Abstract
The unilateral 6-hydroxydopamine (6-OHDA) lesioned rat model is frequently used to study the effects of subthalamic nucleus (STN) deep brain stimulation (DBS) for the treatment of Parkinson’s disease. However, systematic knowledge of the effects of DBS parameters on behavior in this animal model is lacking. The goal of this study was to characterize the effects of DBS on methamphetamine-induced circling in the unilateral 6-OHDA lesioned rat. DBS parameters tested include stimulation amplitude, stimulation frequency, methamphetamine dose, stimulation polarity, and anatomical location of the electrode. When an appropriate stimulation amplitude and dose of methamphetamine were applied, high frequency stimulation (> 130 Hz), but not low frequency stimulation (< 10 Hz), reversed the bias in ipsilateral circling without inhibiting movement. This characteristic frequency tuning profile was only generated when at least one electrode used during bipolar stimulation was located within the STN. No difference was found between bipolar stimulation and monopolar stimulation when the most effective electrode contact was selected, indicating that monopolar stimulation could be used in future experiments. Methamphetamine-induced circling is a simple, reliable, and sensitive behavioral test and holds potential for high-throughput study of the effects of STN DBS in unilaterally lesioned rats.
Index Terms: STN DBS, Parkinson’s disease, 6-OHDA
I. INTRODUCTION
High frequency deep brain stimulation (DBS) of the subthalamic nucleus (STN) is a treatment for motor symptoms in persons with advanced Parkinson’s disease (PD). STN DBS significantly improves motor function and quality of life, and reduces anti-PD medication requirements [1–2].
However, there are few guidelines for the optimal selection of electrode locations and stimulation parameters due to the lack of mechanistic understanding of STN DBS [3]. Thus, programming procedures following surgery are tedious and result in suboptimal treatment in some patients [4]. A clear understanding of the mechanisms underlying the therapeutic efficacy of STN DBS is needed for the further development and optimization of DBS for the treatment of PD, and future applications to other neurological diseases.
The unilateral 6-hydroxydopamine (6-OHDA) lesioned rat is a widely used animal model for PD research, including investigation of STN DBS [5–6]. A number of behavioral tests have been designed to study the motor symptoms of PD in awake and behaving rats [6], and we focused on methamphetamine-induced circling to quantify the effects of STN DBS. Methamphetamine-induced circling has a number of advantages over other behavioral paradigms, including simplicity of implementation [7] and sensitivity to effective DBS [8–10]. Rats with almost complete unilateral dopamine denervation will rotate (circle) continuously ipsilateral to the lesion in a dose dependent manner for a period of 1.5 to 3 hours after administration of amphetamine [7]. Unilaterally lesioned rats typically do not engage in continuous movement over an extended period of time, and administration of amphetamine is necessary to increase the activity of the rat such that the changes in behavior during DBS can be quantified. Although experimental conditions leading to the rapid circling behavior – severe imbalance in dopaminergic innervation between the two hemispheres and exposure to a stimulant drug – are not likely to occur in persons with Parkinson’s, PD patients with asymmetrical disease progression do exhibit spontaneous rotation toward the hemisphere containing less striatal dopaminergic activity [11].
High frequency STN DBS is effective in reducing circling in lesioned rats [8–10], and in other behavioral paradigms [9, 12–15]. However, most of these experiments only reported changes in behavior during high frequency 130 Hz DBS, making it difficult to extrapolate and predict behavior that would result from stimulation with other parameters.
We characterized systematically the effect of parameters of STN DBS on methamphetamine induced circling, including stimulation amplitude, stimulation frequency, methamphetamine dose, monopolar versus bipolar stimulation, and anatomical location of the electrode. Effective STN DBS produced a characteristic frequency profile only with the appropriate stimulation amplitude, methamphetamine dose, and electrode placement. The methamphetamine-induced circling test is a simple, sensitive, and reliable behavioral test for investigating effects of DBS, and the characteristic frequency tuning curve identified in the present study extends the utility of this model.
II. METHODS
We implanted Long Evans rats (250 g – 300 g) with stimulating microelectrodes in the STN and created Parkinsonism via a unilateral lesion of the substantia nigra by injection of 6-OHDA into the medial forebrain bundle (MFB). Stimulation trains with different amplitudes and rates were delivered, and behavior was assessed using methamphetamine-induced circling. Post mortem histology was conducted to determine the anatomical position of the electrodes as well as the extent of degeneration of dopaminergic neurons. All rats were purchased from Charles River Laboratories Inc. (Wilmington, MA), and they were housed in individual cages with free access to food and water, room temperatures between 73 °F–77 °F, and 12 hour light cycles. All experiments were conducted in accordance with a protocol approved by the Duke University Institutional Animal Care and Use Committee.
A. Electrode implantation and 6-OHDA lesioning
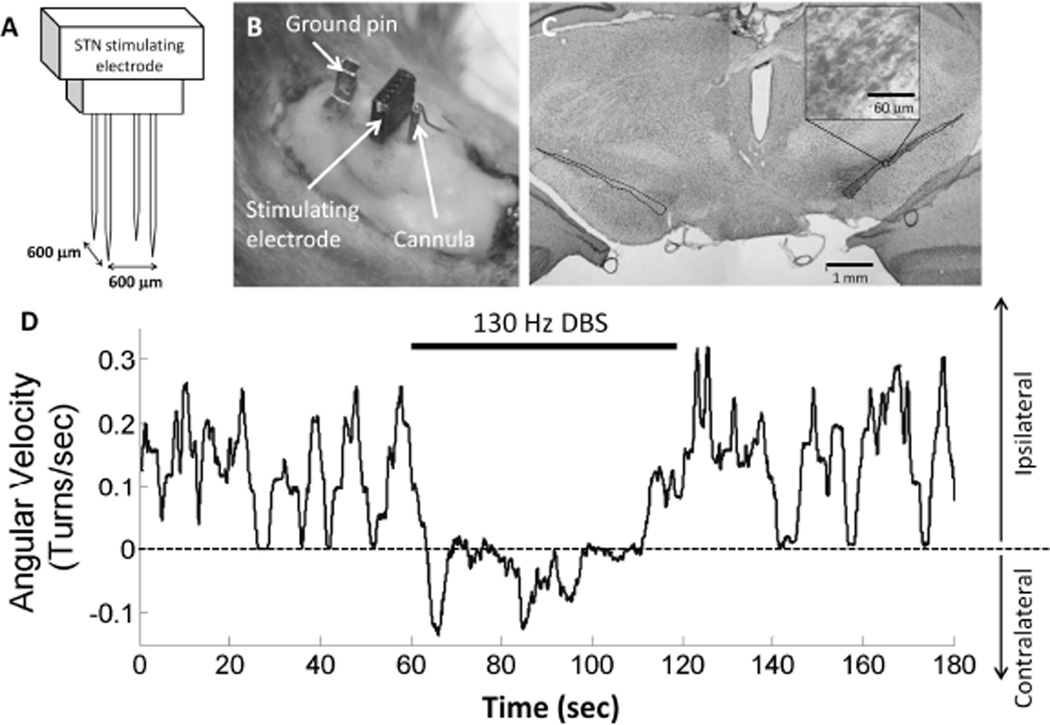
We performed stereotactic surgery using coordinates from the rat brain atlas [16]. The rats were kept under 2 % isoflurane (Halocarbon Products Co., River Edge, NJ) gas anesthesia during surgery and heart rate and oxygen saturation were monitored using a pulse oximeter. A cannula was placed in the MFB [A 2.0 mm from bregma; L 2.0 mm; H 7.5 mm]. Intraoperative microelectode recordings (impedance of 0.5 MΩ) were performed to confirm the position and depth of the STN in each rat prior to chronic implantation of the stimulation electrode array [9–10]. A four-electrode platinum-iridium stimulating microelectrode array (MEA) (Microprobes Inc., Gaithersburg, MD) was implanted targeting the subthalamic nucleus (STN) [A 3.6 mm from bregma; L 2.6 mm; H 6.6 – 6.9 mm]. The stimulating electrodes were 75 µm in diameter, arranged in a two by two array with an interelectrode spacing of 600 µm (Fig. 1A). Each electrode had an impedance of 10 kΩ. Eight stainless steel screws were anchored to the skull, and the implanted microelectrode array and cannula were secured to the screws using dental acrylic (Fig. 1B).
Fig. 1.
