Abstract
Background
Vein graft failure (VGF) is common after coronary artery bypass graft surgery, but its relationship with long-term clinical outcomes is unknown. In this retrospective analysis, we examined the relationship between VGF, assessed by coronary angiography 12 to 18 months after coronary artery bypass graft surgery, and subsequent clinical outcomes.
Methods and Results
Using the Project of Ex Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) trial database, we studied data from 1829 patients who underwent coronary artery bypass graft surgery and had an angiogram performed up to 18 months after surgery. The main outcome measure was death, myocardial infarction, and repeat revascularization through 4 years after angiography. VGF occurred in 787 of 1829 patients (43%). Clinical follow-up was completed in 97% of patients with angiographic follow-up. The composite of death, myocardial infarction, or revascularization occurred more frequently among patients who had any VGF compared with those who had none (adjusted hazard ratio, 1.58; 95% confidence interval, 1.21–2.06; P=0.008). This was due mainly to more frequent revascularization with no differences in death (adjusted hazard ratio, 1.04; 95% confidence interval, 0.71–1.52; P=0.85) or death or myocardial infarction (adjusted hazard ratio, 1.08; 95% confidence interval, 0.77–1.53; P=0.65).
Conclusions
VGF is common after coronary artery bypass graft surgery and is associated with repeat revascularization but not with death and/or myocardial infarction. Further investigations are needed to evaluate therapies and strategies for decreasing VGF to improve outcomes in patients undergoing coronary artery bypass graft surgery.
Keywords: angiography, coronary artery bypass, graft survival, outcome assessment, veins
Saphenous vein grafts remain the most widely used conduits for coronary artery bypass graft (CABG) surgery.1 However, despite major advances in surgical techniques, intraoperative adjuncts, and perioperative care, vein grafts continue to have high failure rates.2,3 In fact, per-graft occlusion rates are estimated to be up to 25% during the first 12 to 18 months after surgery.4–6 Although prior studies have identified patient- and graft-specific variables that are associated with graft patency,4–7 little is known about the relationship between vein graft failure and subsequent clinical outcomes.8–11 The objective of this study was to examine the relationship between vein graft failure, assessed by coronary angiography 12 to 18 months after CABG, and subsequent clinical outcomes.
Methods
Study Population
We conducted these analyses using the Project of Ex Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) trial database. The design and primary results of the PREVENT IV trial have previously been published.12,13 PREVENT IV was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial of ex vivo treatment of vein grafts with the E2F transcription factor decoy edifoligide in patients undergoing CABG surgery. The trial, conducted in 107 US sites, enrolled >3000 patients between 2002 and 2003. Patients were eligible for PREVENT IV if they were 18 to 80 years of age and were undergoing first isolated CABG surgery for atherosclerotic coronary artery disease with at least 2 planned vein grafts.
Major exclusion criteria included prior cardiac surgery or planned concomitant valve surgery, vasculitis or another nonatherosclerotic cause of coronary artery disease, and a comorbid illness that made 5-year survival unlikely. The first 2400 patients enrolled in PREVENT IV were assigned to an angiographic cohort and scheduled to return for angiography 12 to 18 months after surgery. The institutional review boards of participating medical centers approved the PREVENT IV protocol, and all patients gave written informed consent.
This study focuses on the PREVENT IV patients who underwent follow-up angiography and had subsequent clinical follow-up.
Outcomes
The primary outcome of PREVENT IV was death or angiographic vein graft failure as assessed by quantitative coronary angiography 12 to 18 months after surgery. Vein graft failure was defined as a ≥75% stenosis or occlusion at follow-up angiography. All angiograms were analyzed at a core laboratory (PERFUSE Angiographic Core Laboratory, Boston, MA). Vein graft and target vessel quality were rated as good, fair, or poor by the operating surgeon at the time of surgery. All patients were followed up for death, myocardial infarction (MI), and repeat revascularization up to 4 years after the CABG surgery. Major adverse cardiac events, including death, MI, and repeat revascularization, were assessed annually by mail and phone follow-up. For patients who reported potential events, medical records were collected and the events were adjudicated by an independent clinical events committee using prespecified criteria.
The main outcome for this study was the composite of death, MI, and repeat revascularization after angiography. Additional outcomes included death or MI and death after angiography and MI and repeat revascularization occurring between CABG surgery and follow-up angiography.
Statistical Analysis
Baseline characteristics and medications were summarized by medians and 25th and 75th percentiles for continuous variables and by percentages for categorical variables. No statistical tests to assess differences between groups were performed. Event rates for postan-giography clinical outcomes in patients with and without vein graft failure were calculated with Kaplan-Meier methods. When the postangiography clinical outcomes were assessed, the day of the angiography was considered day 1. Although it is impossible to know whether vein graft failure preceded the clinical event, the rates of nonfatal MI and repeat revascularization procedures occurring between CABG surgery and angiographic follow-up are reported among patients with and without vein graft failure on subsequent angiography. Because only the first occurrence of each event type (MI, revascularization) was adjudicated, patients who met an end point before angiography were excluded from the postangiography analyses. Hazard ratios (HRs) and 95% confidence intervals (CIs) to assess the relationship of per-patient vein graft failure and clinical outcomes were calculated with the Cox proportional hazards model. Covariates adjusted for in the postangiography clinical outcome models included sex, age, baseline creatinine clearance, ejection fraction, prior MI, recent MI (last 30 days), peri-index CABG MI, history of congestive heart failure, history of peripheral vascular disease, history of cerebrovascular disease, lung disease, diabetes mellitus, history of atrial fibrillation, use of endoscopic vein graft harvesting technique, use of an internal thoracic artery, duration of cardiopulmonary bypass, cardiac shock, worst vein graft quality, and worst target artery quality. Nominal P values were provided only for prespecified hypotheses.
