Abstract
Context
The safety and durability of endoscopic vein harvest (EVH) in coronary artery bypass graft surgery (CABG) has recently been called into question.
Objective
To compare the long-term outcomes of endoscopic versus open vein graft harvesting for Medicare patients undergoing CABG surgery in the United States of America.
Design, Setting, and Patients
This is an observational study of 235,394 Medicare patients undergoing isolated CABG from 2003 to 2008 at 934 surgical centers participating in the Society of Thoracic Surgeons (STS) national database. STS records were linked to Medicare files to allow longitudinal assessment (median follow-up 3 years) through December 2009.
Main Outcome Measure
Primary: death; Secondary: wound complications and the composite of myocardial infarction (MI) and revascularization.
Results
Based on Medicare Part B coding, 52% of patients received EVH during CABG, less often in females than males (but <2% difference). After propensity score adjustment for clinical characteristics, there were no significant differences between long-term mortality (12,429/122,899 [13.2%] vs. 13,096/112,495 [13.4%]) and the composite of death, MI and revascularization (18,419/122,899 [19.5%] vs. 19,232/112,495 [19.7%])for those receiving EVH vs. open, adjusted hazard ratio [HR] 1.0 (95% confidence interval [CI] 0.97, 1.04) for mortality and 1.0 (95% CI 0.98, 1.05) for the composite outcome. EVH was associated with lower harvest site wound complications relative to open procedure (3,654/122,899 [2.97%] vs. 4,047/112,495 [3.6%]), adjusted HR 0.83 (95 % CI 0.77, 0.89; p<0.0001).
Conclusions
Among patients undergoing CABG, the use of endoscopic vein-graft harvest compared with open vein graft harvest was not associated with increased mortality.
In the mid 1990’s, surgeons began using endoscopic vein-graft harvesting (EVH) techniques as an alternative to large, incision-based open vein harvesting to improve postoperative discomfort and incision-site complications.1–3 EVH involves use of devices cleared by the Food and Drug Administration (FDA) based on substantial equivalence. The perceived advantages of EVH led to widespread adoption of the technique, and the devices have been employed in the majority of the more than 400,000 coronary artery bypass grafting (CABG) procedures performed at U.S. surgical centers each year.4
Carefully conducted randomized controlled trials demonstrated the short-term safety and efficacy of EVH, but did not assess long-term outcomes following EVH. In 2009, a large observational study called into question the safety of endoscopic vein-graft harvesting.5 That study examined 3000 CABG patients enrolled in the PREVENT IV trial and found that those receiving EVH had a higher risk of 1-year angiographic vein graft failure and higher 3-year mortality than those receiving open harvesting.5 Proposed biological mechanisms for EVH harm included potentially greater vessel manipulation, venous stasis during harvest caused by the pressurized subcutaneous tunnel, and larger caliber segments of harvested vein with endoscopic techniques. The PREVENT IV findings were not confirmed in one regional study.6
To further assess EVH use in CABG and risk of death, myocardial infarction, and repeat revascularization, the U.S. FDA issued a request for proposal for use of the Society of Thoracic Surgeons’ Adult Cardiac Surgery Database. This data source was linked to U.S. administrative data sources to provide long-term follow-up and outcome assessment among Medicare patients.
METHODS
Data Sources
The Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database (ACSD) is the largest specialty-specific clinical data registry in the world. The STS ACSD currently houses data from 1,091 participants, representing nearly 90% of the cardiac surgery providers in the U.S. Participating centers report more than 300 data elements for each episode of cardiac surgery using a standardized data collection form. Race and ethnicity data were collected by open-ended patient designation and utilized based on previous literature reporting differences in surgical procedure utilization and outcomes associated with these variables. In collaboration with the Duke Clinical Research Institute’s outcomes research group, the STS has developed mortality, morbidity, and length-of-stay risk models for CABG and other major adult cardiac surgery procedures.4,7 The quality of the STS ACSD data has been assessed in a regional independent chart abstraction study, which documented a 96% correlation between submitted and re-abstracted data elements.8
The STS ACSD has previously been limited to perioperative episodes of care with outcomes truncated at 30 days or hospital discharge. For the purposes of this study, we utilized the capability to link STS ACSD registry files with two administrative databases: the Social Security Administration’s Death Master File and the Centers for Medicare and Medicaid Services (CMS) Parts A and B claims database.9 STS ACSD records were matched with Medicare inpatient claims data using previously validated deterministic matching techniques and indirect identifiers including admission date, discharge date, patient age, and hospital center.10 Linkage with Medicare Part A data afforded the measurement of mid- to long-term outcomes.
Institutional review board approval and the processing of data sharing agreements were achieved prior to proceeding with any analyses. A waiver of patient informed consent was applied for and obtained from the Duke University Institutional Review Board.
Identification of Treatment Assignment
Because the STS ACSD only began collecting information on harvest technique (endoscopic vs. open) on January 1, 2008, linkage to Medicare Part B data (professional billing) was used to determine whether or not CABG cases utilized EVH technique. Endoscopic harvest technique is coded in Medicare Part B claims with Current Procedural Terminology (CPT) code 33508.
Study Population
The study population consisted of primary isolated CABG patients having at least one vein graft and enrolled in the STS ACSD between 2003 and 2008 (Figure 1). Exclusions were the following: (1) emergent/salvage procedure; (2) prior CABG; (3) radial artery or right internal mammary artery grafting; and (4) patients without an internal mammary artery graft. Presence of at least one vein graft was confirmed by STS ACSD records and CMS part B CPT codes 33510 to 33523 occurring on the same day as the CABG procedure in the corresponding CMS part A claim.
Figure 1.
Patient flow chart
Study Outcomes
The primary outcome measure was all-cause mortality, obtained through the linkage of STS registry data to the Social Security Administration’s Death Master File. Inpatient mortality was determined using the STS ACSD and post-discharge mortality was identified using the Medicare denominator file. Myocardial infarction (MI) and revascularization requiring rehospitalization were identified using Medicare Part A data. The International Classification of Diseases, 9th Revision [ICD-9] codes 410.x1 were used to identify MI following hospital discharge. For revascularization, ICD-9 codes 36.10–19 (CABG) and 0066, 36.01–09, 3602 (percutaneous coronary intervention [PCI]) were used.
Wound complication was identified in-hospital following CABG using the STS ACSD. The STS ACSD defines harvest site wound complication by the presence of any of the following: wound opened with excision of tissue (incision and drainage); positive culture; or treated with antibiotics. Leg wound complications occurring following hospital discharge and within the first post-operative month were identified through Medicare Part A data (disruption of operative wound, 998.32; post-operative infection, 998.5x; non-healing surgical wound, 998.83). Any episode of systemic sepsis occurring following hospital discharge and within the first post-operative month was considered surgery-related, and was identified through Medicare Part A data (sepsis/systemic inflammatory response syndrome, 995.90–995.92, 998.59; septicemia, 038.0x-038.9x).
