Abstract
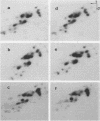
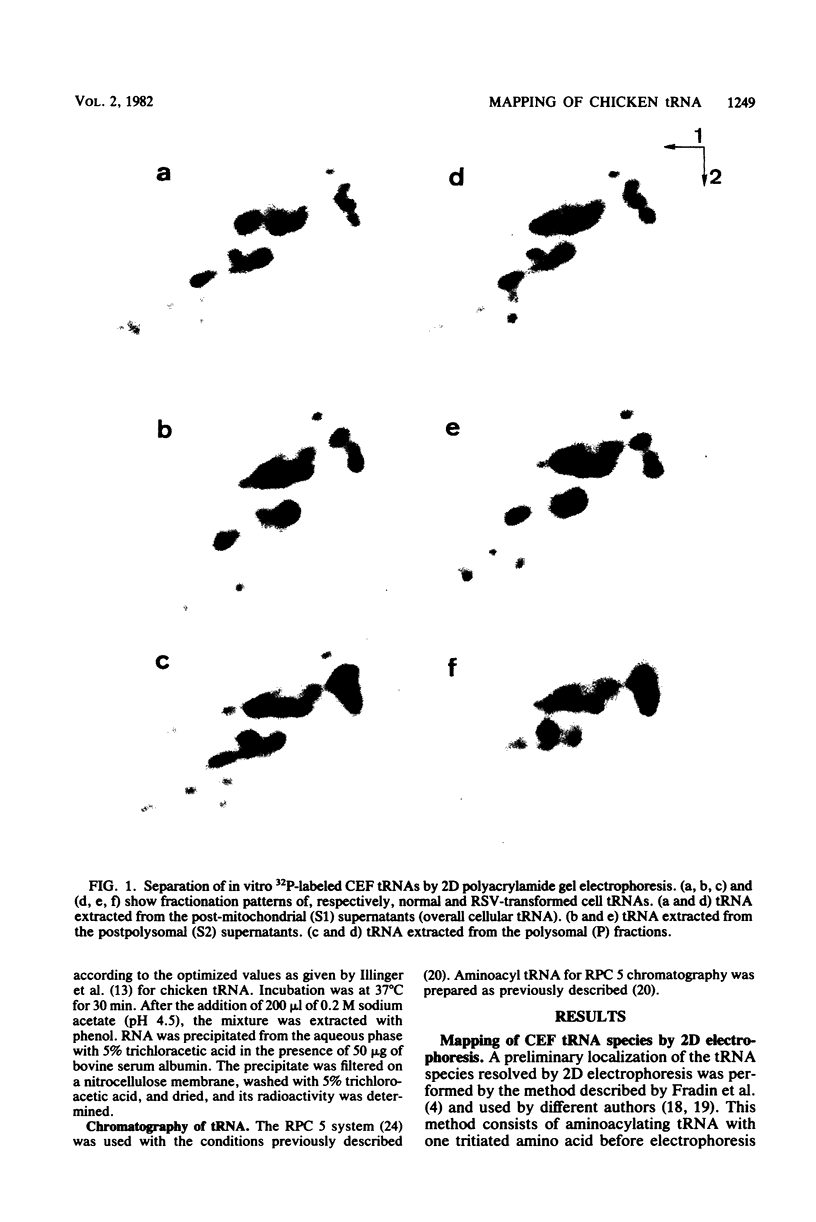
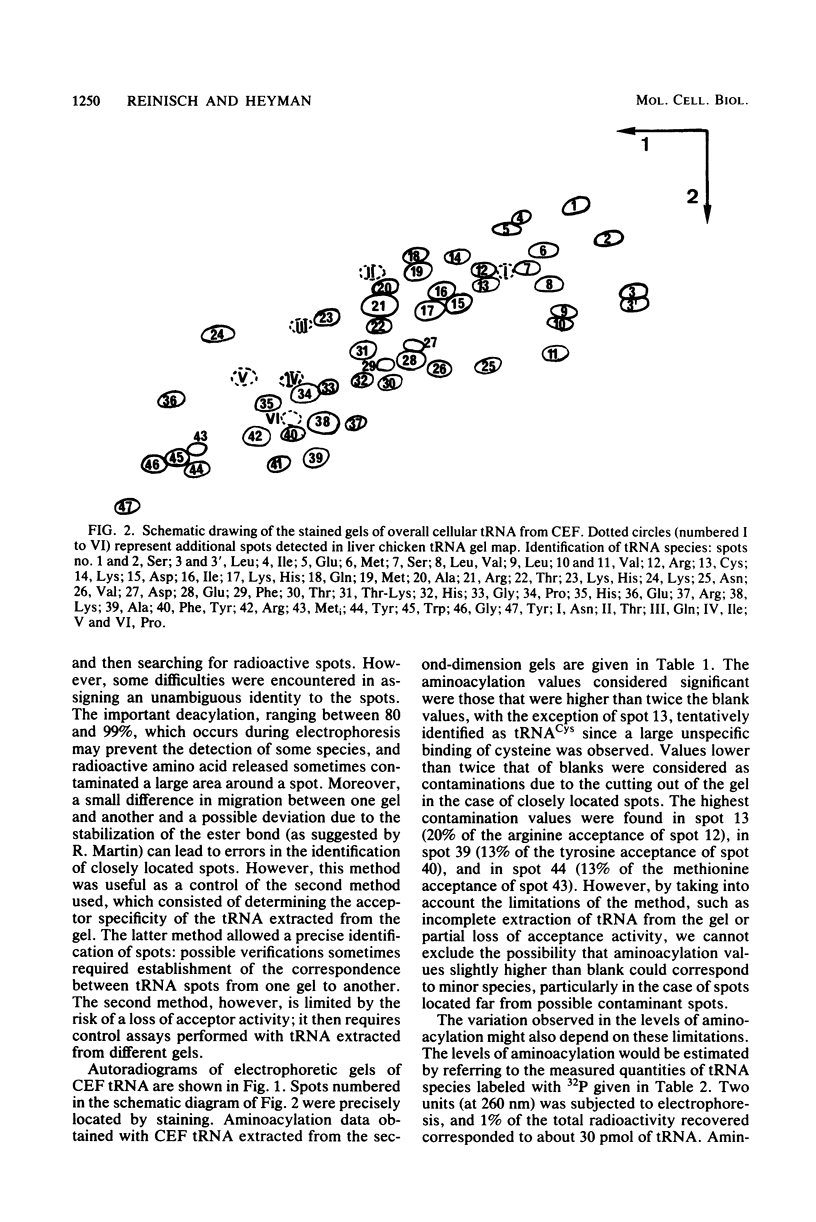
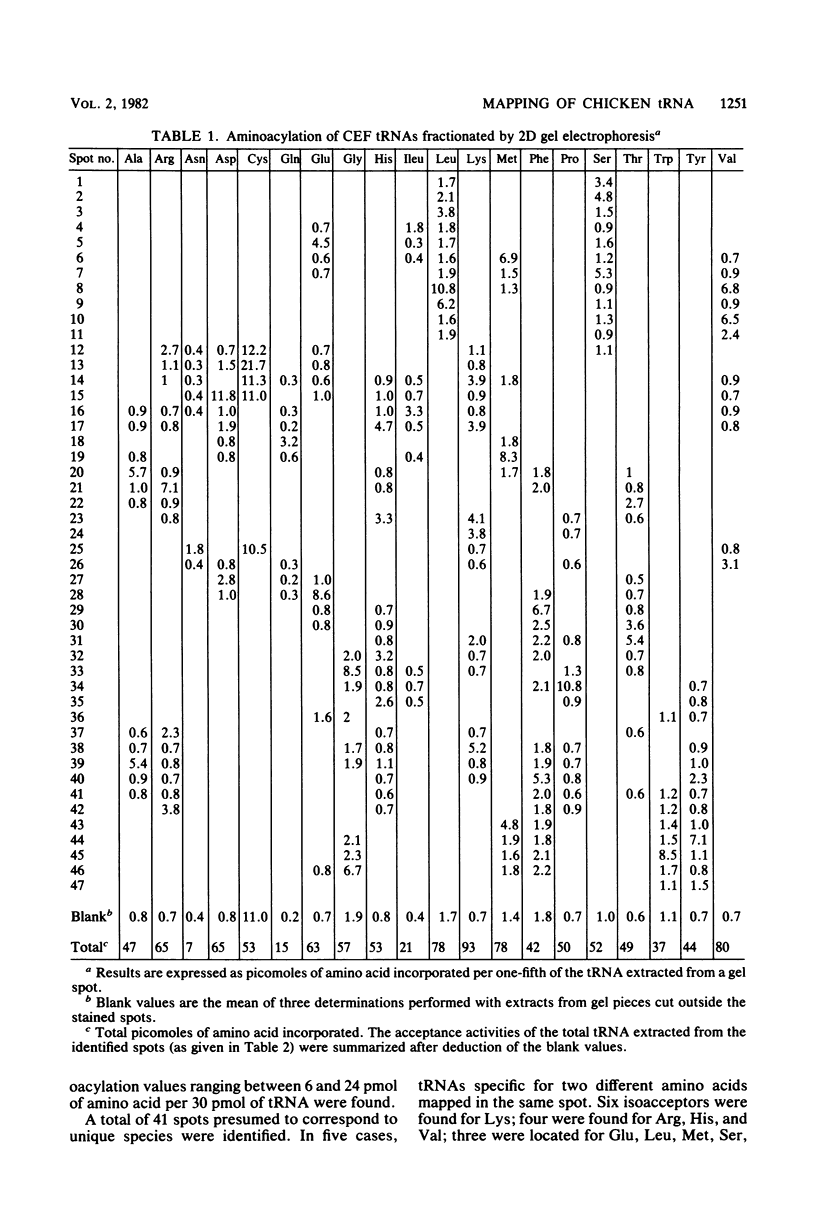
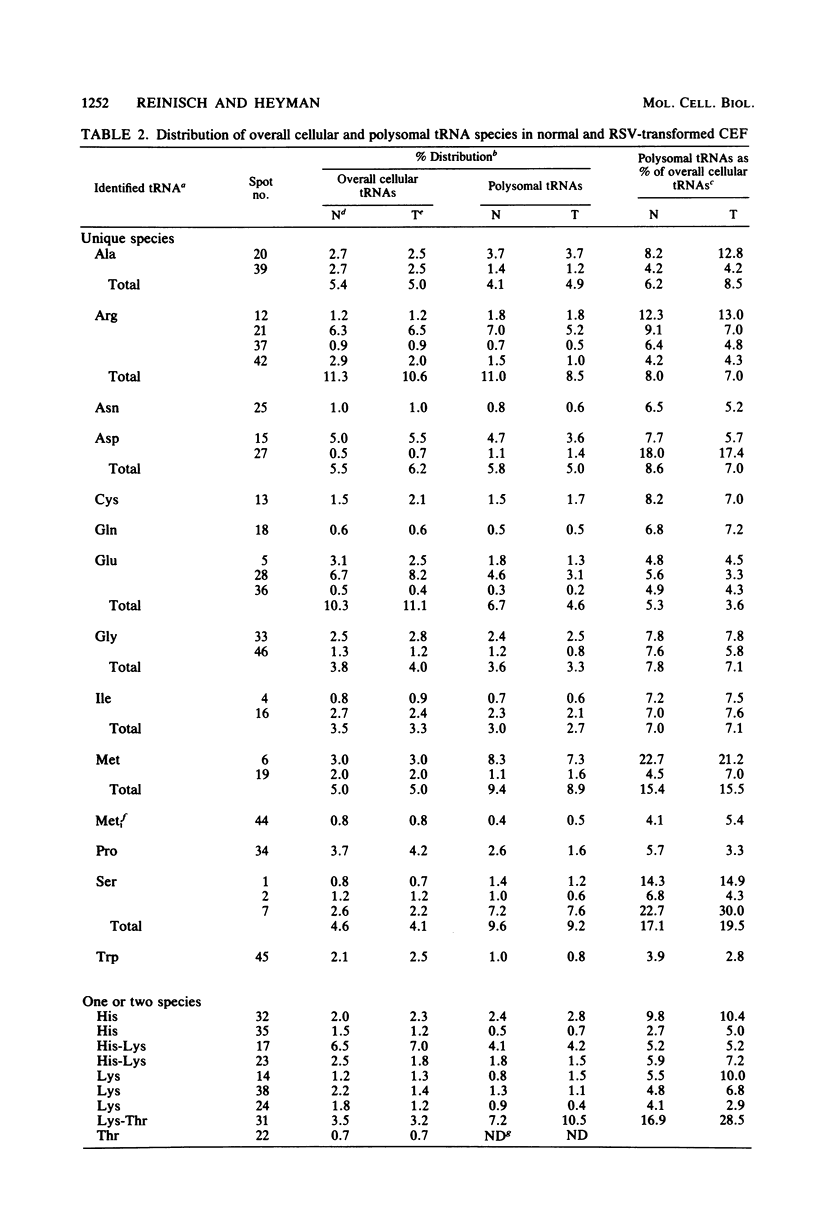
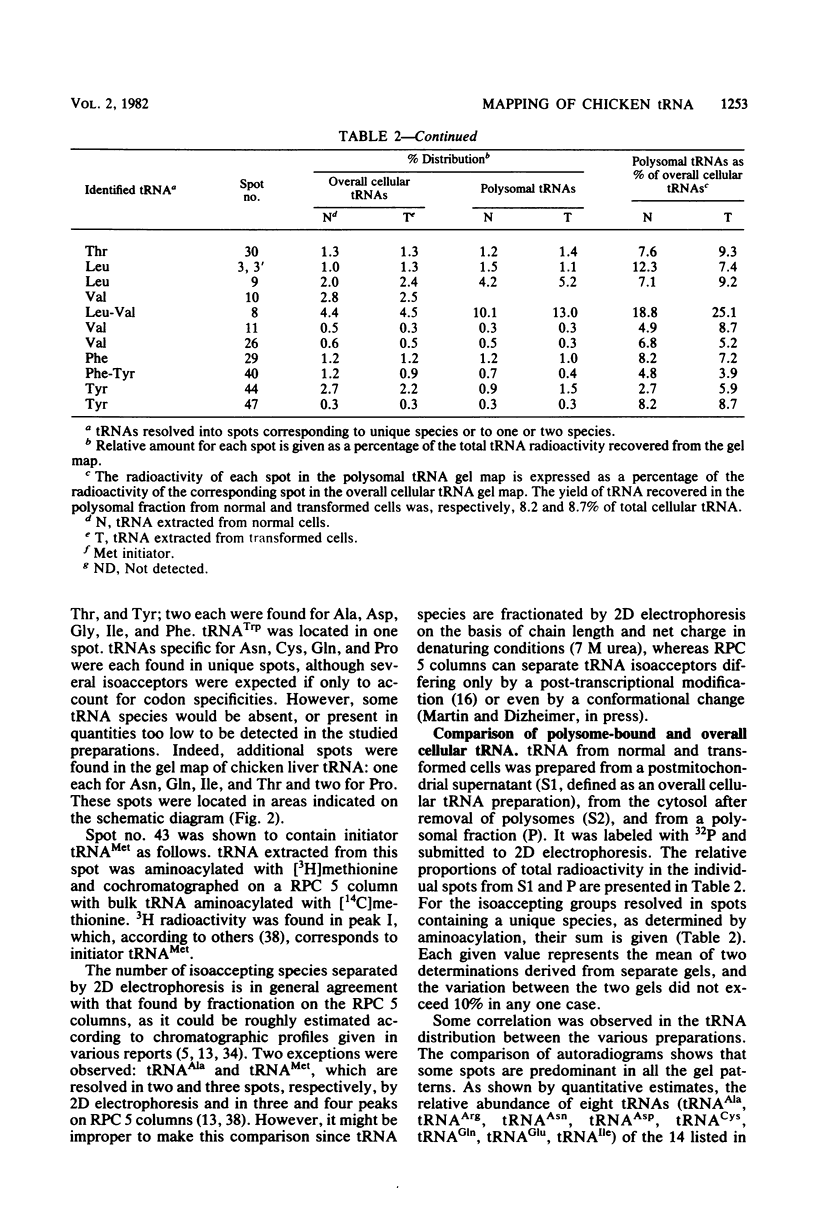
Analysis of the tRNA population from chicken cells was performed by means of polyacrylamide gel mapping. About 60 species were detected; most of these were positively identified by their acceptor specificity. The comparison of polysome-bound and overall cellular tRNA gel patterns from normal and Rous sarcoma virus-infected chicken embryo fibroblasts led us to the following observations: some tRNA species were present in the same relative proportions in all the preparations, and within isoaccepting groups the same species was preponderant; however, although about 8% of whole-cell tRNA was recovered in polysomal preparations, amounts ranging from 3 to 30% were found for individual tRNA species. This points to the absence of a direct correlation between the amount of each mature tRNA species produced and the frequency with which it is used in this case of embryonic cells. No significant difference was observed between the whole-cell tRNA patterns from normal and infected cells. Thus, tRNA transcription appears unaltered when cells are transformed and virus producing. No change was observed in the extent of a post-transcriptional modification of tRNAPhe (the base Y). However, viral