Abstract
Human lung tissue donated for research purposes is a precious resource which can enhance the exploration of mechanisms involved in ventilator-induced lung injury (VILI). The goal of this work was to establish methods and demonstrate the feasibility of obtaining viable primary human type I-like alveolar epithelial cells (AECs) from remnant tissue, even after a significant lapse in post-mortem time, as well as human precision-cut lung slices (PCLSs), and stretch them at magnitudes correlated with mechanical ventilation volumes. Although after 3 days in culture many of the isolated cells stained for the type II AEC marker pro-surfactant Protein C (pro-SPC), after 6 days in culture the monolayer stained only weakly and non-specifically for pro-SPC, and stained brightly for the type I AEC marker aquaporin-5. A strong zona-occludin 1 stain demonstrated the formation of tight junctions between the cells in the epithelial monolayer after only 3 days in culture. To demonstrate the utility of the preparations for the study of lung injury, we stretched the cells and the PCLSs cyclically, uniformly, and equibiaxially and quantified their viability. Our data show that the described methods allow the utilization of human tissue in in vitro stretch studies investigating VILI.
Keywords: Human tissue, Lung injury, Precision-cut lung slice, Pulmonary alveolar epithelial cells
INTRODUCTION
Ventilator-induced lung injury (VILI), associated with pulmonary edema,27,31 occurs when mechanical ventilation overinflates the lungs and overstretches the alveolar walls,14 increasing the epithelial barrier permeability through breaching of the tight junction complexes between alveolar epithelial cells (AECs).3 Thus, laboratory studies of VILI require mechanical stretching of lung tissue in vivo, in vitro or ex vivo. Whereas in vivo models as well as ex vivo whole-lung models have been used in VILI studies,5,29 non-uniform lung expansion and heterogeneous cell composition hinder the identification of specific injury mechanisms or contribution of individual cell types in the injury response.
A common in vitro approach to study VILI in an idealized alveolus is to isolate type II AECs and maintain them in culture until they adopt type I-like features associated with maintaining the alveolar wall mechanical integrity,13,25 and stretch these cells at magnitudes that are analogous to pathological mechanical ventilation tidal inflations.28 Although methods for isolating human AECs for different purposes have been well established,15,17,20 the vast majority of VILI investigators use rodent primary cells2,10 or alveolar type II cell lines,4 likely because of the paucity of fresh human tissue. In fact, to the best of our knowledge there is currently no published study in which stretch is applied to primary human type I-like AECs.
Another in vitro approach to study VILI is stretching of precision cut lung slices (PCLSs), typically obtained from rodent lungs. PCLSs have been shown to stretch non-uniformly when clamped onto a thin and flexible PDMS membrane8 or when depressed into a polyacrylamide gel using an indenter.24 Recently, we demonstrated uniform equibiaxial stretch of rat PCLSs that were stitched onto flexible membranes,9,22 however there are currently no published stretched human PCLS studies.
Translation of VILI investigative methods to human tissue is restricted because the availability of fresh human tissue for research is unusual and erratic. Sharing of the tissue between two or more research laboratories further exacerbates delays in tissue acquisition, and may limit methods for cell isolation, if the sample presents without organized access to the bronchioles for perfusion. Perfusion is commonly employed in cell isolation and purification techniques to remove red blood cells from the tissue and initiate elastase-driven enzymatic digestion of the structural matrix of the tissue. Similarly, bronchioles are used for injecting agarose gel into the airways to facilitate slicing of PCLSs. With short notice prior to human tissue availability, overnight coating of the culture substrates with an extracellular matrix protein, e.g., collagen, to enhance cell adhesion, further delays the cell isolation. The total post-mortem time between lung excision from the donor to cell or tissue plating may be up to 36–48 h, rather than the 1–5 h typical of planned animal tissue isolation.
The goal of this study was to overcome the technical difficulties of human lung tissue investigations by developing methods to isolate and purify human primary type II AECs and culture them on stretchable membranes, and to maintain them in culture conditions that allow their differentiation into type I-like AECs. In addition, we demonstrate the preparation and use of human PCLSs for stretch studies. The tissue used in this work was received as remnant fragments resulting from tissue distribution among several research laboratories, without access to a main bronchiole.