There was a strong indication of nonproportional hazards in the relationship between vein graft failure and the composite outcome of death, MI, or revascularization. Immediately after protocol-mandated angiography, the rate of revascularization was substantially higher than during longer follow-up. To account for this, we estimated different hazard ratios for the periods before and after 14 days for outcomes containing revascularization. The first hazard ratio primarily quantifies the relationship with immediate revascularization, whereas the second hazard ratio represents the relationship with long-term outcomes among patients who were event-free through 14 days. We report separate HRs for each of these 2 time periods.
Finally, to determine whether the number of vein grafts that fail is associated with worse outcomes, Cox proportional hazards models for each of the 3 composite end points were developed, each containing 2 covariates: the number of vein grafts implanted and the proportion of vein grafts that failed.
Results
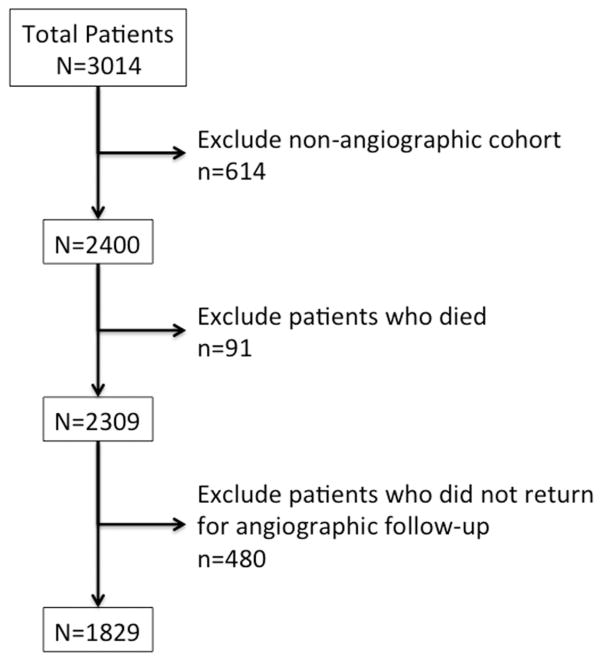
Of the 3014 patients enrolled in PREVENT IV, 614 were not in the angiographic cohort, 91 died before angiography, and 480 did not return for angiography 12 to 18 months after surgery (Figure 1). The 4-year follow-up was complete in 95.1% overall and in 97.0% of the angiographic cohort.
Figure 1.
Flowchart displaying the derivation of the study cohort from the Project of Ex Vivo Vein Graft Engineering via Transfection IV (PREVENT IV) population.
Baseline and Surgery Characteristics
The baseline and surgery characteristics of the PREVENT IV overall and angiographic populations are shown in Table 1. Patients who underwent an angiogram at 1 year and had complete clinical outcomes follow-up after 4 years tended to have similar baseline and surgical characteristics compared with the overall trial population. In general, among the angiographic population, patients with vein graft failure at 12 to 18 months had slightly more comorbidities than those without vein graft failure. In addition, patients with vein graft failure tended to have longer surgery duration and more often had vein grafts harvested endoscopically than patients without vein graft failure.
Table 1.
