SUMMARY
Subtle random deviations from perfect symmetry in bilateral traits are suggested to signal reduced phenotypic and genetic quality of a sender, but little is known about the related receiver mechanisms for discriminating symmetrical from asymmetrical traits. Here, we investigated these mechanisms in behavioural and neurophysiological experiments in the Mediterranean field cricket, Gryllus bimaculatus. A downward frequency modulation at the end of each syllable in the calling song has been suggested to indicate morphological asymmetry in sound radiating structures between left and right forewings. Even under ideal laboratory conditions on a trackball system, female crickets only discriminated between songs of symmetrical and asymmetrical males in two-choice experiments at carrier frequencies of 4.4 kHz and a large modulation depth of 600 and 800 Hz. Under these conditions they preferred the pure-tone calling songs over the modulated (asymmetrical) alternative, whereas no preference was observed at carrier frequencies of 4.9 and 5.2 kHz. These preferences correlate well with the responses of a pair of identified auditory interneurons (AN1), known for their importance in female phonotaxis. The AN1 interneuron is tuned to an average frequency of 4.9 kHz, and the roll-off towards lower and higher frequencies determines the magnitude of responses to pure-tone and frequency-modulated calling songs. The difference in response magnitude between the two neurons appears to drive the decision of females towards the song alternatives. We discuss the relevance of song differences based on asymmetry in the morphology of song-producing structures under natural conditions.
KEY WORDS: symmetry, female choice, sensory processing, cricket, acoustic communication, decision making, phonotaxis
INTRODUCTION
The role of fluctuating asymmetry in sexual selection is much debated. After Møller suggested that small deviations from bilateral symmetry in male sexual ornaments may reliably reveal male quality (Møller, 1990), and the discovery that these differences might predict fitness (Watson and Thornhill, 1994; Møller and Swaddle, 1997; Rantala et al., 2004), the evidence for an actual preference for symmetrical traits was ambiguous (Møller, 1992; Møller, 1993; Grammer and Thornhill, 1994; Oakes and Barnard, 1994; Swaddle and Cuthill, 1995; Kowner, 1996). Swaddle argued in his review on the perceptual processes in visual signalling by asymmetry that fluctuating asymmetry turned out to be an effective signal in most studies in which asymmetry had been manipulated independently of other confounding factors (Swaddle, 1999). However, the experimentally tested asymmetries were much larger than those observed in natural populations, often only in the range of 1% of the trait size. In order to act as a useful signal, the degree of fluctuating asymmetry must be detected and discriminated by receivers under natural conditions. However, the proximate neural mechanisms to detect such small deviations from perfect symmetry are largely unknown.
Whereas the overwhelming number of studies on fluctuating asymmetry deal with visual traits, Simmons and Ritchie reported a case study on asymmetry in the song of field crickets, Gryllus campestris (Simmons and Ritchie, 1996). Male crickets produce an almost pure-tone calling song to attract females from some distance and to repel rivals (reviewed by Gerhardt and Huber, 2002). Sound is produced with modified forewings (tegmina), and an area of the tegmina (harp) is set into vibration at its resonant frequency when the plectrum of the left tegmen acts against the file of the right one (Elliott and Koch, 1985; Bennet-Clark, 1989; Montealegre-Z et al., 2011). Carrier frequency (fc) could be a reliable indicator of male size as the size of the harp correlates with overall body size and determines the fc of the calling song, with larger harps resulting in lower fc values. Indeed, Simmons and Ritchie found a negative correlation between harp size and fc of the calling song (Simmons and Ritchie, 1996). In addition they reported variation among males with respect to degrees of downward frequency modulation (FM) in the second part of each syllable, which they could correlate with asymmetric left and right harp sizes. Based on these data, they argued that these spectral properties, i.e. call fc and FM, might explain findings in natural populations of crickets where larger and more symmetrical males appear to have a mating advantage (Simmons and Zuk, 1992; Simmons, 1995; Brown et al., 1996) (but see Rodríguez-Muñoz et al., 2010; Deb et al., 2012). In a series of playback experiments, Simmons and Ritchie demonstrated a preference of females for songs of lower fc (4.25 kHz over 5 kHz) (Simmons and Ritchie, 1996). Furthermore, females also preferred downward FMs in the range 600–800 Hz, depending on the fc of the initial frequency in the first part of the syllable. The authors proposed a proximate explanation for the choice of females on the basis of the tuning of ears at 4 kHz (Nocke, 1972), which has, however, never been tested experimentally. In addition, recent data provide evidence for large individual differences in female preference [e.g. for grasshoppers (von Helversen et al., 2004) and crickets (Kostarakos et al., 2009)], requiring a more detailed analysis of female tuning than Nocke's original data.
Field crickets represent an ideal model system for such a detailed study of the proximate basis of the perception of asymmetry-associated trait variation. They posess almost pure-tone calling songs, with fc varying within populations (Simmons and Ritchie, 1996; Bennet-Clark, 1999; Ferreira and Ferguson, 2002). This fc is crucial in eliciting female phonotaxis (Thorson et al., 1982; Simmons and Ritchie, 1996; Hunt et al., 2005; Verburgt et al., 2008), and receivers are best tuned to particular fc (Nocke, 1972; Kostarakos et al., 2008). Single, identified interneurons responsible for the steering towards an acoustic stimulus during positive phonotaxis are known (Schildberger and Hörner, 1988; Atkins et al., 1992) and hence the proximate basis of such tuning can be well characterised. The tuning of an identified interneuron (ascending neuron 1, hereafter AN1 neuron) in a field cricket (Gryllus bimaculatus) correlates strongly with the preference of females in two-choice trials (Kostarakos et al., 2008), in agreement with predictions of the ‘matched filter hypothesis’ (Capranica and Moffat, 1983). The pair of AN1-interneurons can be considered the ‘hard-wired’ preference for phonotaxis (for differences in frequency and intensity, but not for pattern quality) in this insect, as it exhibits a tuning that explains about 80% of the variation in the direction and strength of directional steering in two-choice trials (Kostarakos et al., 2008). Furthermore, new technologies for the quantitative study of phonotaxis (Hedwig and Poulet, 2004; Hedwig and Poulet, 2005) guarantee constant acoustic conditions for a receiver throughout the entire simulated phonotactic approach, eliminating the influence of other acoustic parameters, such as changing sound pressure level during the phonotactic approach affecting the perception of subtle differences between two stimuli, as is the case in arena trials. This allows us to determine the perceptual thresholds for acoustic asymmetry detection at the behavioural and neuronal level, and draw conclusions about the likelihood of symmetry as a signal in acoustic signalling in field crickets.
Hypotheses about the expected outcome of phonotactic trials are based on the assumption that the perceptual processes in female receivers constitute a tuned hearing system (Kostarakos et al., 2008) (schematically summarized in Fig. 1). Depending on the steepness of the tuning, we predict (1) that females will prefer fc corresponding to the best frequency (fb) of the neural tuning and thus reject both lower and higher than average fc. For the same reason, females will (2) detect the difference between FM and non-modulated calling songs only at lower than average fc, and will prefer non-modulated calling songs.