infection led to some changes in the relative proportions of individual species from polysomal preparations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustyniak H., Pawełkiewicz J. Preferential binding of isoaccepting species of tRNALys and tRNAIle from lupin cotyledons to polyribosomes. Biochim Biophys Acta. 1979 Nov 22;565(1):148–153. doi: 10.1016/0005-2787(79)90090-x. [DOI] [PubMed] [Google Scholar]
- Chevallier A., Garel J. P. Studies on tRNA adaptation, tRNA turnover, precursor tRNA and tRNA gene distribution in Bombyx mori by using two-dimensional polyacrylamide gel electrophoresis. Biochimie. 1979;61(2):245–262. doi: 10.1016/s0300-9084(79)80070-x. [DOI] [PubMed] [Google Scholar]
- Dingermann T., Pistel F., Kersten H. Functional role of ribosylthymine in transfer RNA. Preferential utilization of tRNAs containing ribosylthymine instead of uridine at position 54 in protein synthesis of Dictyostelium discoideum. Eur J Biochem. 1980 Feb;104(1):33–40. doi: 10.1111/j.1432-1033.1980.tb04396.x. [DOI] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Gallagher R. E., Gallo R. C. Chromatographic analyses of isoaccepting tRNAs from avian myeloblastosis virus. J Virol. 1973 Sep;12(3):449–457. doi: 10.1128/jvi.12.3.449-457.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel J. P. Functional adaptation of tRNA population. J Theor Biol. 1974 Jan;43(1):211–225. doi: 10.1016/s0022-5193(74)80054-8. [DOI] [PubMed] [Google Scholar]
