Abstract
Although research unanimously maintains that exercise can ward off cardiovascular disease (CVD), the optimal type, duration, intensity, and combination of forms are yet not clear. In our review of existing rodent-based studies on exercise and cardiovascular health, we attempt to find the optimal forms, intensities, and durations of exercise. Using Scopus and Medline, a literature review of English language comparative journal studies of cardiovascular benefits and exercise was performed. This review examines the existing literature on rodent models of aerobic, anaerobic, and power exercise and compares the benefits of various training forms, intensities, and durations. The rodent studies reviewed in this article correlate with reports on human subjects that suggest regular aerobic exercise can improve cardiac and vascular structure and function, as well as lipid profiles, and reduce the risk of CVD. Findings demonstrate an abundance of rodent-based aerobic studies, but a lack of anaerobic and power forms of exercise, as well as comparisons of these three components of exercise. Thus, further studies must be conducted to determine a truly optimal regimen for cardiovascular health.
Keywords: aerobic, anaerobic, training, physical activity, cardiac, vascular, lipid profile
Unlike many other fatal diseases, cardiovascular disease (CVD) risk is highly modifiable through lifestyle changes that can virtually eliminate the initiation and progression of atherosclerotic plaque formation. A large body of evidence supports the hypothesis that CVD is drastically influenced by diet and exercise.1 2 3 Exercise serves to counteract excess caloric consumption, thereby reducing the risks of obesity. Exercise may also have direct physiologic effects that attenuate CVD, but it is not clear what type, intensity, and duration of exercise is most beneficial to cardiovascular fitness and metabolic optimization.
In this review, we will examine three different categories of exercise and compare the effects of each form of activity on cardiac, vascular, and lipid measures of health. Information from athletes or patients is difficult to analyze because of variability of human subjects and confounding comorbidities and medications. Rodent models, on the other hand, often allow for more invasive, extensive, and homogenous experimental designs than human models. These rodent-based exercise studies may provide more information and help to elucidate the benefits of different training regimens to reduce the risk of CVD.
Methods
Data retrieval was performed using Medline and Scopus. In Medline, the search term “cardiac or vascular or lipid” AND “exercise” AND “English Language” was entered, then the resulting literature was restricted to comparative studies using rats or mice, published as journal articles. In Scopus, “Cardiac benefits and endurance exercise and rodents or mice or rats,” “Cardiac benefits and sprint exercise or interval exercise and rodents or mice or rats,” and “Cardiac benefits and power exercise or weight exercise and rodents or mice or rats” were entered to look at cardiac effects. For vascular and metabolic effects, the same three searches were performed using “vascular” and “lipid profile” in place of cardiac. In both searches, studies that did not specifically address either cardiac, vascular, or lipid alterations, where exercise was not employed as the main experimental condition, or in which rodents received a supplement or drug were excluded. Any literature examining rodents with other systemic disorders (diabetes, hypertension, obese, etc.) were also excluded. The 78 remaining articles were then utilized in this analysis (Fig. 1).
Fig. 1.
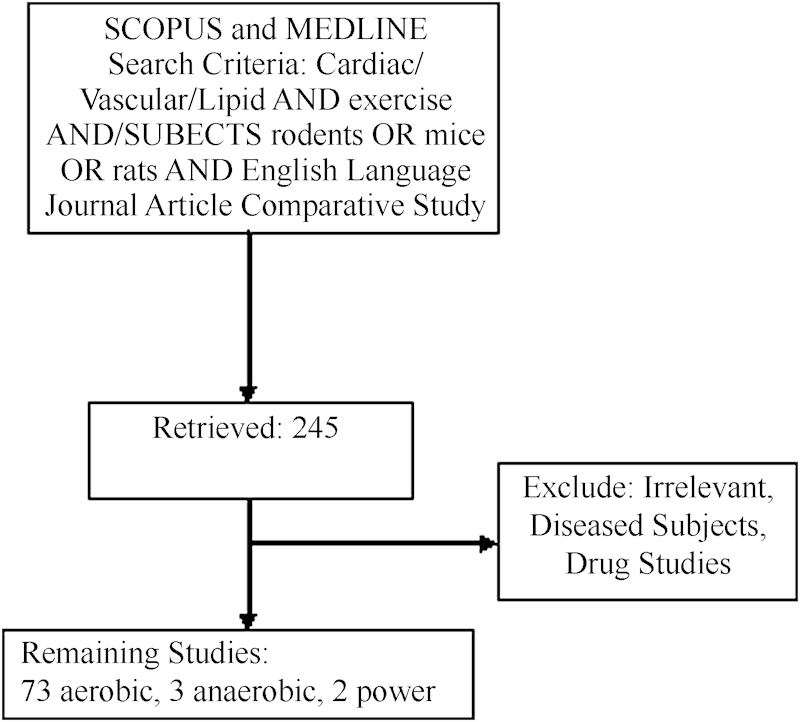
Summary of literature retrieval. Scopus and Medlin searches were performed as outlined above. Of the 245 retrieved articles, 185 were excluded for the following reasons: not examining the impact of exercise on cardiovascular parameters (e.g., looked at impact of exercise on testicular arteries) and thus were irrelevant, employing only diseased exercising subjects and sedentary controls with no healthy exercising comparisons, and or examining the effects of drugs or supplements on exercise-induced adaptations. The remaining studies were then categorized as aerobic, anaerobic, or power.
Results
Experimental Design
For these studies, exercise was categorized as either dynamic (i.e., aerobic or anaerobic) or static (i.e., resistance-based training) (see Table 1). Aerobic or endurance exercise was defined as a physical exertion that raises the heart to 60 to 90% of an individual's max heart rate, or that which is below the “lactic acid threshold” (LAT). The LAT is a subject-specific level of exertion which is just below that which would lead to a buildup of lactic acid in the muscles. Theoretically, if an exercise is entirely aerobic, the animal would be able to continue it forever without muscle failure. In many of the published rodent studies, aerobic activity is produced as either running or swimming for anywhere between 20 and 60 minutes.