Baseline Characteristics Among the Overall Population and the Angiographic Population With and Without Vein Graft Failure
| Characteristics | Total (n=3014) | Angiographic Population (n=1829) | Angiographic Population With VGF (n=787) | Angiographic Population Without VGF (n=1042) |
|---|---|---|---|---|
| Age, median, y | 64.0 (56.0, 71.0) | 63.0 (55.0, 70.0) | 63.0 (55.0, 69.0) | 63.0 (56.0, 70.0) |
| Male, % | 79.1 | 81.3 | 80.1 | 82.2 |
| Race/ethnicity, % | ||||
| White | 90.9 | 90.5 | 89.7 | 91.2 |
| Black | 4.6 | 4.7 | 5.7 | 3.9 |
| Asian | 1.1 | 0.9 | 1.5 | 0.5 |
| Hispanic | 2.4 | 2.7 | 2.4 | 3.0 |
| Native American | 0.4 | 0.6 | 0.4 | 0.8 |
| Other | 0.6 | 0.5 | 0.3 | 0.7 |
| Weight, median, kg | 88.0 (76.7, 100.0) | 88.0 (77.3, 100.0) | 88.9 (77.0, 100.0) | 88.0 (78.0, 100.0) |
| Height, median, cm | 175.0 (167.63, 180.0) | 175 (168.0, 180.0) | 175.0 (167.6, 180.0) | 175.0 (168.0, 180.3) |
| BMI, median, kg/m2 | 28.9 (26.0, 32.6) | 29.0 (26.3, 32.6) | 29.0 (26.2, 32.7) | 29.0 (26.3, 32.6) |
| Systolic BP, median, mm Hg | 134.0 (120.0, 149.0) | 134.0 (120.0, 148.0) | 134.0 (120.0, 149.0) | 132.0 (120.0, 146.0) |
| Diastolic BP, median, mm Hg | 75.0 (67.0, 82.0) | 75.0 (68.0, 82.0) | 76.0 (68.0, 82.0) | 74.0 (68.0, 82.0) |
| Heart rate, median, bpm | 70.0 (62.0, 80.0) | 70.0 (61.0, 80.0) | 71.0 (61.0, 80.0) | 70.0 (62.0, 80.0) |
| Current NYHA heart failure class, % | ||||
| I | 40.2 | 40.8 | 40.4 | 41.1 |
| II | 33.3 | 34.4 | 34.9 | 34.1 |
| III | 18.0 | 17.0 | 17.1 | 16.9 |
| IV | 8.5 | 7.8 | 7.6 | 7.9 |
| Hypertension, % | 75.1 | 73.0 | 73.2 | 72.8 |
| History of diabetes mellitus, % | 37.8 | 36.2 | 37.7 | 35.0 |
| Hypercholesterolemia, % | 76.3 | 76.9 | 78.4 | 75.7 |
| Prior myocardial infarction, % | 42.2 | 42.4 | 44.0 | 41.2 |
| Prior stroke, % | 5.5 | 3.9 | 4.6 | 3.4 |
| Renal failure, % | 2.2 | 1.3 | 0.8 | 1.6 |
| Peripheral vascular disease, % | 12.2 | 11.0 | 11.2 | 10.8 |
| Prior PCI, % | 25.9 | 27.3 | 28.2 | 26.6 |
| Pacemaker implantation, % | 1.2 | 0.9 | 0.5 | 1.2 |
| Cigarette smoker, % | ||||
| Never | 31.4 | 32.6 | 32.9 | 32.3 |
| Former | 45.7 | 45.3 | 44.2 | 46.2 |
| Current | 22.9 | 22.1 | 22.9 | 21.5 |
| Creatinine clearance, median, mL/min | 88.5 (69.6, 112.1) | 90.1 (72.5, 113.1) | 88.9 (71.1, 112.9) | 90.8 (72.8, 113.4) |
| Total CK, median, IU/L | 0.4 (0.3, 0.7) | 0.4 (0.3, 0.7) | 0.4 (0.3, 0.7) | 0.4 (0.3, 0.7) |
| CK-MB, median, IU/L | 0.4 (0.2, 0.8) | 0.4 (0.2, 0.9) | 0.4 (0.2, 0.9) | 0.4 (0.2, 0.9) |
| Surgery | ||||
| Urgent surgery, % | 48.5 | 46.7 | 47.6 | 45.9 |
| Endoscopic vein graft, % | 58.4 | 54.8 | 60.0 | 51.0 |
| Internal thoracic artery graft, % | 92.3 | 92.7 | 92.5 | 92.9 |
| Surgery duration, median, min | 231.0 (193.0, 272.0) | 229.0 (192.0, 270.0) | 240.0 (200.0, 284.0) | 221.0 (187.0, 261.0) |
| CPB duration, median, min | 100.0 (79.0, 123.0) | 100.0 (80.0, 122.0) | 107.0 (85.0, 128.0) | 95.0 (77.0, 117.0) |
| Postoperative duration, median Ventilator, h | 7.5 (4.6, 13.8) | 7.0 (4.5, 12.0) | 7.0 (4.5, 13.0) | 7.0 (4.3, 11.0) |
| Intensive care unit stay, h | 26.0 (22.0, 47.0) | 25.0 (21.5, 45.5) | 25.0 (21.0, 46.0) | 26.0 (22.0, 45.0) |
| Hospital stay, d | 6.0 (5.0, 8.0) | 60. (5.0, 7.0) | 6.0 (5.0, 7.0) | 6.0 (5.0, 7.0) |
VGF indicates vein graft failure; BMI, body mass index; BP, blood pressure; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; CK, creatine kinase; and CPB, cardiopulmonary bypass. Values in parentheses are 25th and 75th percentiles.
Medications at 30 Days and 1 Year
Medications used 30 days and 1 year after the CABG surgery among the angiographic population with and without vein graft failure are shown in Table 2. At 30 days, similar proportions of patients with and without vein graft failure were on aspirin, angiotensin-converting enzyme inhibitors, β-blockers, and statins. At 1 year, the proportion of patients on angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statins tended to be higher than at 30 days but was similar between patients with and without vein graft failure. Use of clopidogrel tended to be higher among patients with vein graft failure at both 30 days and 1 year.
Table 2.