- Garel J. P., Garber R. L., Siddiqui M. A. Transfer RNA in posterior silk gland of Bombyx mori: polyacrylamide gel mapping of mature transfer RNA, identification and partial structural characterization of major isoacceptor species. Biochemistry. 1977 Aug 9;16(16):3618–3624. doi: 10.1021/bi00635a018. [DOI] [PubMed] [Google Scholar]
- Gielkens A. L., Berns T. J., Bloemendal H. An efficient procedure for the isolation of polyribosomes from tissue culture. Eur J Biochem. 1971 Oct 26;22(4):478–484. doi: 10.1111/j.1432-1033.1971.tb01566.x. [DOI] [PubMed] [Google Scholar]
- Grunberger D., Weinstein I. B., Mushinski J. F. Deficiency of the Y base in a hepatoma phenylalanine tRNA. Nature. 1975 Jan 3;253(5486):66–67. doi: 10.1038/253066a0. [DOI] [PubMed] [Google Scholar]
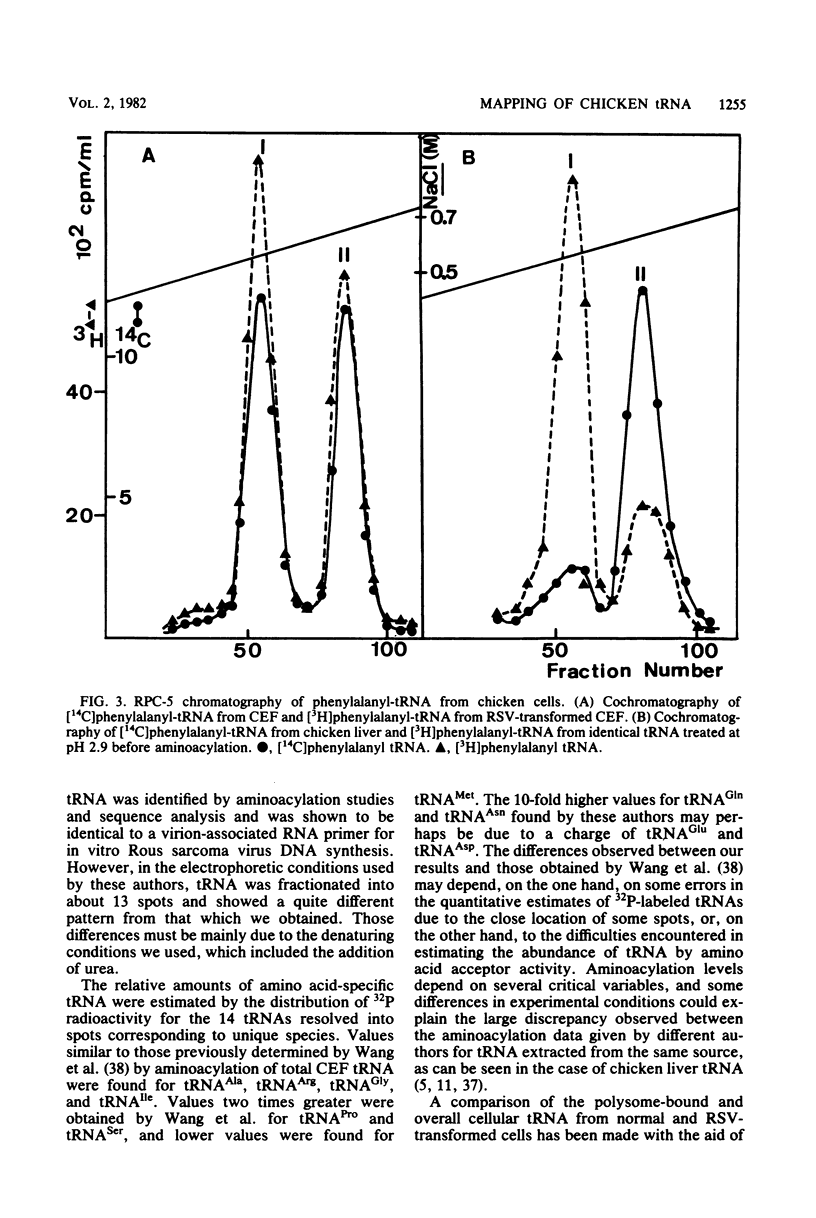
- Harada F., Sawyer R. C., Dahlberg J. E. A primer ribonucleic acid for initiation of in vitro Rous sarcarcoma virus deoxyribonucleic acid synthesis. J Biol Chem. 1975 May 10;250(9):3487–3497. [PubMed] [Google Scholar]
- Holmes W. M., Goldman E., Miner T. A., Hatfield G. W. Differential utilization of leucyl-tRNAs by Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1393–1397. doi: 10.1073/pnas.74.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illinger D., Le Meur M. A., Gerlinger P., Ebel J. P. Essai de mise en évidence d'un rôle régulateur des tRNA dans la synthése des protéines de l'oeuf de poule. I. Etude comparative des acides ribonucléiques de transfert extraits du foie et de l'oviducte de poule en ponte. Biochimie. 