Statistical Analysis
Demographic and operative characteristics were categorized by endoscopic vs. open harvesting technique (Table 1). Baseline characteristics were summarized as percentages for categorical variables and as medians with 25th and 75th percentiles for continuous variables. Baseline characteristics were also summarized for STS ACSD patients meeting study inclusion criteria who were successfully linked versus not linked with Medicare files.
Table 1.
Selected patient demographic and operative variables for 235,394 study patients before and after propensity score adjustment using inverse probability weighting
| Before Propensity Score Adjustment |
After Propensity Score Adjustment |
|||
|---|---|---|---|---|
| Variable | Endoscopic Harvest |
Open Harvest | Endoscopic Harvest |
Open Harvest |
| Number of patients | 122,899 | 112,495 | 122,899 | 112,495 |
| Age in years (SD) | 73.6 (5.70) | 73.6 (5.67) | 73.6 (5.69) | 73.6 (5.68) |
| Female | 30.7 | 32.3 | 31.4 | 31.5 |
| Race | ||||
| White | 90.1 | 89.0 | 89.6 | 89.6 |
| Black | 4.3 | 4.3 | 4.3 | 4.3 |
| Asian | 1.1 | 1.2 | 1.1 | 1.1 |
| Other | 2.9 | 3.2 | 3.1 | 3.1 |
| Ethnicity: Hispanic | 1.5 | 2.3 | 1.9 | 1.9 |
| Height in cm (SD) | 170.7 (10.9) | 170.4 (10.9) | 170.6 (10.89) | 170.6 (10.83) |
| BMI in kg/m2 (SD) | 28.5 (5.36) | 28.4 (5.30) | 28.5 (5.32) | 28.5 (5.32) |
| Peripheral vascular disease | 17.9 | 18.0 | 18.0 | 17.9 |
| Current smoker | 13.9 | 13.5 | 13.7 | 13.7 |
| Dyslipidemia | 78.4 | 74.4 | 76.4 | 76.4 |
| Hypertension | 84.5 | 82.7 | 83.6 | 83.6 |
| Non-insulin dependent diabetes mellitus | 27.6 | 27.2 | 27.4 | 27.4 |
| Insulin dependent diabetes mellitus | 10.9 | 9.9 | 10.0 | 10.0 |
| Immunosuppressive treatment | 2.3 | 2.1 | 2.2 | 2.2 |
| Atrial fibrillation | 6.8 | 7.2 | 7.0 | 7.0 |
| History of stroke or TIA | 12.5 | 12.2 | 12.4 | 12.4 |
| History of CHF | 14.4 | 15.2 | 14.8 | 14.8 |
| Preoperative dialysis | 1.5 | 1.5 | 1.5 | 1.5 |
| Unstable angina | 25.5 | 25.3 | 25.4 | 25.4 |
| Previous MI within 24 hrs. | 1.6 | 1.5 | 1.5 | 1.5 |
| Any prior PCI | 18.8 | 18.2 | 18.5 | 18.5 |
| Preoperative IABP or inotrope | 5.4 | 6.1 | 5.8 | 5.8 |
| Procedure status: elective | 51.1 | 50.8 | 51.0 | 51.1 |
| Procedure status: urgent | 48.9 | 49.2 | 49.0 | 48.9 |
| Preoperative shock | 0.58 | 0.70 | 0.65 | 0.64 |
| Left main coronary artery disease |
33.4 | 31.9 | 32.6 | 32.6 |
| Diseased coronary vessel: <2 | 1.5 | 1.9 | 1.7 | 1.7 |
| Diseased coronary vessel: 2 | 17.1 | 18.4 | 17.8 | 17.7 |
| Diseased coronary vessel: 3 | 81.4 | 79.7 | 80.5 | 80.6 |
| Academic center location | 11.8 | 9.7 | 10.6 | 10.6 |
| Geographic region | ||||
| Midwest | 29.7 | 38.1 | 33.8 | 33.8 |
| Northeast | 14.6 | 12.6 | 13.6 | 13.6 |
| South | 43.8 | 35.0 | 39.3 | 39.5 |
| West | 11.9 | 14.3 | 13.2 | 13.1 |
| Surgical year | ||||
| 2003 | 9.0 | 24.1 | 16.4 | 16.3 |
| 2004 | 13.3 | 19.9 | 16.5 | 16.5 |
| 2005 | 17.0 | 17.0 | 17.0 | 17.0 |
| 2006 | 19.5 | 14.8 | 17.2 | 17.2 |
| 2007 | 19.9 | 12.9 | 16.6 | 16.6 |
| 2008 | 21.1 | 11.3 | 16.4 | 16.4 |
Abbreviation: CABG, coronary artery bypass grafting; MI, myocardial infarction; IABP, intra-aortic balloon pump; SD, standard deviation; BMI, body mass index; CHF, congestive heart failure; PCI, percutaneous coronary intervention; GFR, glomerular filtration rate.
Propensity scores with inverse probability-weights (IPW) were developed to adjust for differences in baseline characteristics between the two treatment groups. The propensity score represents the estimated probability of patients receiving endoscopic vs. direct vein harvest as a function of the covariates in the propensity model.11 Propensity scores were estimated using a non-parsimonious logistic regression model, including each of the variables listed in Table 1.
The ability of the propensity model to balance the two treatment groups was assessed in two ways. First, we compared the distribution of estimated propensity scores in the two treatment groups to ensure that there was a high degree of overlap. The 5-number summaries (minimum, 25th, 50th, 75th, maximum) of the propensity distributions in each treatment group were similar (endoscopic: 14.5%, 48.6%, 58.7%, 65.8%, 84.7%); open: 14.2%, 35.7%, 49.1%, 59.8%, 80.0%), suggesting that comparisons based on the propensity score were statistically appropriate. To further increase the comparability between the two groups, patients with propensity scores that were not in the range of overlapping propensity distributions (i.e. <14.5% or >80.0%) were removed from the risk adjusted analysis. Next, we compared the distribution of patient characteristics across the two treatment groups before and after weighting the observations based on the propensity score. After propensity weighting, the observed differences in covariates were small, and in all cases were less than 1% of the estimated standard deviation.12
For comparison of wound complications within the first post-operative month, the unadjusted odds ratio (OR) was estimated using generalized estimating equation (GEE) models to account for potential clustering of similar patients within sites and using a logit link function having a single covariate for treatment group.13 The adjusted OR was estimated by fitting a similar GEE model and weighting each observation by the inverse of the estimated propensity score (IPW).14 Robust sandwich variance estimates were used to obtain 95% confidence intervals. Statistical tests were 2-sided and performed at the 5% level of significance.