Medications at 30 Days and 1 Year After Coronary Artery Bypass Graft Surgery Among the 1829 Patients in the Angiographic Population With and Without Vein Graft Failure
| Medications | At 30 Days With VGF* (n=787), n (%) | At 30 Days Without VGF* (n=1042), n (%) | At 1 Year With VGF* (n=770), n (%) | At 1 Year Without VGF* (n=1020), n (%) |
|---|---|---|---|---|
| Aspirin | 715 (91.3) | 967 (92.9) | 716 (93.0) | 960 (94.1) |
| Clopidogrel | 204 (26.1) | 199 (19.1) | 230 (29.9) | 190 (18.6) |
| Angiotensin-converting enzyme inhibitor | 281 (35.9) | 368 (35.4) | 332 (43.1) | 430 (42.2) |
| Angiotensin receptor blocker | 48 (6.1) | 68 (6.5) | 99 (12.9) | 154 (15.1) |
| β-Blocker | 639 (81.6) | 811 (77.9) | 574 (74.5) | 739 (72.5) |
| Nitrate | 45 (5.7) | 65 (6.3) | 73 (9.5) | 49 (4.8) |
| Diuretic | 240 (30.7) | 258 (24.8) | 259 (33.6) | 315 (30.9) |
| Statin | 583 (74.5) | 780 (74.9) | 603 (78.3) | 838 (82.2) |
| Other lipid-lowering drug | 75 (9.6) | 112 (10.8) | 164 (21.3) | 212 (20.8) |
| Corticosteroid | 15 (1.9) | 17 (1.6) | 12 (1.6) | 15 (1.5) |
Vein graft failure (VGF) assessed at the angiogram at 12 to 18 months.
Events Between CABG Surgery and Angiographic Follow-Up
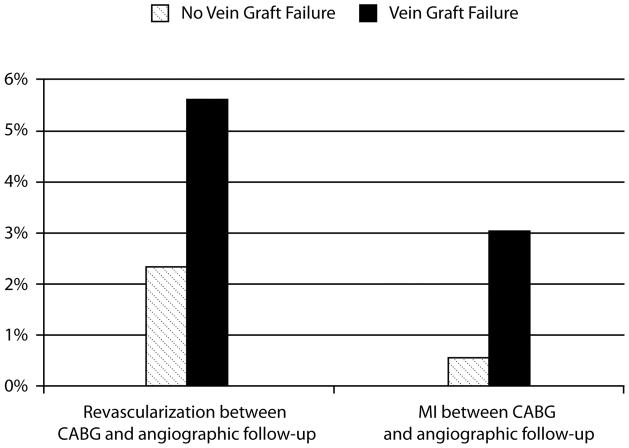
Of the 787 patients with vein graft failure assessed on the 1-year angiogram, 44 (5.6%) had a repeat revascularization procedure and 24 (3.0%) had an MI between CABG surgery and angiographic follow-up. Of the 1042 patients without vein graft failure on angiography, 24 (2.3%) had a repeat revascularization procedure and 5 (0.5%) had an MI between CABG surgery and angiographic follow-up (Figure 2).
Figure 2.
Rates of myocardial infarction (MI) and revascularization between coronary artery bypass graft (CABG) surgery and 12- to 18-month angiographic follow-up among patients with and without subsequent angiographic vein graft failure. The timing of vein graft failure in relationship to these events is unknown.
Vein Graft Failure According to Graft Quality, Target Vessel, and Target Vessel Quality
Vein graft failure rates according to graft quality, target vessel, and target vessel quality are shown in Table 3. Compared with better-quality grafts, patients with poor-quality grafts had higher rates of vein graft failure (vein graft failure was defined as 75% or total occlusion). Similar results were seen for target artery quality. Vein graft failure was more common in grafts with multiple targets, followed by circumflex artery and diagonal artery grafts.
Table 3.
Vein Graft Failure and Occlusion According to Graft Quality, Target Vessel, and Target Vessel Quality
| Vein Graft Failure, % (n)
|
||
|---|---|---|
| 75% Stenosis | Occluded | |
| Graft quality | ||
| Good (n=3581) | 28 (994) | 25 (889) |
| Fair (n=826) | 32 (268) | 29 (239) |
| Poor (n=153) | 37 (56) | 33 (51) |
| Graft target vessel | ||
| LAD (n=110) | 25 (27) | 23 (25) |
| Diagonal (n=707) | 29 (206) | 26 (185) |
| LCx (n=148) | 29 (43) | 28 (41) |
| RI (n=167) | 25 (41) | 22 (36) |
| OM (n=1135) | 28 (316) | 25 (284) |
| RCA (n=493) | 24 (118) | 22 (106) |
| PDA (n=921) | 29 (269) | 25 (226) |
| Multiple (n=757) | 34 (260) | 31 (238) |
| Target artery quality | ||
| Good (n=2413) | 25 (598) | 22 (535) |
| Fair (n=985) | 29 (289) | 26 (254) |
| Poor (n=397) | 43 (169) | 38 (150) |
LAD indicates left anterior descending; LCx, left circumflex; RI, ramus intermedius; OM, obtuse marginal; RCA, right coronary artery; and PDA, posterior descending artery.