(A) Schematic of the subthalamic nucleus (STN) stimulating electrode showing a two by two array with an interelectrode distance of 600 µm. (B) Stimulating electrode, cannula, and ground pin embedded in dental acrylic. (C) Tyrosine hydroxylase immunohistochemical staining. Rat was successfully lesioned, showing > 90 % dopaminergic denervation in the substantia nigra pars compacta (SNc) on one side of the brain. The borders of the SNc on each side of the brain are highlighted with dotted lines. Inset shows individual stained dopaminergic neurons. (D) Example of one trial of STN deep brain simulation (DBS) on methamphetamine-induced circling in a 6-OHDA lesioned rat. Rat turned ipsilaterally to the 6-OHDA lesion during pre-stimulation and post-stimulation periods, but made turns both ipsilaterally and contralaterally during 130 Hz DBS.
After one week of recovery, lesioning was conducted to produce unilateral degeneration of the dopaminergic neurons in the substantia nigra pars compacta (SNc) (Fig. 1C). Rats were pretreated 30 minutes prior to infusion of 6-OHDA by intraperitoneal injections of 50 mg/kg pargyline (Sigma-Aldrich Co., St. Louis, MO) to inhibit monoamine oxidase and 5 mg/kg desipramine (Sigma-Aldrich Co., St. Louis, MO) to protect noradrenergic neurons. 6-OHDA (Sigma-Aldrich Co., St. Louis, MO) was dissolved in ice cold 0.2 % ascorbic acid (Sigma-Aldrich Co., St. Louis, MO) immediately before use. 10 µl of 10 mM 6-OHDA was infused through the cannula at a rate of 2 µl/min. During infusion of 6-OHDA, the rats were anesthetized with 2 % isoflurane gas and heart rate and oxygen saturation were monitored using a pulse oximeter. The rats recovered for one week before initiation of stimulation and behavioral experiments. In cases when a rat did not exhibit motor deficits following lesioning, we performed up to two additional 6-OHDA injections.
B. Assessment of methamphetamine-induced circling during DBS
Methamphetamine solution was prepared by dissolving 5 mg of powdered methamphetamine (Sigma-Aldrich Co., St. Louis, MO) in normal saline, and diluting to the desired concentration. During each experiment, the rat was administered methamphetamine through an intraperitoneal injection and then placed in a dark 30 cm diameter cylinder chamber equipped with an infrared camera that captured the rat’s activity for two hours. During this time, we delivered four blocks of ten different amplitudes or frequencies of DBS, with presentation order randomized within each block. One-minute epochs of stimulation (Fig. 1D) were delivered three minutes apart, allowing two minutes between each pattern to minimize carry-over effects. Biphasic pulses, with a pulse width of 90 µs were delivered to the STN through the stimulating MEA via an isolated voltage to current converter (A-M Systems, Carlsborg, WA). Experiments were performed at least two days apart to allow for a rest period between each drug dose.
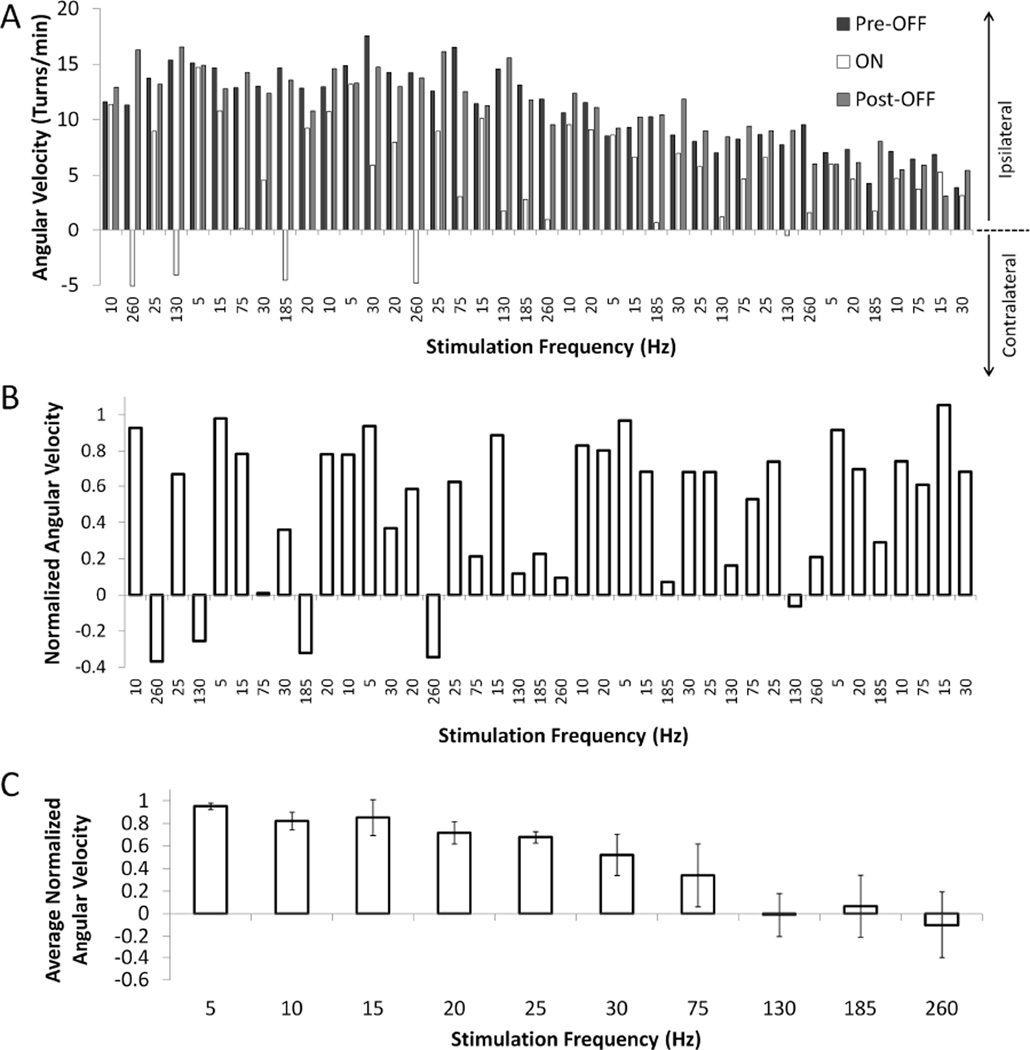
Behavioral analysis software (Clever Systems Inc., Reston, VA) was used for automatic animal tracking, and angular velocities and total distances travelled were calculated from the tracking data using MATLAB (version R2009a, Mathworks Inc., Natick, MA) (Fig. 2A). Normalized angular velocity for each trial was determined by dividing the angular velocity during the one minute ‘ON’ period by the average angular velocities of the one minute ‘Pre-OFF’ and ‘Post-OFF’ periods immediately before and after the ‘ON’ period (Fig. 2B). Normalized angular velocity and total distance traveled for each stimulation amplitude or frequency were then calculated by averaging across the four randomized blocks (Fig. 2C). Normalization reduced variability due to differences in circling rates across different rats and within each rat during an experimental session due to the time course of the drug effect.
Fig. 2.
Example of the effects of STN DBS at various frequencies on methamphetamine-induced circling in a 6-OHDA lesioned rat. (A) Turning rates (angular velocity) during pre-stimulation off, stimulation on, and post-stimulation off periods across 40 consecutive trials in one rat. Four randomized blocks of 10 different frequencies were used. Turning rates during stimulation off periods were ≥ 3 turns/min for all 40 trials. Turns made towards the right (ipsilateral to the lesion in this example) were counted as positive and turns made towards the left (contralateral to the lesion in this example) were counted as negative. (B) Normalized angular velocity (AV) for each trial calculated from turning rates in A. Normalized AV=AVON/[0.5(AVPre-OFF+AVPost-OFF)]. (C) Normalized AVs from four different trials at each stimulation frequency in B were averaged to obtain the average normalized AV. Means ± SD are shown.