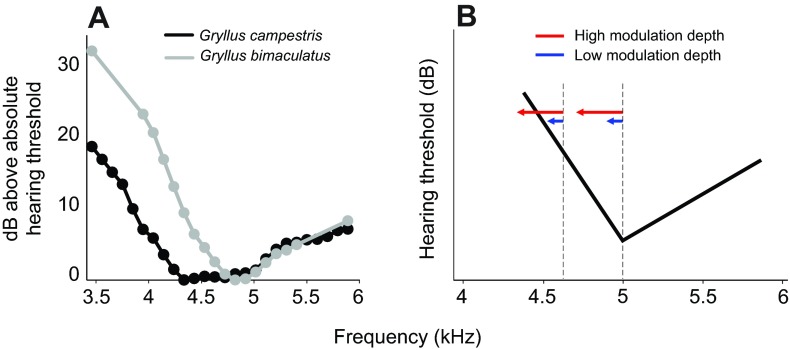
Fig. 1.
Tuning of the AN1 neuron and its implications for the hypothesis of detecting a signal trait associated with fluctuating asymmetry. (A) Comparison of the average tuning curves of AN1 in G. campestris and G. bimaculatus (modified from Kostarakos et al., 2009). (B) Schematic illustration of the effect of frequency modulation (FM) for the perceived loudness at two different carrier frequencies (fc). For further explanation see Introduction.
MATERIALS AND METHODS
Animals and phonotaxis on the trackball system
Female crickets (Gryllus bimaculatus de Geer) were taken from a colony at the Department of Zoology, University of Graz. Animals were reared at an ambient temperature of 25–31°C and a photo cycle of 12 h:12 h L:D. In the first 2 weeks after eclosion, we provided a rearing concentrate (Nekton Grillenzuchtkonzentrat, Pforzheim, Germany), and fresh water, fish food and oat flakes ad libitum. Final instars were raised individually to adulthood to maintain phonotactic responsiveness. Behavioural experiments started 1 week after the final moult.
Phonotactic behaviour was studied using a highly sensitive trackball system, which allowed measurement of the walking behaviour of females at the level of the insect stepping movement (see Hedwig and Poulet, 2004; Hedwig and Poulet, 2005). In short, tethered females stood in a normal walking position on top of a Rohacell ball (66 mm diameter, 5.0 g) floating on a moderate airstream. Phonotactically responding females moved the trackball with their legs, and an optical mouse sensor (Logitech, MX518) positioned at the south pole of the trackball recorded its movements in the forward–backward x-axis, and lateral left–right y-axis on two separated channels. The trackball data were sampled online at 10 kHz, controlled by custom-programmed software. In this way, the movement of females could be quantified with respect to steering towards one or more sound sources, its speed and the total distance covered. The advantage of this set-up is that while females moved the trackball with their legs, their body position relative to the speakers, and thus the acoustic conditions for the ears, remained constant throughout the phonotactic walking.
Acoustic stimulation
Sound stimuli were broadcast via an Edirol Firewire Audio Capture FA-101 soundcard, stereo power amplifier (NAD 214), KAY 837 attenuators and two mid-range speakers (Tonsil GTC 10/60). Signal intensity of each model song was calibrated to 80 dB re. 20 μPa at the walking position of the female cricket with a sound level meter CEL-414 and Larson Davis 2540 ½ in free-field microphone. To be sure that the frequency characteristics of the model songs were correctly broadcast to the animal, we corrected all stimuli with transfer functions due to slight non-linear speaker characteristics in the used frequency range from 3–6 kHz. Speakers were placed at a distance of 50 cm and an angle of 30 deg to either side of the longitudinal body axis of the female.
We generated male calling songs with Cool Edit Pro software (version 2.00; Syntrillium, Phoenix, AZ, USA) at a sampling rate of 48 k-samples s−1 by mimicking the temporal structure and frequency of male songs measured in natural cricket populations (Ferreira and Ferguson, 2002; Verburgt and Ferguson, 2010). We digitally generated sound pulses of a defined duration of 23 ms, constant fc and a 2 ms rise/fall time. To create a conspecific chirp, four pulses were grouped together separated by an inter-pulse interval of 16 ms, resulting in a chirp duration of 140 ms. A constant inter-chirp interval of 230 ms produced a rate of 162 chirps min−1 for each calling song. Model songs were generated with three different fc of 4.4, 4.9 and 5.2 kHz; 4.9 kHz matched the average fb of female receivers, and 4.4 and 5.2 kHz cover the range of variation in fc of male calling songs in populations of G. bimaculatus (Kostarakos et al., 2008; Ferreira and Ferguson, 2002). In addition, we generated model songs at the same fc, but differing in the degree of downward FM, beginning at the second half of each syllable (Fig. 2A). For each fc, we generated model songs with a downward FM of 200, 400, 600 and 800 Hz, again representing the maximal range of FM in natural populations (Leroy, 1966; Simmons, 1988; Simmons and Ritchie, 1996; Ferreira and Ferguson, 2002). The resulting power spectra of these stimuli, as analysed from the playback at the position of the female, are shown in Fig. 2B. For each fc, the downward FM resulted in a broadening of the narrow spectrum of the respective calling song towards the lower frequency end. It should be noted, however, that a FM of e.g. 800 Hz does not result in a corresponding increase in the width of the spectrum, but approximately only to 400 Hz, when measured 20 dB below the maximum at the fc (see Fig. 2B, dashed line).
Fig. 2.
(A) Spectrogram of a male calling song (upper panel) and a synthetic model song (lower panel) with a fc of 4.9 kHz and 600 Hz downward FM. (B) Power spectrum of model songs for each tested fc and depth of FM. Note that even a downward modulation of 800 Hz does not result in a corresponding increase in the width of the spectrum towards lower frequencies, but approximately only to 400 Hz, when measured 20 dB below the maximum at the fc (dashed line).
In female choice experiments, the temporal pattern of the alternative stimulus was presented via the opposite speaker in a time-shifted fashion, so that females were exposed to alternating chirps from the left and right speaker, with one chirp being broadcast at one speaker during the inter-chirp interval of the alternative stimulus.
Behavioural experiments
Experiments were conducted in total darkness in an anechoic chamber (180×180×210 cm) at room temperature (21–23°C). For measurement of phonotactic behaviour, females were tethered on the trackball device, with their legs in a normal walking position and free to move the trackball, and with their longitudinal body axis aligned exactly between the two speakers. Before the start of the experiment, females were left for 5 min without stimulation to adapt to the new conditions. The test series started with a no-choice experiment (signal broadcast from one speaker only) with the standard signal (fc 4.9 kHz, non-modulated) from one speaker. By recording the rotation of the trackball, we obtained the lateral steering velocity by which the female turned to either side, and calculated the lateral deviation of the animal from a straight, forward path. The steering to one of two alternative stimuli indicates the preference for a given song model. Unresponsive females were excluded from further testing the same day, but were retested the next 2 days. After successful phonotaxis towards the standard stimulus, females were given a choice between two simultaneously presented song models differing in the degree of downward FM, but at the same fc and sound pressure level (SPL) (see Table 1).