Table 1. Design summary of exercise protocols.
| Definition | Methods | References | |
|---|---|---|---|
| Aerobic | Constant moderate intensity exertion O2 consumption below LAT Employs slow-twitch muscle fibers |
Treadmill/wheel running at constant pace Swimming |
6 7 8 9 10 12 13 14 15 16 17 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 |
| Anaerobic | Shorter high intensity exercise that often occurs in intervals O2 consumption cannot be met by the body Employs fast-twitch fibers |
Treadmill running at fast pace for short intervals | 8 11 18 |
| Power | Weight or resistance opposing movements Muscles employ force through concentric or eccentric contraction |
Attachment of weights to rodents and then let them hang from ledge | 4 5 |
Note: The working definitions of each form of exercise and the methods used to engage rodents in the specific activity are listed for each study included in our literature search.
Anaerobic activity was the second form of dynamic exercise and was defined as movement that raises the heart rate above 90% of the max, employs fast-twitch muscle fibers, and occurs above the LAT. In contrast to the steady, prolonged manner of aerobic movement, anaerobic activity occurs in short, high-intensity spurts and cannot be sustained as lactic acid accumulation occurs rapidly. A typical anaerobic regimen would be for rodents to run as fast as possible for intervals of 10 to 20 seconds.
Resistance training, or strength training, is a static form of exercise that engages muscles in a very different manner from the dynamic forms of endurance and sprint activities. This form of training employs a force that opposes muscular contraction, such as the use of metal weights or an individual's body weight to “resist” the contraction. Designing a weight-lifting experiment with rodents can be difficult, and this certainly can account for the paucity of power studies. Previous studies have attached small weights to rodents placed in a “hanging” climb position4 or have weighted rats climb a 1-m wire.5
Cardiac, Vascular, and Metabolic Benefits of Different Forms of Exercise
As indicated in Table 2, heart function was the most studied component of exercise-induced CV health, especially utilizing aerobic exercise protocols. The rodent models revealed that endurance activity improved left ventricle size, diastolic and systolic functions, cardiac output, and stroke volume.6 No studies directly measured resting heart rate or blood pressure, which are common indicators of human cardiac well-being.
Table 2. Cardiac, vascular, and metabolic benefits of different exercise types.
| Aerobic | Anaerobic | Power | |
|---|---|---|---|
|
Cardiac
LV size LV diastolic/systolic Fnx Cardiac output Stroke volume Myocardial health Cardioprotection Resting HR Resting BP |
+++ +++ +++ +++ ++— ++— ? ? |
? ? ? ? +++ +++ ? ? |
? ? ? ? ? ? ? ? |
|
Vascular
VEGF mRNA Endothelial Fnx AS lesions Inflammation Vasodilation |
+++ +++ ++— +++ +++ |
? +++ ? ? +++ |
? +++ ? ? +++ |
|
Metabolic
LDL levels Triacylglycerol lipids Fatty acids Visceral fat Subcutaneous fat Leptin |
+++ +++ +— +++ +++ +++ |
? ? ? ? ? ? |
? ? ? ? ? ? |
Note: The benefits of each form of exercise on three set of cardiovascular measures are given as follows: ++ + , all studies retrieved beneficial effects; ++—, more beneficial findings than not; +—, half beneficial effects and half not; ?, no data.
Anaerobic exercise was found only to have positive influence on two factors found to be equivocal with aerobic exercise. Specifically, chronic interval exercise was found to increase protein kinase C–mediated cardioprotection against ischemia-reperfusion7 and anaerobic interval training induced adaptive enzymatic and free radical changes in the myocardium of rodents.8 There were no articles for the effects of sprint training on other cardiac health components. Similarly, the search for power exercise and cardiac benefits yielded no relevant papers.
Studies on the vascular benefits of aerobic exercise indicated a positive effect on VEGF mRNA,9 10 endothelial function,11 12 and inflammatory markers.13 There was some controversy on whether aerobic activity diminished atherosclerotic lesions. Young14 found that aerobic exercise did not decrease markers associated with plaque build-up, nor did it reduce lesion development. Conversely, researchers have observed reduced atherosclerotic lesion size and improved markers of vascular health in exercising rodents.13 15 16 17 Anaerobic exercise's influence on vascular components of health is understudied; there were no research articles on its effect on VEGF mRNA expression, atherosclerotic lesions, inflammatory markers, and resting blood pressure. However, both endothelial function and vasodilation were found to improve with interval training.8 11 18 Although the influence of power exercise on most vascular health factors had not been studied, both Figard4 and Harris5 did find weight-bearing exercise to improve endothelial function and vasodilation in mice.
The effect of aerobic exercise on metabolic profiles was well studied and most of the literature was congruent in their findings. Endurance activity improved LDL levels,13 triacylglycerol levels,15 17 visceral and subcutaneous fat deposits,17 and leptin levels.17 However, there were contradictory results regarding fatty acid serum levels and metabolism. Two separate studies found no effect of exercise on these two components,16 19 whereas another team of researchers found such exercise lowered the levels.15 Our search yielded no published studies on the lipid profile effects of either anaerobic or power exercise.
Studies Directly Comparing Various Exercise Protocols
Kemi et al11 examined different intensities of aerobic running on the cardiovascular health of healthy rats (Table 3). The higher intensity, which was performed at 85 to 90% of VO2max, was at the brink of becoming classified as an anaerobic effort. The lower intensity was performed at 65 to 70% of VO2max and was entirely aerobic. The study showed significantly greater cardiomyocyte hypertrophy and functional improvements, carotid endothelial functional enhancement, and vasorelaxation and dilation improvements in the higher intensity group compared with the lower intensity. These experiments demonstrated an intensity-dependent adaptation to endurance exercise and suggest that higher levels of intensity are more beneficial to cardiovascular health in a healthy animal but both groups were better than the no exercise group.