C. Experimental variables
We investigated the effects of five variables on the change in circling behavior during DBS. First, we quantified the change in circling rate when different amplitudes of stimulation (15 µA – 130 µA) were applied at 130 Hz. Next, we quantified the effect of varying the frequency of stimulation (5 Hz – 260 Hz) on circling behavior using fixed stimulation amplitudes. Third, we measured the effect of methamphetamine dose on changes in circling due to DBS. The application of methamphetamine only affected the baseline turning rates of rats, and was not used to treat PD or enhance the effects of DBS. Two different doses of methamphetamine [2.5 mg/kg (high dose) versus 1.25 or 1.875 mg/kg (low dose)] were administered in each rat to observe the relationship between methamphetamine dose and effectiveness of DBS at a given amplitude. 1.25 mg/kg methamphetamine was used for the low dose experiments, except in two rats which did not maintain a turning rate of 3 turns/min at this drug concentration, and 1.875 mg/kg methamphetamine was used instead.
Next, we studied the differences between bipolar and monopolar stimulation by delivering current either through two electrode tips for bipolar stimulation or through one electrode tip and a skull screw for monopolar stimulation. Finally, through post-mortem histology, we determined how the anatomical position of the stimulating electrodes affected the rats’ circling behavior during DBS. With the exception of the amplitude tests, all other experiments were conducted using a range of stimulation frequencies between 5 Hz and 260 Hz, with a fixed stimulation amplitude. The amplitude of stimulation for each rat was determined based on tuning immediately following methamphetamine injection. The stimulation amplitude chosen for each experiment elicited sustained motor responses to 130 Hz stimulation, which included decreased ipsilateral turning, increased contralateral turning, increased activity, increased rearing, as well as a lack of motor side effects such as involuntary muscle contractions of the limbs and neck.
A total of 16 rats were used in our study, and subsets of these rats were used in each experiment. Only rats that showed effective response to 130 Hz stimulation and electrode placement within the STN based on post-mortem histology were included in the analysis of stimulation amplitude, frequency, methamphetamine dose, and electrode polarity.
D. Perfusion and Histology
Rats were deeply anesthetized with pentobarbital (Virbac Co., Fort Worth, TX, 1.2 ml Euthasol solution injected intraperitoneally), and intracardiac perfusion was conducted with 10 % paraformaldehyde (Azer Scientific, Morgantown, PA). The head was removed and postfixed overnight in 10 % paraformaldehyde at 4 °C. The brain was removed the following day and placed in 30 % sucrose solution at 4 °C until it sunk to the bottom. The brain was then placed in optimal cutting temperature compound (O.C.T., Ted Pella Inc., Redding, CA) and sectioned coronally at 50 µm using a cryostat.
Tyrosine hydroxylase (TH) immunohistochemistry was used to evaluate the extent of degeneration of dopaminergic neurons (Fig. 1C). Tissue sections were first blocked for 1 hour at 4 °C in blocking solution (8 % horse serum, 10 µg/ml avidin, and 0.1 % Triton-X), then incubated with TH primary monoclonal antibody (T1299, Sigma-Aldrich Co., St. Louis, MO, diluted 1:1000 with 2 % horse serum and 50 µg/ml biotin) overnight at 4 °C. Next, they were incubated with biotinylated horse anti-mouse secondary antibody (BA-2011, Vector Laboratories Inc., Burlingame, CA, diluted 1:200 with 2 % horse serum) for 1 hr at 4 °C. The sections were then stained using a VECTASTAIN Elite ABC kit (Vector Laboratories Inc., Burlingame, CA) and ImmPACT DAB solution (Vector Laboratories Inc., Burlingame, CA), and finally counterstained with 0.2 % Crysel violet (Sigma-Aldrich Co., St. Louis, MO). TH labeled SNc cells on both the lesioned and non-lesioned sides were counted under a light microscope. The percentage of dopaminergic cell loss was calculated using the following formula.
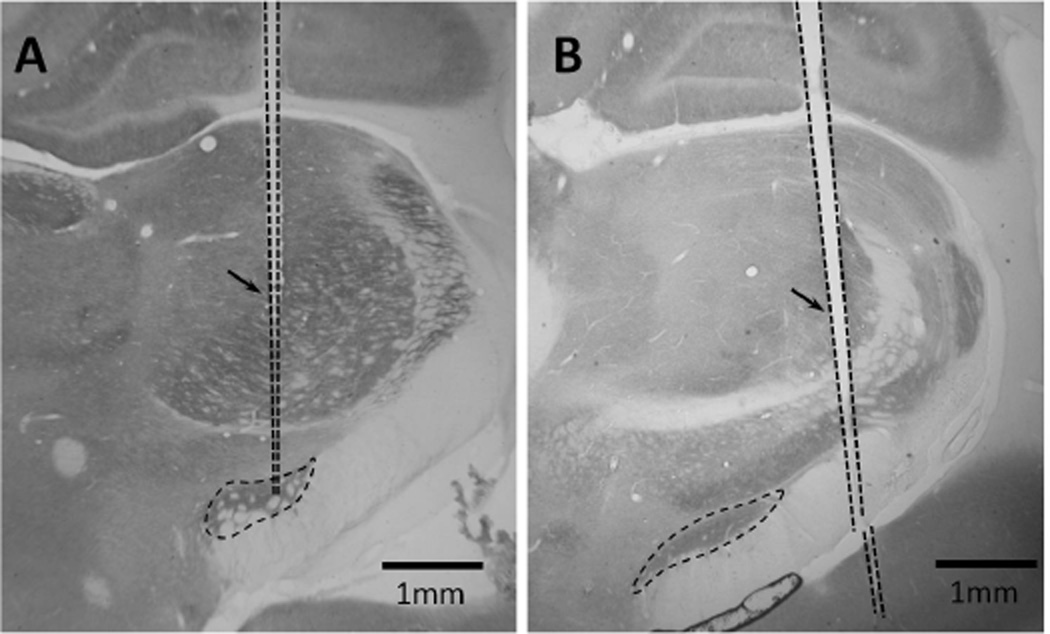
Cytochrome oxidase (CO) staining was used to identify the STN and determine electrode placement (Fig. 8A, 8B). CO is a mitochondrial enzyme correlated with neuronal activity, and staining for CO activity allowed clear visualization of the STN against surrounding white matter tracts. Tissue sections were suspended in CO staining solution (10 mg DAB (Sigma-Aldrich Co., St. Louis, MO), 5mg cytochrome C (Sigma-Aldrich Co., St. Louis, MO), and 0.8 g sucrose dissolved in 0.1 M phosphate buffer) for at least 6 hours.
Fig. 8.
(A) Example of accurate placement of electrode within STN. The borders of the subthalamic nucleus (STN), identified by staining for cytochrome oxidase, and the track formed by the electrode is shown by dotted lines. The arrow points to the electrode track. The tip of the electrode was inside the STN. (B) Example of incorrect placement of electrode. The tip of this electrode was implanted lateral to the STN, and ventral to both the STN and internal capsule.
III. RESULTS
We measured the effect of STN DBS on methamphetamine induced circling in unilateral 6-OHDA lesioned rats. All rats included in this study had greater than 90 % loss of TH-immunoreactive neurons in the SNc. Following administration of methamphetamine these well-lesioned rats made turns ipsilateral to the lesion at an average rate of at least 3 turns/min over a period of two hours with no stimulation. Effective DBS reversed this bias by decreasing ipsilateral turning and / or increasing contralateral turning, thus bringing the average normalized turning rate to zero. However, effective DBS did not cause decreased mobility or freezing behavior in the rats, and the normalized total distance travelled was unaffected by stimulation. The effect of DBS on circling was observed immediately following the onset of stimulation, and continuous ipsilateral turning resumed immediately after stimulation was turned off.
A. Effects of stimulation amplitude on circling behavior
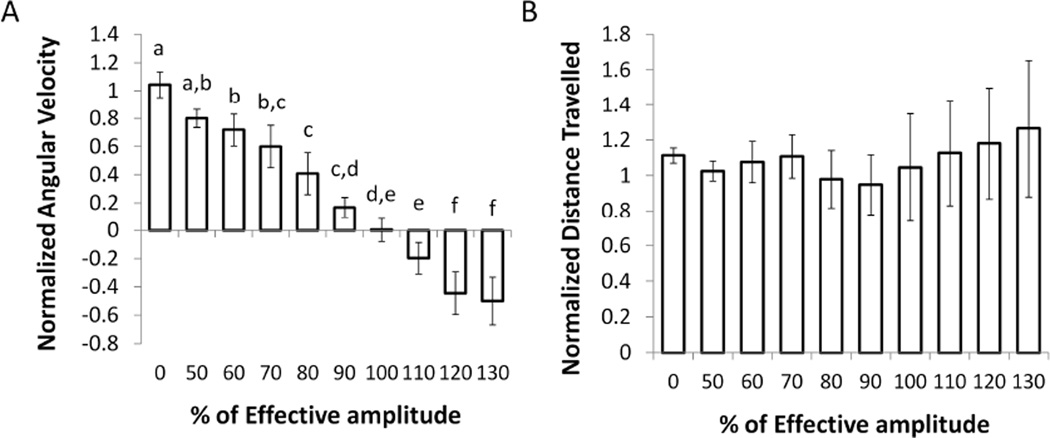
Different amplitudes of 130 Hz bipolar DBS were applied following administration of a low dose of methamphetamine (n=6). The range of amplitudes used for each rat was a percentage (50 % to 130 %) of the minimum amplitude required to elicit a sustained decrease in ipsilateral turning for 30 s. Minimum effective amplitudes (100 %) were 30 µA - 100 µA (mean 62 µA).
