Abstract
Prevention and treatment of postoperative pain continues to be a major challenge in postoperative care. Opioid analgesics, with their well-known side effects, continue to represent a cornerstone in postoperative pain control. Anticonvulsant medications are established treatments for neuropathic pain. Pregabalin (S-[+]-3-isobutylgaba), a structural analog of gamma-Aminobutyric acid, has been used for the treatment of various neuropathic pain and also as an adjunctive therapy for adults with partial onset seizures. This study was thus taken up to primarily assess and compare the analgesic and anxiolytic effects of administering pregabalin and tramadol preoperatively for patients undergoing elective decompressive lumbar laminectomy. The study group included 75 patients between the ages of 20–60 years belonging to American Society of Anesthesiology-1 (ASA) and ASA-2 patients. The patients were randomly allocated into three groups of 25 patients each. The placebo group received a placebo capsule, the tramadol group received a 100 mg capsule, while the pregabalin group received a 150 mg capsule orally 1 hour before anesthetic induction. Pregabalin showed statistically significant analgesic effects compared to placebo, but the effect was found to be less prevalent compared to tramadol. The need for rescue analgesia was the least prevalent in tramadol patients followed by pregabalin patients, and reached a maximum in the control group. Pregabalin showed statistically significant anxiolytic effects compared to placebo, and this was associated with less sedation in comparison to tramadol. Pregabalin had fewer numbers of postoperative complications of nausea, vomiting, and drowsiness in comparison to tramadol. The results of this study support the clinical use of pregabalin in the postsurgical setting for pain relief, as it is well tolerated, and usually presents with transient adverse effects.
Keywords: pregabalin, tramadol, postoperative pain, lumbar laminectomy
Video abstract
Introduction
Postoperative management of a patient includes pain management,1 prevention and treatment of postoperative complications,2 and recovery of preoperative function.3 Prevention and treatment of postoperative pain still remains a major challenge in postoperative care in spite of significant advancements in options for pain assessment and therapy.4 It helps in early mobilization of the patient and improves his or her well-being. It has been reported that around 80% of patients undergoing surgical procedures experience postoperative pain.4 Postoperative pain at rest is responsive to opioid therapy;5 however, movement-evoked pain is considerably less responsive to opioids,5 and is related to postoperative pulmonary,6 cardiac,7 and thromboembolic complications.8–10
Opioid analgesics, in spite of their side effects, continue to represent a cornerstone in postoperative pain control. Hence, the search for new analgesics, as well as combinations of analgesics with the same potency as that of opioids (but without the side effects) continues. In this context, anticonvulsant drugs gabapentin and pregabalin have been targeted by researchers.
The early success in the treatment of trigeminal neuralgia with anticonvulsant drugs has led to many studies that have assessed their analgesic potency in treating neuropathic pain associated with diabetic peripheral neuropathy and postherpetic neuralgia.11–16 Also, their analgesic efficacy after a variety of surgical procedures has also been studied.17,18 Two such drugs, especially pregabalin and gabapentin, which are alfa-2-delta (α2-δ) subunit calcium channel ligands, have been widely studied. Pregabalin binds potently to the α2-δ subunit and modulates calcium influx at nerve terminals, and thus reduces the release of several neurotransmitters, including glutamate, noradrenaline, serotonin, dopamine, and substance P.19–23
Pregabalin has been shown to have greater analgesic efficacy in rodent models of neuropathic pain, exhibits linear pharmacokinetics across the therapeutic dose range, and demonstrates low intersubject variability.24
The present study was thus taken up to test the hypothesis of the utility of pregabalin for the relief of postoperative pain in a prospective double-blind, randomized, placebo-controlled trial in which we aimed to compare and assess the analgesic and anxiolytic efficacy of pregabalin and tramadol in patients undergoing lumbar laminectomy.