1974;56(4):529–536. doi: 10.1016/s0300-9084(74)80069-6. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Isoaccepting species differences between polysome-bound and total cellular tRNA in SVT2 cells. Biochim Biophys Acta. 1975 Nov 4;407(4):399–406. doi: 10.1016/0005-2787(75)90292-0. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Relation of cell type and cell density to the degree of post-transcriptional modification of tRNALys and tRNAPhe. Biochim Biophys Acta. 1975 Nov 4;407(4):392–398. doi: 10.1016/0005-2787(75)90291-9. [DOI] [PubMed] [Google Scholar]
- Keith G., Rogg H., Dirheimer G., Menichi B., Heyham T. Post-transcriptional modification of tyrosine tRNA as a function of growth in Bacillus subtilis. FEBS Lett. 1976 Jan 15;61(2):120–123. doi: 10.1016/0014-5793(76)81017-4. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Arpin M., Reboud J. P. A simple water-colled apparatus for two-dimensional gel electrophoresis. Anal Biochem. 1977 Nov;83(1):304–310. doi: 10.1016/0003-2697(77)90539-5. [DOI] [PubMed] [Google Scholar]
- Martin R. P., Schneller J. M., Stahl A. J., Dirheimer G. Study of yeast mitochondrial tRNAs by two-dimensional polyacrylamide gel electrophoresis: characterization of isoaccepting species and search for imported cytoplasmic tRNAs. Nucleic Acids Res. 1977 Oct;4(10):3497–3510. doi: 10.1093/nar/4.10.