The difference between treatment groups in long term all-cause mortality and the composite of death, MI, and revascularization were compared by time to event analyses. Patient follow-up was considered to be censored at the end of the study period (December 31, 2008). The unadjusted hazard ratio (HR) for endoscopic versus open vein harvest, was estimated in a Cox regression model with a single treatment group indicator, stratified by surgical year to allow year-specific baseline hazard. The risk adjusted HR was estimated by fitting the similar model and weighting each observation by the inverse of the estimated propensity score.15 To account for the correlation of patients’ failure time within the same participant site (cluster), a robust sandwich covariance estimator with an independent working covariance matrix was used to obtain 95% confidence intervals of coefficients under the assumption of a common baseline hazard within a cluster.16 The unadjusted mortality cumulative incidence rate was estimated for each treatment group using the product-limit method of Kaplan and Meier;17 the propensity-adjusted incidence rate was calculated for each treatment group using the Breslow estimator based on the IPW Cox model.18
Sensitivity Analyses
Because the STS ACSD only began collecting information on harvest technique (endoscopic vs. open) on January 1, 2008, the 2008 data have EVH coding from both CMS carrier claim and STS ACSD. For each individual center performing CABG, the sensitivity and specificity of Medicare Part B capture of endoscopic vs. open vein harvest was calculated using 2008 STS ACSD reporting of EVH as the reference standard. Centers having >80% sensitivity and specificity for EVH coding, which included 44,423 patients at 165 sites, were also identified for a planned sensitivity analysis to evaluate the potential effect of EVH data collection error. Among these 165 sites, the sensitivity and specificity of identifying EVH was 93% and 96% respectively.
Because our primary Cox model analysis did not account for measurement error in the Medicare-derived EVH variable, we re-fit the model using the estimation technique of corrected score estimation as described by Zucker and Spiegelman.19 The published corrected score estimation methodology was modified to allow for tied observations, stratum variables, and center-level clustering and the simplifying assumption was made that measurement error was nondifferential and could be modeled by two parameters, sensitivity and specificity. The association of interest was then estimated across a range of plausible estimates for sensitivity (0.60 to 1.00) and specificity (0.85 to 1.00) based upon our previous study of EVH coding accuracy using the PREVENT IV database and 2008 STS data.
The influence of unmeasured confounders on the estimated HR of death or the composite of death, MI, or revascularization for the endoscopic vs. open vein harvest groups was further evaluated using the method of Lin et al.20 This method utilizes a regression model including the exposure of interest (vein harvest method) as well as measured and unmeasured confounders to make statistical inferences about the true exposure effect by specifying distributions of an unmeasured confounder in the study groups along with effects of an unmeasured confounder on outcomes. The effect of departure from randomization due to unbalanced prevalence of an unmeasured confounder was assessed upon a range of possible hazard ratio values of that confounder for the exposure variable.
Subpopulation Analyses
Subpopulations were identified using STS data files, including: diabetes mellitus (yes or no), body mass index (BMI; <30, 30 to 35, and ≥35), and number of vein grafts (1, 2 to 3, and ≥3). Separate propensity models were fit within these subgroups. To estimate strata-specific treatment effects, the inverse probability weighted Cox and logistic models were applied, as previously described, within each stratum of these three pre-specified subpopulations.
RESULTS
Study Population
A cohort of 235,394 patients from 934 U.S. sites met study inclusion criteria and was available for analysis, including 122,899 endoscopic and 112,495 open vein-harvest cases (Figure 1). Table 1 presents demographic and operative variables before and after propensity score adjustment among these patients. The mean age is 74 years for both the endoscopic (EVH) and open (OVH) vein-harvest groups, representing a Medicare population as expected given study inclusion of patients eligible for Medicare claims at the time of CABG operation. Baseline patient characteristics were generally balanced across treatment groups, including: age (EVH 74 yrs [95% confidence interval (CI) 69,78 yrs]. vs. OVH 74 yrs [95% CI 69,78 yrs]), body mass index (28.5 kg/m² [95% CI 25,31 kg/m²] vs. 28.4 kg/m² [95% CI 25,31 kg/m²]), prevalence of peripheral vascular disease (17.9% vs. 18.0%), active smoking (13.9% vs. 13.5%), diabetes mellitus requiring insulin (10.1% vs. 9.9%), and urgent case status (48.9% vs. 49.2%). Notably, the year of surgery (2003 to 2008) was imbalanced between treatment groups, with later years of study reporting more endoscopic harvest cases than earlier years. The year 2003 accounted for 9% of the EVH cases captured in the study, and the proportion increased each year with 21% of the EVH cases being performed in 2008. This trend was reversed for open cases, with 24% of the open cases being performed in 2003 as opposed to only 11% in 2008. Overall, in 2008, 70% of the vein harvests captured in this study were performed endoscopically. Following propensity score adjustment, all observed covariates were balanced across treatment groups (Table 1). The complete list of all patient demographic and operative variables before and after propensity score adjustment using inverse probability weighting is provided in Appendix 1.
A comparison of demographic characteristics and in-hospital outcomes for Medicare linked vs. not-linked STS ACSD patients meeting study inclusion criteria revealed these groups of patients to be generally similar with regard to most variables of interest. However, the successfully linked STS to Medicare patients were less likely to be urgently (vs. electively) operated (49.2% linked vs. 53.9% not-linked, P<0.001) and less likely to suffer in-hospital MI (.99% vs. 1.1%, P<0.001), wound complication (3.0% vs. 3.6%, P<0.001), and death (2.3% vs. 2.5%, P=0.001). A summary of pertinent baseline variables and short term outcomes according to Medicare linkage for all STS ACSD patients meeting study inclusion criteria is provided in Appendix 2.