Table 3. Randomized control trials comparing various types and intensities of exercise.
| Exercise groups | Exercise regimens | Differences in findings | Conclusion |
|---|---|---|---|
| HI vs. LI vs. Sed11 | Treadmill running 1 h/d, 5 d/wk at: 85–90% VO2max (HI) vs. 65–70% VO2max (LI) vs. No exercise |
HI group had greater increase in VO2max, cardiomyocyte hypertrophy, cardiomyocyte function, carotid artery endothelial function | Higher intensity > lower intensity for certain cardiovascular benefits |
| IEI vs. ITI vs. Sed8 | IEI ran on treadmill at 26.8 m/min for 1 min at the gradient of 10°, then 5 m/min at the gradient of 0°, 3 minutes rest, 20x for 1 day vs. ITI ran the above protocol call 5 d/wk, for 6 weeks vs. No exercise |
ITI had more adaptive change of antioxidant enzymes and free radical clearing in the myocardium | Sustained training > single exercise session Anaerobic interval training can reduce inflammatory components associated with atherosclerosis |
| CT vs. IN vs. Sed21 | Swimming with 5% body weight load for 5 d/wk for 8 weeks for: 90 min/d (CT) vs. 3 × 30 min/d with 4 hours between sets (IN) vs. No exercise |
IN rodents exhibited greater ↓ visceral and central adipose tissue ↓ Cholesterol and triacylglycerol ↑ Energy expenditure than CT, who exhibited greater benefits than Sed |
Interval > continuous > sedentary for serum lipid levels and body fat composition |
| YL, YM, YH for 20, 40, or 60 vs. OL, OM, OH for 20 or 40a 22 | Swimming 6 d/wk for 4 weeks: Young and old rodents ran at L, M, H intensities (2, 3, and 5% of body weight added in weights) for group-specific time duration of 20, 40, or 60 minutes |
↓ cholesterol, LDLs, triglycerides, and ↑ HDLs in both Y and O at L and M for 20 and 40 min/d. L 20 min/d improved lipid profile L60 derived greater benefits Higher intensity did not alter lipid profile for any time duration |
Low intensity for even 20 min/d can improve lipid profile and markers of cardiovascular health Higher intensity elicits less lipid changes |
Abbreviations: HI, high intensity; LI, low intensity; Sed, sedentary; IEI, intermittent exercise interval; ITI, intermittent training interval; CT, continuous; IN, interval.
Notes: Groups of aerobically exercised rodents (n = 8 per group) were compared to determine differences in advantageous cardiovascular responses to various intensities,11 the efficacy of a single session versus longer term training in eliciting cardiovascular adaptations,8 and the differences between continuous and intermittent swimming exercise on serum lipid levels.21 Finally, age, various intensities, and duration were cross-compared by attaching weights of 2, 3, or 5%, respectively, of the animal's body weight to the tail (n = 4 rodents per subset group).22
Young (Y) or old (O), at low (L), medium (M), or high (H) intensity for 20, 40, or 60 minutes.
Qiao8 examined the differences in adaptations between a single bout of anaerobic interval exercise and a 6-week program of anaerobic training. The longer training produced much greater enzymatic and oxidative adaptations, which in turn support greater myocardial health. However, it is important to note that a single exercise session did provide some degree of benefit, as evidenced by the resulting increase in antioxidant enzyme levels and free radical clearing in the myocardium.
Gute et al20 directly compared sprint interval training (SIT) and low-intensity training (LET). These researchers found that while both modes of training increased capillarization of muscle tissue, SIT increased that in white and mixed muscle tissue, while LET increased that in red and mixed muscle tissue.
In another study team, the effect of continuous versus intermittent aerobic exercise was examined by subjecting rodents to either a session of 90 minutes or three 30-minute sessions of swimming, 5 days a week for 8 weeks.21 The intervals of swimming elicited more profound changes in adipose tissue, lipid profile, and lipid metabolism than continuous swimming.
The intensity and duration of training in young and old rodents were also examined.22 Young rodents swam for a duration of 20, 40, or 60 minutes, and in each of the three durations they swam with a weight of either 2, 3, or 5% their body weight attached to for a low, moderate, or high intensity, respectively. Older rodents swam for either 20 or 40 minutes with different groups for each of the different intensities. Low and moderate intensity exercise for only 20 or 40 minutes improved lipid profile, whereas higher intensity did not seem to impact the profile. Likewise, 60 minutes of exercise did not appear to have significantly greater impact than the shorter durations.
A final study compared the different cardiovascular impacts of two different types of endurance exercise: swimming and running.23 Although both groups exhibited enhanced markers of ventricular function, such as increased stroke volume, stroke work, and maximum power, as well as enhanced ejection fractions and fiber shortening. However, the swim group demonstrated faster rates of cardiac relaxation.
Discussion
Our review of the relevant rodent studies clearly document the cardiac, vascular, and lipid profile benefits derived from aerobic exercise. Studies on anaerobic benefits were less abundant, but consistent. Power-induced benefits were almost completely neglected in rodent models. Despite the scarcity of studies, each form of exercise appears to provide some form of CV protection and decrease factors involved in CVD.