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazabraud A., Garel J. P. Analysis of tRNA population from Drosophila melanogaster by means of polyacrylamide gel mapping. FEBS Lett. 1979 Sep 1;105(1):70–76. doi: 10.1016/0014-5793(79)80889-3. [DOI] [PubMed] [Google Scholar]
- Menichi B., Heyman T. Study of tyrosine transfer ribonucleic acid modification in relation to sporulation in Bacillus subtilis. J Bacteriol. 1976 Jul;127(1):268–280. doi: 10.1128/jb.127.1.268-280.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerjee H., Goldfeder A. Transfer RNA species in tumors of different growth rates. Cancer Res. 1976 Sep;36(9 PT1):3330–3338. [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Osterman L. A. Participation of tRNA in regulation of protein biosynthesis at the translational level in eukaryotes. Biochimie. 1979;61(3):323–342. doi: 10.1016/s0300-9084(79)80126-1. [DOI] [PubMed] [Google Scholar]
- Pearson R. L., Weiss J. F., Kelmers A. D. Improved separation of transfer RNA's on polychlorotrifuoroethylene-supported reversed-phase chromatography columns. Biochim Biophys Acta. 1971 Feb 11;228(3):770–774. doi: 10.1016/0005-2787(71)90748-9. [DOI] [PubMed] [Google Scholar]
- Raba M., Limburg K., Burghagen M., Katze J. R., Simsek M., Heckman J. E., Rajbhandary U. L., Gross H. J. Nucleotide sequence of three isoaccepting lysine tRNAs from rabbit liver and SV40-transformed mouse fibroblasts. Eur J Biochem. 1979 Jun;97(1):305–318. doi: 10.1111/j.1432-1033.1979.tb13115.x. [DOI] [PubMed] [Google Scholar]