MATERIALS AND METHODS
The human tissue used to derive epithelial cell cultures and precision-cut lung slices is obtained from deidentified materials from National Disease Research Interchange (NDRI). De-identified tissue and cells without the ability to link to research subjects are considered non-human as per the University of Pennsylvania Committee on Studies Involving Human Subjects and the National Institutes of Health. Distal lung tissue chunks weighing ~20 g in total from each of 6 donors, were generously provided to us by Dr. Reynold A. Panettieri (University of Pennsylvania, Philadelphia, PA).
Isolation of Human Primary Type II Alveolar Epithelial Cells
Pieces of remnant human lung tissue (approximately 2 cm3 in volume) were kept at 4 °C in phosphate buffered saline (PBS) prior to the isolation, for 20–24 h, during which Silastic membranes (Specialty Manufacturing, Saginaw, MI) were coated with collagen as detailed below. The cell isolation procedure was based on previously published protocols with modifications.15,21 The tissue was transferred into a tissue culture plastic dish containing a water solution with 6 mM Glucose, 0.5 mM EGTA, 140 mM NaCl, 5 mM KCL, 2.5 mM Na2HPO4 and 10 mM HEPES (Solution A) and kept on ice. Because we could not perfuse these small tissue pieces, we increased the tissue surface area that is exposed to the different solutions by first cutting the pieces into smaller fragments, roughly 0.5 cm3 in size, followed by five washes with Solution A to remove red blood cells and debris. The pieces were then transferred into another plastic dish on ice and washed thoroughly with a water solution containing 2 mM CaCl2, 1.3 mM MgSO4, 0.5 mM EGTA, 140 mM NaCl, 5 mM KCL, 2.5 mM Na2HPO4 and 10 mM HEPES (Solution B).
After washing, the pieces were transferred into elastase solution (13 units/mL, Worthington Biochemical, LS002292) and incubated at 37 °C for 1 h while stirring very slowly on a magnetic stirrer using a magnetic bar in the bottom of the flask to enhance cell detachment from the basal membrane. After this enzymatic digestion, the tissue pieces were transferred into Dulbecco’s Modified Eagle Medium (DMEM, MediaTeach, MT10-017-CV) supplemented with 10% fetal bovine serum (FBS, Sigma, F6178), 0.25 lg/mL Amphotericin B (Gibco, 1529-018) and 50 lg/mL Gentamicin (Gibco, 15750-060) and kept at room temperature. Several pieces were subsequently transferred into a small beaker and minced with scissors. This step was repeated until all the pieces were minced. The minced tissue was then transferred into an Erlenmeyer flask with Solution B supplemented with 200 lg/mL DNase (Sigma, DN25-1G) and the flask was vigorously swirled in a 37 °C water bath for 2 min.
Purification and Culture of Human Primary Type II Alveolar Epithelial Cells
Because we could not perfuse our tissue, many cell types were still present in the cell suspension after the DNase step and the AECs needed to be purified using two different purification methods. First, the cell suspension in the DNase solution was filtered consecutively through 405, 160, and 28 μm nylon meshes on ice. The resulting filtrate was spun at 1500 g for 10 min at room temperature. During this the step, a Percoll (Sigma, P4937) gradient was prepared by gently pouring a Percoll light layer (at a density of 0.31 g/mL in PBS) onto the Percoll heavy layer (0.73 g/mL in PBS) in order to minimize the mixing between the layers. After spinning the cell filtrate, the solution was aspirated and the cell pellet was resuspended in 3 mL of DMEM with FBS and antibiotics (see details above). The cell suspension was gently placed onto the Percoll gradient. The gradient was spun at 300 g for 30 min at 4 °C, after which three distinct cell layers were formed: red blood cell layer at the bottom of the gradient tube, a white creamy band of alveolar epithelial cells between the heavy and light layers, and a third layer of cell debris above the light layer.
The layers above the white creamy AEC layer were gently removed from the gradient so that the AEC band could be collected and resuspended in DMEM with FBS and antibiotics (see details above). The resulting cell solution was spun at 1500 g for 10 min and the pellet was resuspended in Small Airway Epithelial Cell Growth Medium (SAGM; Lonza, CC-3118) containing 10% FBS, 0.25 μg/mL Amphotericin B, 100 units/mL and 100 μg/mL Penicillin/Streptomycin, respectively (Invitrogen 15140122), and 10 units/mL Nystatin (Sigma, N1638). AECs were then counted and seeded on flexible Silastic membranes in custom-designed wells,28 at a density of 2.2 × 106 cells/cm2 and cultured at 37 °C and 5% CO2 for 9 days. Prior to seeding, the membranes were coated overnight with 20 μg/cm2 rat tail type-I collagen18 (BD Biosciences 354236) at 37 °C, 5% CO2.