Table 1.
Results of two-choice tests examining phonotactic responses of 14 female field crickets
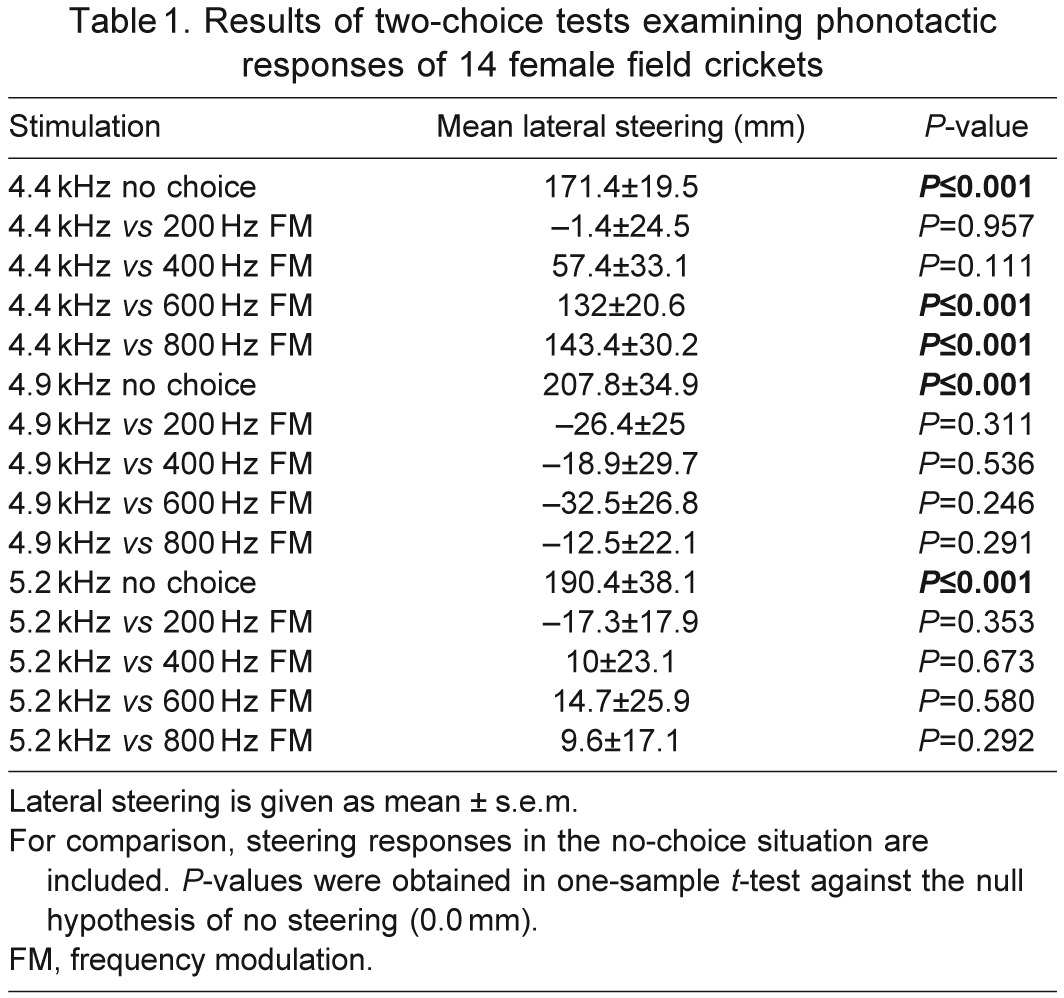
Phonotactic tracks were recorded for 1 min, so that the average lateral steering towards the model songs could be calculated by summing up the rotational left–right displacement of the trackball over 60 s of stimulation. In order to slightly smooth the illustration of phonotactic tracks, a running average of the previous and next 5 ms was calculated. Positive and negative values indicated steering to the right or left speaker, respectively. We used the phonotactic paths of females under symmetrical stimulation (standard song model at fc of 4.9 kHz from both speakers) as a reference for lateral deviation when females started to steer significantly towards one of the two alternative song models. Under these conditions, females should track a phonotactic path in a direction exactly straight ahead (lateral deviation zero), but with some random and meandering steering towards either side. In preliminary experiments, we therefore evaluated the amount of variation in lateral steering for 17 females when they moved in the forward direction. A mean (±s.d.) lateral steering (the deviation from an exactly straight, forward direction) of 8.2±57 mm was measured over 1 min. Because of this random deviation of females, we defined a preference for a song model when females exceeded a threshold value of 122.2 mm, which represents the mean + 2 s.d. towards the respective speaker.
Each of the 14 females was tested once in every single choice situation, which we presented in randomised order after the control for phontactic responsiveness with the standard stimulus had been made. This control was randomly broadcast either from the left or from the right speaker in order to evaluate potential spatial asymmetries in the set-up. Furthermore, in two-choice situations where the two competing stimuli were presented in an alternating fashion, the non-modulated calling song was randomly chosen to be presented first or second to control for a possible preceding effect. We conducted four, two-choice experiments at each of the three carrier frequencies of 4.4, 4.9 and 5.2 kHz: non-modulated versus 200, 400, 600 and 800 Hz FM (Table 1). After completion of all choice experiments we re-tested the females with a final no-choice experiment. If a female in the final test did not respond phonotactically we excluded the data of the previous set of experiments. For statistical analysis, we used SigmaPlot statistical software (version 12.0; Systat Software Inc., Chicago, IL, USA). In two-choice preference experiments we tested the lateral deviation for each choice situation against a null hypothesis of no lateral steering (0.0 mm) using one-sample t-tests. To further compare the lateral deviation for each experiment, we employed a repeated measures ANOVA and a post hoc comparison using the Holm–Sidak method. In order to standardize the steering of females towards the various song models at different fc, the amount of lateral steering towards the non-modulated song model in the no-choice test was taken as the reference for the two-choice trials at the respective fc.
Neurophysiology
Because of the prominent role of the pair of AN1 neurons in phonotaxis (Nolen and Hoy, 1984; Schildberger and Hörner, 1988; Atkins et al., 1992; Kostarakos et al., 2008), we recorded the extracellular action potential activity of this neuron in response to the various song models in 17 females. The techniques for extracellular AN1 recording are described in detail elsewhere (Kostarakos et al., 2008). Recordings were amplified with a custom-made preamplifier and visualised on an Agilent 54616 oscilloscope. Each song model was broadcast for a total duration of 60 s. Stimuli were calibrated to 80 dB re. 20 μPa at the position of the preparation. A Raveland MHX 138 speaker was placed 40 cm in front of the preparation at an angle of 30 deg off the longitudinal body axis in the horizontal plane. Signals were broadcast using a Heinecken HF amplifier and a digital attenuator (PA5 Tucker Davis Technologies, Alachua, FL, USA). We used Spike2 version 5.21 software (Cambridge Design Limited, Cambridge, UK) to analyse the recorded spike trains. We quantified the neural response to a specific song model taking the average response strength [action potentials (APs) per chirp] over a period of 60 s. These average responses were compared with Kruskal–Wallis one-way ANOVA after evaluating the data by performing Shapiro–Wilk normality tests as well as equal variance tests. The reactive steering hypothesis for phonotactic orientation predicts that auditory orientation emerges from single steering events, by turning towards individual sound pulses (Hedwig and Poulet, 2004). The effect of many fast steering decisions made on a stepping cycle is then accumulated over time. With respect to the sensory information necessary for these decisions, sufficient differences in the responses of the two AN1 neurons involved are required for such fast reactive steering events. Therefore, we calculated the difference in the number of AN1 spikes evoked by the various song models and cumulated these differences for 135 chirp responses over time. The differences in neural response were matched with a hypothetical threshold of two APs per chirp, representing the minimal difference at which females steer reliably to one side more than the other (Trobe et al., 2011).