There are some limitations of our approach to study analysis. First, in our review, we focused exclusively on the small animal model utilizing the rodent primarily because of the greater number of studies performed. Second, in an effort to avoid confounding effects, several types of studies were excluded from our data retrieval process, including those in which a secondary variable (such as administration of a drug) was employed in combination with the exercise, studies that did not examine exercise or cardiovascular-related adaptations, and those that utilized rodents models with systemic diseases. Our research focused on healthy populations because in disease models, there are conflicting reports on whether aerobic exercise provides benefits or impairs the cardiovascular system and such findings confound the results for disease-free animals.24 25 Consequently, we neglected most studies of Harold Laughlin, Timothy Musch, and Joseph Cheung, experts in the field of rat-based models of cardiovascular adaptations to anaerobic physical activity, as the researchers often use myocardial infarction model rodents. Only original studies were included in our review, and database-based analyses, protocol proposals, and reviews were excluded.
One of the limitations of our review of the relevant rodent studies is that there are no exact, all-encompassing components that define aerobic activity employed in research. In some studies, aerobic activity bordered on the brink of anaerobic exercise, potentially confounding the experiments and calling into question where the line between the two dynamic forms of exercise lies. Heart rate, duration of each activity, lengths of continuous movement and rest varied considerably between different exercise models which can confound the analysis of the CV benefits. It cannot be determined which parameters, or combination thereof, provide the greatest cardiovascular benefits. A better understanding of the physical adaptations that endurance exercise fosters is needed and studies comparing the duration, intensities, and type of exercise performed.
The studies we retrieved provided solid evidence that aerobic exercise enhanced gene expression of endothelial growth and angiogenesis markers.10 12 13 18 However, there were several confusing and somewhat contradicting results. Two studies assessed rodents running 1 hour/day but one had rodents running 7 days/week for 3 weeks and the other for 5 days/week for 8 weeks.17 19 The former study found that the fatty acid profile of cardiac phospholipids was not significantly altered, although resting heart rat, aortic flow, and cardiac output were reduced.19 In contrast, the results of the 8-week study indicated that exercise significantly reduced levels of triacylglycerol and free fatty acids in the blood, as well as subcutaneous and visceral fat deposits.17
Review of the rodent studies provides interesting information on the duration of exercise needed to bolster cardiovascular health.12 22 26 In a study that compared the benefits of running for 20 minutes/day, 5 days/week for a period of either 6 or 12 weeks, cardiac function was most improved after 12 weeks.26 While longer training regimes and longer bouts of exercise do seem to produce greater benefits, those that lasted only 3 weeks or less than 1 hour/day still resulted in significantly improved cardiovascular markers of health.6 9 12 14 In one study comparing the benefits of swimming for either 15 minutes or 30 minutes daily for 16 weeks, both groups displayed decreased levels of molecular CVD risk factors, including lessened atherosclerotic lesion development, without any significant differences between groups.14
Furthermore, longer exercise may not always be beneficial. In a study that compared the effects of running for 1 hour a day, 5 days a week for a period or 4, 8, or 16 weeks, all three training durations were found to increase cardiac mass; however, after the 8-week mark, the authors note exercise produced unfavorable alterations, including increased arrhythmia inducibility and markers of fibrosis in both atria and right ventricle.6 The authors argue that their findings support the idea that long-term endurance training can, in certain situations, promote adverse remodeling and perhaps lead to cardiac arrhythmias, which are detrimental to CV health. This is in stark contrast to previously mentioned studies arguing that long-term exercise bolsters cardiac health and reduces the risk of disease.6 9 12 14 The contradiction in results may be a result of the type of running performed and underscores the disparity in exercise protocols. Although groups of rats ran 5 days a week, one group ran for 60 minutes a day27 and the other for 30 minutes.26 In addition, the study that reported negative effects shocked the rats when they ceased movement, and thereby engaged the rats in “forced” exercise. Voluntary exercise elicits more favorable physiological outcomes such as reduction of glucocorticoid levels,28 whereas forced exercise appears to upregulate the production of these stress hormones.29 Because stress is a well-documented factor in myocardial infarctions, as well as a component of CVD risk, the detrimental cardiovascular changes observed may be a consequence of the forced nature of the exercise.
Analysis of rodent exercise research is also hampered by the varying degrees of intensity used in different investigations. In our review, only two studies directly compared higher intensity aerobic exercise to lower intensity ones. In one study, healthy rodents in a high-intensity group ran at 85 to 90% of their VO2max and rodents in a moderate intensity group ran at 65 to 70% of their VO2max for 1 hour/day, 5 days/week for 10 weeks.11 The rodents in the higher intensity group displayed greater cardiomyocyte hypertrophy and function, as well as better carotid artery endothelial function than the low-intensity mice. In the second study,22 low, moderate, and high intensities of swimming were examined. These researchers found that while low and moderate intensities produced beneficial effects on the lipid profile of rodents, high intensity did not. The reasons for these findings are complex but may likely be related to the differing energy sources for different degrees of exertion. During low or moderate exertion, fat stores are used as a source of fuel, but during higher levels of exertion, the body switches mainly to glycolytic stores or free glucose for energy. Thus, the animals in the high-intensity group may have switched from depleting lipid stores for energy to using carbohydrate-based sources. Although higher intensity may not lower cholesterol levels as effectively, exercise that raises the heart rate to at least 70% of maximum has been shown to elicit significant cardiac benefits.10 11 30 Running between 50 and 70% of maximum heart rate improves markers of CV health and reduces mortality rate.10 The type of aerobic exercise commonly performed by the rodents were running, either in a wheel or on a treadmill, and sometimes swimming. We only found one article directly comparing the effects of these two forms on cardiovascular adaptations.31 Despite the matched duration and workload of the exercises, and despite similar effects on lipid profile, some of the cardiovascular-related physiological changes appeared to be form-dependent. Specifically, peripheral serotonergic system modulation was more influenced by running, whereas sympathetic nervous system modulation was more highly impacted by swimming.