Materials and methods
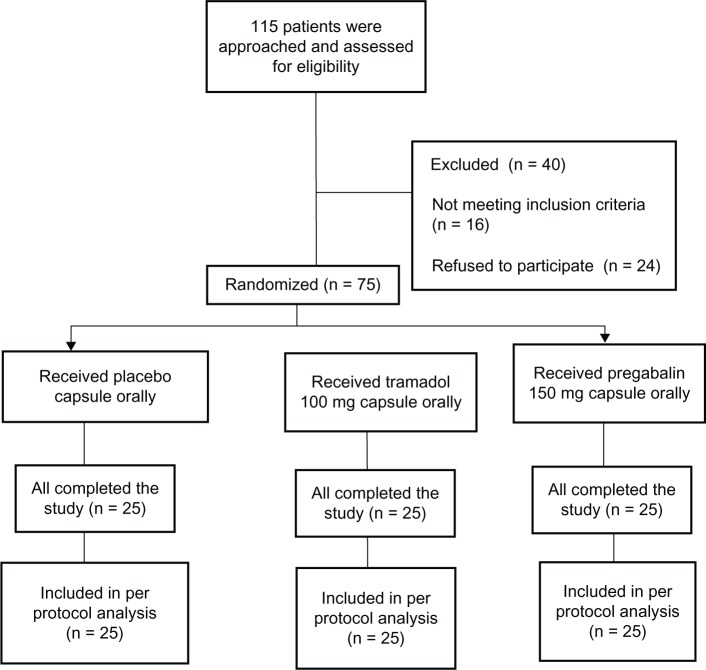
After institutional ethical committee approval and obtaining informed consent, the study was conducted on 75 patients of either sex and age group between 20–60 years belonging to American Society of Anesthesiology-1 (ASA) and ASA-2 patients undergoing elective decompressive lumbar laminectomy under general anesthesia (Figure 1). The patients were randomized into three groups of 25 patients each by a computer-generated random number table and the sealed opaque envelope technique. The person administering the drug was blinded to the drug used. Exclusion criteria included known allergy or sensitivity to the drugs, renal insufficiency, ongoing therapy with sustained release opioids, and seizure disorders.
Figure 1.
Enrollment and randomization.
The primary outcome measure was to study the analgesic and anxiolytic efficacy of pregabalin and tramadol for postoperative pain, while the secondary outcome was to assess their adverse effects.
Study design
The details of patient enrollment and randomization are given in Figure 1. The study medications consisted of placebo, tramadol 100 mg, and pregabalin 150 mg capsules.
Group 1 (placebo): received a placebo capsule orally 1 hour before anesthetic induction.
Group 2 (tramadol): received a 100 mg capsule orally 1 hour before the anesthetic induction.
Group 3 (pregabalin): received a 150 mg capsule orally 1 hour before the anesthetic induction.
All patients were orally premedicated with 0.5 mg of alprazolam at 9.00 pm on the day before surgery.
Anesthesia protocol
The patient was premedicated with an injection of midazolam (0.5 mg/kg intravenously [IV]) and an injection of glycopyrolate (0.02 mg/kg). Induction with thiopentone sodium (4–5 mg/kg of 2.5% solution) was titrated to the loss of eyelash reflex. Analgesia was provided with fentanyl 1 mic/kg, endotracheal intubation was facilitated by using vecuronium bromide as muscle relaxant in the dose of 0.1 mg/kg, and anesthesia was maintained with isoflurane (0.4%–0.8% vaporizer dial settings) with N2O:O2 (66:33). Standard monitoring included electrocardiogram, noninvasive blood pressure, end tidal concentration of carbon dioxide, and pulse oximetry. Intravenous fluids, Ringer’s lactate and normal saline, were administered at the rate of 60–80 mL/hour. There was minimal blood loss during the surgery. All patients were given antiemetic injected ondansetron 4 mg IV during surgery. At the end of surgery, patients were extubated after the reversal of residual neuromuscular blockade with injectable neostigmine (0.05 mg/kg) and glycopyrolate (0.01 mg/kg). Postoperatively, whenever patients complained of pain (Visual Analog Score25 greater than three) they received 0.5 mic/kg of fentanyl or diclofenac 50 mg IV as rescue analgesia, which was repeated until the pain subsided. The bispectral index was used for level of consciousness, and changes in heart rate and blood pressure were considered to be indirect measures of pain intraoperatively. The following parameters were studied and compared after being double blinded to the patients’ group assignments.