We also performed a direct response-by-response comparison to chirps in these two-choice trials and asked whether the response to a non-modulated chirp was stronger than that to a modulated chirp (threshold difference 1 AP per chirp). For this comparison, we evaluated the frequency of a stronger activation to non-modulated chirps than to modulated chirps using Kruskal–Wallis one-way ANOVA.
RESULTS
Behavioural responses to FM songs
In the no-choice situation, females exhibited no difference in lateral steering towards the song model at the three fc tested (Kruskal–Wallis one-way ANOVA on ranks: H=0.464, N=14, P=0.793). At the most preferred frequency (4.9 kHz), females showed no preference for pure-tone or FM songs, independent of the degree of FM (Fig. 3) (one-sample t-tests; all P>0.05). The same was true for a fc of 5.2 kHz. In contrast, females preferred pure-tone songs with a fc of 4.4 kHz over model songs with a FM at modulation depths of 600 Hz (one-sample t-test: t=6.391, N=14, P<0.001) and 800 Hz (one-sample t-test: t=4.750, N=14, P<0.001), but not at smaller modulation depths of 200 or 400 Hz (one-sample t-tests; P>0.05). A comparison of differences in lateral deviation for each test condition revealed a significantly stronger steering in choice situations at 4.4 kHz, and FM of 600 and 800 Hz [repeated measures ANOVA: P<0.001; post hoc Holm–Sidak against control group (fc 4.4 kHz and FM of 200 Hz): P<0.001 for a choice between non-modulated song at 4.4 kHz, FM of 800 Hz; P=0.003 for a choice between non-modulated song at 4.4 kHz, FM of 600 Hz].
Fig. 3.
Mean (+s.e.m.) lateral steering response of phonotactic tracks (N=14). Positive values indicate a preference for the non-modulated signal. Note that increasing modulation depth caused a significant increase in lateral steering towards symmetrical signals only for a fc of 4.4 kHz. Asterisks denote mean lateral steering significantly different from zero (one-sample t-tests, see Materials and methods).
We also averaged the accumulated steering responses of 14 females (Fig. 4). The steering response appears as a steady increase of lateral deviation over the 60 s stimulation period (see accumulated values for the control situation in a no-choice test). At a fc of 4.9 and 5.2 kHz, none of the modulated stimuli resulted in a significant steering towards one side when presented in a choice with the non-modulated stimulus. Only at a fc of 4.4 kHz did the cumulated steering responses exceed the threshold value for steering in the forward direction, when the depth of modulation was 600 or 800 Hz.
Fig. 4.
Averaged lateral steering (N=14 females) during 60 s of stimulation. Positive values indicate steering towards the non-modulated model song in two-choice experiments. Dashed lines indicate the predefined threshold value for steering in the forward direction. For comparison, the averaged lateral steering of females to the standard stimulus in the no-choice experiments is also given.
Neurophysiology
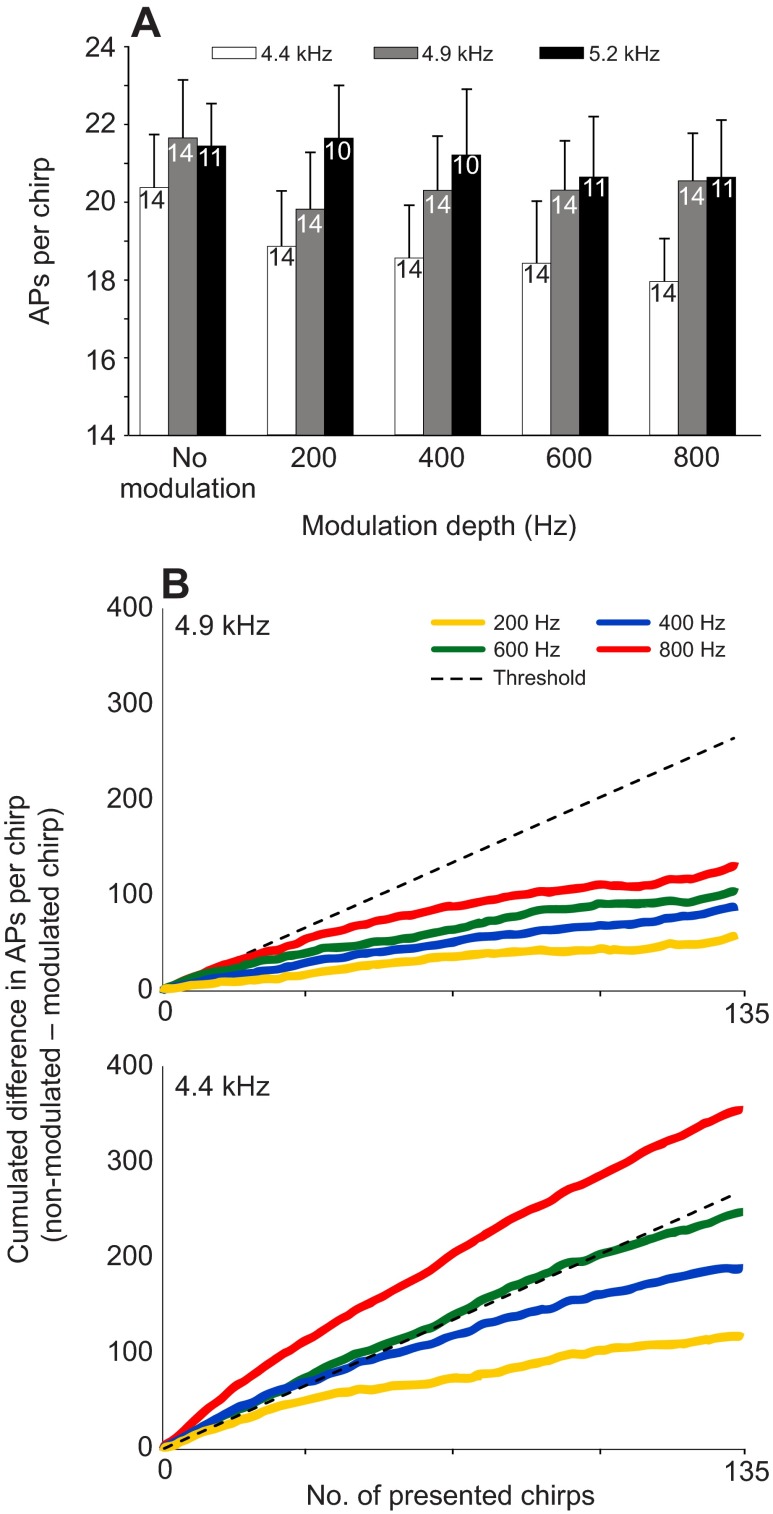
In order to quantify the possible sensory mechanism underlying the behavioural responses to the various song models, we measured the discharge of the AN1 neuron in response to these stimuli and determined the number of APs per chirp for each song model (shown in Fig. 5A for all tested fc of 5.2, 4.9 and 4.4 kHz). At a fc of 4.9 or 5.2 kHz, the response magnitude decreased only slightly with increasing modulation depth compared with that for the non-modulated model. The decrease in neural response for all FM songs was not statistically significant (Kruskal–Wallis one-way ANOVA on ranks: H=1.037, N=14, P=0.904). In contrast, there was a continuous and stronger decrease in the response strength in FM song at a fc of 4.4 kHz with increasing modulation depth. The mean response decreased from 20.4±1.44 APs per chirp for the pure-tone song model to 17.9±1.1 APs per chirp for the 800 Hz modulated counterpart.