A surprising finding of our review was the relative paucity of anaerobic and power models. Anaerobic training models were highly understudied, due in part to the difficulty in making mice voluntarily run multiple repetitions at an all-out pace. Power exercise is even more understudied—how can a rat lift weights? One clever study attached weights to the tails of rodents and placed them in a hanging position.4 This forced the rats to utilize muscle to oppose the weights in much the same way a bicep must oppose a dumbbell's weight during a curl or the body's weight during a push up. The investigators demonstrated that the rats had enhanced vasodilation and endothelial function, suggesting that resistance training may provide benefits that prevent vascular dysfunction.
In summary, research on the effects of aerobic exercise suggests that in disease-free conditions moderate to high-intensity exercise is beneficial to cardiac, vascular, and lipid health. However, lipid levels may not be affected by exercise, and in this case, perhaps a more stringent diet is required, or a longer duration of exercise training. The literature on cardiovascular benefits of anaerobic training is limited, although existing studies on cardiac and vascular parameters indicate highly beneficial adaptations occur. If any such studies examine lipid profile, we may find similar beneficial effects. Power exercise needs to be further studied as only two groups of researchers have sought to understand its impact. The existing evidence does propose health benefits in disease models, but this must be confirmed with future studies.
The rodent studies reviewed in this article correlate with reports on human subjects that suggest regular aerobic exercise can improve cardiac and vascular structure and function, as well as lipid profiles, and reduce the risk of CVD.32 33 34 35 39 40 46 51 60 62 63 71 73 74 75 76 77 78 79 Studies on endurance athletes have repeatedly shown exercise to result in morphological and functional adaptations that correlate with a reduction in heart disease. Long-term exercise induces hemodynamic changes and alters the loading conditions of the heart, with specific effects depending on the type of sport and intensity.1 2 6
Conclusion
The rodent studies provide a useful resource to help elucidate the beneficial effects of exercise on cardiovascular health. By studying a homogenous population of animals and subjecting them to defined exercise regiments, we are able to garner objective evidence with regard to the role of type of exercise, duration, and intensity on quantifiably cardiac, vascular, and metabolic parameters. Although some limitations still exist in being able to translate the rodent model to human relevance, these studies provide valuable mechanical insight that help direct future research in this area.
Acknowledgments
This article was supported in part by grants to Bauer E. Sumpio from National Institute of Health (RO1-HL 47345) and Veterans Administration Merit Review Board.
Footnotes
Conflicts of Interest The authors report no conflicts of interest, financial or otherwise, and state that the results of the present study do not constitute endorsement by ACSM.
References
- 1.Thompson P D. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Arterioscler Thromb Vasc Biol. 2003;23(8):1319–1321. doi: 10.1161/01.ATV.0000087143.33998.F2. [DOI] [PubMed] [Google Scholar]
- 2.Fox A A, Thompson J L, Butterfield G E, Gylfadottir U, Moynihan S, Spiller G. Effects of diet and exercise on common cardiovascular disease risk factors in moderately obese older women. Am J Clin Nutr. 1996;63(2):225–233. doi: 10.1093/ajcn/63.2.225. [DOI] [PubMed] [Google Scholar]
- 3.Hellénius M L, de Faire U, Berglund B, Hamsten A, Krakau I. Diet and exercise are equally effective in reducing risk for cardiovascular disease. Results of a randomized controlled study in men with slightly to moderately raised cardiovascular risk factors. Atherosclerosis. 1993;103(1):81–91. doi: 10.1016/0021-9150(93)90042-s. [DOI] [PubMed] [Google Scholar]
- 4.Figard H, Gaume V, Mougin F. et al. Effects of an isometric strength training on endothelial function in ovariectomized female rats. Sci Sports. 2006;21:101–103. [Google Scholar]
- 5.Harris M B, Slack K N, Prestosa D T, Hryvniak D J. Resistance training improves femoral artery endothelial dysfunction in aged rats. Eur J Appl Physiol. 2010;108(3):533–540. doi: 10.1007/s00421-009-1250-z. [DOI] [PubMed] [Google Scholar]
- 6.Benito B, Gay-Jordi G, Serrano-Mollar A. et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123(1):13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 7.Peng F, Zhang L, Chen D. The effect of interval exercises on expression of protein kinase C in rat's ischemic-reperfusion cardiac Myocyte. Chin J Rehab Med. 2010;25:544–547. [Google Scholar]
- 8.Qiao X F. Effects of anaerobic interval training on free radical metabolism of skeletal muscle, myocardium and liver in rats. J Clin Rehab Tissue Eng Res. 2009;13:9129–9132. [Google Scholar]
- 9.Wu G, Rana J S, Wykrzykowska J. et al. Exercise-induced expression of VEGF and salvation of myocardium in the early stage of myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296(2):H389–H395. doi: 10.1152/ajpheart.01393.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jorge L, Rodrigues B, Rosa K T. et al. Cardiac and peripheral adjustments induced by early exercise training intervention were associated with autonomic improvement in infarcted rats: role in functional capacity and mortality. Eur Heart J. 2011;32(7):904–912. doi: 10.1093/eurheartj/ehq244. [DOI] [PubMed] [Google Scholar]
