ejcts.2004.12.064. [DOI] [PubMed] [Google Scholar]
- 42.Kearon C, Hirsh J. Management of anticoagulation before and after elective surgery. N Engl J Med. 1997;336:1506–1511. doi: 10.1056/NEJM199705223362107. [DOI] [PubMed] [Google Scholar]
- 43.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 44.Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 45.Garcia DA, Regan S, Henault LE, et al. Risk of thrombo-embolism with short-term interruption of warfarin therapy. Arch Intern Med. 2008;168:63–69. doi: 10.1001/archinternmed.2007.23. [DOI] [PubMed] [Google Scholar]
- 46.Wijdicks EF, Schievink WI, Brown RD, Mullany CJ, et al. The dilemma of discontinuation of anticoagulation therapy for patients with intracranial hemorrhage and mechanical heart valves. Neurosurgery. 1998;42:769–773. doi: 10.1097/00006123-199804000-00053. [DOI] [PubMed] [Google Scholar]
- 47.Babikian VL, Kase CS, Pessin MS, Caplan LR, Gorelick PB. Resumption of anticoagulation after intracranial bleeding in patients with prosthetic heart valves. Stroke. 1988;19:407–408. [PubMed] [Google Scholar]
- 48.Roudaut R, Lafitte S, Roudeaut MF, et al. Management of prosthetic heart valve obstruction: fibrinolysis versus surgery. Early results and long-term follow-up in a single-centre study of 263 cases. Arch Cardiovasc Dis. 2009;102:269–277. doi: 10.1016/j.acvd.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Gupta D, Kothari SS, Bahl VK, et al. Thrombolytic therapy for prosthetic valve thrombosis: short- and long-term results. Am Heart J. 2000;140:906–916. doi: 10.1067/mhj.2000.111109. [DOI] [PubMed] [Google Scholar]
- 50.Caceres-Loriga FM, Perez-Lopez H, Morlans-Hernandez K, et al. Thrombolysis as first choice therapy in prosthetic heart valve thrombosis. A study of 68 patients. J Thromb Thrombolysis. 2006;21:185–190. doi: 10.1007/s11239-006-4969-y. [DOI] [PubMed] [Google Scholar]
- 51.Karthikeyan G, Math RS, Mathew N, et al. Accelerated infusion of streptokinase for the treatment of left-sided prosthetic valve thrombosis: a randomized controlled trial. Circulation. 2009;120:1108–1114. doi: 10.1161/CIRCULATIONAHA.109.876706. [DOI] [PubMed] [Google Scholar]
- 52.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 53.Kulik A, Le May M, Wells GA, Mesana TG, Ruel M. The clopidogrel after surgery for coronary artery disease (CASCADE) randomized controlled trial: clopidogrel and aspirin versus aspirin alone after coronary bypass surgery [ NCT00228423] Curr Control Trials Cardiovasc Med. 2005;6:15. doi: 10.1186/1468-6708-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 55.Sun JC, Davidson MJ, Lamy A, Eikelboom JW. Anti-thrombotic management of patients with prosthetic heart valves: current evidence and future trends. Lancet. 2009;374:565–576. doi: 10.1016/S0140-6736(09)60780-7. [DOI] [PubMed] [Google Scholar]