Pain score
Pain quantification was done on a modified Visual Analog Score between 0 and 10 (0 = no pain; 10 = worst imaginable pain).25 Sedation scores were given as awake and alert or tense (4), awake and not alert (3), drowsy (2), asleep (1), and asleep and not arousable (0).26 Anxiety scores were given as frightened/terrified (4), very upset and worried (3), worried and anxious (2), uneasy (1), calm and comfortable (0).26
Postoperative blood pressure (Datex-Ohmeda S5 noninvasive blood pressure monitor; NIBP, GE Healthcare, Germany), heart rate (Datex-Ohmeda S5 monitor [GE Healthcare Europe GmbH, Freiburg, Germany]), respiratory rate, postoperative pain, level of sedation as measured by sedation scoring, amount of rescue analgesia, number of doses, and total analgesic consumption were recorded at the end of 6 hours. Side effects like nausea, vomiting, constipation, drowsiness, and other complications, if any, were also recorded preoperatively, as well as 1 hour, 2 hours, 4 hours, and 6 hours after extubation.
Statistical analysis
Sample size was estimated by conducting a pilot study in 12 patients. Power analysis of variance (ANOVA) was done with Power Analysis and Sample Size (PASS) software (trial version, NCSS, Kaysville, Utah, USA), taking mean pain scores as 6, 5, and 4 in the three groups, standard deviation of 2, power of 80%, and alpha at 0.05. According to the analysis, 63 patients (21 in each group) was found to be the adequate sample size; however, we studied 75 patients (25 in each group) as there may have been patient drop-outs during the study. Statistical analysis was performed by one-way repeated measures ANOVA (NCSS statistical software, trial version) for pain, anxiety, sedation scales, and other parameters like heart rate, blood pressure, and analgesic requirements. If the difference was found to be statistically significant (P < 0.05), post hoc analysis was done by using the Tukey–Kramer multiple comparison test. Demographic data and adverse effects were analyzed by ANOVA or χ2 test, as appropriate.
Results
The demographic data of all three groups is shown in Table 1. The groups were matched in terms of age, gender, weight, duration of surgery, and spinal levels of laminectomy (P > 0.05). No significant difference was observed in the heart rate and respiratory rate recorded preoperatively (1 minute and 5 minutes after intubation), immediately after extubation (1 hour, 2 hours, 4 hours, and 6 hours postoperatively) among the groups (P > 0.05). Similarly, no significant difference was observed in the mean systolic and diastolic blood pressures preoperatively, 1 minute after intubation, as well as 1 hour, 2 hours, 4 hours, and 6 hours postoperatively among all the three groups. However, in the patients who received tramadol, both the systolic and diastolic blood pressure changes were significantly lower 5 minutes after intubation and immediately after extubation in comparison to both placebo and pregabalin patients (P > 0.05).
Table 1.
Demographic variables in all the three groups
| Variables | Group1 (Mean ± SD) | Group 2 (Mean ± SD) | Group 3 (Mean ± SD) |
|---|---|---|---|
| Age (years) | 45.64 ± 11.10 | 41.8 ± 12.43* | 45.36 ± 11.04* |
| Weight (kg) | 61.76 ± 6.82 | 61.44 ± 7.51* | 61.4 ± 7.91* |
| Sex ratio (male:female) | 8:17 | 9:18 | 8:17 |
| Duration of surgery (minutes) | 194.8 ± 48.61 | 210.4 ± 51.17* | 230.6 ± 38.72* |
| Spinal levels | |||
| 1 level | 10 | 11 | 13 |
| 2 level | 15 | 14 | 12 |
Note:
P > 0.05 compared to placebo group.
Abbreviation: SD, standard deviation.
The pain scores, anxiety scores, and sedation score in the three groups at different time intervals are depicted in Table 2. No significant differences were observed in the pain scores of all three groups preoperatively; however, after extubation and at 1 hour, 2 hours, 4 hours, and 6 hours postoperatively, a significant decrease in the pain scores of the patients who received tramadol and pregabalin in comparison to the placebo group was noted (P < 0.05). After post hoc analysis, it was found that pain relief in the tramadol group was better than among the pregabalin patients (P < 0.05). The pain scores were low at all time intervals in the tramadol group. The pain scores increased at 4 hours and 6 hours postoperatively in the pregabalin group, but they were still lower than that of the placebo group (P < 0.05) (Figure 2).
Table 2.