Fig. 5.
(A) Mean (+s.e.m.) response strength [action potentials (APs) per chirp; N=135 chirps] for song models at a fc of 4.4 and 4.9 kHz and different modulation depths (numbers indicate the number of experiments). (B) Cumulated difference in response strength (APs per chirp; non-modulated versus modulated chirps). The dashed line indicates an empirically derived threshold of a discharge difference of 2.0 APs necessary for significant steering towards one of two alternative stimuli (Trobe et al., 2011).
By assuming that the lateral steering in two-choice experiments is based on the continuous evaluation of the AN1 responses to the model songs presented on both sides of the female, we calculated the differences in response strength over the total time of the phonotactic trials. These differences in response strength are shown as cumulated differences over all presented chirps in Fig. 5B. For a fc of 4.9 kHz, these differences slowly accumulate over time, and there is also a gradual increase of these cumulated differences with increasing modulation depth. However, even for a modulation depth of 800 Hz the accumulated difference was much smaller than the empirically derived threshold of 2 APs per chirp calculated from previous experiments (Trobe et al., 2011) (dashed line in Fig. 5B). For a fc of 4.4 kHz, however, the cumulated differences in response strength also gradually increased with increasing modulation depth, but exceeded this threshold for the two highest modulation depths of 600 and 800 Hz.
In addition, we performed a direct response-by-response comparison to chirps in these two-choice trials and asked how often in the successive responses to 135 presented chirps the magnitude of the response (in APs per chirp) was stronger with the non-modulated signal compared with the same series of responses to a modulated one. Thus, rather than resulting in averaged response magnitudes, this analysis gives information about the probability of a superior response to one signal compared with another, irrespective of the magnitude of the difference. At a fc of 4.9 kHz, the non-modulated song caused a stronger response in 54% of chirps compared with its 200 Hz modulated alternative. Higher modulation depths of 400, 600 and 800 Hz only slightly increased this value to 58%, 59% and 60%, respectively, in favour of the non-modulated signal, without any statistical significance (for absolute values: Kruskal–Wallis one-way ANOVA on ranks: H=2.490, N=16, P=0.477). For a fc of 4.4 kHz, the percentage of chirp responses with a stronger activation compared with downward modulated alternatives was 56% (200 Hz), 62% (400 Hz), 65% (600 Hz) and 72% (800 Hz) (for absolute values: Kruskal–Wallis one-way ANOVA on ranks: H=6.014, N=17, P=0.111).
DISCUSSION
The aim of the present paper was to analyse the ability of female receivers to discriminate FM in male calling song. This FM constitutes small deviations in fc, which were thought to result from morphological asymmetries in the structure of the left and right forewings producing the song (Simmons and Ritchie, 1996). These authors assumed that female receivers may detect asymmetry in males based on the downward FM at the second half of each syllable. The underlying proximate mechanism for the FM should be based on differences in the size of the harps, producing different resonant frequencies, with the left and right wing contributing to the first and second part of a syllable, respectively. A recent detailed analysis of sound production and of the resonances of left and right cricket wings by Montealegre-Z and colleagues (Montealegre-Z et al., 2011) does not support this assumption. Although the authors could confirm a lower resonant frequency of the left wing compared with the right wing, they also showed that the two wings are simultaneously involved in controlling the fc of the calling song, with the left wing dominating the combined frequency output. Thus, the proximate reason for the FM in the second part of most field cricket songs remains unsolved, but, as pointed out by Montealegre-Z and colleagues (Montealegre-Z et al., 2011), it is specific for every individual, and could be caused by a combination of several factors inherent to the features of wings. Thus, the original assumption of Simmons and Ritchie (Simmons and Ritchie, 1996) concerning the underlying mechanism for cues in the song relating to asymmetry in the signaller appears to be wrong. Nevertheless, the particular differences, such as the amount of FM, are characteristic for each individual, and could be used in a choice between males.
For the behavioural assay, we used a trackball system for the precise control of the relevant acoustic parameters, as previous studies in different cricket species had revealed a FM in the range of maximally 10–15% of the fc, representing rather small deviations from the fc (Leroy, 1966; Koch et al., 1988; Simmons and Ritchie, 1996; Bennet-Clark, 2003). Such small differences, when tested in two-choice trials in an arena, may easily be ‘masked’ by changes in other cues of the signal, such as their relative intensity, when the female is free to turn and move after the start of the trial. Under the controlled conditions of the trackball, the fast reactive steering hypothesis (Hedwig and Poulet, 2005) predicts that two identical stimuli with a species-specific temporal pattern will result in similarly large steering of the insect to either side, producing a net direction of steering straight ahead. Indeed, this prediction matches our observation (Fig. 3). For the same reasoning we can assume that only when subtle differences between two stimuli have been detected by a receiver and translated into a behavioural steering response does the phonotactic path deviate significantly from the forward direction (Hedwig and Poulet, 2005; Trobe et al., 2011).
Hypotheses about the expected outcome of these trials are based on the assumption that the perceptual processes in female receivers constitute a tuned hearing system. Fig. 1 summarizes the empirical data on this tuning for the AN1 neuron in G. bimaculatus and G. campestris, known for its prominent role in providing sensory information to the brain (Schildberger and Hörner, 1988; Atkins et al., 1992; Kostarakos et al., 2008). If we assume that the neural tuning of AN1 indeed reflects female preference, this allows us to make predictions on female preference in choice experiments. Average G. bimaculatus females are tuned to a fb of 4.9 kHz, and the sensitivity of receivers decreases towards lower frequencies more than towards higher frequencies (24 compared with 8 dB kHz−1) (Kostarakos et al., 2009). Thus, the characteristics of the tuning curve predict that average females prefer a fc in calling songs at 4.9 kHz over lower or higher fc, as their sensory system will be stimulated less strongly at frequencies away from the fb of receivers. This would result in stabilizing selection for fc at the fb of females. For the same reason, one would predict a preference of females for symmetrical (non-modulated) songs, in particular if downward FM occurs at lower fc, because this modulation makes the signal much less intense for the receiver as a result of the steep slope of the tuned system (Fig. 1B). Such a preference can only be expected if females are sensitive to these small changes in perceived relative loudness. By contrast, the same modulation should have little or no effect at 4.9 kHz (the fb of females) or even at a fc higher than the fb (5.2 kHz) on the female's preference.