Phenotype Studies
The cultured cells were fixed on days 3, 6, and 9 and stained immunofluorescently with phenotype markers. For detecting type I AECs, we used an antibody for aquaporin-5 (AQP5), a water channel localized to the apical membrane of type I cells.12,23,26 For detecting type II AECs, we used an antibody for pro-surfactant Protein C (pro-SPC), a member of the pulmonary surfactant family of proteins and a widely used marker for type II AECs.1,19,30 In addition, we stained for vimentin to determine the presence of mesenchymal cells in the cultures and for zona-occludin 1 (ZO-1) to mark the tight junctional complexes between adjacent AECs. In cells from two donors, we used two additional antibodies made and provided as a gift by Dr. Leland G. Dobbs (University of California, San Francisco, San Francisco, CA): an antibody for 56-kDa human type I protein (HTI-56)11 and an antibody specific for the apical surface of human type II cells (HTII-280).20
Briefly, the cultured cells were fixed with 4% paraformaldehyde in PBS for 10 min and blocked with 5% normal goat serum in PBS for 1 h at room temperature. The cells were then incubated overnight with mouse anti-AQP5 antibody (1:200) and rabbit anti-pro-SPC antibody (1:200), or with mouse anti-vimentin antibody (1:50) and rabbit anti-ZO-1 antibody (1:50). FITC-conjugated anti-mouse (1:100) and Texas Red-conjugated anti-rabbit (1:50) secondary antibodies were used as appropriate and the incubation was for 4 h at room temperature. For the HTI-56/HTII-280 stain, we used mouse IgG anti-HTI-56 (1:25) and mouse IgM anti-HTII-280 (1:25), followed by incubation with Texas Red-conjugated anti-mouse IgG (1:300) and Alexa 488 anti-mouse IgM (1:300). Thereafter, the membranes were cut and mounted onto microscope slides using Prolong Gold DAPI mounting medium (Invitrogen P-36931), and images were captured on an epifluorescent microscope (Nikon Eclipse TE300).
Preparation of Human Precision-Cut Lung Slices
Human PCLSs were prepared from lung tissue donated by three donors, as detailed elsewhere.6,7 PCLSs were prepared from distal pieces of the smallest lobe of lungs that had been inflated with low melting point agarose via the main bronchiole by Dr. Panettieri’s laboratory staff, who provided us with the human tissue. Briefly, as soon as possible after transferring the tissue, cylindrical pieces were cut from the lung lobes using a 15 mm diameter corer while care was taken to exclude major airways and blood vessels. Subsequently, 250 μm-thick PCLSs were sliced in ice-cold PBS using a Leica VT 1000S tissue slicer. The PCLSs were then incubated floating in serum-free minimal essential medium (MEM) supplemented with 50 μg/mL Gentamicin and 0.25 μg/mL Amphotericin B and kept in a 5% CO2 incubator at 37 °C. To remove residual agarose and cellular debris, the medium was changed every 30 min for the first 2 h, every 1 h for the next 2 h, and every 24 h thereafter.
Stretch experiments were conducted with PCLSs that were maintained floating in culture for up to 3 days. Prior to stretch experiments, the PCLSs were stitched to the flexible Silastic membranes mounted in custom-designed wells,28 using a 5–0 non-absorbable silk suture (Surgik LC, Broken Arrow, OK), in a star-shaped pattern, with an inner diameter of 5 mm and an outer diameter of 12 mm (Fig. 1a).9,22
FIGURE 1.