Clinical Outcomes
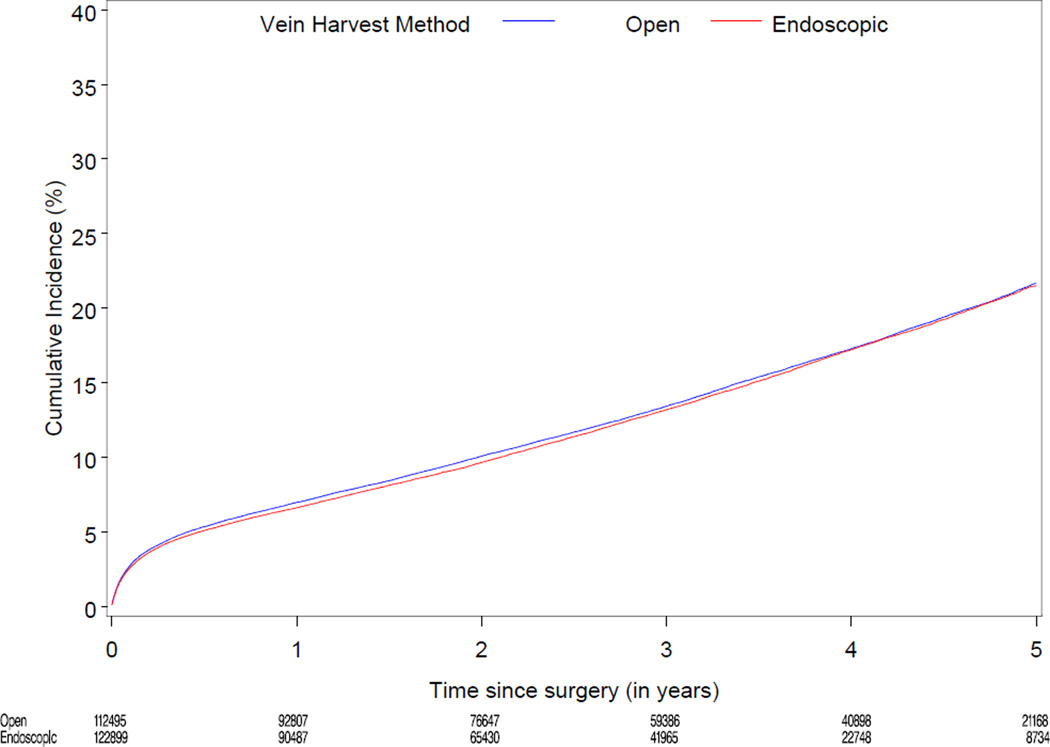
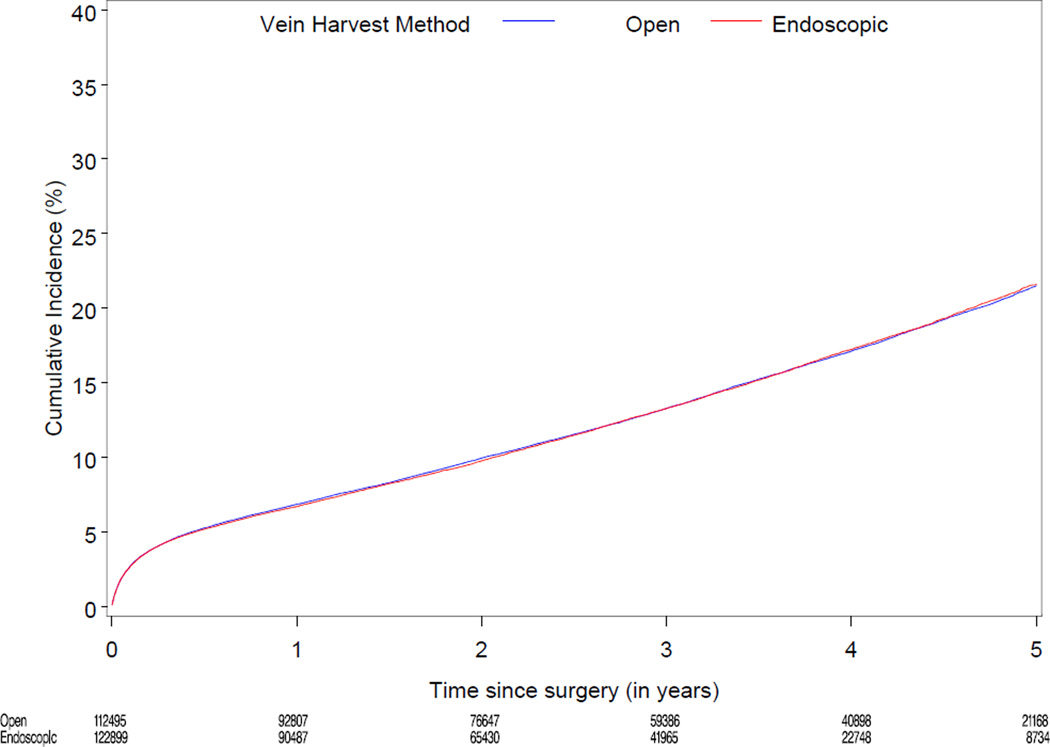
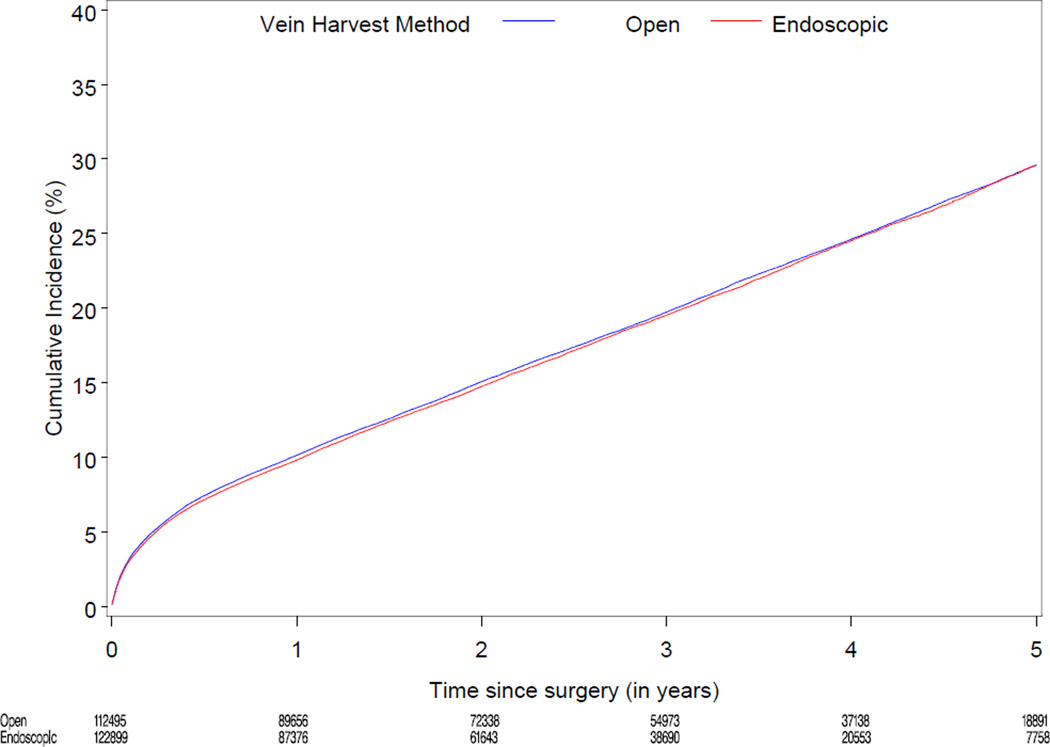
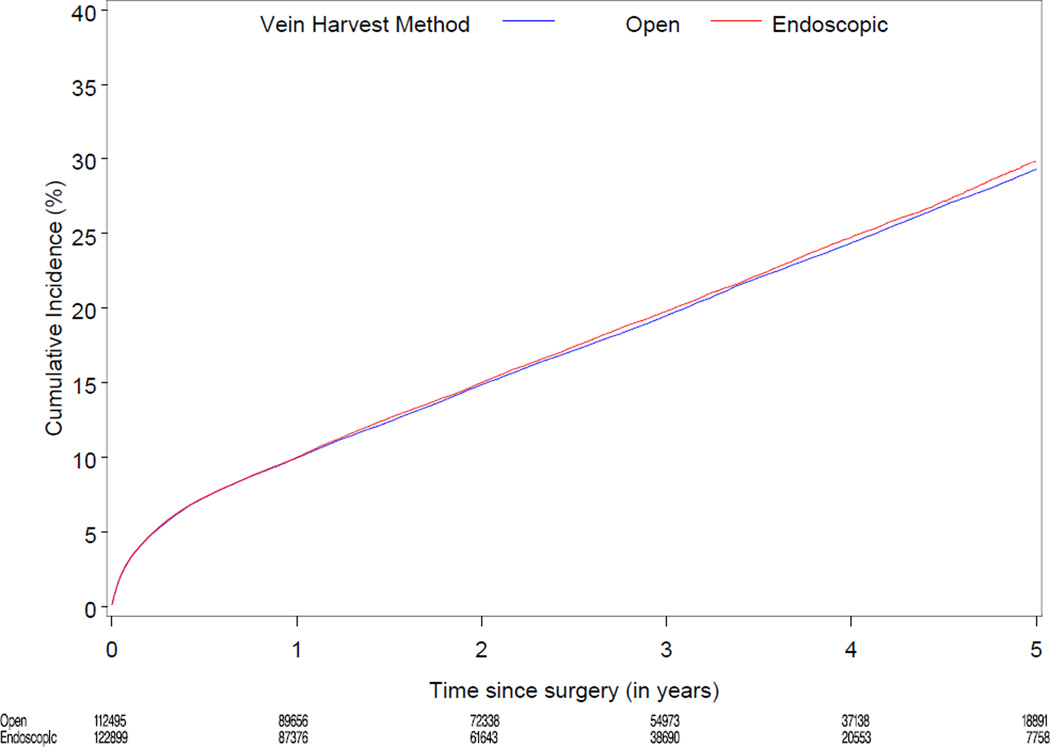
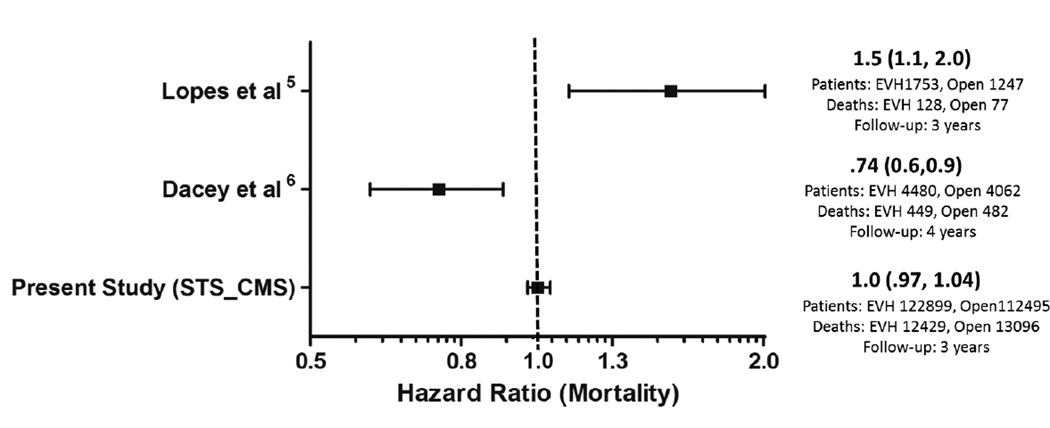
Median follow-up was 3.0 years (range 0–6). Table 2 displays cumulative incidence rates for death, the composite of death, MI, or revascularization, and wound complications for endoscopic vs. open vein-graft harvest among the 274,404 study patients. There were no significant differences between the unadjusted cumulative incidence rate for death through 3 years for the EVH (12,429/122,899 [13.2%]) and the OVH groups (13,096/112,495 [13.4%]). There were no significant differences between the cumulative incidence through 3 years for the composite of death, MI, or revascularization, among the EVH vs. OVH group (18,419/122,899 [19.5%] vs. 19,232/112,495 [19.7%]). The unadjusted 30 day rate for wound complication was 3.0% (3,654/122,899) for EVH vs. 3.6% (4,047/112,495) for OVH. Figure 2 displays the unadjusted and risk-adjusted mortality curves comparing CABG with EVH vs. OVH. The risk-adjusted hazard ratio (HR) for long-term mortality was 1.0 (95% CI 0.97, 1.04; p=1.00). Figure 3 displays unadjusted and risk-adjusted event curves for the composite endpoint of death, MI, or revascularization. The adjusted HR for the composite of death, MI and revascularization was 1.0 (95% CI 0.98, 1.05; p=0.34).
Table 2.