Vein Graft Failure and Subsequent Clinical Outcomes
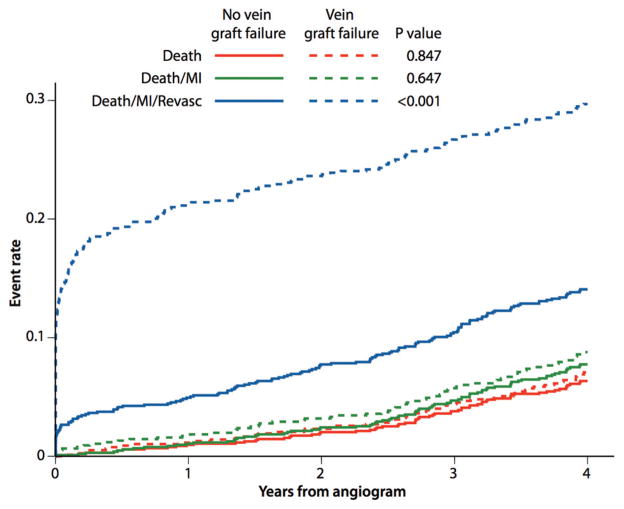
After angiography, there was a markedly higher rate of death, MI, or revascularization within 14 days of angiography among patients who had vein graft failure compared with those who did not (adjusted HR, 5.52; 95% CI, 3.55–8.56; P<0.001). After the exclusion of patients who underwent revascularization within the first 14 days of the angiogram, the composite of death, MI, and revascularization occurred more frequently among patients who had vein graft failure compared with those who did not (adjusted HR, 1.58; 95% CI, 1.21–2.06; P=0.008). This was due mainly to more frequent revascularization. There was no relationship between vein graft failure and the composite of death or MI or death alone (Table 4). Figure 3 illustrates the occurrence of clinical events through 4 years from follow-up angiography among patients with and without vein graft failure. Most patients who underwent repeat revascularization had the procedure early after follow-up angiography (Figure 3). There was no significant relationship between the number of vein grafts implanted and outcome; however, after the number of vein grafts implanted was accounted for, there was a relationship between the proportion with vein graft failure and the composite outcome of death, MI, and revascularization (adjusted HR, 5.23; 95% CI, 3.98–6.87; P<0.001). This relationship was not significant for death (adjusted HR, 1.24; 95% CI, 0.73–2.10; P=0.423) and death or MI (adjusted HR, 1.38; 95% CI, 0.86–2.21; P=0.184).
Table 4.
Relationship of Vein Graft Failure (per Patient) to 4-Year Major Adverse Cardiac Event Outcomes
| n | Events, n | Events Occurring ≤14 Days After Angiogram
|
Events Occurring >14 Days After Angiogram
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Death, MI, or revascularization | 1751 | 351 | 5.52 (3.55– 8.56) | <0.001 | 1.58 (1.21–2.06) | 0.008 |
| Death or MI* | 1800 | 138 | 1.08 (0.77–1.53) | 0.647 | ||
| Death or revascularization | 1761 | 341 | 5.42 (3.49–8.42) | <0.001 | 1.57 (1.20–2.06) | 0.001 |
| Death* | 1829 | 113 | 1.04 (0.71–1.52) | 0.847 | ||
| Revascularization | 1761 | 245 | 5.71 (3.65, 8.94) | <0.001 | 2.29 (1.59–3.30) | <0.001 |
HR indicates hazard ratio; CI, confidence interval; and MI, myocardial infarction. All models are adjusted for variables in the associated 5-y model.
The proportional hazards assumption applies for death and death or MI, so the statistics presented for these end points apply to the entire postangiogram period.
Figure 3.
Relationship between vein graft failure and clinical outcomes over 4 years after angiographic follow-up. MI indicates myocardial infarction; Revasc, revascularization.
Discussion
Vein graft failure, assessed by angiography 12 to 18 months after CABG surgery, was strongly associated with an increased risk for the composite of death, MI, or repeat revascularization at 4 years after the angiogram. This relationship was driven mostly by repeat revascularization with no differences in either death or the composite of death and MI between patients with and without vein graft failure. The association between vein graft failure and an increased risk of subsequent revascularization persisted even after the exclusion of patients who underwent revascularization within the first 14 days after protocol-driven angiography. This suggests that the increased risk of later repeat revascularization resulted not only from incidental findings on protocol-mandated angiography but also from clinical symptoms and/or objective evidence of ischemia.
Most studies that evaluated outcomes of patients undergoing CABG surgery found a relationship between the use of arterial conduits, particularly the internal mammary arteries, and improved long-term survival. Although complete arterial revascularization remains an ideal and optimistic goal, saphenous vein conduit is used ubiquitously in contemporary coronary artery bypass surgery with an average of 2 vein grafts per CABG procedure. It has been intuitively assumed that the loss of bypass conduit (arterial or venous) that results in a decrease in the blood supply beyond a stenotic coronary lesion should adversely affect long-term outcomes. Prior studies have shown that the outcomes of patients with more severe coronary artery disease are worse than those with lesser degrees of coronary artery disease.14 In addition, patients with more severe coronary artery disease get a greater benefit from CABG, and more complete revascularization during CABG is associated with better outcomes than incomplete revascularization.14 Despite this intuitive logic, few studies have provided insight into whether patients with vein graft failure after CABG surgery actually have worse subsequent long-term clinical outcomes. Recent data from the Fractional Flow Reserve Versus Angiography for Multivessel Evaluation (FAME) study indicate that in 20% of coronary arteries with angiographic stenosis between 70% and 90%, typical for an aortocoronary bypass, there was no fractional flow reserve evidence of ischemia.15 In such cases, if a surgical vein graft target is not associated with ischemia, graft failure may occur without clinical consequence, and vein graft failure at these targets may be accelerated for functional reasons.