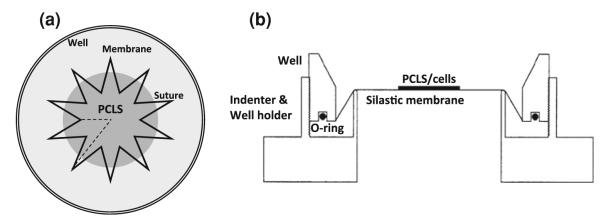
(a) Top-view drawing of the star-shaped stitching pattern of PCLS onto the Silastic membrane in the custom-designed well. The suture pattern inner diameter is 5 mm and the outer diameter is 12 mm.9,22 (b) Side-view drawing of previously developed cell/PCLS stretching device.28
Viability Studies
Cell viability was evaluated in isolated and cultured day 9 type I-like alveolar epithelial cells as well as in day 2–3 PCLSs with and without exposure to cyclic stretch. Prior to stretch, the cells and the stitched PCLSs were incubated in serum-free Dulbecco’s minimum essential medium (DMEM) containing 20 mM Hepes for 1 h at 37 °C. Subsequently, 0.6 μM calcein AM and 0.28 μM ethidium homodimer-1 (LIVE/DEAD, Molecular Probes, Grand Island, NY) were added to the wells to stain for live and dead cells, respectively. The cells and PCLSs then either remained unstretched or were stretched for 10 or 60 min using a stretching device that was previously designed in our lab (Fig. 1b),28 at a frequency of 0.25 Hz and at a stretch magnitude of 37% change in surface area (ΔSA), roughly corresponding to 100% total lung capacity.28 After stretch, at least three regions per preparation were randomly chosen for imaging. Cells were imaged on an epifluorescent microscope (Nikon Eclipse TE300), while the PCLSs were imaged on a confocal microscope (Leica TCS SP5) and a vertical stack of ten 1 μm images from the mid-thickness region of the PCLS was captured.
An image processing algorithm was then implemented in Matlab (R2010b, version 7.11) to semi-quantify the percent of dead cells in each AEC and PCLS image. For each image, we computed separately the total number of green (Calcein AM) and red (ethidium homodimer-1) pixels. Then, assuming the area of live and dead stained cells was approximately the same, we calculated the percent of area of dead cells in each image. To obtain only the dead cells attributable to stretch, we subtracted the averaged percent of area of dead cells in unstretched preparations from the same study day from the values obtained from images of stretched AEC cultures or PCLSs.
RESULTS
Isolation and Culture of Human Type I-like Alveolar Epithelial Cells
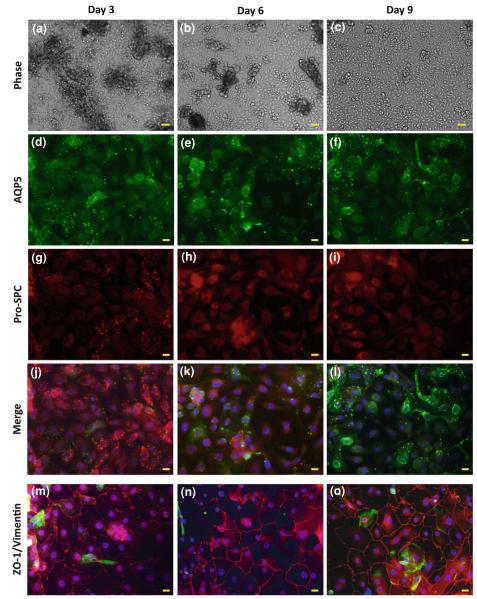
Primary human AECs were isolated from lung tissues donated from 5 different subjects. The average yield was 1.89 ± 0.68 million cells per one gram of wet tissue, similar to other studies where human AECs were isolated.16,17 The cells were cultured on flexible collagen coated Silastic membranes. The cells were fixed 3, 6, and 9 days after seeding and stained for phenotypic markers, and phase images were taken on the same days prior to fixing (Fig. 2). The phase images show fewer cell clusters and less debris, and more uniform distribution of the epithelial cells on the membranes with increasing culturing time (Figs. 2a–2c). In addition, more cells gained type I-like phenotype while almost all the cells lost their type II characteristics with time in culture, as evidenced by the AQP5 and Pro-SPC stains (Figs. 2d–2l). In fact, starting from day 6, the monolayer stained weakly and non-specifically for Pro-SPC, and conversely, they stained brightly for the type I AEC marker, AQP5. Altogether these data suggest that the differentiation timeline is over several days in culture. We confirmed these results using another set of antibodies, namely HTI-56 and HTII-280, which showed similar results, however the signal-to-noise ratio for this stain was poor (data not shown). The strong ZO-1 stain (Figs. 2m–2o, in red) demonstrates the formation of tight junctions between the cells in the epithelial monolayer as early as after 3 days in culture. The vimentin stain (Figs. 2m–2o, in green) demonstrates that there was only a small amount of mesenchymal cells and other non-epithelial cells in the culture.
FIGURE 2.