Of course, differences in the tuning of individual females would have an effect on their behavioural response towards modulated songs. Kostarakos and colleagues (Kostarakos et al., 2008) described the individual variation in fb as ranging from 4.5 to 5.2 kHz. For females at the low and high frequency end of this distribution, a fc of 4.4 kHz with a FM of 800 Hz would have rather different effects according to the hypothesis presented in Fig. 1: either little or no effect in a choice with a non-modulated model, or an even stronger choice for the non-modulated model for a female tuned to 5.2 kHz. This does not affect our general interpretation, as we averaged the steering of all tested individuals in Fig. 4, assuming a similar variance in the fb of tuning.
Our results clearly indicate a strong correlation between the perception of differences in fc and differences in the depth of FM. Female G. bimaculatus did not deviate significantly from a straight, forward direction in a choice between stimuli with a fc of 4.9 and 5.2 kHz, irrespective of the amount of FM. Only at the lowest tested fc of 4.4 kHz did FM of 600 Hz or higher cause a significant deviation of the average steering of females towards the non-modulated alternative (Fig. 3). Based on the known tuning curve of female receivers (Fig. 1A), this result was expected as only a downward FM at the lower end of the fb would reduce the relative intensity of the perceived signal strongly enough compared with the non-modulated alternative. This is favoured by the steeper slope of the tuning curve towards lower frequencies.
Exactly the same underlying perceptual mechanism appears to determine the female decisions in two-choice trials between stimuli varying in their fc (Kostarakos et al., 2008). The fc of 4.9 kHz is preferred over all other fc in the male population, 4.5 kHz is preferred over 4 kHz, and 4 kHz is only preferred over 3.5 kHz. The strong correlation of these choices with the individual tuning of the relevant AN1 neuron in each female further indicates that this choice is determined by the threshold differences, and thus by the perceived relative loudness of the signal options.
Surprisingly, the cricket neural circuit for discriminating signals differing in fc or FM does not possess a population of receptors or interneurons each differing in its fb, as in vertebrates for instance. In the best-studied species, Teleogryllus oceanicus, the majority of receptors are tuned around the fb of the calling song; only one-quarter are tuned to either high frequencies >18 kHz or intermediate frequencies of 9–12 kHz (Imaizumi and Pollack, 1999; Imaizumi and Pollack, 2001). The receptor information is mainly communicated to the brain via two ascending interneurons, AN1 and AN2 (ascending neuron 2) (Wohlers and Huber, 1982; Hennig, 1988; Boyd et al., 1984). As AN2 is tuned to higher and ultrasonic frequencies, the behaviourally relevant information about fc and FM in a two-choice situation with spatially separated sources can only be carried to the brain by activity in AN1, in the form of a discharge difference between the left and right neuron of the pair. As the role of the pair of AN1 for phonotactic steering has been demonstrated by manipulating the amount of discharge by one neuron (Schildberger and Hörner, 1988) or by destruction of one neuron (Atkins et al., 1992), we searched in our neurophysiological survey for an imbalance in the responses of this pair of neurons that might cause the significant steering of the female towards one of the two alternatives. Our results clearly show that (1) such small response differences are elicited in a comparison between non-modulated and modulated calling songs, (2) the differences are graded with modulation depth, and (3) the differences are larger for signals at a fc of 4.4 kHz compared with other fc. If (4) we assume a ‘threshold difference’ based on previous studies for significant steering towards one side, the differences are larger than this threshold criterion only for signals with a fc of 4.4 kHz and a modulation depth of 600 and 800 Hz (Fig. 5B). These neurophysiological results are mirrored in the behaviour of females, as females steered significantly towards the non-modulated signal only for the fc of 4.4 kHz and larger modulation depths (Figs 3, 4).
Thus, the available data in field crickets point to a tuned preference for the fc of the male calling song. The ‘hard-wired’ preference function for this parameter is a single sensory interneuron pair (AN1), as it exhibits a frequency tuning accounting for 80% of the variation in the direction and strength of phonotactic steering in a choice situation (Kostarakos et al., 2008). The amount of neuronal activity in response to one or more signals in the pair of neurons determines the amount of reactive motor steering to either side (Hedwig and Poulet, 2005; Hedwig, 2006). If there is some asymmetry in the neuronal responses of the two AN1 neurons due to differences in fc, FM or SPL, the female will eventually arrive at the signal providing the greater neuronal response.
The sensitivity and the choice of G. bimaculatus females for non-modulated calling songs at a 4.4 kHz fc relative to a 4.9 kHz fc appears to be consistent with results of choice experiments in the sister species G. campestris, as pure-tone songs at a fc of 4.25 kHz were preferred relative to FM ones, but not at a 5 kHz carrier (Simmons and Ritchie, 1996). Although the tuning of the homologous AN1 neuron in G. campestris is less selective than that of G. bimaculatus (Fig. 1A) (Kostarakos et al., 2009), the same proximate mechanism as suggested here for G. bimaculatus may account for this choice. However, Simmons and Ritchie also found a strong preference for calling songs with a pure-tone fc of 4.25 kHz over higher fc (Simmons and Ritchie, 1996). Such a low-frequency (large male) advantage does not occur in G. bimaculatus, where only one out of 20 tested individuals steered towards a 4.0 kHz calling song compared with a 4.9 kHz alternative. These differences between the two Gryllus species may result from a significantly lower mean AN1 tuning in G. campestris at a fb of 4.4 kHz (and thus close to the preferred fc of 4.25 kHz), and from a reduced roll-off towards lower frequencies in G. campestris compared with G. bimaculatus (Kostarakos et al., 2009). The ambiguity of results concerning female preference for larger males with lower fc may be partly explained by the fact that females may prefer larger males, and size is correlated negatively with fc in the calling song, but females do not choose males based on lower fc (Simmons and Zuk, 1992; Shaw and Herlihy, 2000; Deb et al., 2012).