Average normalized angular velocity decreased significantly with increasing stimulation amplitude (Fig. 3A). Increasing amplitude between 50 % and 100 % gradually decreased the average rate of ipsilateral turning, at 100 % the normalized angular velocity was close to zero, indicating that the amplitude had reached a level that caused the rat to turn equally in both directions, and beyond 100 %, the normalized angular velocity was negative, showing that at higher amplitudes, the rats turned contralateral to the lesion. The average normalized total distance travelled, however, was not significantly different across amplitudes (Fig. 3B). The variability of normalized distance travelled was larger during stimulation at high amplitudes because some rats responded with a slightly decreased total distance travelled, while others showed slightly increased distance travelled during high amplitude stimulation. Nonetheless, all rats exhibited a significant decrease in angular velocity, and the mean normalized distance travelled was close to one for all amplitudes tested. Thus, although the bias toward ipsilateral turning was reversed with increasing amplitudes, the rats still engaged in the same average amount of movement at all amplitudes, and the change in angular velocity was not due to decreased mobility or freezing.
Fig. 3.
Effects of DBS amplitude on average normalized angular velocity (AV) and average normalized distance travelled. 100 % was determined for each rat as the minimum amplitude that resulted in a sustained decrease in ipsilateral turning for 30 s without the presence of any side effects. (A) Normalized AV decreased with increasing amplitude (p<0.0001, one-way repeated measures ANOVA. Amplitudes labeled with the same letters did not produce effects that were significantly different, while those that do not share letters exhibited significant differences p<0.05, post-hoc Fisher’s PLSD). (B) Normalized total distance travelled was not significantly affected by stimulation amplitude (p=0.61, one-way repeated measures ANOVA). Means ± SE are shown (n=6).
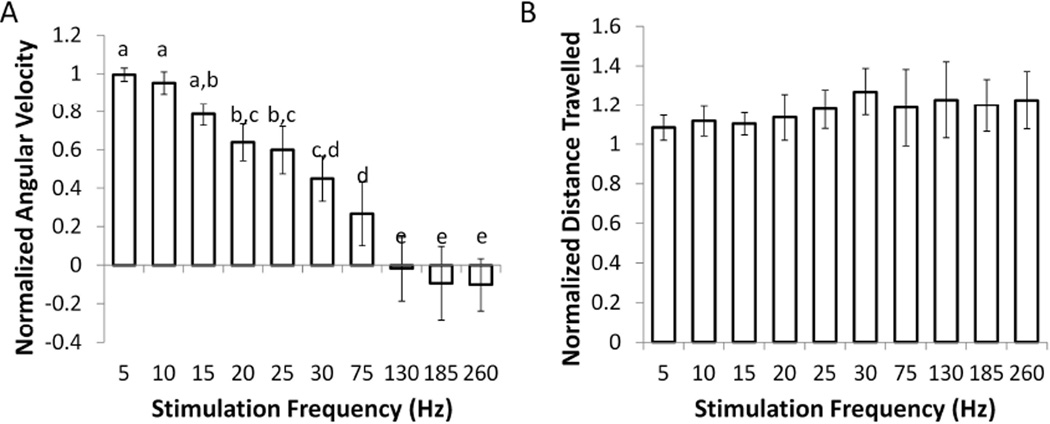
B. Effects of stimulation frequency on circling behavior
Ten different frequencies of bipolar DBS were applied following administration of a low dose of methamphetamine (n=7). The amplitude of stimulation was fixed for each rat at the minimum effective amplitude at 130 Hz (range 50 µA – 125 µA). There was a decrease in normalized angular velocity with an increase in stimulation frequency (Fig. 4A). Frequencies ≤ 10 Hz did not result in a significant decrease in ipsilateral turning, frequencies between 15 Hz and 75 Hz caused a progressive decrease in normalized angular velocity, and frequencies ≥ 130 Hz resulted in normalized angular velocities close to zero. The normalized total distance travelled did not depend on stimulation frequency (fig. 4B), and all frequencies produced normalized distance travelled close to one, indicating that the overall amount of movement did not change between stimulation on and off for any stimulation frequency.
Fig. 4.
Effects of DBS frequency on average normalized angular velocity (AV) and average normalized distance travelled. (A) Characteristic frequency profile during STN DBS. Normalized AV decreased with the increase in frequency (p<0.0001, one-way repeated measures ANOVA. Frequencies labeled with the same letters did not produce effects that were significantly different, while those that do not share letters exhibited significant differences p<0.05, post-hoc Fisher’s PLSD). (B) Normalized total distance travelled was not significantly affected by stimulation frequency (p=0.95, one-way repeated measures ANOVA). Means ± SE are shown (n=7).
This change in circling behavior during high frequency DBS, including a reduction in normalized angular velocity and no change in normalized total distance travelled, was the characteristic frequency profile of effective STN DBS. Such frequency profiles were observed only when the stimulating electrodes were placed within the STN, and the presence of this characteristic frequency profile served as a good predictor of accurate placement of the stimulating electrode (see Results section E).
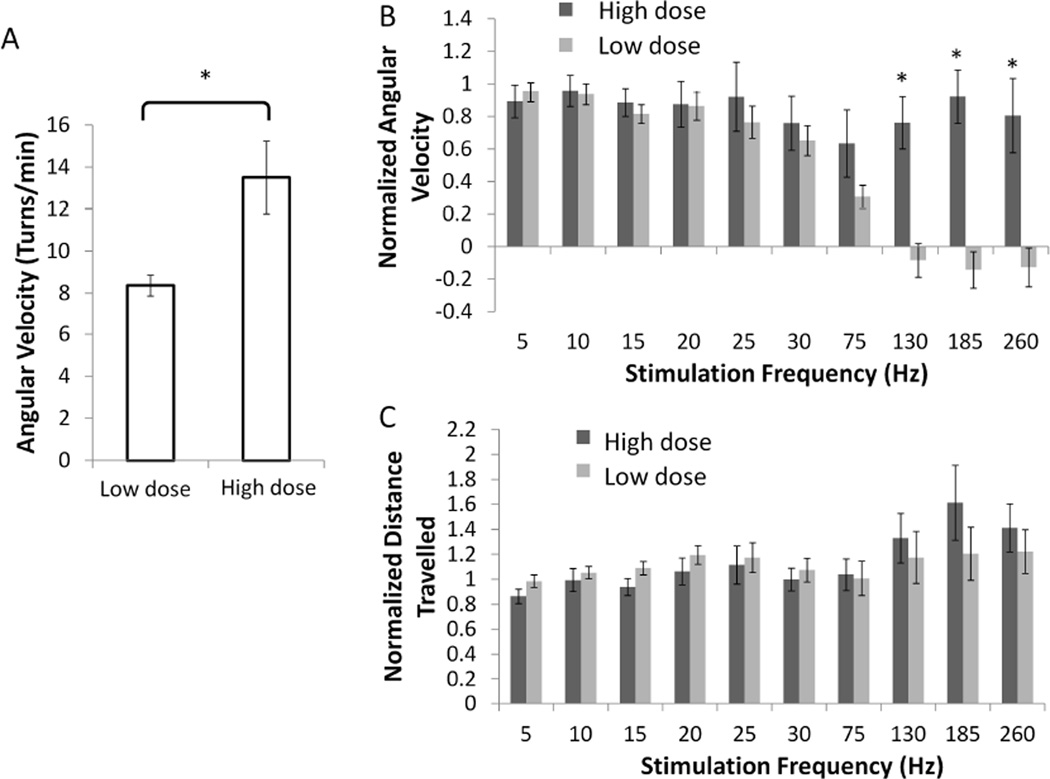
C. Effects of methamphetamine dose on circling behavior
We compared the effects of high and low doses of methamphetamine on circling during bipolar DBS at different frequencies (5 Hz – 260 Hz) (n=7). High doses of methamphetamine resulted in a higher turning rate compared to low doses of methamphetamine (Fig. 5A). High frequency STN DBS did not result in decreased normalized angular velocity or total distance travelled following high doses of methamphetamine (Fig. 5B, 5C). However, when a lower dose of methamphetamine was administered to the same rats, high frequency STN DBS decreased normalized angular velocities and produced no significant change in normalized distance travelled (Fig. 5B, 5C).