- Roe B. A., Tsen H. Y. Role of ribothymidine in mammalian tRNAPhe. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3696–3700. doi: 10.1073/pnas.74.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Hanafusa H. Comparison of the small RNAs of polymerase-deficient and polymerase-positive Rous sarcoma virus and another species of avian retrovirus. J Virol. 1979 Mar;29(3):863–871. doi: 10.1128/jvi.29.3.863-871.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer R. C., Harada F., Dahlberg J. E. Virion-associated RNA primer for Rous sarcoma virus DNA synthesis: isolation from uninfected cells. J Virol. 1974 Jun;13(6):1302–1311. doi: 10.1128/jvi.13.6.1302-1311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Sehulster L. M., Varricchio F., Raska K Jun Synthesis of transfer ribonucleic acid in KB cells infected with adenovirus type 2. J Gen Virol. 1978 Jul;40(1):183–194. doi: 10.1099/0022-1317-40-1-183. [DOI] [PubMed] [Google Scholar]
- Sekiya T., Oda K. I. The altered patterns of transfer RNA in SV40-infected and transformed cells. Virology. 1972 Jan;47(1):168–180. doi: 10.1016/0042-6822(72)90250-4. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. The use of nuclease P1 in sequence analysis of end group labeled RNA. Nucleic Acids Res. 1977 Dec;4(12):4091–4108. doi: 10.1093/nar/4.12.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Kano-Sueoka T. Transfer RNA and cell differentiation. Prog Nucleic Acid Res Mol Biol. 1970;10:23–55. doi: 10.1016/s0079-6603(08)60560-7. [DOI] [PubMed] [Google Scholar]