The relevance of ‘symmetrical’ songs for female choice in crickets
Leaving aside the question of whether the variation in downward FM in the songs of males is an indicator for asymmetry and carries information about fitness-relevant traits, we can still ask how relevant these variations in the male population might be in relation to other signal parameters, in particular when we consider the acoustic and ecological situation under which female choice occurs in nature. We come to the conclusion that they are largely irrelevant, for the following reasons. (1) We have shown here that under the best-controlled choice situation on the trackball, only FM at 4.4 kHz, and larger modulations of 600–800 Hz, cause a small but significant steering towards the pure-tone alternative. When females are free to orient and approach a sound source, such as in arena trials and outdoors, the changes in the female's position relative to the sound sources and some random deviations from a straight line to one source produce larger deviations in the amplitude of the two signals than those inherent to pure-tone or modulated signals. (2) Under natural conditions in the field, the properties of the transmission channel result in even larger random variations in the signal amplitude of alternative signals (Römer, 1998; Römer, 2001; Kostarakos and Römer, 2010), so that the minute differences between pure tone and modulated songs should play no role. For example, in an ongoing outdoor study, female G. bimaculatus needed a 5 dB difference in call amplitude for a significant approach to the louder signal (S.H. and H.R., unpublished), whereas on the trackball the minimum difference was 1–2 dB (Hedwig and Poulet, 2005). Even under closed-loop conditions in the laboratory, Mhatre and Balakrishnan (Mhatre and Balakrishnan, 2007) found that a difference of 6 dB between song alternatives was required for clear orientation towards the louder call, whereas a 3 dB difference was insufficient. Interestingly, even when the difference between the two speakers was as high as 9 dB, a few females still approached the softer speaker. (3) In our studied population of males, the whole range of variation in the calling song fc was 4.3–5.2 kHz [data for other populations is given elsewhere (see Ferreira and Ferguson, 2002; Verburgt and Ferguson, 2010)], and only 2 out of 36 males would have been in the category with a low fc and a FM of >600 Hz. The data in Simmons and Ritchie (Simmons and Ritchie, 1996) also indicate that only 3 out of 63 males exhibited a modulation >700 Hz, at unknown fc. (4) The amount of FM in spectrograms like those in Fig. 2A is open to measurement error. Zollinger and colleagues (Zollinger et al., 2012) discussed in detail the pitfalls associated with the use of spectrograms, and the extraction of quantitative values such as depth of FM, from sound recordings not calibrated carefully for amplitude. The latter was the case for the analysis of FM in cricket song by Simmons and Ritchie (Simmons and Ritchie, 1996) and may have resulted in an overestimation of FM. In a power spectrum of these signals, the downward FM appears as a low-frequency shoulder in the narrow spectrum. The resulting increase in the width of the spectrum by the largest modulation tested is, however, only about 400 Hz, 20 dB below the maximum at the fc, and thus less than expected from the spectrogram (see Fig. 2).
ACKNOWLEDGEMENTS
We thank Michael Ritchie and Fernando Montealegre-Z for helpful comments on an earlier draft of the manuscript, and Jan Clemens and Manfred Hartbauer for support in the analysis of neuronal data. We are grateful to the reviewer's valuable comments on the manuscript.
FOOTNOTES
COMPETING INTERESTS
No competing interests declared.
FUNDING
This project was supported by the Austrian Science Fund [FWF P20882-B09] to H.R., and the Karl-Franzens-University of Graz to S.H. Deposited in PMC for immediate release.
REFERENCES
- Atkins G., Henley J., Handysides R., Stout J. (1992). Evaluation of the behavioral roles of ascending auditory interneurons in calling song phonotaxis by the female cricket (Acheta domesticus). J. Comp. Physiol. A 170, 363-372 [Google Scholar]
- Bennet-Clark H. C. (1989). Songs and the physics of sound production. In Cricket Behavior and Neurobiology (ed. Huber F., Moore T. E., Loher W.), pp. 227-261 Ithaca, NY: Cornell University Press; [Google Scholar]
- Bennet-Clark H. C. (1999). Resonators in insect sound production: how insects produce loud pure-tone songs. J. Exp. Biol. 202, 3347-3357 [DOI] [PubMed] [Google Scholar]
- Bennet-Clark H. C. (2003). Wing resonances in the Australian field cricket Teleogryllus oceanicus. J. Exp. Biol. 206, 1479-1496 [DOI] [PubMed] [Google Scholar]
- Boyd P., Kühne R., Silver S., Lewis B. (1984). Two-tone suppression and song coding by ascending neurones in the cricket Gryllus campestris L. J. Comp. Physiol. A 154, 423-430 [Google Scholar]
- Brown W. D., Wideman J., Andrade M. C. B., Mason A. C., Gwynne D. T. (1996). Female choice for an indicator of male size in the song of the black-horned tree cricket, Oecanthus nigricornis (Orthoptera: Gryllidae: Oecanthinae). Evolution 50, 2400-2411 [DOI] [PubMed] [Google Scholar]
- Capranica R. R., Moffat A. J. M. (1983). Neurobehavioral correlates of sound communication in anurans. In Advances in Vertebrate Neuroethology (ed. Ewert J. P., Capranica R. R., Ingle D.), pp. 701-730 New York, NY: Plenum; [Google Scholar]
- Deb R., Bhattacharya M., Balakrishnan R. (2012). Females of a tree cricket prefer larger males but not the lower frequency male calls that indicate large body size. Anim. Behav. 84, 137-149 [Google Scholar]
- Elliott C. J. H., Koch U. T. (1985). The clockwork cricket. Naturwissenschaften 72, 150-153 [Google Scholar]
- Ferreira M., Ferguson J. W. H. (2002). Geographic variation in the calling song of the field cricket Gryllus bimaculatus (Orthoptera: Gryllidae) and its relevance to mate recognition and mate choice. J. Zool. (Lond.) 257, 163-170 [Google Scholar]
- Gerhardt H. C., Huber F. (2002). Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago, IL: University of Chicago Press; [Google Scholar]
- Grammer K., Thornhill R. (1994). Human (Homo sapiens) facial attractiveness and sexual selection: the role of symmetry and averageness. J. Comp. Psychol. 108, 233-242 [DOI] [PubMed] [Google Scholar]
- Hedwig B. (2006). Pulses, patterns and paths: neurobiology of acoustic behaviour in crickets. J. Comp. Physiol. A 192, 677-689 [DOI] [PubMed] [Google Scholar]
- Hedwig B., Poulet J. F. A. (2004). Complex auditory behaviour emerges from simple reactive steering. Nature 430, 781-785 [DOI] [PubMed] [Google Scholar]
- Hedwig B., Poulet J. F. A. (2005). Mechanisms underlying phonotactic steering in the cricket Gryllus bimaculatus revealed with a fast trackball system. J. Exp. Biol. 208, 915-927 [DOI] [PubMed] [Google Scholar]
- Hennig R. M. (1988). Ascending auditory interneurons in the cricket Teleogryllus commodus (Walker): comparative physiology and direct connections with afferents. J. Comp. Physiol. A 163, 135-143 [DOI] [PubMed] [Google Scholar]