Pain scores, anxiety scores, and sedation scores among the three groups at different time intervals
| Variables | Time point | Group 1: Placebo (Mean ± SD) | Group 2: Tramadol (Mean ± SD) | Group 3:Pregabalin (Mean ± SD) |
|---|---|---|---|---|
| Pain scores | Preoperatively | 1.68 ± 1.46 | 0.8 ±1.35 | 1.44 ± 1.44 |
| After extubation | 4.8 ± 1.32 | 1.88 ± 0.78* | 3.12 ± 1.09*# | |
| 1 hr after extubation | 5.48 ± 1.26 | 2 ± 1.08* | 2.92 ± 1.23* | |
| 2 hr after extubation | 6.04 ± 0.93 | 2.32 ± 1.18* | 3.64 ± 1.28* | |
| 4 hr after extubation | 5.76 ± 0.77 | 2.36 ± 0.91* | 4.28 ± 1.1*# | |
| 6 hr after extubation | 5.8 ± 1.08 | 2.68 ± 1.22* | 4.12 ± 1.16*# | |
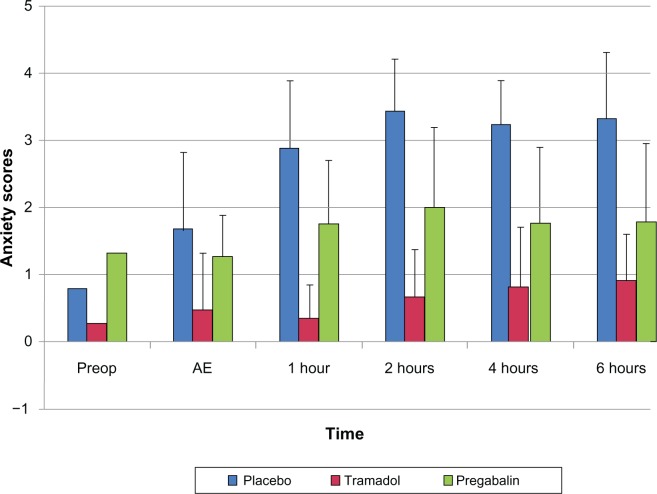
| Anxiety score | Preoperatively | 1.8 ± 1.15 | 0.28 ± 0.84* | 1.32 ± 0.62* |
| After extubation | 1.68 ± 1.02 | 0.48 ± 0.50* | 1.28 ± 0.97*# | |
| 1 hr after extubation | 2.88 ± 0.78 | 0.36 ± 0.7* | 1.76 ± 1.2*# | |
| 2 hr after extubation | 3.44 ± 0.65 | 0.68 ± 0.85* | 2 ± 1.15*# | |
| 4 hr after extubation | 3.24 ± 1.01 | 0.84 ± 0.68* | 1.76 ± 1.16* | |
| 6 hr after extubation | 3.32 ± 0.94 | 0.92 ± 0.90* | 1.8 ± 1.15* | |
| Sedation scores | Preoperatively | 4 ± 0 | 2.88 ± 1.09* | 3.56 ± 2.12* |
| After extubation | 3.72 ± 0.73 | 2.12 ± 0.66* | 3 ± 2.06 | |
| 1 hr after extubation | 3.52 ± 0.58 | 1.8 ± 0.91* | 2.24 ± 1.33*# | |
| 2 hr after extubation | 3.44 ± 0.76 | 2.08 ± 0.95* | 2.48 ± 1.26*# | |
| 4 hr after extubation | 3.4 ± 0.81 | 2 ± 1* | 2.6 ± 1.15*# | |
| 6 hr after extubation | 3.32 ± 0.85 | 2.24 ± 0.92* | 2.64 ± 1.25*# |
Note:
P < 0.05 compared to placebo group,
P < 0.05 compared to tramadol group.
Abbreviations: hr, hour(s); SD, standard deviation.
Figure 2.