Unadjusted cumulative incidence rates at median follow-up of 3.0 years among 235,394 North American patients undergoing isolated CABG 2003 to 2008, including 122,899 with endoscopic and 112,495 with open vein harvest
| Endoscopic Harvest | Open Harvest | |||
|---|---|---|---|---|
| Outcomes | Events | Cumulative incidence rate (95% CI) |
Events | Cumulative incidence rate (95% CI) |
| Death (through 3 years) |
12,429 | 13.2% (13.0, 13.4) |
13,096 | 13.4% (13.2, 13.7) |
| Death, MI, or revascularization (through 3 years) |
18,419 | 19.5% (19.3, 19.8) |
19,232 | 19.7% (19.5, 20.0) |
| Wound complication (through 30 days) |
3,654 | 2.97% (2.93, 3.01) |
4,047 | 3.60% (3.56, 3.64) |
Abbreviation: CABG, coronary artery bypass grafting; MI, myocardial infarction.
Figure 2.
Unadjusted (A) and risk-adjusted (B) Kaplan-Meier curves for death according to endoscopic versus open vein-graft harvest technique among 235,394 North American patients undergoing isolated CABG 2003–2008
A. Unadjusted mortality curve
B. Risk-adjusted mortality curve
Figure 3.
Unadjusted (A) and risk-adjusted (B) Kaplan-Meier curves for the composite outcomes of death, MI, or revascularization according to endoscopic versus open vein-graft harvest technique among 235,394 North American patients undergoing isolated CABG 2003 to 2008
A. Unadjusted curve for the composite outcomes of death, MI, or revascularization
B. Risk-adjusted curve for the composite outcomes of death, MI, or revascularization
EVH was associated with a significantly lower rate of wound complications: adjusted HR = 0.83 (95% CI 0.77, 0.89; p<0.001) for EVH vs. OVH (Table 3).
Table 3.