Lytle and colleagues16,17 retrospectively studied 723 patients who underwent angiography that documented a stenosis of 20% to 99% in at least 1 saphenous vein graft after CABG surgery and did not undergo reoperation or percutaneous coronary intervention within 1 year after that catheterization. After a mean follow-up of 83 months, they found that patients with vein graft stenoses within 5 years after operation and patients with no stenotic vein grafts had similar outcomes. However, patients with significant stenosis in saphenous vein grafts to the left anterior descending coronary artery had higher rates of death and cardiac ischemic events.
Fitzgibbon et al18 described clinical outcomes and vein graft failure rates among 1388 patients who underwent a first CABG surgery from 1969 to 1994. This study showed that both mortality and vein graft failure rates increased over time, particularly 7 years after the CABG surgery. At 5 years, survival rates were ≈94% and the vein graft failure rate was 25%. However, this study did not relate vein graft failure with subsequent clinical outcomes. In a single-center study, Halabi et al19 demonstrated that for patients presenting for coronary angiography, up to 20% of vein grafts failed within 2 years of CABG surgery. These investigators also found that early vein graft failure was associated with worse long-term outcomes of death, MI, and revascularization and that, similar to our findings, these results were driven by early revascularization. In their study, vein graft failure was the strongest predictor of the composite clinical outcome at 10 years.
In our study, we did not see the significant differences in death and cardiac ischemic event rates seen in the study by Lytle et al.16,17 This may be related to the fact that in the more contemporary PREVENT IV study, nearly all grafts to the left anterior descending artery (93%) were internal mammary arterial grafts with low failure rates. In the studies by Lytle et al16,17 and Halabi et al,19 follow-up angiography was driven by clinical symptoms or a positive functional study rather than by protocol at 12 to 18 months after surgery. Thus, unlike in our study, these 2 studies did not capture vein graft failure in most patients in whom angiography was not performed, thereby underestimating the true incidence of vein graft failure in patients undergoing CABG surgery and precluding the evaluation of the true association between vein graft failure and clinical outcomes. Perhaps early vein graft failure or vein graft failure accompanied by associated clinical symptoms is more prognostically important.
We did not observe a relationship between vein graft failure and subsequent death and death or MI; in many instances, however, a clinical event resulting from vein graft failure may precede the detection of vein graft failure on protocol-driven angiography 12 to 18 months after CABG surgery. Although we do not know whether vein graft failure preceded the clinical event, we observed higher rates of both MI and repeat revascularization in the period between CABG surgery and the protocol-driven angiography among patients with vein graft failure. Similarly, we excluded patients who did not survive until their first angiogram. Some of these deaths also may have been related to vein graft failure. By excluding these early events that occurred before the detection of vein graft failure, we likely biased our results toward the null (ie, no difference in death and death or MI in patients with vein graft failure). It is also possible that the protocol-driven angiography and the subsequent revascularization of patients who had vein graft failure may have reduced the rate of subsequent clinical events in patients with vein graft failure.
In addition to reducing hard clinical events such as death, MI, and revascularization, CABG surgery has been shown to decrease angina, rehospitalization for congestive heart failure, and ischemic ventricular tachyarrhythmias while improving functional status and quality of life. Vein graft failure may have an impact on these outcomes; however, these data were not collected in PREVENT IV.
Our study has both clinical and research implications. Efforts to prolong vein graft patency should be an integral part of the CABG procedure. Although striving to achieve complete arterial revascularization remains ideal, this goal is generally not achievable. Emphasis should be directed toward the selection of good-quality vein conduit and preventing vein graft injury during harvesting. Beyond surgery, optimal secondary prevention, including medications and lifestyle modification, may have significant influence on prolonging vein graft patency and/or reducing clinical event rates in patients undergoing CABG surgery. These measures are likely to reduce vein graft failure and to improve clinical outcomes through both preserving graft patency and limiting the progression of native coronary disease. In PREVENT IV, more than a quarter of patients were receiving clopidogrel postoperatively, with higher use among patients who developed vein graft failure. Although aspirin has been shown to improve graft patency, there are no randomized data with clopidogrel to support its use after elective CABG surgery.20–22 Clopidogrel is more likely to be used in patients with a recent acute coronary syndrome, those with more extensive disease, and those undergoing off-pump CABG surgery and thus may be a marker of a sicker patient.23–27 The high vein graft failure rate and its relationship with worse clinical outcomes call for more research, including randomized clinical trials, on how to improve both short- and long-term vein graft patency, including more optimal identification of targets based on both anatomic and functional (ischemic) characteristics and elucidation of the ideal secondary prevention medical regimen after CABG surgery.20–22
Limitations
Our study has several limitations. We measured vein graft failure at 1 year, but it is possible that failure happened earlier. We did not have information on the timing of vein graft failure before angiography to determine whether it occurred before, coincident with, or after nonfatal events that occurred between CABG surgery and angiographic follow-up. Protocol-driven angiography may have influenced revascularization rates and clinical events. We did not assess the impact of internal mammary artery failure on the relationship between vein graft failure and clinical outcomes. The study may have been underpowered to detect a modest but clinically important relationship between vein graft failure and subsequent death and death or MI. Finally, it is likely that not all vein grafts are equal and that, depending on the myocardium that a vein graft supplies, failure of some vein grafts may have a greater influence on outcomes than failure of other vein grafts.