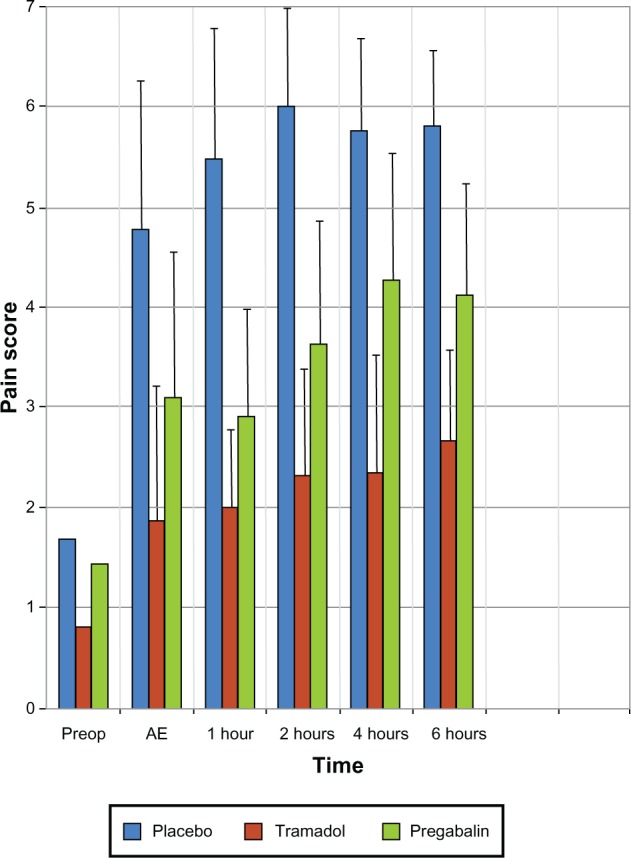
Pain scores in the three groups at different time intervals.
Abbreviations: Preop, preoperatively; AE, after extubation.
The mean anxiety scores were significantly lower in pregabalin and tramadol patients in comparison to placebo patients at all time points of the study (P < 0.05) (Figure 3). After post hoc analysis, anxiety scores in the pregabalin group were found to be significantly higher compared to those of the tramadol patients (P < 0.05), but they were lower than that of the placebo group (P < 0.05).
Figure 3.
Anxiety scores among all the three groups at different time intervals.
Abbreviations: Preop, preoperatively; AE, after extubation.
Preoperatively, the sedation scores were significantly lower in tramadol patients when compared to the pregabalin and placebo patients (P < 0.05). After extubation and postoperatively, the level of sedation was significantly increased in both tramadol and pregabalin patients when compared to placebo (P < 0.05). Following post hoc analysis, the degree of sedation in the pregabalin group was found to be significantly less in comparison to tramadol patients, but higher than that of the placebo group (P < 0.05) (Figure 4).
Figure 4.
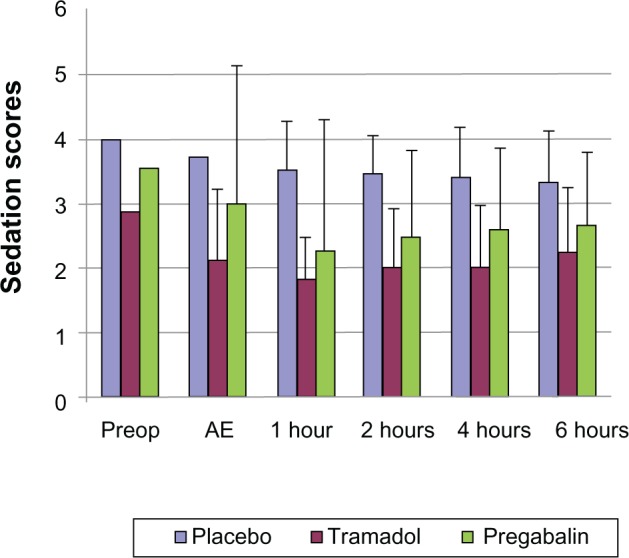
Sedation scores among all the three groups at different time intervals.
Abbreviations: Preop, preoperatively; AE, after extubation.
The mean doses of fentanyl and diclofenac given for the frst 6 hours of the postoperative period are given in Table 3. The analgesic requirements were significantly reduced in the tramadol and pregabalin groups when compared to placebo. The adverse effects observed in the three groups are given in Table 4. Complications like nausea, vomiting, and drowsiness were the highest in the tramadol patients and the least in the pregabalin patients, though this finding was statistically insignificant (P > 0.05).
Table 3.