Results of marginal common baseline hazard model, before and after risk adjustment, for endoscopic versus open vein-graft harvesting. Primary analysis of the overall population is presented first, followed by results for the sensitivity analysis.
| Unadjusted | Risk adjusted | ||||||
|---|---|---|---|---|---|---|---|
| Outcomes | Events by Harvest Technique |
HR | 95% CI | P value |
HR | 95% CI | P value |
|
Overall population, all 989 sites N=235,394 patients including 122,899 endoscopic (EVH) and 112,495 open vein harvest | |||||||
| Mortality (through 3 years) |
EVH 12,429 Open 13,096 |
0.99 | 0.95,1.02 | 0.454 | 1.0 | 0.97,1.04 | 1.000 |
| Death, MI, or revascularization (through 3 years) |
EVH 18,419 Open 19,232 |
1.0 | 0.97,1.03 | 1.000 | 1.0 | 0.98,1.05 | 0.344 |
| Wound complication (through 30 days) |
EVH 3,654 Open 4,047 |
0.82 | 0.78,0.86 | <0.001 | 0.83 | 0.77,0.89 | <0.001 |
|
Patients at the 165 sites with sensitivity and specificity > 80% for EVH N=44,423 patients including 25,199 endoscopic(EVH) and 19,244 open vein harvest | |||||||
| Mortality (through 3 years) |
EVH 2,474 Open 2307 |
0.96 | 0.90,1.03 | 0.249 | 0.95 | 0.89,1.01 | 0.097 |
| Death, MI, or revascularization (through 3 years) |
EVH 3707 Open 3350 |
1.0 | 0.94,1.07 | .924 | 1.0 | 0.94,1.06 | 0.883 |
| Wound complication (through 30 days) |
EVH 778 Open 737 |
0.80 | 0.72,0.89 | <0.001 | 0.75 | 0.64,0.89 | <0.001 |
Abbreviation: CABG, coronary artery bypass grafting; MI, myocardial infarction.
Sensitivity Analyses
To evaluate the effect of treatment misclassification resulting from under-reporting of the EVH CPT code, we replicated our primary analysis in a center-level subgroup of the overall cohort. We examined the cohort of sites having > 80% sensitivity and specificity for EVH reporting. Among 44,423 patients at 165 sites, the unadjusted incidence of mortality, the composite of death, MI, or revascularization, and wound complications were 14%, 20%, and 3.3%, respectively. No risk-adjusted difference was observed between patients undergoing endoscopic vs. open harvest in mortality, adjusted HR 0.95 (95% CI 0.89, 1.0; p=0.10), or the composite of death, MI, and revascularization, adjusted HR 1.0 (95% CI 0.94, 1.1; p=0.88); but lower rates of wound complications were observed among EVH patients, adjusted HR 0.75 (0.64, 0.89; p<0.001). Table 3 displays the results of the marginal common baseline hazard model, before and after risk adjustment, for EVH vs. OVH for the overall population as well as for the sensitivity cohort.
Sensitivity analysis for measurement error in the coding of EVH findings revealed no possibility of a large difference between the treatment groups for the mortality endpoint. Point estimates for the hazard ratio of EVH vs. OVH ranged across scenarios from 1.00 to 1.02. The upper limit of the 95% CI was ≤1.05 for 19 of 36 scenarios tested, ≤1.10 for 31 of 36 scenarios, and ≤1.16 for all 36 scenarios.
Subpopulation Analyses
No difference in study endpoints were observed when comparing open vs. endoscopic vein harvest among patients with (n=41,745) or without (n=70,698) diabetes mellitus. Similarly, within three BMI strata (<30, 30 to 35, and >35) there was no difference in mortality or the composite outcome between the open and endoscopic vein graft harvest groups, and no difference was observed based on the number of vein grafts utilized.
COMMENT
In 2009, the FDA issued a request for proposal to evaluate the safety of endoscopic vein harvest for CABG surgery. Investigators from the Society of Thoracic Surgeons and the Duke Clinical Research Institute answered this request and, in partnership with the FDA, we conducted this nationally representative observational comparison of the long-term outcomes of endoscopic vein-graft harvesting in CABG. Our study found that EVH was the most commonly used technique for vein graft harvesting, with approximately 70% of CABG cases in the STS ACSD utilizing this technique in 2008, the most recent year examined. After adjustment for baseline clinical factors, we found no evidence of increased long-term mortality or the composite of death, MI, or revascularization associated with endoscopic (EVH) vs. open (OVH) vein-graft harvesting in isolated CABG patients. Consistent with previous randomized comparisons use of EVH was associated with a significant reduction in wound complications relative to open procedures (risk-adjusted HR 0.83; 95% CI 0.77, 0.89).
Since the introduction of EVH techniques and device systems in the 1990’s,1,21 multiple randomized controlled trials have demonstrated the short-term advantages of EVH with respect to morbidities (predominantly wound infections) and patient satisfaction.3,22–26 The wound complication rates reported in these randomized comparisons are generally lower than those observed in the present study, likely due to protocol-driven inspection of harvest sites and more liberal definitions of wound complications in the prospective trial setting. One small RCT by Allen et al reported that 112 CABG patients randomized to EVH vs. open harvesting had similar 5 year likelihood of death, myocardial infarction, or recurrent angina (75% for EVH versus 74% for OVH; P=0.85).27 In 2005, based on these limited data, a consensus statement from the International Society for Minimally Invasive Cardiothoracic Surgery (ISMICS) recommended that EVH be the preferred technique given its proven benefit in wound-related complications, postoperative pain, and consumption of outpatient wound-management resources.28
In 2009 an observational study by Lopes et al challenged the use of EVH as the preferred technique for vein-graft harvest,5 and generated considerable debate in the cardiovascular community.6,29–33 Looking at patients undergoing first-time isolated CABG as part of a multicenter PREVENT IV (PRoject of Ex-vivo Vein graft ENgineering via Transfection IV) trial, Lopes et al compared outcomes of 1753 EVH versus 1247 OVH procedures.34 All veins harvested in the PREVENT IV trial underwent ex-vivo manipulation with pressurized delivery of study drug or placebo, and overall vein graft failure rates were higher than for other CABG trials. Nonetheless, the post-hoc analysis by Lopes et al using propensity adjustment found EVH was associated with a higher adjusted risk of death (HR 1.5; 95% CI 1.1, 2.0; p<0.005) as well as higher risk for death, MI, or repeat revascularization (HR 1.22; p=0.04). Based on this finding, the United Kingdom’s National Institute for Health and Clinical Excellence (NICE) published new recommendations relating to the use of EVH for CABG, advising that this procedure should only be used with special arrangements for “clinical governance, consent and audit or research”.35 The NICE group recommends in this statement that clinicians ensure that their patients understand the uncertain balance regarding the known benefits of EVH versus the potential risks of inferior cardiovascular clinical outcomes.
In contrast to those finding from Lopes et al, the Northern New England study group found no safety concerns with EVH. The Northern New England group included 8542 patients from 2001 to 2004 having isolated CABG, including 53% with EVH.6 EVH was associated with a 20% reduced risk of mortality at 4 years following CABG (adjusted HR 0.74; 95% CI 0.60, 0.92 for those patients surviving 90 days). EVH was not found to be associated with a higher rate of repeat revascularization (adjusted HR 1.10, 95% CI 0.96, 1.74).