Freshly isolated human alveolar epithelial cells were cultured on collagen coated Silastic membranes, and phase images were taken after (a) 3, (b) 6, and (c) 9 days in culture. On the same days, the cells were fixed and immunostained for (d–f) AQP5, a type I AEC marker, and (g–i) pro-SPC, a type II AEC marker. (j–l) Merge of the AQP5/pro-SPC stains with DAPI stain for the nuclei (in blue). Other cultures that were fixed after (m) 3, (n) 6, and (o) 9 days in culture were stained for ZO-1 (red), vimentin (green), and the nuclei were stained with DAPI (blue). Bar for phase images = 50 μm; Bar for immunofluorescent images = 10 μm.
Viability of Stretched Human Lung Tissue Preparations
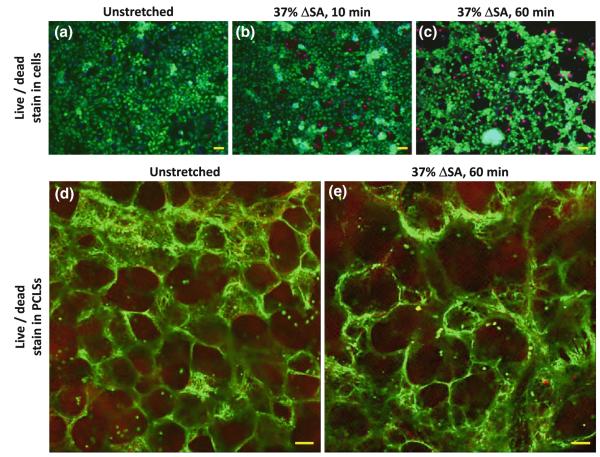
Human AEC viability was quantified in live/dead images of unstretched cells and cells that were cyclically stretched for 10 and 60 min at 37% ΔSA (Figs. 3a–3c). After subtraction of the average percent of area of dead cells in unstretched AEC cultures, the average percent of dead cells in cultures that were stretched at 37% ΔSA for 10 or 60 min was −0.66 ± 3.46% and 4.97 ± 1.56%, respectively. While the 10 min data were not significantly different than zero, the 60 min data were. In addition, we compared the percent of dead cells in the 60 min stretched human AEC cultures to our previously published data in rat type I-like AEC cultures (8.9 ± 3.9%),28 and found that cell death in the human AEC culture was significantly lower than in the rat AEC cultures. However, unlike with stretched cultured primary rat AEC, stretch at 37% ΔSA partially compromised the human AEC adhesion to the collagen-coated Silastic membrane (Figs. 3a–3c), as evidenced by small gaps that were observed in the monolayers after 10 min of stretch with average size of 0.16 ± 0.03% out of the total image area, and larger gaps that were found after 60 min with average size of 0.50 ± 0.06% out of the total image area. The gaps at the 60 min stretched culture were significantly larger in size compared to unstretched cell cultures which presented almost confluent monolayers with gap size of 0.11 ± 0.01% out of the total image area.
FIGURE 3.
Representative live/dead stain images for human AECs after 9 days in culture, which were then (a) remained unstretched, or stretched at 37% ΔSA for (b) 10 min, or (c) 60 min, and human PCLSs (d) left unstretched, or (e) stretched at 37% ΔSA for 60 min. Bar for AEC images = 50 μm; Bar for PCLS images = 100 μm.
Similar viability studies were conducted with 12 human day 2–3 PCLSs, obtained from 3 different lung donors. The PCLSs were fastened onto the stretchable membranes by stitching (Fig. 1a), and either remained unstretched or were cyclically stretched for 60 min at a magnitude of 37% ΔSA. After subtraction of the averaged percent of area of dead cells in unstretched PCLSs, the average percent of dead cells in the stretched PCLSs was 8.61 ± 3.09% (Figs. 3d, 3e), not significantly different than zero, and similar to stretched rat PCLSs (0.9 ± 8.3%).9
DISCUSSION
The goal of the current work was to establish human lung cell and tissue isolation methods and demonstrate the feasibility of stretching primary human AECs and human PCLSs as a translational research platform to investigate the mechanisms responsible for VILI. The methods used in this paper attempt to overcome the limitations of using human tissue, such as post-mortem delays and limited access to main bronchioles. Although the total time between lung excision from the donor to cell seeding was as long as 36–48 h, which is significantly longer than other published post-mortem delay times,17 the cell isolation yield we obtained was very similar to results from other published human AEC isolation studies.15,17 Future studies may correlate the time between lung excision and cell isolation with the isolation yield to determine a threshold after which it is no longer worthwhile to isolate these cells.