Conclusions
Vein graft failure 1 year after CABG surgery is associated with an increased risk of death, MI, or revascularization at 4 years after the angiogram. This association is driven mostly by revascularization with little relationship between vein graft failure and death and death or MI. Further investigation is warranted to devise strategies for detecting and preventing vein graft failure and to gain a better understanding of the clinical consequences and appropriate management of patients with vein graft failure after CABG surgery.
CLINICAL PERSPECTIVE.
In our study, we showed that the composite of death, myocardial infarction, or revascularization occurred more frequently among patients who had any vein graft failure compared with those who had none (adjusted hazard ratio, 1.58; 95% confidence interval, 1.21–2.06; P=0.008). This was due mainly to more frequent revascularization in patients with vein graft failure with no differences in death (adjusted hazard ratio, 1.04; 95% confidence interval, 0.71–1.52; P=0.85) or death or myocardial infarction (adjusted hazard ratio, 1.08; 95% confidence interval, 0.77–1.53; P=0.65) between patients with and without vein graft failure. Our findings suggest that the increased risk of repeat revascularization was not only from incidental findings on protocol-mandated angiography but also from clinical symptoms and/or objective evidence of ischemia. Vein graft failure rates were higher in patients with poor graft and target artery quality. The absolute number of failed grafts did not correlate with long-term clinical outcomes; however, the proportion of grafts with vein graft failure did. Emphasis should be directed toward selection of a good-quality vein conduit and preventing vein graft injury during harvesting. Beyond surgery, optimal secondary prevention, including medications and lifestyle modification, may have significant influence on prolonging vein graft patency and/or reducing clinical event rates in patients undergoing coronary artery bypass graft surgery. The high vein graft failure rate and its relationship with worse clinical outcomes call for more research on how to improve both short- and long-term vein graft patency in patients undergoing coronary artery bypass graft surgery.
Acknowledgments
Sources of Funding
The PREVENT IV trial was funded by Corgentech Inc, San Francisco, CA. This analysis was supported by the Duke Clinical Research Institute. Drs Williams, Ferguson, and Alexander are supported in part by grant U01-HL088953 from the National Institutes of Health Cardiothoracic Surgical Trials Network.
Footnotes
Disclosures
Dr Mack has received consulting fees from Maquet, Inc. The other authors report no conflicts.
References
- 1.Allen K, Cheng D, Cohn W, Conolly M, Edgerton J, Falk V, Martin J, Ohtsuka T, Vitali R. Endoscopic vascular harvest in coronary artery bypass grafting surgery: a consensus statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) Innovations (Phila) 2005;1:51–60. doi: 10.1097/01.gim.0000196315.32179.82. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson TB, Jr, Peterson ED, Coombs LP, Eiken MC, Carey ML, Grover FL, DeLong ER. Use of continuous quality improvement to increase use of process measures in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. JAMA. 2003;290:49–56. doi: 10.1001/jama.290.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Holman WL, Sansom M, Kiefe CI, Peterson ED, Hubbard SG, Delong JF, Allman RM. Alabama Coronary Artery Bypass Grafting Project: results from phase II of a statewide quality improvement initiative. Ann Surg. 2004;239:99–109. doi: 10.1097/01.sla.0000103065.17661.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjork VO, Ekestrom S, Henze A, Ivert T, Landou C. Early and late patency of aortocoronary vein grafts. Scand J Thorac Cardiovasc Surg. 1981;15:11–21. doi: 10.3109/14017438109101020. [DOI] [PubMed] [Google Scholar]
- 5.Roth JA, Cukingnan RA, Brown BG, Gocka E, Carey JS. Factors influencing patency of saphenous vein grafts. Ann Thorac Surg. 1979;28:176–183. doi: 10.1016/s0003-4975(10)63777-0. [DOI] [PubMed] [Google Scholar]
- 6.Cataldo G, Braga M, Pirotta N, Lavezzari M, Rovelli F, Marubini E. Factors influencing 1-year patency of coronary artery saphenous vein grafts: Studio Indobufene nel Bypass Aortocoronarico (SINBA) Circulation. 1993;88(suppl):II-93–II-98. [PubMed] [Google Scholar]
- 7.Paz MA, Lupon J, Bosch X, Pomar JL, Sanz G. Predictors of early saphenous vein aortocoronary bypass graft occlusion: the GESIC Study Group. Ann Thorac Surg. 1993;56:1101–1106. doi: 10.1016/0003-4975(95)90024-1. [DOI] [PubMed] [Google Scholar]