In a predetermined secondary analysis of the Randomized On/Off Bypass (ROOBY) Trial,36 short-term and 1-year clinical composite outcomes (death or major perioperative complication defined as reoperation, new mechanical support, cardiac arrest, coma, stroke, or renal failure requiring dialysis) were compared between patients who underwent EVH and those who underwent OVH.37 This ROOBY sub study captured 1471 patients enrolled from 2003 (the first year the trial began recording vein harvest technique) to 2007. EVH was used in only 38% of the cases among the 18 US Veterans Affairs medical centers studied. There were no significant differences in both short-term and 1-year composite outcomes between the endoscopic and open groups. No interaction was found between EVH and off-pump coronary artery bypass grafting treatment.
Our current analysis from the STS ACSD extends the findings of Northern New England study for reduced wound complications.6 However, unlike the Northern New England study, our results do not suggest an associated survival advantage with EVH. On the other hand, unlike the Lopes et al study, our analysis did not identify harm associated with EVH (Figure 4).5 Several differences between the present study and the preceding clinical studies are worth noting. Compared to the studies by Lopes et al and Northern New England group, the present study includes patients over 65 years old and a more contemporary cohort, perhaps further along the EVH learning curve with fewer traction or electrocautery injuries in recent years. The present study is also 10-fold greater in size than the Lopes et al and the Northern New England group analyses combined (Figure 4). The present study includes a diverse, more representative group of large and small community programs and university and non-university affiliated centers.
Figure 4.
Forest plot displaying hazard ratio point estimates (weighted by study sample size) and 95% confidence intervals for long term mortality from present and recent large observational studies comparing endoscopic and open vein graft harvesting
As with both the Lopes et al and Northern New England studies, the present analysis was unable to account for differences in conduit caliber between the EVH and OVH groups, a potentially critical confounding variable in comparing EVH and OVH techniques. Several trials have compared blinded tissue specimens between segments of vein harvested conventionally and endoscopically and found no histologic difference.23,25 Vein-grafts harvested endoscopically are commonly taken above the knee, whereas vein-grafts harvested by open techniques are commonly taken beginning from the ankle (where the vein is smallest) and then upwards as needed. The diameter of the vein grows along its cephalad course up the lower extremity when it is harvested. Several reports have shown that large vein caliber is associated with poorer patency,22,38,39 likely the result of reduced flow velocity within a larger-diameter conduit. Thus, the exact level from which the saphenous vein is harvested might be important. In addition, no study has specifically addressed the effect of the use of carbon dioxide insufflation (either the carbon dioxide itself or the gas pressure) on the quality of SVGs. All endoscopic vein harvests are not the same. The present observational study, as with previous studies, is unable to assess for particulars of technique such as carbon dioxide insufflations, use of electro-cautery, nor the experience of the endoscopic harvester.
This study has other important limitations. First, there was only 3 year median follow-up and no direct clinical identification of EVH use in the STS before 2008. It is also possible for surgeons to convert from endoscopic to open harvesting during the course of the operation. This occurs most often when speed is required or a vein is difficult to harvest. If conversion from endoscopic to open occurred in the present analysis, the case would be identified as endoscopic by our methods and at least some of the harvested vessel exposed to any inherent risks of EVH and therefore properly allocated in the analysis. We used billing (professional fees) to identify EVH and non-differential misclassification of EVH status could bias our estimate of the treatment effect toward the null effect. Our analysis of the CPT code accuracy suggested that if EVH was coded, it was likely performed. However, if EVH was not coded, it may or may not have been performed. This degree of treatment group misclassification would serve to obscure any existing difference between the two treatment groups, biasing the results of the study toward the null. Because of this, we cannot be certain that no difference in mortality or the composite of mortality, MI, or revascularization exists. However, we performed sensitivity analyses utilizing those sites with >80% sensitivity and specificity for EVH reporting in order to address this potential bias. The persistence of the null effect at centers with more EVH billing precision makes the explanation of misclassification causing the null effect less likely. Moreover, based on our sensitivity analysis for measurement error in the coding of EVH findings, we are able to rule out any large difference between the treatment groups for the mortality endpoint. The significant and consistent finding of an association of EVH with improved wound complications, an expected effect confirmed by multiple randomized studies, also supports a reasonable degree of sensitivity to our analytic assay. Finally, information regarding the type of device used for EVH was lacking for this analysis.
In conclusion, this observational study found no evidence of an association of endoscopic vein-graft harvest with long-term mortality or a composite of death, MI, or repeat revascularization. Endoscopic technique was found to be associated with significantly reduced wound complications.
Acknowledgments
Funding/Support: This study was funded by the United States Food and Drug Administration through a contract with the Society of Thoracic Surgeons. Dr. Williams is supported in part by training grant T32-HL069749 from the National Institutes of Health and is a Cardiothoracic Surgical Trials Network Scholar supported by grant U01-HL088953 from the National Heart, Lung and Blood Institute which also supports Drs. Smith, Alexander, Thourani, and Michler as investigators of the Cardiothoracic Surgical Trials Network (CTSN).
Role of Sponsor: The sponsor (United States Food and Drug Administration) provided scientific input regarding the design of the study, the interpretation of the data, and approval of the manuscript. The sponsor played no direct role in data collection, management, or performance of the final analyses.
Footnotes
Author Contributions: Dr Smith had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Statistical Analysis: Drs. Zhao and O’Brien performed the statistical analyses for this study.
Financial Disclosures: Dr. Thourani: Maquet, honorarium for lectures, advisory board. Dr. Smith: Society of Thoracic Surgeons, honorarium.