To compensate for this delay and increase the number of cells obtained in the isolation, we enhanced the enzymatic digestion by cutting the tissue into fine pieces and stirring the tissue during incubation with elastase at 37 °C. To increase the purity of the cells in absence of the ability to perform a pulmonary lung perfusion for removing red blood cells, we used two consecutive filtration steps; first with meshes of different pore sizes, and second, with a Percoll gradient. Similar to others,16 we promoted cell adhesion to the culture substrates by coating them with collagen. However, the Silastic stretchable membrane substrates differ from other substrates used to culture primary human AECs (e.g., permeable Transwells or culture dishes) in two aspects: they are not permeable and they are elastic. Both features present challenges to cell culture and adhesion, requiring modified methods.
The isolated and purified primary human AECs were cultured over 9 days. The immunofluorescent stain used for specific phenotypic markers showed that within several days in culture the cells differentiated into type I-like AECs (Figs. 2d–2l) and formed tight junctions (Figs. 2m–2o), which are essential for maintaining the mechanical integrity of the epithelial barrier. As early as day 3, the cells developed into a confluent monolayer of alveolar epithelial cells (Fig. 2m), mostly maintaining type II cell phenotype (Fig. 2g). On day 6 the alveolar monolayer stained only non-specifically for pro-SPC (Fig. 2h), indicating the loss of type II AEC phenotype. On the same day, a strong stain for AQP5 marker appeared, although not in all cells (Fig. 2e). On day 9, almost all cells presented a strong AQP5 stain, demonstrating the adoption of type I-like AEC characteristics. The simultaneous loss of type II cell marker and emergence of type I-like cell marker up to day 6 in culture indicates the differentiation timeline for primary human AECs in culture, which is completed by day 9 in culture. Future studies may test the presence of other surfactant proteins (A, B or D) as type II AEC markers to completely exclude the possibility of mixed type I and type II AEC cultures.
Quantification human AEC viability showed no significant increase in the percent of dead cells after 10 min of stretch at 37% ΔSA compared to unstretched cultures (Fig. 3b). However, in AEC cultures that were stretched for 60 min, a significant increase of cell death was found with the presence of significantly larger gaps between the cells compared to unstretched preparations (Figs. 3a, 3c). However, the percent of dead cells in the human cell cultures was significantly lower than in the rat cell cultures28 but there were no changes in confluency observed in the rat cells, suggesting stronger cell adhesion in the rat preparations. Future studies may explore improved adhesion techniques using additional extracellular proteins for the human AEC cultures.
The viability data found here for stretched and unstretched human PCLSs were very similar to data from rat PCLSs.9 Although 37% ΔSA is a relatively high magnitude (corresponding to 100% total lung capacity),28 we show here, as well as previously with primary rat type I-like AECs,28 that the amount of stretch-induced cell death in cells and in PCLSs is not very high. However, we have shown previously in cell monolayers that these magnitudes of stretch induce increases in permeability that are associated with edema in VILI.3,10 This suggests that cell death is not the primary mechanism through which the epithelial barrier function is damaged under stretch conditions.
To summarize, the methods presented demonstrate the ability to isolate and culture AECs and PCLSs from human remnant lung tissue in a way that allows their stretching, which is an integral part of VILI studies in vitro. Furthermore, the described methods allow utilization of human AECs and PCLSs in a large array of studies with and without stretch, allowing investigators to take the dynamic biomechanical environment of lung tissue during breathing into account. For example, one can compare drug uptake in cell monolayers and PLCSs with and without stretch, study basic cellular responses in specific types of AECs in culture and in PCLSs, and for the PCLSs one can also compare responses between samples from normal and asthmatic or emphysematous tissues without stretch and with different stretch profiles. The use of remnant tissue is necessary because the isolated lung tissue is commonly shared among several laboratories, but delay times affect the tissue viability and the isolation method options. We have developed alternative techniques which overcome these limitations. The cells isolated from the human tissue were purely epithelial cells and they differentiated into type I-like cells within several days in culture. The human cells and tissue PCLSs remained viable in culture for at least 9 and 3 days, respectively, and were successfully stretched at a high magnitude of stretch. These methods lay the foundation for future studies with human lung AECs and PCLSs to identify mechanisms of VILI and explore therapeutic interventions in human in vitro preparations.