- Hunt J., Brooks R., Jennions M. D. (2005). Female mate choice as a condition-dependent life-history trait. Am. Nat. 166, 79-92 [DOI] [PubMed] [Google Scholar]
- Imaizumi K., Pollack G. S. (1999). Neural coding of sound frequency by cricket auditory receptors. J. Neurosci. 19, 1508-1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K., Pollack G. S. (2001). Neural representation of sound amplitude by functionally different auditory receptors in crickets. J. Acoust. Soc. Am. 109, 1247-1260 [DOI] [PubMed] [Google Scholar]
- Koch U. T., Elliott C. J. H., Schäffner K.-H., Kleindienst H.-U. (1988). The mechanics of stridulation of the cricket Gryllus campestris. J. Comp. Physiol. A 162, 213-223 [Google Scholar]
- Kostarakos K., Römer H. (2010). Sound transmission and directional hearing in field crickets: neurophysiological studies outdoors. J. Comp. Physiol. A 196, 669-681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarakos K., Hartbauer M., Römer H. (2008). Matched filters, mate choice and the evolution of sexually selected traits. PLoS ONE 3, e3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostarakos K., Hennig M. R., Römer H. (2009). Two matched filters and the evolution of mating signals in four species of cricket. Front. Zool. 6, 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowner R. (1996). Facial asymmetry and attractiveness judgement in developmental perspective. J. Exp. Psychol. Hum. Percept. Perform. 22, 662-675 [DOI] [PubMed] [Google Scholar]
- Leroy Y. (1966). Signaux acoustiques, comportement et systématique de quelques espéces de Gryllidae (Orthoptères, Ensifères). Bull. Biol. Fr. Belg. 100, 1-134 [PubMed] [Google Scholar]
- Mhatre N., Balakrishnan R. (2007). Phonotactic walking paths of field crickets in closed-loop conditions and their simulation using a stochastic model. J. Exp. Biol. 210, 3661-3676 [DOI] [PubMed] [Google Scholar]
- Møller A. P. (1990). Fluctuating asymmetry in male sexual ornaments may reliably reveal male quality. Anim. Behav. 40, 1185-1187 [Google Scholar]
- Møller A. P. (1992). Female swallow preference for symmetrical male sexual ornaments. Nature 357, 238-240 [DOI] [PubMed] [Google Scholar]
- Møller A. P. (1993). Female preference for apparently symmetrical male sexual ornaments in the barn swallow Hirundo rustica. Behav. Ecol. Sociobiol. 32, 371-376 [Google Scholar]
- Møller A. P., Swaddle J. P. (1997). Asymmetry, Developmental Stability, and Evolution. New York, NY: Oxford University Press; [Google Scholar]
- Montealegre-Z F., Jonsson T., Robert D. (2011). Sound radiation and wing mechanics in stridulating field crickets (Orthoptera: Gryllidae). J. Exp. Biol. 214, 2105-2117 [DOI] [PubMed] [Google Scholar]
- Nocke H. (1972). Physiological aspects of sound communication in crickets (Gryllus campestris L.). J. Comp. Physiol. A 80, 141-162 [Google Scholar]
- Nolen T. G., Hoy R. R. (1984). Initiation of behavior by single neurons: the role of behavioral context. Science 226, 992-994 [DOI] [PubMed] [Google Scholar]
- Oakes E. J., Barnard P. (1994). Fluctuating asymmetry and mate choice in paradise whydahs, Vidua paradisaea: an experimental manipulation. Anim. Behav. 48, 937-943 [Google Scholar]
- Rantala M. J., Ahtiainen J. J., Suhonen J. (2004). Fluctuating asymmetry and immune function in a field cricket. Oikos 107, 479-484 [Google Scholar]
- Rodríguez-Muñoz R., Bretman A., Slate J., Walling C. A., Tregenza T. (2010). Natural and sexual selection in a wild insect population. Science 328, 1269-1272 [DOI] [PubMed] [Google Scholar]
- Römer H. (1998). The sensory ecology of acoustic communication in insects. In Comparative Hearing: Insects, Vol. 10 (ed. Hoy R. R., Popper A. N., Fay R. R.), pp. 63-96 New York, NY: Springer; [Google Scholar]
- Römer H. (2001). Ecological constraints for sound communication: from grasshoppers to elephants. In Ecology of Sensing (ed. Barth F. G., Schmid A.), pp. 59-77 Berlin, Heidelberg, New York, NY: Springer; [Google Scholar]
- Schildberger K., Hörner M. (1988). The function of auditory neurons in cricket phonotaxis. J. Comp. Physiol. A 163, 621-631 [Google Scholar]
- Shaw K. L., Herlihy D. P. (2000). Acoustic preference functions and song variability in the Hawaiian cricket Laupala cerasina. Proc. R. Soc. B 267, 577-584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. W. (1988). The calling song of the field cricket Gryllus bimaculatus (de Geer): constraints on transmission and its role in intermale competition and female choice. Anim. Behav. 36, 380-394 [Google Scholar]
- Simmons L. W. (1995). Correlates of male quality in the field cricket, Gryllus campestris L.: age, size, and symmetry determine pairing success in field populations. Behav. Ecol. 6, 376-381 [Google Scholar]
- Simmons L. W., Ritchie M. G. (1996). Symmetry in the songs of crickets. Proc. R. Soc. B 263, 1305-1311 [Google Scholar]
- Simmons L. W., Zuk M. (1992). Variability in call structure and pairing success of male field crickets, Gryllus bimaculatus: the effects of age, size and parasite load. Anim. Behav. 44, 1145-1152 [Google Scholar]
- Swaddle J. P. (1999). Visual signalling by asymmetry: a review of perceptual processes. Philos. Trans. R. Soc. B 354, 1383-1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaddle J. P., Cuthill I. C. (1995). Asymmetry and human facial attractiveness: symmetry may not always be beautiful. Proc. R. Soc. B 261, 111-116 [DOI] [PubMed] [Google Scholar]
- Thorson J., Weber T., Huber F. (1982). Auditory behavior of the cricket. J. Comp. Physiol. A 146, 361-378 [Google Scholar]
- Trobe D., Schuster R., Römer H. (2011). Fast and reliable decisions for a dynamic song parameter in field crickets. J. Comp. Physiol. A 197, 131-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburgt L., Ferguson J. W. H. (2010). Mate choice in field crickets: can females acoustically detect male body size? J. Ethol. 28, 141-151 [Google Scholar]
- Verburgt L., Ferguson J. W. H., Weber T. (2008). Phonotactic response of female crickets on the Kramer treadmill: methodology, sensory and behavioural implications. J. Comp. Physiol. A 194, 79-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Helversen D., Balakrishnan R., von Helversen O. (2004). Acoustic communication in a duetting grasshopper: receiver response variability, male strategies and signal design. Anim. Behav. 68, 131-144 [Google Scholar]
- Watson P. J., Thornhill R. (1994). Fluctuating asymmetry and sexual selection. Trends Ecol. Evol. 9, 21-25 [DOI] [PubMed] [Google Scholar]
- Wohlers D. W., Huber F. (1982). Processing of sound signals by six types of neurons in the prothoracic ganglion of the cricket, Gryllus campestris L. J. Comp. Physiol. A 146, 161-173 [Google Scholar]
- Zollinger S. A., Podos J., Nemeth E., Goller F., Brumm H. (2012). On the relationship between, and measurement of, amplitude and frequency in birdsong. Anim. Behav. 84, e1-e9 [Google Scholar]