Fig. 5.
Effect of methamphetamine dose on turning rates and frequency profiles. (A) Angular velocity without stimulation was decreased at low dose compared to high dose (* p=0.0274 one-way ANOVA). Rats without methamphetamine injections did not display sustained activity beyond 10 minutes of initial exploration of the cylinder. (B) There was a significant difference between normalized angular velocities during high frequency stimulation following low and high doses (two-way ANOVA, frequency × dose interaction term p<0.0001, * p<0.05 post-hoc Fisher’s PLSD analysis). (C) No significant differences were found between normalized distance travelled following low and high doses (two-way ANOVA, frequency × dose interaction term p = 0.4073). Means ± SE are shown (n=6).
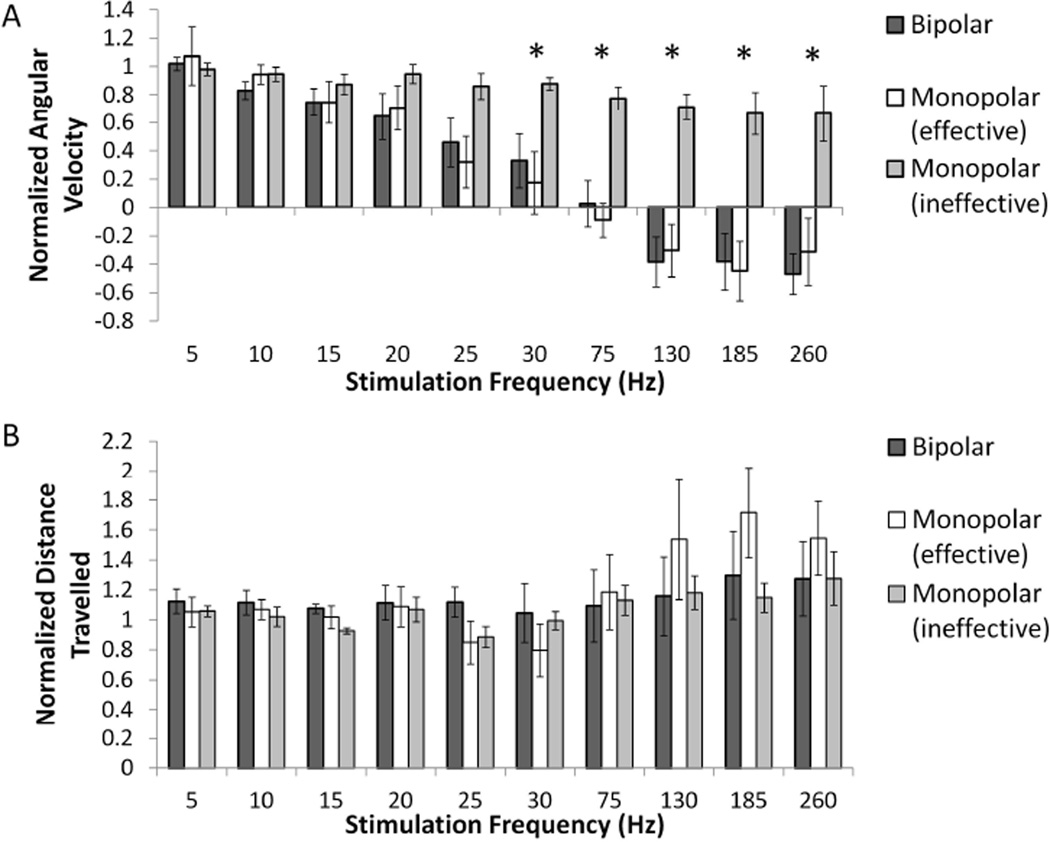
D. Effects of stimulation polarity on circling behavior
The difference between bipolar and monopolar biphasic stimulation was determined (n=5). Two electrodes within the stimulating MEA were selected for each rat during initial contact testing to locate the pair of electrodes that produced the most effective behavioral response and the fewest side effects during bipolar stimulation. The amplitudes used for the monopolar stimulation experiments were the same as those for the bipolar experiments, and ten frequencies from 5 Hz to 260 Hz were applied following low dose methamphetamine.
Bipolar stimulation generated the typical frequency profile for effective STN DBS, with decreased normalized angular velocity and no change in normalized distance travelled with increased stimulation frequency (Fig. 6). Monopolar stimulation delivered through each of the two electrodes resulted in two different frequency profiles, and the two electrodes were sub-categorized as “effective” and “ineffective” based on the behavioral results. Monopolar stimulation through effective electrodes resulted in a frequency profile that was no different than bipolar stimulation. In contrast, monopolar high frequency stimulation using ineffective electrodes did not reverse ipsilateral turning.
Fig. 6.
Differences in frequency tuning curves between bipolar and monopolar STN DBS. Monopolar stimulation was applied using each of the poles of the bipolar pair. (A) There was a significant difference between normalized angular velocity during monopolar and bipolar DBS at higher frequencies (two-way ANOVA, frequency × polarity interaction term p<0.0001, * p<0.05 post-hoc Fisher’s PLSD analysis between bipolar and ineffective monopolar). (B) No significant difference was found between normalized distance travelled during monopolar and bipolar DBS (two-way ANOVA, frequency × polarity interaction term p = 0.4496). Means ± SE are shown (n=5).
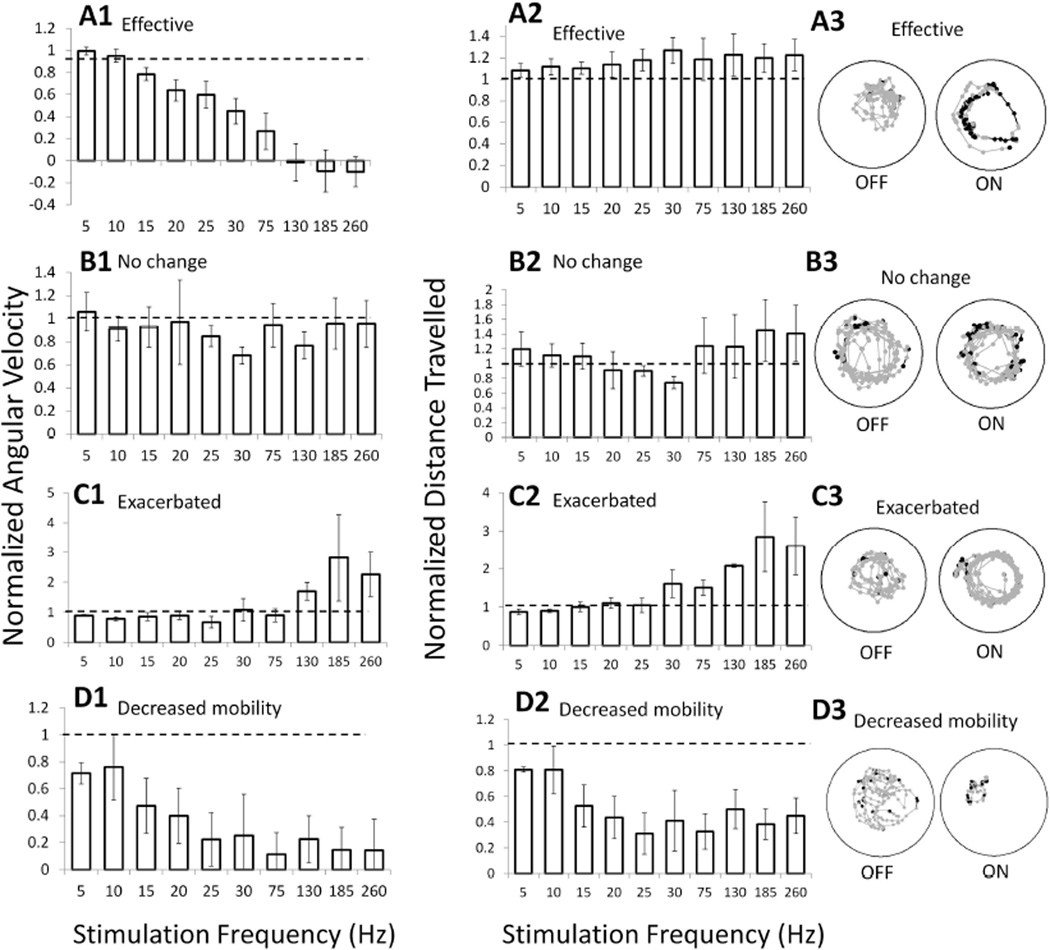
E. Effects of anatomical location of electrodes on stimulation-induced changes in circling behavior
We correlated the behavioral response to stimulation with frequencies between 5 Hz – 260 Hz with the anatomical position of stimulating electrodes (n=16). The frequency profiles were classified into four different categories. The first group (effective, n=7) exhibited a frequency profile showing decreased normalized angular velocity and no change in normalized distance travelled with increased stimulation frequency (Fig. 7A). The second group (no response, n=3) exhibited no difference in either normalized angular velocity or normalized distance travelled with increased stimulation frequency (Fig. 7B), indicating stimulation had little effect on these rats. The third group (exacerbated, n=3) exhibited an increase in both normalized angular velocity and normalized distance travelled at high stimulation frequencies (Fig. 7C). The last group (decreased mobility, n=3) showed a decrease in both normalized angular velocity and normalized distance travelled (Fig. 7D) in response to stimulation at ≥ 15 Hz due to freezing or decreased movement.