ACKNOWLEDGMENTS
The authors thank Dr. Reynold A. Panettieri for generously providing us with unused human lung tissue for cell isolation and PCLS preparation, and to Gladys G. Lawrence for assistance with perfecting the procedure for isolating primary human alveolar epithelial cells. This study was supported by NIH NHLBI R01-HL057204
ABBREVIATIONS
- VILI
Ventilator-induced lung injury
- AECs
Alveolar epithelial cells
- PCLSs
Precision-cut lung slices
- pro-SPC
Pro-surfactant Protein C
- AQP5
Aquaporin-5
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no commercial associations or sources of support that might pose a conflict of interest.
N. Davidovich and P. Chhour are joint first authors.
REFERENCES
- 1.Alcorn JL, Smith ME, Smith JF, Margraf LR, Mendelson CR. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am. J. Respir. Cell Mol. Biol. 1997;17:672–682. doi: 10.1165/ajrcmb.17.6.2858. [DOI] [PubMed] [Google Scholar]
- 2.Budinger GR, Urich D, DeBiase PJ, Chiarella SE, Burgess ZO, Baker CM, Soberanes S, Mutlu GM, Jones JC. Stretch-induced activation of AMP kinase in the lung requires dystroglycan. Am. J. Respir. Cell Mol. Biol. 2008;39:666–672. doi: 10.1165/rcmb.2007-0432OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanaugh KJ, Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 2001;25:584–591. doi: 10.1165/ajrcmb.25.5.4486. [DOI] [PubMed] [Google Scholar]
- 4.Chapman KE, Sinclair SE, Zhuang D, Hassid A, Desai LP, Waters CM. Cyclic mechanical strain increases reactive oxygen species production in pulmonary epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L834–L841. doi: 10.1152/ajplung.00069.2005. [DOI] [PubMed] [Google Scholar]
- 5.Chiang CH, Chuang CH, Liu SL, Lee TS, Kou YR, Zhang H. Apocynin attenuates ventilator-induced lung injury in an isolated and perfused rat lung model. Intensive Care Med. 2011;37:1360–1367. doi: 10.1007/s00134-011-2251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper PR, Panettieri RA., Jr. Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J. Allergy Clin. Immunol. 2008;122:734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 7.Damera G, Druey KM, Cooper PR, Krymskaya VP, Soberman RJ, Amrani Y, Hoshi T, Brightling CE, Panettieri RA., Jr. An RGS4-mediated phenotypic switch of bronchial smooth muscle cells promotes fixed airway obstruction in asthma. PLoS ONE. 2012;7:e28504. doi: 10.1371/journal.pone.0028504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dassow C, Wiechert L, Martin C, Schumann S, Muller-Newen G, Pack O, Guttmann J, Wall WA, Uhlig S. Biaxial distension of precision-cut lung slices. J. Appl. Physiol. 2010;108:713–721. doi: 10.1152/japplphysiol.00229.2009. [DOI] [PubMed] [Google Scholar]
- 9.Davidovich N, Huang J, Margulies SS. Reproducible uniform equibiaxial stretch of precision-cut lung slices. In review. [DOI] [PMC free article] [PubMed]
- 10.DiPaolo BC, Lenormand G, Fredberg JJ, Margulies SS. Stretch magnitude and frequency-dependent actin cytoskeleton remodeling in alveolar epithelia. Am. J. Physiol. Cell Physiol. 2010;299:C345–C353. doi: 10.1152/ajpcell.00379.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobbs LG, Gonzalez RF, Allen L, Froh DK. HTI56, an integral membrane protein specific to human alveolar type I cells. J. Histochem. Cytochem. 1999;47:129–137. doi: 10.1177/002215549904700202. [DOI] [PubMed] [Google Scholar]
- 12.Dobbs LG, Gonzalez R, Matthay MA, Carter EP, Allen L, Verkman AS. Highly water-permeable type I alveolar epithelial cells confer high water permeability between the airspace and vasculature in rat lung. Proc. Natl Acad. Sci. USA. 1998;95:2991–2996. doi: 10.1073/pnas.95.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. The great big alveolar TI cell: evolving concepts and paradigms. Cell. Physiol. Biochem. 2010;25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- 14.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am. J. Respir. Crit. Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhardt C, Kim KJ, Lehr CM. Human cell culture protocols. In: Picot J, editor. Methods in Molecular Medicine. 2nd ed Humana Press; Totowa, NJ: 2005. p. xi.p. 359. [Google Scholar]