- 11.Kemi O J, Haram P M, Loennechen J P. et al. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67(1):161–172. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Reboul C, Tanguy S, Dauzat M, Obert P. Altitude negates the benefits of aerobic training on the vascular adaptations in rats. Med Sci Sports Exerc. 2005;37(6):979–985. [PubMed] [Google Scholar]
- 13.Fukao K, Shimada K, Naito H. et al. Voluntary exercise ameliorates the progression of atherosclerotic lesion formation via anti-inflammatory effects in apolipoprotein E-deficient mice. J Atheroscler Thromb. 2010;17(12):1226–1236. doi: 10.5551/jat.4788. [DOI] [PubMed] [Google Scholar]
- 14.Young C G, Knight C A, Vickers K C. et al. Differential effects of exercise on aortic mitochondria. Am J Physiol Heart Circ Physiol. 2005;288(4):H1683–H1689. doi: 10.1152/ajpheart.00136.2004. [DOI] [PubMed] [Google Scholar]
- 15.Nikolaidis M G, Mougios V. Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med. 2004;34(15):1051–1076. doi: 10.2165/00007256-200434150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Tsalouhidou S, Petridou A, Mougios V. Effect of chronic exercise on DNA fragmentation and on lipid profiles in rat skeletal muscle. Exp Physiol. 2009;94(3):362–370. doi: 10.1113/expphysiol.2008.045732. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier M S, Couturier K, Charbonneau A, Lavoie J M. Effects of introducing physical training in the course of a 16-week high-fat diet regimen on hepatic steatosis, adipose tissue fat accumulation, and plasma lipid profile. Int J Obes Relat Metab Disord. 2004;28(8):1064–1071. doi: 10.1038/sj.ijo.0802628. [DOI] [PubMed] [Google Scholar]
- 18.Laughlin M H, Woodman C R, Schrage W G, Gute D, Price E M. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol. 2004;96(1):233–244. doi: 10.1152/japplphysiol.00105.2003. [DOI] [PubMed] [Google Scholar]
- 19.Demaison L, Blet J, Sergiel J P, Gregoire S, Argaud D. Effect of dietary polyunsaturated fatty acids on contractile function of hearts isolated from sedentary and trained rats. Reprod Nutr Dev. 2000;40(2):113–125. doi: 10.1051/rnd:2000124. [DOI] [PubMed] [Google Scholar]
- 20.Gute D, Laughlin M H, Amann J F. Regional changes in capillary supply in skeletal muscle of interval-sprint and low-intensity, endurance-trained rats. Microcirculation. 1994;1(3):183–193. doi: 10.3109/10739689409148273. [DOI] [PubMed] [Google Scholar]
- 21.Sene-Fiorese M, Duarte F O, Scarmagnani F RR. et al. Efficiency of intermittent exercise on adiposity and fatty liver in rats fed with high-fat diet. Obesity (Silver Spring) 2008;16(10):2217–2222. doi: 10.1038/oby.2008.339. [DOI] [PubMed] [Google Scholar]
- 22.Ravi Kiran T, Subramanyam M VV, Prathima S, Asha Devi S. Blood lipid profile and myocardial superoxide dismutase in swim-trained young and middle-aged rats: comparison between left and right ventricular adaptations to oxidative stress. J Comp Physiol B. 2006;176(8):749–762. doi: 10.1007/s00360-006-0096-5. [DOI] [PubMed] [Google Scholar]
- 23.Schaible T F, Scheuer J. Effects of physical training by running or swimming on ventricular performance of rat hearts. J Appl Physiol. 1979;46(4):854–860. doi: 10.1152/jappl.1979.46.4.854. [DOI] [PubMed] [Google Scholar]
- 24.de Waard M C, van Haperen R, Soullié T, Tempel D, de Crom R, Duncker D J. Beneficial effects of exercise training after myocardial infarction require full eNOS expression. J Mol Cell Cardiol. 2010;48(6):1041–1049. doi: 10.1016/j.yjmcc.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Soares E R, Lima W G, Machado R P. et al. Cardiac and renal effects induced by different exercise workloads in renovascular hypertensive rats. Braz J Med Biol Res. 2011;44(6):573–582. doi: 10.1590/s0100-879x2011007500049. [DOI] [PubMed] [Google Scholar]
- 26.Fenning A, Harrison G, Dwyer D, Rose'Meyer R, Brown L. Cardiac adaptation to endurance exercise in rats. Mol Cell Biochem. 2003;251(1–2):51–59. doi: 10.1007/978-1-4419-9238-3_8. [DOI] [PubMed] [Google Scholar]
- 27.Benito B, Gay-Jordi G, Serrano-Mollar A. et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011;123(1):13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 28.Kannangara T S, Lucero M J, Gil-Mohapel J. et al. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging. 2011;32(12):2279–2286. doi: 10.1016/j.neurobiolaging.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagita S, Amemiya S, Suzuki S, Kita I. Effects of spontaneous and forced running on activation of hypothalamic corticotropin-releasing hormone neurons in rats. Life Sci. 2007;80(4):356–363. doi: 10.1016/j.lfs.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Høydal M A, Wisløff U, Kemi O J. et al. Nitric oxide synthase type-1 modulates cardiomyocyte contractility and calcium handling: association with low intrinsic aerobic capacity. Eur J Cardiovasc Prev Rehabil. 2007;14(2):319–325. doi: 10.1097/hjr.0b013e3280128bef. [DOI] [PubMed] [Google Scholar]
- 31.Baptista S, Piloto N, Reis F. et al. Treadmill running and swimming imposes distinct cardiovascular physiological adaptations in the rat: focus on serotonergic and sympathetic nervous systems modulation. Acta Physiol Hung. 2008;95(4):365–381. doi: 10.1556/APhysiol.2008.0002. [DOI] [PubMed] [Google Scholar]