Dosage of rescue analgesic drugs in the three groups
| Drugs | Group 1: Placebo (Mean ± SD) | Group 2: Tramadol (Mean ± SD) | Group 3: Pregabalin (Mean ± SD) |
|---|---|---|---|
| Diclofenac (mg) | 38 ± 21.79* | 12 ± 21.8* | 30 ± 20.80*,# |
| Fentanyl (mic) | 31.2 ± 27.73* | 7.2 ± 16.20* | 24.8 ± 22.0*,# |
Note:
P <0.05 compared to placebo group,
P < 0.05 compared to tramadol group.
Abbreviation: SD, standard deviation.
Table 4.
Adverse effects in the three groups
| Group 1: Placebo N(%) | Group 2: Tramadol N(%) | Group 3: Pregabalin N(%) | |
|---|---|---|---|
| Nausea | 2(8%) | 5(20%) | 1(4%) |
| Vomiting | 3(12%) | 5(20%) | 1(4%) |
| Drowsiness | 1(4%) | 8(32%) | 1(4%) |
Discussion
In the present study, there was a significant decrease in the pain scores of the patients who received tramadol and pregabalin in comparison to the placebo group. The tramadol group had lower pain scores compared to the pregabalin and placebo groups. We also observed that the analgesic efficacy of tramadol was superior to that of pregabalin, but when compared to placebo, pregabalin was more effective in reducing pain. The control group required a greater amount of rescue analgesia, and hence the total dose of fentanyl and diclofenac given for the first 6 hours during the postoperative period was relatively more when compared to the pregabalin and tramadol groups. The patients in the tramadol group required significantly less rescue analgesia than the pregabalin patients. This indicates that pregabalin has some opioid sparing effects; this is in agreement with previous studies.27–33
Pregabalin has previously been shown to have good analgesic efficacy in patients with spinal cord injury,34 postherpetic neuralgia,35 dental surgery,36 gynecological surgery,32 and in patients following lumbar laminectomy and discectomy.37 However, the doses required for analgesia varied in these studies from 75 mg to 300 mg per day.
In our study, the anxiety scores were significantly lower in the pregabalin and tramadol groups when compared to the placebo group. However, the anxiety scores in the pregabalin group were found to be significantly higher in comparison to the tramadol group, but lower than the placebo group. This shows that pregabalin also has an anxiolytic effect, although it is to a lesser degree when compared to tramadol. Our observation is in line with previous studies.37,38
We observed that preoperatively, the sedation scores were significantly lower in the tramadol group when compared to the pregabalin and placebo groups. After extubation and postoperatively, the level of sedation was significantly increased in both the tramadol and pregabalin groups when compared to placebo; however, the degree of sedation in the pregabalin group was found to be significantly less in comparison to the tramadol group, but more than the placebo group. From this, we infer that pregabalin has a good anxiolytic effect without resulting in excessive sedation.
Pregabalin had no effect on heart rate, which is consistent with animal experiments showing that intrathecal administration of the related compound, gabapentin, does not alter resting or acutely evoked autonomic outflow.39 However, the systolic and diastolic blood pressures were reduced at 5 minutes after intubation and immediately after extubation in the tramadol patients and the pregabalin patients when compared to placebo, though the decrease in pregabalin was not statistically significant as in tramadol patients. This observation is in agreement with earlier reports that tramadol attenuates the pressor response to endotracheal intubation.40
As shown in Table 4, drowsiness was less frequent with pregabalin and was seen in 4% of the patients compared to 32% in the tramadol group. Fewer patients experienced nausea (4%) and vomiting (4%) in the pregabalin group compared to the placebo group (nausea 8%, vomiting 12%) and the tramadol group (nausea 20%, vomiting 20%), implying that the incidence of nausea and vomiting is more in the tramadol group and the placebo group than in the pregabalin group.
This is in agreement with previous reports,37 which assessed the efficacy and tolerability of pregabalin and found pregabalin to be well tolerated at all doses, while dizziness, drowsiness, nausea, vomiting, and headache were the common adverse effects observed after tramadol.41 A higher incidence of these adverse effects was seen at higher doses, due to a dose–response effect.42
The following conclusions can be drawn from the study.
Pregabalin has a statistically significant analgesic effect when compared to placebo, but this effect is less when compared to tramadol.
The need for rescue analgesia is the least in tramadol patients followed by pregabalin, and it reached a maximum in the control group.