- 16.Ehrhardt C, Kim KJ, Lehr CM. Isolation and culture of human alveolar epithelial cells. Methods Mol. Med. 2005;107:207–216. doi: 10.1385/1-59259-861-7:207. [DOI] [PubMed] [Google Scholar]
- 17.Elbert KJ, Schafer UF, Schafers HJ, Kim KJ, Lee VH, Lehr CM. Monolayers of human alveolar epithelial cells in primary culture for pulmonary absorption and transport studies. Pharm. Res. 1999;16:601–608. doi: 10.1023/a:1018887501927. [DOI] [PubMed] [Google Scholar]
- 18.Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J. Biol. Chem. 2010;285:26211–26222. doi: 10.1074/jbc.M110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res. 2003;311:31–45. doi: 10.1007/s00441-002-0653-5. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez RF, Allen L, Gonzales L, Ballard PL, Dobbs LG. HTII-280, a biomarker specific to the apical plasma membrane of human lung alveolar type II cells. J. Histochem. Cytochem. 2010;58:891–901. doi: 10.1369/jhc.2010.956433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoet PH, Lewis CP, Demedts M, Nemery B. Putrescine and paraquat uptake in human lung slices and isolated type II pneumocytes. Biochem. Pharmacol. 1994;48:517–524. doi: 10.1016/0006-2952(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Davidovich N, Margulies SS. Rat precision-cut lung slices as a model for deformation-induced lung injury studies. 38th Annual Northeast Bioengineering Conference; Philadelphia, PA, USA. 2012. [Google Scholar]
- 23.Kreda SM, Gynn MC, Fenstermacher DA, Boucher RC, Gabriel SE. Expression and localization of epithelial aquaporins in the adult human lung. Am. J. Respir. Cell Mol. Biol. 2001;24:224–234. doi: 10.1165/ajrcmb.24.3.4367. [DOI] [PubMed] [Google Scholar]
- 24.Lavoie TL, Krishnan R, Siegel HR, Maston ED, Fredberg JJ, Solway J, Dowell ML. Dilatation of the constricted human airway by tidal expansion of lung parenchyma. Am. J. Respir. Crit. Care Med. 2012;186:225–232. doi: 10.1164/rccm.201202-0368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lubman RL, Kim KJ, Crandall ED. Alveolar epithelial barrier properties. In: Crystal RG, West JB, editors. The Lung: Scientific Foundations. Lippincott, Raven; Philadelphia: 1997. pp. 585–602. [Google Scholar]
- 26.Nielsen S, King LS, Christensen BM, Agre P. Aquaporins in complex tissues. II. Subcellular distribution in respiratory and glandular tissues of rat. Am. J. Physiol. 1997;273:C1549–C1561. doi: 10.1152/ajpcell.1997.273.5.C1549. [DOI] [PubMed] [Google Scholar]
- 27.Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit. Care Med. 1993;21:131–143. doi: 10.1097/00003246-199301000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Tschumperlin DJ, Margulies SS. Equibiaxial deformation-induced injury of alveolar epithelial cells in vitro. Am. J. Physiol. 1998;275:L1173–L1183. doi: 10.1152/ajplung.1998.275.6.L1173. [DOI] [PubMed] [Google Scholar]
- 29.Vaneker M, Halbertsma FJ, van Egmond J, Netea MG, Dijkman HB, Snijdelaar DG, Joosten LA, van der Hoeven JG, Scheffer GJ. Mechanical ventilation in healthy mice induces reversible pulmonary and systemic cytokine elevation with preserved alveolar integrity: an in vivo model using clinical relevant ventilation settings. Anesthesiology. 2007;107:419–426. doi: 10.1097/01.anes.0000278908.22686.01. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Edeen K, Manzer R, Chang Y, Wang S, Chen X, Funk CJ, Cosgrove GP, Fang X, Mason RJ. Differentiated human alveolar epithelial cells and reversibility of their phenotype in vitro. Am. J. Respir. Cell Mol. Biol. 2007;36:661–668. doi: 10.1165/rcmb.2006-0410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware LB, Matthay MA. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]