REFERENCES
- 1.Lumsden AB, Eaves FF, 3rd, Ofenloch JC, Jordan WD. Subcutaneous, video-assisted saphenous vein harvest: report of the first 30 cases. Cardiovasc Surg. 1996 Dec;4(6):771–776. doi: 10.1016/s0967-2109(96)00055-5. [DOI] [PubMed] [Google Scholar]
- 2.Allen KB, Griffith GL, Heimansohn DA, et al. Endoscopic versus traditional saphenous vein harvesting: a prospective, randomized trial. Ann Thorac Surg. 1998 Jul;66(1):26–31. doi: 10.1016/s0003-4975(98)00392-0. discussion 31-22. [DOI] [PubMed] [Google Scholar]
- 3.Puskas JD, Wright CE, Miller PK, et al. A randomized trial of endoscopic versus open saphenous vein harvest in coronary bypass surgery. Ann Thorac Surg. 1999 Oct;68(4):1509–1512. doi: 10.1016/s0003-4975(99)00952-2. [DOI] [PubMed] [Google Scholar]
- 4.Shahian D, O'Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88(1 Suppl):S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 5.Lopes RD, Hafley GE, Allen KB, et al. Endoscopic versus open vein-graft harvesting in coronary-artery bypass surgery. N Engl J Med. 2009 Jul 16;361(3):235–244. doi: 10.1056/NEJMoa0900708. [DOI] [PubMed] [Google Scholar]
- 6.Dacey LJ, Braxton JH, Jr., Kramer RS, et al. Long-term outcomes of endoscopic vein harvesting after coronary artery bypass grafting. Circulation. 2011 Jan 18;123(2):147–153. doi: 10.1161/CIRCULATIONAHA.110.960765. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien SM, Shahian DM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 8.Welke K, Ferguson TB, Jr., Schroeder M, Coombs LP, Dokholyan RS, Peterson ED. Validity of the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg. 2004;77(4):1137–1139. doi: 10.1016/j.athoracsur.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs JP, Edwards FH, Shahian DM, et al. Successful linking of the Society of Thoracic Surgeons adult cardiac surgery database to Centers for Medicare and Medicaid Services Medicare data. Ann Thorac Surg. 2010 Oct;90(4):1150–1156. doi: 10.1016/j.athoracsur.2010.05.042. discussion 1156-1157. [DOI] [PubMed] [Google Scholar]
- 10.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009 Jun;157(6):995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 12.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med. 2008 May 30;27(12):2037–2049. doi: 10.1002/sim.3150. [DOI] [PubMed] [Google Scholar]
- 13.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986 Mar;42(1):121–130. [PubMed] [Google Scholar]
- 14.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007 Oct;45(10 Supl 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 15.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004 Jul;75(1):45–49. doi: 10.1016/j.cmpb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Lin DY, Wei LJ. The Robust Inference for the Proportional Hazard Model. Journal of the American Statistical Association. 1989;(84):1074–1078. [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;(53):457–481. [Google Scholar]
- 18.Breslow NE. Discussion of Professor Cox’s Paper. J. Royal Stat. Soc. 1972;(34):216–217. [Google Scholar]
- 19.Zucker DM, Spiegelman D. Corrected score estimation in the proportional hazards model with misclassified discrete covariates. Stat Med. 2008 May 20;27(11):1911–1933. doi: 10.1002/sim.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998 Sep;54(3):948–963. [PubMed] [Google Scholar]
- 21.Lumsden AB, Eaves FF., III . Vein harvest in endoscopic plastic surgery. In: Bostwick EIFJ, Nahai F, editors. Endoscopic Plastic Surgery. St. Louis MO: Quality Medical; 1995. pp. 535–545. [Google Scholar]
- 22.Yun KL, Wu Y, Aharonian V, et al. Randomized trial of endoscopic versus open vein harvest for coronary artery bypass grafting: six-month patency rates. J Thorac Cardiovasc Surg. 2005 Mar;129(3):496–503. doi: 10.1016/j.jtcvs.2004.08.054. [DOI] [PubMed] [Google Scholar]
- 23.Kiaii B, Moon BC, Massel D, et al. A prospective randomized trial of endoscopic versus conventional harvesting of the saphenous vein in coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2002 Feb;123(2):204–212. doi: 10.1067/mtc.2002.118682. [DOI] [PubMed] [Google Scholar]
- 24.Andreasen JJ, Nekrasas V, Dethlefsen C. Endoscopic vs open saphenous vein harvest for coronary artery bypass grafting: a prospective randomized trial. Eur J Cardiothorac Surg. 2008 Aug;34(2):384–389. doi: 10.1016/j.ejcts.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Tang H, Wilkinson V, et al. Saphenous vein harvest with SaphLITE system versus conventional technique: a prospective, randomized study. Ann Thorac Surg. 2005 Jun;79(6):2018–2023. doi: 10.1016/j.athoracsur.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 26.Schurr UP, Lachat ML, Reuthebuch O, et al. Endoscopic saphenous vein harvesting for CABG -- a randomized, prospective trial. Thorac Cardiovasc Surg. 2002 Jun;50(3):160–163. doi: 10.1055/s-2002-32412. [DOI] [PubMed] [Google Scholar]
- 27.Allen KB, Heimansohn DA, Robison RJ, Schier JJ, Griffith GL, Fitzgerald EB. Influence of endoscopic versus traditional saphenectomy on event-free survival: five-year follow-up of a prospective randomized trial. Heart Surg Forum. 2003;6(6):E143–E145. [PubMed] [Google Scholar]
- 28.Allen K, Cheng D, Cohn W, et al. Endoscopic vascular harvest in coronary artery bypass grafting surgery: a consensus statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) Innovations: Technology and Techniques in Cardiothoracic and Vascular Surgery. 2005;1:51–60. doi: 10.1097/01.gim.0000196315.32179.82. [DOI] [PubMed] [Google Scholar]
- 29.Markar SR, Kutty R, Edmonds L, Sadat U, Nair S. A meta-analysis of minimally invasive versus traditional open vein harvest technique for coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg. 2010 Feb;10(2):266–270. doi: 10.1510/icvts.2009.222430. [DOI] [PubMed] [Google Scholar]
- 30.Patel NN, Angelini GD. Surgery: Open or endoscopic vein graft harvesting-this is the question! Nat Rev Cardiol. 2009 Dec;6(12):738–740. doi: 10.1038/nrcardio.2009.190. [DOI] [PubMed] [Google Scholar]
- 31.Connolly MW, Poston RS. Endoscopic versus open vein-graft harvesting. N Engl J Med. 2009 Nov 5;361(19):1907–1908. author reply 1909-1910. [PubMed] [Google Scholar]
- 32.Barnard JB, Keenan DJ. Endoscopic saphenous vein harvesting for coronary artery bypass grafts: NICE guidance. Heart. 2011 Feb;97(4):327–329. doi: 10.1136/hrt.2010.209668. [DOI] [PubMed] [Google Scholar]
- 33.Ouzounian M, Hassan A, Buth KJ, et al. Impact of endoscopic versus open saphenous vein harvest techniques on outcomes after coronary artery bypass grafting. Ann Thorac Surg. 2010 Feb;89(2):403–408. doi: 10.1016/j.athoracsur.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 34.Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005 Nov 16;294(19):2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 35.NICE. Endoscopic saphenous vein harvest for coronary artery bypass grafting. Interventional procedure guidance. London, UK: Z: Y;NICE; 2010. [Google Scholar]
- 36.Shroyer AL, Grover FL, Hattler B, et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009 Nov 5;361(19):1827–1837. doi: 10.1056/NEJMoa0902905. [DOI] [PubMed] [Google Scholar]
- 37.Zenati MA, Shroyer AL, Collins JF, et al. Impact of endoscopic versus open saphenous vein harvest technique on late coronary artery bypass grafting patient outcomes in the ROOBY (Randomized On/Off Bypass) Trial. J Thorac Cardiovasc Surg. 2011 Feb;141(2):338–344. doi: 10.1016/j.jtcvs.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Cook RC, Crowley CM, Hayden R, et al. Traction injury during minimally invasive harvesting of the saphenous vein is associated with impaired endothelial function. J Thorac Cardiov Sur. 2004 Jan;127(1):65–71. doi: 10.1016/s0022-5223(03)01024-9. [DOI] [PubMed] [Google Scholar]
- 39.Rousou LJ, Taylor KB, Lu XG, et al. Saphenous Vein Conduits Harvested by Endoscopic Technique Exhibit Structural and Functional Damage. Annals of Thoracic Surgery. 2009 Jan;87(1):62–70. doi: 10.1016/j.athoracsur.2008.08.049. [DOI] [PubMed] [Google Scholar]