Pregabalin has a statistically significant anxiolytic effect compared to placebo.
The anxiolytic effect of pregabalin is associated with less sedation when compared to tramadol.
Pregabalin has a lower number of postoperative complications of nausea, vomiting, and drowsiness when compared to tramadol.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cousins MJ, Power I. Acute and postoperative pain. In: Wall PD, Melzack R, editors. Textbook of Pain. 4th ed. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 2.Warner DO. Preventing postoperative pulmonary complications. The role of the anesthesiologist. Anesthesiology. 2000;92(5):1467–1472. doi: 10.1097/00000542-200005000-00037. [DOI] [PubMed] [Google Scholar]
- 3.Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care. 2005;8(1):23–32. doi: 10.1097/00075197-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Apfelbaun JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 5.Tverskoy M, Oren M, Dashkovsky I, Kissin I. Alfentanil dose-response relationships for relief of postoperative pain. Anesth Analg. 1996;83(2):387–393. doi: 10.1097/00000539-199608000-00032. [DOI] [PubMed] [Google Scholar]
- 6.Joris JL, Hinque VL, Laurent PE, Desaive CJ, Lamy ML. Pulmonary function and pain after gastroplasty performed via laparotomy or laparoscopy in morbidly obese patients. Br J Anaesth. 1998;80(3):283–288. doi: 10.1093/bja/80.3.283. [DOI] [PubMed] [Google Scholar]
- 7.Garnett RL, MacIntyre A, Lindsay P, et al. Perioperative ischaemia in aortic surgery: combined epidural/general anaesthesia and epidural analgesia vs general anaesthesia and i.v. analgesia. Can J Anaesth. 1996;43(8):769–777. doi: 10.1007/BF03013027. [DOI] [PubMed] [Google Scholar]
- 8.Modig J, Borg T, Karlström G, Maripuu E, Sahlstedt B. Thromboembolism after total hip replacement: role of epidural and general anesthesia. Anesth Analg. 1983;62(2):174–180. [PubMed] [Google Scholar]
- 9.Lawrence VA, Hilsenbeck SG, Mulrow CD, Dhanda R, Sapp J, Page CP. Incidence and hospital stay for cardiac and pulmonary complications after abdominal surgery. J Gen Intern Med. 1995;10(12):671–678. doi: 10.1007/BF02602761. [DOI] [PubMed] [Google Scholar]
- 10.Caprini JA, Botteman MF, Stephens JM, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health. 2003;6(1):59–74. doi: 10.1046/j.1524-4733.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- 11.Iannone A, Baker AB, Morrell F. Dilantin in the treatment of trigeminal neuralgia. Neurology. 1958;8(2):126–128. doi: 10.1212/wnl.8.2.126. [DOI] [PubMed] [Google Scholar]
- 12.Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29(3):265–267. doi: 10.1136/jnnp.29.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104–2110. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 14.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain. 2005;6(4):253–260. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280(21):1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- 16.Dworkin RH, Corbin AE, Young JP, Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60(8):1274–1283. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- 17.Dahl JB, Mathiesen O, Møiniche S. ‘Protective premedication’: an option with gabapentin and related drugs? A review of gabapentin and pregabalin in the treatment of post-operative pain. Acta Anesthesiol Scand. 2004;48(9):1130–1136. doi: 10.1111/j.1399-6576.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 18.Gilron I, Orr E, Tu D, O’Neill P, Zamora JE, Bell AC. A placebo-controlled randomized clinical trial of perioperative administration of gabapentin, rofecoxib and their combination for spontaneous and movement-evoked pain after abdominal hysterectomy. Pain. 2005;113(1–2):191–200. doi: 10.1016/j.pain.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Dooley DJ, Donovan CM, Pugsley TA. Stimulus-dependent modulation of [(3)H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J Pharmacol Exp Ther. 2000;295(3):1086–1093. [PubMed] [Google Scholar]