- 8.Cho KR, Kim JS, Choi JS, Kim KB. Serial angiographic follow-up of grafts one year and five years after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2006;29:511–516. doi: 10.1016/j.ejcts.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Kim KB, Cho KR, Jeong DS. Midterm angiographic follow-up after off-pump coronary artery bypass: serial comparison using early, 1-year, and 5-year postoperative angiograms. J Thorac Cardiovasc Surg. 2008;135:300–307. doi: 10.1016/j.jtcvs.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor NJ, Morton JR, Birkmeyer JD, Olmstead EM, O’Connor GT. Effect of coronary artery diameter in patients undergoing coronary bypass surgery: Northern New England Cardiovascular Disease Study Group. Circulation. 1996;93:652–655. doi: 10.1161/01.cir.93.4.652. [DOI] [PubMed] [Google Scholar]
- 11.Shah PJ, Gordon I, Fuller J, Seevanayagam S, Rosalion A, Tatoulis J, Raman JS, Buxton BF. Factors affecting saphenous vein graft patency: clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J Thorac Cardiovasc Surg. 2003;126:1972–1977. doi: 10.1016/s0022-5223(03)01276-5. [DOI] [PubMed] [Google Scholar]
- 12.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 13.Alexander JH, Ferguson TB, Jr, Joseph DM, Mack MJ, Wolf RK, Gibson CM, Gennevois D, Lorenz TJ, Harrington RA, Peterson ED, Lee KL, Califf RM, Kouchoukos NT. The PRoject of Ex-vivo Vein graft ENgineering via Transfection IV (PREVENT IV) trial: study rationale, design, and baseline patient characteristics. Am Heart J. 2005;150:643–649. doi: 10.1016/j.ahj.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 14.European Association for Percutaneous Cardiovascular Interventions. Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 15.Tonino PA, Fearon WF, DeBruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, MacCarthy PA, Van’t Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the FAME study: Fractional Flow Reserve Versus Angiography in Multivessel Evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 16.Lytle BW, Loop FD, Taylor PC, Simpfendorfer C, Kramer JR, Ratliff NB, Goormastic M, Cosgrove DM. Vein graft disease: the clinical impact of stenoses in saphenous vein bypass grafts to coronary arteries. J Thorac Cardiovasc Surg. 1992;103:831–840. [PubMed] [Google Scholar]
- 17.Lytle BW, Loop FD, Taylor PC, Goormastic M, Stewart RW, Novoa R, McCarthy P, Cosgrove DM. The effect of coronary reoperation on the survival of patients with stenoses in saphenous vein bypass grafts to coronary arteries. J Thorac Cardiovasc Surg. 1993;105:605–614. [PubMed] [Google Scholar]
- 18.Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996;28:616–626. doi: 10.1016/0735-1097(96)00206-9. [DOI] [PubMed] [Google Scholar]
- 19.Halabi AR, Alexander JH, Shaw LK, Lorenz TJ, Liao L, Kong DF, Milano CA, Harrington RA, Smith PK. Relation of early saphenous vein graft failure to outcomes following coronary artery bypass surgery. Am J Cardiol. 2005;96:1254–1259. doi: 10.1016/j.amjcard.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz RL, Schacky CV, Weber M, Meister W, Kotzur J, Reichardt B, Theisen K, Weber PC. Improved aortocoronary bypass patency by low-dose aspirin (100 mg daily): effects on platelet aggregation and thromboxane formation. Lancet. 1984;1:1261–1264. doi: 10.1016/s0140-6736(84)92446-2. [DOI] [PubMed] [Google Scholar]
- 21.Goldman S, Copeland J, Mortiz T, Henderson W, Zadina K, Ovitt T, Kern KB, Sethi G, Sharma GV, Khuri S. Long-term graft patency (3 years) after coronary artery surgery: effects of aspirin: results of a VA Cooperative Study. Circulation. 1994;89:1138–1143. doi: 10.1161/01.cir.89.3.1138. [DOI] [PubMed] [Google Scholar]
- 22.Collaborative overview of randomised trials of antiplatelet therapy. II: maintenance of vascular graft of arterial patency by antiplatelet therapy. BMJ. 1994;308:159–168. [PMC free article] [PubMed] [Google Scholar]
- 23.Tricoci P, Roe MT, Mulgund J, Newby LK, Smith SC, Jr, Pollack CV, Jr, Fintel DJ, Cannon CP, Bhatt DL, Gibler WB, Ohman EM, Peterson ED, Harrington RA. Clopidogrel to treat patients with non-ST-segment elevation acute coronary syndromes after hospital discharge. Arch Intern Med. 2006;166:806–811. doi: 10.1001/archinte.166.7.806. [DOI] [PubMed] [Google Scholar]
- 24.Gurbuz AT, Zia AA, Vuran AC, Cui H, Aytac A. Postoperative clopidogrel improves mid-term outcome after off-pump coronary artery bypass graft surgery: a prospective study. Eur J Cardiothorac Surg. 2006;29:190–195. doi: 10.1016/j.ejcts.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Gao G, Zheng Z, Pi Y, Lu B, Lu J, Hu S. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery: a single-center, randomized, controlled trial. J Am Coll Cardiol. 2010;56:1639–1643. doi: 10.1016/j.jacc.2010.03.104. [DOI] [PubMed] [Google Scholar]
- 26.A randomised, blinded trial of Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) Lancet. 1996;348:1329–1339. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]