- Taylor M. W., Wang S., Kothari R. M., Hung P. P. Chromatographic analyses of isoaccepting tRNAs from avian tumor viruses. J Virol. 1974 Nov;14(5):1092–1098. doi: 10.1128/jvi.14.5.1092-1098.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebe R., Zachau H. G. A specific modification next to the anticodon of phenylalanine transfer ribonucleic acid. Eur J Biochem. 1968 Sep 24;5(4):546–555. doi: 10.1111/j.1432-1033.1968.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Tockman J., Vold B. S. In vivo aminoacylation of transfer ribonucleic acid in Bacillus subtilis and evidence for differential utilization of lysine-isoaccepting transfer ribonucleic acid species. J Bacteriol. 1977 Jun;130(3):1091–1097. doi: 10.1128/jb.130.3.1091-1097.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Kothari R. M., Taylor M. W., Hung P. P. Selective incorporation of host cell methionyl-transfer RNA by RNA tumor viruses. Biochim Biophys Acta. 1974 Feb 27;340(1):52–63. doi: 10.1016/0005-2787(74)90173-7. [DOI] [PubMed] [Google Scholar]
- Wang S., Kothari R. M., Taylor M., Hung P. Transfer RNA activities of Rous sarcoma and Rous associated viruses. Nat New Biol. 1973 Apr 4;242(118):133–135. doi: 10.1038/newbio242133a0. [DOI] [PubMed] [Google Scholar]