Fig. 7.
Different categories of behavioral responses as determined by frequency profiles during STN DBS. Example trajectories from individual rats with different categories of behavior during DBS are shown. Grey lines indicate ipsilateral turning, while black lines indicate contralateral turning. (A) Effective DBS showing a decrease in angular velocity (AV) (A1), but no change in distance travelled (A2) with increasing frequency of stimulation. Same data as in Fig. 4 (n=7). Trajectories show even occurrences of ipsilateral and contralateral turning during 130 Hz DBS (A3). (B) No change response, showing no trend in AV (B1) or distance travelled (B2) with increases in stimulation frequency (n=3). Little change observed in trajectories during 130 Hz DBS (B3). (C) Exacerbated response, showing increases in AV (C1) and increases in distance travelled (C2) during high frequency stimulation (n=3). Trajectories show increased ipsilateral turning during 130 Hz DBS (C3). (D) Decreased mobility response, showing a decrease in both AV (D1) and distance travelled (D2) with increases in stimulation frequency (n=3). Trajectories show decreased movement during 130 Hz DBS (D3). Means ± SE are shown.
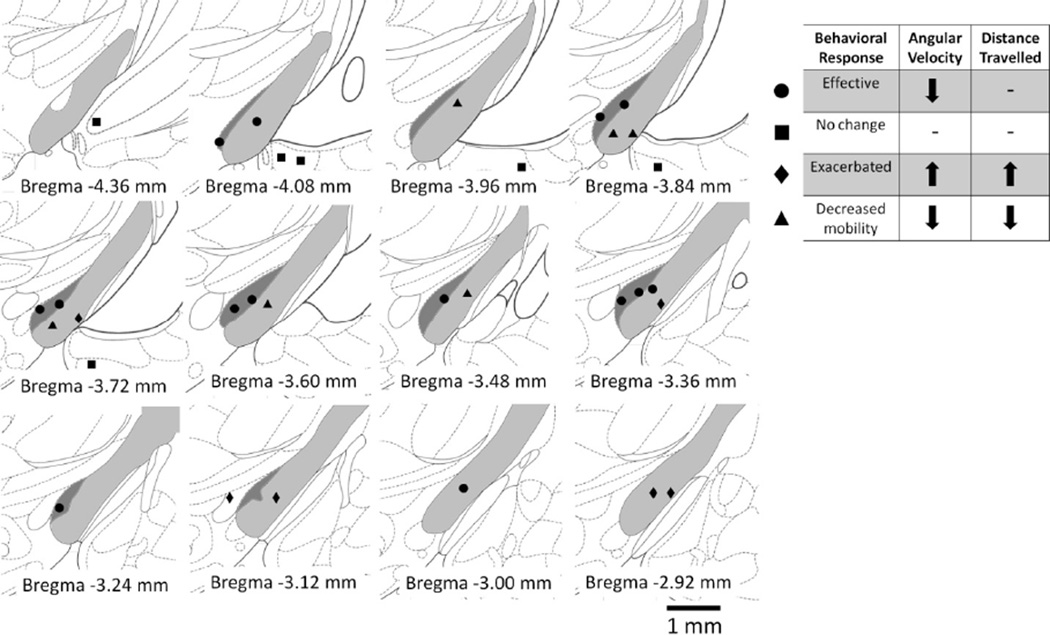
The behavioral findings were correlated with the location of the electrode tips identified from histology (Fig. 8) and mapped onto a rat brain atlas (Fig. 9). All rats exhibiting an effective response to DBS had one or both electrodes used in bipolar stimulation within the STN. In rats that had no responses to stimulation, electrodes were ventral to both the STN and internal capsule in the ventral hippocampus, amygdala, or other areas within the ventral cortex. In rats showing either an exacerbated response or decreased mobility, electrodes were located in the internal capsule. Electrodes in animals exhibiting exacerbated ipsilateral turning were located toward the anterior internal capsule, while electrodes in animals exhibiting decreased mobility were located toward the posterior internal capsule.
Fig. 9.
Positions of electrode tips corresponding to behavioral responses. Anatomy of the rat brain, showing posterior (top left) to anterior (bottom right) coronal sections (Paxinos and Watson 2007). The subthalamic nucleus (STN) is highlighted in dark gray and the internal capsule ventral to the STN is highlighted in light gray. Circles show the positions of tips of electrodes that produced effective behavioral response as indicated by frequency tuning curves. Most of these tips were within the STN. Although some tips corresponding to effective behavior were within the internal capsule, the other tip of the pair used in bipolar stimulation was always within the STN. Squares show the positions of electrode tips that produced no change in behavior. These tips were found ventral to both the STN and internal capsule. Diamonds show the positions of electrode tips that produced exacerbation of pathological behavior. These tips clustered within the anterior internal capsule. Triangles show the positions of electrode tips that produced decreased mobility. These tips clustered within the posterior internal capsule.
IV. DISCUSSION
We characterized the effects of DBS parameters, including stimulation amplitude, stimulation frequency, methamphetamine dose, monopolar versus bipolar stimulation, and electrode placement, on methamphetamine-induced circling in rats following a unilateral lesion of the substantia nigra. We quantified changes in both angular velocity and distance travelled across parameters, compared to previous studies that recorded only the change in number of rotations [8–10]. Effective STN stimulation resulted in a characteristic frequency profile showing a decrease in ipsilateral circling during stimulation at high frequencies but not low frequencies, indicating a reversal of the bias in turning direction and return to the healthy condition during high frequency DBS. Importantly, this decreased ipsilateral circling rate during high frequency stimulation did not decrease the mobility of the rat, and was only observed for electrodes positioned correctly within the STN.
The amplitude of stimulation greatly infuenced the effect of DBS on circling, and the amplitude was individually adjusted for each rat. Amplitudes ranging from 15 µA to 200 µA were used in all of our experiments, and a narrower range of amplitudes from 30 µA to 125 µA was able to elicit effective behavioral responses. This range is comparable to amplitudes used in previous studies of STN DBS in awake animals [8–9, 12–15]. Spieles-Engemann et al. [5] estimated that at 100 µA, current spread from the STN electrode affects a sphere with a radius of 250 µm, and highlighted the possible generation of side effects with larger amplitudes. However, these measurements were made during acute experiments in anesthetized animals, and did not take into account the effect of scar formation in the brain around the electrode following chronic implantation [17]. The location of the electrode contact in the STN, tissue damage during implantation, and increased impedance resulting from gliosis may all impact the stimulation amplitude necessary for successful STN activation. The presence of side effects during stimulation, including myoclonic jerks of the forepaw and neck, is an accurate indicator that current has spread to fibers and structures outside of the STN [5]. However, amplitudes resulting in side effects varied among rats and can only be determined during acute amplitude testing in awake animals. All amplitudes used in our experiments were below the threshold that caused side effects. Amplitudes that did not induce side effects, but were higher than required to reverse ipsilateral circling, produced contralateral turning, which might be considered as a form of dyskinesia [18].