- 32.Lavie C J, Thomas R J, Squires R W, Allison T G, Milani R V. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84(4):373–383. doi: 10.1016/S0025-6196(11)60548-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yung L M, Laher I, Yao X, Chen Z Y, Huang Y, Leung F P. Exercise, vascular wall and cardiovascular diseases: an update (part 2) Sports Med. 2009;39(1):45–63. doi: 10.2165/00007256-200939010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Leung F P, Yung L M, Laher I, Yao X, Chen Z Y, Huang Y. Exercise, vascular wall and cardiovascular diseases: an update (Part 1) Sports Med. 2008;38(12):1009–1024. doi: 10.2165/00007256-200838120-00005. [DOI] [PubMed] [Google Scholar]
- 35.Swain D P, Franklin B A. Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am J Cardiol. 2006;97(1):141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 36.Moien-Afshari F, Ghosh S, Elmi S. et al. Exercise restores coronary vascular function independent of myogenic tone or hyperglycemic status in db/db mice. Am J Physiol Heart Circ Physiol. 2008;295(4):H1470–H1480. doi: 10.1152/ajpheart.00016.2008. [DOI] [PubMed] [Google Scholar]
- 37.Husain K. Physical conditioning modulates rat cardiac vascular endothelial growth factor gene expression in nitric oxide-deficient hypertension. Biochem Biophys Res Commun. 2004;320(4):1169–1174. doi: 10.1016/j.bbrc.2004.06.058. [DOI] [PubMed] [Google Scholar]
- 38.Kemi O J, Haram P M, Wisløff U, Ellingsen Ø. Aerobic fitness is associated with cardiomyocyte contractile capacity and endothelial function in exercise training and detraining. Circulation. 2004;109(23):2897–2904. doi: 10.1161/01.CIR.0000129308.04757.72. [DOI] [PubMed] [Google Scholar]
- 39.O'Brien D W. The effect of prolonged physical training and high fat diet on heart size and body weight in rats. Can J Physiol Pharmacol. 1981;59(3):268–272. doi: 10.1139/y81-042. [DOI] [PubMed] [Google Scholar]
- 40.Becker L K, Santos R AS, Campagnole-Santos M J. Cardiovascular effects of angiotensin II and angiotensin-(1-7) at the RVLM of trained normotensive rats. Brain Res. 2005;1040(1–2):121–128. doi: 10.1016/j.brainres.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 41.Haram P M, Kemi O J, Lee S J. et al. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009;81(4):723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei J Y, Li Y, Ragland J. Effect of exercise training on resting blood pressure and heart rate in adult and aged rats. J Gerontol. 1987;42(1):11–16. doi: 10.1093/geronj/42.1.11. [DOI] [PubMed] [Google Scholar]
- 43.Yan Z C, Liu D Y, Zhang L L. et al. Exercise reduces adipose tissue via cannabinoid receptor type 1 which is regulated by peroxisome proliferator-activated receptor-delta. Biochem Biophys Res Commun. 2007;354(2):427–433. doi: 10.1016/j.bbrc.2006.12.213. [DOI] [PubMed] [Google Scholar]
- 44.Donato A J, Lesniewski L A, Delp M D. The effects of aging and exercise training on endothelin-1 vasoconstrictor responses in rat skeletal muscle arterioles. Cardiovasc Res. 2005;66(2):393–401. doi: 10.1016/j.cardiores.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Higa-Taniguchi K T, Silva F CP, Silva H MV, Michelini L C, Stern J E. Exercise training-induced remodeling of paraventricular nucleus (nor)adrenergic innervation in normotensive and hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(4):R1717–R1727. doi: 10.1152/ajpregu.00613.2006. [DOI] [PubMed] [Google Scholar]
- 46.Jackson K, Silva H MV, Zhang W, Michelini L C, Stern J E. Exercise training differentially affects intrinsic excitability of autonomic and neuroendocrine neurons in the hypothalamic paraventricular nucleus. J Neurophysiol. 2005;94(5):3211–3220. doi: 10.1152/jn.00277.2005. [DOI] [PubMed] [Google Scholar]
- 47.Schweitzer N B, Alessio H M, Hagerman A E. et al. Access to exercise and its relation to cardiovascular health and gene expression in laboratory animals. Life Sci. 2005;77(18):2246–2261. doi: 10.1016/j.lfs.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 48.Ray C J, Marshall J M. Elucidation in the rat of the role of adenosine and A2A-receptors in the hyperaemia of twitch and tetanic contractions. J Physiol. 2009;587(Pt 7):1565–1578. doi: 10.1113/jphysiol.2008.163683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quiles J L, Huertas J R, Mañas M, Battino M, Mataix J. Physical exercise affects the lipid profile of mitochondrial membranes in rats fed with virgin olive oil or sunflower oil. Br J Nutr. 1999;81(1):21–24. [PubMed] [Google Scholar]
- 50.Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. J Am Coll Cardiol. 2004;44(6):1320–1327. doi: 10.1016/j.jacc.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 51.Werner C, Fürster T, Widmann T. et al. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120(24):2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. [DOI] [PubMed] [Google Scholar]
- 52.Jansakul C. Effect of swimming on vascular reactivity to phenylephrine and KC1 in male rats. Br J Pharmacol. 1995;115(4):587–594. doi: 10.1111/j.1476-5381.1995.tb14972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitani Y, Maruyama J, Maruyama K, Sakurai M. Exercise training does not alter acetylcholine-induced responses in isolated pulmonary artery from rat. Eur Respir J. 1999;13(3):622–625. doi: 10.1183/09031936.99.13362299. [DOI] [PubMed] [Google Scholar]
- 54.Elkouri S, Demers P, Sirois M G, Couturier A, Cartier R. Effect of chronic exercise and angiotensin-converting enzyme inhibition on rodent thoracic aorta. J Cardiovasc Pharmacol. 2004;44(5):582–590. doi: 10.1097/00005344-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 55.Donato A J, Lesniewski L A, Delp M D. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol. 2007;579(Pt 1):115–125. doi: 10.1113/jphysiol.2006.120055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding Y-H, Li J, Zhou Y, Rafols J A, Clark J C, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3(1):15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- 57.Mac Gabhann F, Ji J W, Popel A S. VEGF gradients, receptor activation, and sprout guidance in resting and exercising skeletal muscle. J Appl Physiol. 2007;102(2):722–734. doi: 10.1152/japplphysiol.00800.2006. [DOI] [PubMed] [Google Scholar]