The frequency tuning of STN DBS on methamphetamine-induced circling paralleled to a remarkable degree the effects of STN DBS on motor symptoms in persons with PD [19–23], as well as in a computational model of PD [24]. However, lower frequencies of stimulation (≥ 15 Hz) showed some effectiveness in the rat, while clinically, only frequencies ≥ 50 Hz begin to show effectiveness [19]. We did not observe an exacerbation of pathological behavior during low frequency stimulation (< 20 Hz) reported previously in rats [10] and humans [20, 22], except for decreased mobility caused by malpositioned electrodes. In persons with PD, the average baseline firing rate of the STN is ~ 40 Hz [25–26], while in 6-OHDA lesioned rats, the average baseline STN firing rate is 10 Hz to 15 Hz [27], and this difference in intrinsic firing rate could account for stimulation becoming effective at lower frequencies in rats than in humans [23].
Although the total charge delivered was higher for both high amplitude and high frequency stimulation, total charge is not the critical variable for relief of symptoms by DBS. Charge (controlled either by the amplitude or duration of the stimulation pulse) determines the spatial extent of stimulation and therefore the number of activated neurons. On the other hand, frequency determines how often those neurons are stimulated. Moro et al. [19] tested the dependence of motor symptom alleviation on stimulation frequency, amplitude, and pulsewidth, and found that stimulation frequency, not the total charge, was the primary factor determining efficacy. Similarly, Kuncel et al. [3] investigated the effects of amplitude and frequency of thalamic DBS on tremor over a wide parameter space, and found that low frequency exacerbated tremor even at high amplitudes (i.e., high charge).
STN DBS had no effect in some animals when using high methamphetamine doses (2.5 mg/kg), while stimulation was effective at the same or lower amplitudes after administering a lower dose of methamphetamine (1.25 or 1.875 mg/kg). Amphetamine-induced circling is known to be dose dependent [7], with higher concentrations of amphetamines resulting in a higher rate of rotation. Different rates of turning could also be affected by the extent of degeneration of dopaminergic neurons in the SNc. To reduce the variability in lesioning, we performed up to two re-lesions to ensure that all rats had severe lesions with greater than 90 % dopaminergic cell loss on the side of the lesion. Further, differences in turning rates across rats could be attributed to different intrinsic striatal dopamine activity [28], or different rates of compensatory mechanisms after lesioning, including development of hypersensitivity of postsynaptic dopaminergic receptors in the lesioned striatum [28] or reinnervation through dopaminergic sprouting [29].
Both monopolar and bipolar DBS have been used clinically, and the configuration for optimal treatment could be either monopolar or bipolar [4]. DBS experiments in rats have mostly used bipolar stimulation [8–9, 12–15]. Unlike clinical DBS electrodes that have four contacts along the length of the electrode shank, the electrodes used in this study were two-by-two arrays with the active conducting contacts at the tip of each electrode. The two-by-two array was used to maximize the chances of placing at least one electrode tip within the STN.
Using this electrode design, we quantified differences between monopolar and bipolar stimulation during STN DBS in rats. We found that when each of the electrode contacts used during bipolar stimulation was individually stimulated (monopolar stimulation), one contact was often more effective than the other in producing a behavioral response. Monopolar stimulation using the effective contact resulted in behavioral effects, as reflected in the frequency profiles, similar to those when bipolar stimulation was used, while stimulation using the ineffective contact resulted in little effect on behavior. Our results suggest that the effect of bipolar stimulation was due to a superposition, and not an interaction of the effects from stimulation at each pole. Apparently, the electrode contacts were far enough from each other (0.6 mm) that the volumes of tissue activated at each electrode tip did not interact during bipolar stimulation, unlike that seen using contacts from clinical electrodes [30]. When the inter-electrode spacing between two contacts during bipolar stimulation is large, the contacts do not influence each other, and the area of activation is greatest surrounding the contacts, and not between the contacts [31].
Correct placement of the STN electrode was critical to observe reduced ipsilateral turning during STN DBS. Investigators have employed a number of methods to improve the placement of electrodes within the STN, including using an array of electrodes [12, 14] and performing acute extracellular recordings [9–10]. However, targeting the small STN in the rat [0.8 mm3 in rats compared to 240 mm3 in humans [32]] remains a challenge and the electrode placement cannot be determined prior to post-mortem histology. The characteristic frequency profile was only produced when at least one of the two electrodes used for bipolar stimulation was within the STN, while incorrect placement resulted in frequency profiles that were classified into three sub-groups, all with deviations from the characteristic profile. Stimulation of regions ventral to both the STN and internal capsule, including the ventral hippocampus and amgydala, had little effect on motor function. Stimulation of the anterior internal capsule, which contains both motor tracts exiting the midbrain and sensory tracts from the thalamus to the pre-frontal cortex [33], resulted in hyperactivity with exacerbation of ipsilateral turning during high frequency stimulation. Stimulation of fibers within the anterior internal capsule may lead to sensory discomfort, or fear and panic [34–35] that could increase ipsilateral turning in the rat. Stimulation of the posterior internal capsule, which contains the corticospinal tracts primarily carrying efferent motor signals [33], decreased both angular velocity and distance travelled. Stimulation of these fibers could result in muscle contractions, disrupting normal motor movements and causing the rat to freeze, resulting in decreased movement. Regardless of the mechanisms behind the different motor responses, the frequency profile was strongly dependent on the position of the electrode contacts, and could serve as a predictive indicator of correct placement of STN DBS electrodes.
The present results demonstrate that methamphetamine induced circling in unilateral 6-OHDA lesioned rats is a simple, sensitive, and reliable means to study effects of STN DBS. The methamphetamine dose and stimulation amplitude require tuning to achieve desired behavioral outcomes, and the use of monopolar stimulation may be as effective as bipolar stimulation. Since the time course to observe the behavioral effects of stimulation is short, this paradigm allows acute testing to choose appropriate parameters such as amplitude and stimulation contact. A characteristic frequency profile was produced during effective stimulation, showing decreased angular velocity but no change in distance travelled during high frequency stimulation. This characteristic frequency profile may be used as a predictor of correct positioning of electrodes within the STN. Methamphetamine induced circling in unilaterally lesioned rat replicated behaviorally the trends of DBS effectiveness seen clinically, and is a useful model to study the neural mechanism underlying the effectiveness of DBS.
Acknowledgments
This work was supported in part by the U.S. National Institutes of Health under Grant R01-NS040894 and by the A*STAR NSS-Ph.D. scholarship program.
REFERENCES
- 1.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD. Bilateral Deep Brain Stimulation vs Best Medical Therapy for Patients With Advanced Parkinson Disease: A Randomized Controlled Trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moro E, Lozano AM, Pollak P, Agid Y, Rehncrona S, Volkmann J, Kulisevsky J, Obeso JA, Albanese A, Hariz MI, Quinn NP, Speelman JD, Benabid AL, Fraix V, Mendes A, Welter ML, Houeto JL, Cornu P, Dormont D, Tornqvist AL, Ekberg R, Schnitzler A, Timmermann L, Wojtecki L, Gironell A, Rodriguez-Oroz MC, Guridi J, Bentivoglio AR, Contarino MF, Romito L, Scerrati M, Janssens M, Lang AE. Long-Term Results of a Multicenter Study on Subthalamic and Pallidal Stimulation in Parkinson’s disease. Movement Disorders. 2010;5:578–586. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- 3.Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clinical Neurophysiology. 2004;115(11):2431–2441. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Moro E, Poon YY, Lozano AM, Saint-Cyr JA, Lang AE. Subthalamic nucleus stimulation: improvements in outcome with reprogramming. Archives of Neurology. 2006;63(9):1266–1272. doi: 10.1001/archneur.63.9.1266. [DOI] [PubMed] [Google Scholar]
- 5.Spieles-Engemann AL, Collier TJ, Sortwell CE. A functionally relevant and long-term model of deep brain stimulation of the rat subthalamic nucleus: advantages and considerations. European Journal of Neuroscience. 2010;32(7):1092–1099. doi: 10.1111/j.1460-9568.2010.07416.x. [DOI] [PubMed] [Google Scholar]
- 6.Nowak K, Mix E, Gimsa J, Strauss U, Sriperumbudur KK, Benecke R, Gimsa U. Optimizing a rodent model of Parkinson's disease for exploring the effects and mechanisms of deep brain stimulation. Parkinson's disease. 2011;2011:414682. doi: 10.4061/2011/414682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Research. 1970;24(3):485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 8.Meissner W, Harnack D, Paul G, Reum T, Sohr R, Morgenster R, Kupsch A. Deep brain stimulation of subthalamic neurons increases striatal dopamine metabolism and induces contralateral circling in freely moving 6-hydroxydopamine-lesioned rats. Neuroscience letters. 2002;328(2):105–108. doi: 10.1016/s0304-3940(02)00463-9. [DOI] [PubMed] [Google Scholar]
- 9.Fang X, Sugiyama K, Akamine S, Nambu H. Improvements in motor behavioral tests during deep brain stimulation of the subthalamic nucleus in rats with different degrees of unilateral parkinsonism. Brain Research. 2006;1120(1):202–210. doi: 10.1016/j.brainres.2006.08.073. [DOI] [PubMed] [Google Scholar]
- 10.Gradinaru V, Mogri M, Thompson K, Henderson J, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bracha HS, Shults C, Glick D. Spontaneous Asymmetric Circling Behavior in Hemi-Parkinsonism: A Human Equivalent of the Lesioned-circling Rodent Behavior. Life Sciences. 1986;40(11):1127–1130. doi: 10.1016/0024-3205(87)90576-5. [DOI] [PubMed] [Google Scholar]
- 12.Chang JY, Shi LH, Luo F, Woodward DJ. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Research. 2003;983:174–184. doi: 10.1016/s0006-8993(03)03053-1. [DOI] [PubMed] [Google Scholar]
- 13.Darbaky Y, Forni C, Amalric M, Baunez C. High frequency stimulation of the subthalamic nucleus has beneficial antiparkinsonian effects on motor functions in rats, but less efficiency in a choice reaction time task. European Journal of Neuroscience. 2003;18(4):951–956. doi: 10.1046/j.1460-9568.2003.02803.x. [DOI] [PubMed] [Google Scholar]
- 14.Shi LH, Woodward DJ, Luo F, Anstrom K, Schallert T, Chang JY. High-frequency stimulation of the subthalamic nucleus reverses limb-use asymmetry in rats with unilateral 6-hydroxydopamine lesions. Brain Research. 2004;1013:98–106. doi: 10.1016/j.brainres.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 15.Vlamings R, Visser-Vandewalle V, Koopmans G, Joosten EA, Kozan R, Kaplan S, Steinbusch HW, Temel Y. High frequency stimulation of the subthalamic nucleus improves speed of locomotion but impairs forelimb movement in Parkinsonian rats. Neuroscience. 2007;148(3):815–823. doi: 10.1016/j.neuroscience.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen MS, Bjarkam CR, Søensen JC, Bojsen-Møler M, Sunde NA, Øtergaard K. Chronic subthalamic high-frequency deep brain stimulation in Parkinson's disease--a histopathological study. Eureopean Journal of Neurology. 2007;14(2):132–138. doi: 10.1111/j.1468-1331.2006.01569.x. [DOI] [PubMed] [Google Scholar]
- 18.Konitsiotis S, Tsironis C. Levodopa-induced dyskinesia and rotational behavior in hemiparkinsonian rats: Independent features or components of the same phenomenon? Behavioral Brian Research. 2006;170:337–341. doi: 10.1016/j.bbr.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Moro E, Esselink RJ, Xie J, Hommel M, Benabid AL, Pollak P. The impact on Parkinson's disease of electrical parameter settings in STN stimulation. Neurology. 2002;59(5):706–713. doi: 10.1212/wnl.59.5.706. [DOI] [PubMed] [Google Scholar]
- 20.Timmermann L, Wojtecki L, Gross J, Lehrke R, Voges J, Maarouf M, Treuer H, Sturm V, Schnitzler A. Ten-Hertz stimulation of subthalamic nucleus deteriorates motor symptoms in Parkinson's disease. Movement Disorders. 2004;19(11):1328–1333. doi: 10.1002/mds.20198. [DOI] [PubMed] [Google Scholar]
- 21.Fogelson N, Kün AA, Silberstein P, Limousin PD, Hariz M, Trottenberg T, Kupsch A, Brown P. Frequency dependent effects of subthalamic nucleus stimulation in Parkinson’s disease. Neuroscience Letters. 2005;382:5–9. doi: 10.1016/j.neulet.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 22.Eusebio A, Chen CC, Lu CS, Lee ST, Tsai CH, Limousin P, Hariz M, Brown P. Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson's disease. Experimental Neurology. 2008;209:125–130. doi: 10.1016/j.expneurol.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birdno MJ, Grill WM. Mechanisms of deep brain stimulation in movement disorders as revealed by changes in stimulus frequency. Neurotherapeutics. 2008;5(1):14–25. doi: 10.1016/j.nurt.2007.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.So RQ, Kent AR, Grill WM. Relative contributions of local cell and passing fiber activation and silencing to changes in thalamic fidelity during deep brain stimulation and lesioning: a computational modeling study. Journal of Computational Neuroscience. 2011 doi: 10.1007/s10827-011-0366-4. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy R, Hutchison WD, Lozano AM, Dostrovsky JO. High-frequency synchronization of neuronal activity in the subthalamic nucleus of Parkinsonian patients with limb tremor. Journal of Neuroscience. 2000;20:7766–7775. doi: 10.1523/JNEUROSCI.20-20-07766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience. 2000;96:549–564. doi: 10.1016/s0306-4522(99)00583-7. [DOI] [PubMed] [Google Scholar]
- 27.Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulate. FASEB Journal. 2003;17:1820–1830. doi: 10.1096/fj.03-0163com. [DOI] [PubMed] [Google Scholar]
- 28.Glick SD, Jerussi TP, Fleisher LN. Turning in circles: the neuropharmacology of rotation. Life Sciences. 1976;18(9):889–896. doi: 10.1016/0024-3205(76)90405-7. [DOI] [PubMed] [Google Scholar]
- 29.Blanchard V, Anglade P, Dziewczapolski G, Savasta M, Agid Y, Raisman-Vozari R. Dopaminergic sprouting in the rat striatum after partial lesion of the substantia nigra. Brain Research. 1996;709(2):319–325. doi: 10.1016/0006-8993(95)01391-1. [DOI] [PubMed] [Google Scholar]
- 30.Butson C, McIntyre C. Current Steering to Control the Volume of Tissue Activated During Deep Brain Stimulation. Brain Stimulation. 2008;1:7–11. doi: 10.1016/j.brs.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wongsarnpigoon A, Grill WM. Computational modeling of epidural cortical stimulation. Journal of Neural Engineering. 2008;5(4):443–454. doi: 10.1088/1741-2560/5/4/009. [DOI] [PubMed] [Google Scholar]
- 32.Hardman CD, Henderson JM, Finkelstein DI, Horne MK, Paxinos G, Halliday GM. Comparison of the basal ganglia in rats, marmosets, macaques, baboons, and humans: volume and neuronal number for the output, internal relay, and striatal modulating nuclei. Journal of Comparative Neurology. 2002;445:238–255. doi: 10.1002/cne.10165. [DOI] [PubMed] [Google Scholar]
- 33.Chowdhury F, Haque M, Sarkar M, Ara S, Islam M. White fiber dissection of brain; the internal capsule: a cadaveric study. Turkish Neurosurgery. 2010;20(3):314–322. doi: 10.5137/1019-5149.JTN.3052-10.2. [DOI] [PubMed] [Google Scholar]
- 34.Okun MS, Mann G, Foote KD, Shapira NA, Bowers D, Springer U, Knight W, Martin P, Goodman WK. Deep brain stimulation in the internal capsule and nucleus accumbens region: responses observed during active and sham programming. Journal of Neurology, Neurosurgery, and Psychiatry. 2007;78(3):310–4. doi: 10.1136/jnnp.2006.095315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapira NA, Okun MS, Wint D, Foote KD, Byars JA, Bowers D, Springer US, Lang PJ, Greenberg BD, Haber SN, Goodman WK. Panic and fear induced by deep brain stimulation. Journal of Neurology, Neurosurgery, and Psychiatry. 2006;77(3):410–412. doi: 10.1136/jnnp.2005.069906. [DOI] [PMC free article] [PubMed] [Google Scholar]