- 58.Lash J M, Sherman W M, Betts J J, Hamlin R L. Training-induced vascular and metabolic adaptations in normo(11 week)- and hyper(18 week)-glycemic obese Zucker rats. Int J Obes. 1989;13(6):777–789. [PubMed] [Google Scholar]
- 59.Konhilas J P, Maass A H, Luckey S W, Stauffer B L, Olson E N, Leinwand L A. Sex modifies exercise and cardiac adaptation in mice. Am J Physiol Heart Circ Physiol. 2004;287(6):H2768–H2776. doi: 10.1152/ajpheart.00292.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nylander E, Sigvardsson K, Kilbom A. Training-induced bradycardia and intrinsic heart rate in rats. Eur J Appl Physiol Occup Physiol. 1982;48(2):189–199. doi: 10.1007/BF00422980. [DOI] [PubMed] [Google Scholar]
- 61.Böhm M, Dorner H, Htun P, Lensche H, Platt D, Erdmann E. Effects of exercise on myocardial adenylate cyclase and Gi alpha expression in senescence. Am J Physiol. 1993;264(3, Pt 2):H805–H814. doi: 10.1152/ajpheart.1993.264.3.H805. [DOI] [PubMed] [Google Scholar]
- 62.Ljungqvist A, Unge G. Capillary proliferative activity in myocardium and skeletal muscle of exercised rats. J Appl Physiol. 1977;43(2):306–307. doi: 10.1152/jappl.1977.43.2.306. [DOI] [PubMed] [Google Scholar]
- 63.Borensztajn J, Rone M S, Babirak S P, McGarr J A, Oscai L B. Effect of exercise on lipoprotein lipase activity in rat heart and skeletal muscle. Am J Physiol. 1975;229(2):394–397. doi: 10.1152/ajplegacy.1975.229.2.394. [DOI] [PubMed] [Google Scholar]
- 64.Melo R M, Martinho E Jr, Michelini L C. Training-induced, pressure-lowering effect in SHR: wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension. 2003;42(4):851–857. doi: 10.1161/01.HYP.0000086201.27420.33. [DOI] [PubMed] [Google Scholar]
- 65.Negrão C E, Moreira E D, Brum P C, Denadai M L, Krieger E M. Vagal and sympathetic control of heart rate during exercise by sedentary and exercise-trained rats. Braz J Med Biol Res. 1992;25(10):1045–1052. [PubMed] [Google Scholar]
- 66.Husain K. Interaction of regular exercise and chronic nitroglycerin treatment on blood pressure and rat aortic antioxidants. Biochim Biophys Acta. 2004;1688(1):18–25. doi: 10.1016/j.bbadis.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Siu P M, Bryner R W, Martyn J K, Alway S E. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J. 2004;18(10):1150–1152. doi: 10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]
- 68.Judge S, Jang Y M, Smith A. et al. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 69.Iemitsu M, Maeda S, Otsuki T, Goto K, Miyauchi T. Time course alterations of myocardial endothelin-1 production during the formation of exercise training-induced cardiac hypertrophy. Exp Biol Med (Maywood) 2006;231(6):871–875. [PubMed] [Google Scholar]
- 70.Choi S-Y, Chang H-J, Choi S-I. et al. Long-term exercise training attenuates age-related diastolic dysfunction: association of myocardial collagen cross-linking. J Korean Med Sci. 2009;24(1):32–39. doi: 10.3346/jkms.2009.24.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tibbits G, Koziol B J, Roberts N K, Baldwin K M, Barnard R J. Adaptation of the rat myocardium to endurance training. J Appl Physiol. 1978;44(1):85–89. doi: 10.1152/jappl.1978.44.1.85. [DOI] [PubMed] [Google Scholar]
- 72.Advani S V, Geenen D, Malhotra A, Factor S M, Scheuer J. Swimming causes myosin adaptations in the rat cardiac isograft. Circ Res. 1990;67(3):780–783. doi: 10.1161/01.res.67.3.780. [DOI] [PubMed] [Google Scholar]
- 73.Wei J Y, Li Y, Lincoln T, Grossman W, Mendelowitz D. Chronic exercise training protects aged cardiac muscle against hypoxia. J Clin Invest. 1989;83(3):778–784. doi: 10.1172/JCI113957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacIntosh A M, Baldwin K M, Herrick R E, Mullin W M. Effects of training on biochemical and functional properties of rodent neonatal heart. J Appl Physiol. 1985;59(5):1440–1445. doi: 10.1152/jappl.1985.59.5.1440. [DOI] [PubMed] [Google Scholar]
- 75.Musch T I, Terrell J A, Hilty M R. Effects of high-intensity sprint training on skeletal muscle blood flow in rats. J Appl Physiol. 1991;71(4):1387–1395. doi: 10.1152/jappl.1991.71.4.1387. [DOI] [PubMed] [Google Scholar]
- 76.Penpargkul S, Scheuer J. The effect of physical training upon the mechanical and metabolic performance of the rat heart. J Clin Invest. 1970;49(10):1859–1868. doi: 10.1172/JCI106404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schaible T F, Penpargkul S, Scheuer J. Cardiac responses to exercise training in male and female rats. J Appl Physiol. 1981;50(1):112–117. doi: 10.1152/jappl.1981.50.1.112. [DOI] [PubMed] [Google Scholar]
- 78.Hasser E M, Dowell R T, Haithcoat J L. Hemodynamic responses to methoxamine in exercise-conditioned and aorta-constricted rats. J Appl Physiol. 1982;52(4):967–975. doi: 10.1152/jappl.1982.52.4.967. [DOI] [PubMed] [Google Scholar]
- 79.Kivelä R, Silvennoinen M, Lehti M, Jalava S, Vihko V, Kainulainen H. Exercise-induced expression of angiogenic growth factors in skeletal muscle and in capillaries of healthy and diabetic mice. Cardiovasc Diabetol. 2008;7:13. doi: 10.1186/1475-2840-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