- 20.Dooley DJ, Mieske CA, Borosky SA. Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett. 2000;280(2):107–110. doi: 10.1016/s0304-3940(00)00769-2. [DOI] [PubMed] [Google Scholar]
- 21.Fink K, Dooley DJ, Meder WP, et al. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42(2):229–236. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 22.Maneuf YP, Hughes J, McKnight AT. Gabapentin inhibits the substance P-facilitated K(+)-evoked release of [(3)H]glutamate from rat caudal trigeminal nucleus slices. Pain. 2001;93(2):191–196. doi: 10.1016/S0304-3959(01)00316-5. [DOI] [PubMed] [Google Scholar]
- 23.Errante LD, Petroff OA. Acute effects of gabapentin and pregabalin on rat forebrain cellular GABA, glutamate, and glutamine concentrations. Seizure. 2003;12(5):300–306. doi: 10.1016/s1059-1311(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 24.Frampton JE, Scott LJ. Pregabalin: in the treatment of painful diabetic peripheral neuropathy. Drugs. 2004;64(24):2813–2820. doi: 10.2165/00003495-200464240-00006. discussion 2821. [DOI] [PubMed] [Google Scholar]
- 25.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 26.Raval DL, Mehta MK. Oral clonidine pre medication for attenuation of haemodynamic response to laryngoscopy and intubation. Indian J Anaesth. 2002;46(2):124–129. [Google Scholar]
- 27.Pandey CK, Sahay S, Gupta D, Ambesh SP, et al. Preemptive gabapentin decreases postoperative pain after lumbar discoidectomy. Can J Anaesth. 2004;51(10):986–989. doi: 10.1007/BF03018484. [DOI] [PubMed] [Google Scholar]
- 28.Turan A, Karamanlioğlu B, Memiş D, et al. Analgesic effects of gabapentin after spinal surgery. Anesthesiology. 2004;100(4):935–938. doi: 10.1097/00000542-200404000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Pandey CK, Navkar DV, Giri PJ, et al. Evaluation of the optimal preemptive dose of gabapentin for postoperative pain relief after lumbar diskectomy: a randomized, double-blinded, placebo-controlled study. J Neurosurg Anesthsiol. 2005;17(2):65–68. doi: 10.1097/01.ana.0000151407.62650.51. [DOI] [PubMed] [Google Scholar]
- 30.Turan A, Karamanlioğlu B, Memiş D, Usar P, Pamukçu Z, Türe M. The analgesic effects of gabapentin after total abdominal hysterectomy. Anesth Analg. 2004;98(5):1370–1373. doi: 10.1213/01.ane.0000108964.70485.b2. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.Fassoulaki A, Patris K, Sarantapoulos C, Hogan Q. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesth Analg. 2002;95(4):985–991. doi: 10.1097/00000539-200210000-00036. table of contents. [DOI] [PubMed] [Google Scholar]
- 32.Jokela R, Ahonen J, Tallgren M, Haanpää M, Korttila K. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008;134(1–2):106–112. doi: 10.1016/j.pain.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Gianesello L, Pavoni V, Barboni E, Galeotti I, Nella A. Perioperative pregabalin for postoperative pain control and quality of life after major spinal surgery. J Neurosurg Anesthesiol. 2012;24(2):121–126. doi: 10.1097/ANA.0b013e31823a885b. [DOI] [PubMed] [Google Scholar]
- 34.Tzellos TG, Papazisis G, Amaniti E, Kouvelas D. Efficacy of pregabalin and gabapentin for neuropathic pain in spinal-cord injury: an evidence-based evaluation of the literature. Eur J Clin Pharmacol. 2008;64(9):851–858. doi: 10.1007/s00228-008-0523-5. [DOI] [PubMed] [Google Scholar]
- 35.Achar A, Chakraborty PP, Bisai S, Biswas A, Guharay T. Comparative study of clinical efficacy of amitriptyline and pregabalin in postherpetic neuralgia. Acta Dermatovenerol Croat. 2012;20(2):89–94. [PubMed] [Google Scholar]
- 36.Hill CM, Balkenohl M, Thomas DW, Walker R, Mathé H, Murray G. Pregabalin in patients with postoperative dental pain. Eur J Pain. 2001;5(2):119–124. doi: 10.1053/eujp.2001.0235. [DOI] [PubMed] [Google Scholar]
- 37.Ozgencil E, Yalcin S, Tuna H, Yorukoglu D, Kecik Y. Perioperative administration of gabapentin 1,200 mg day-1 and pregabalin 300 mg day-1 for pain following lumbar laminectomy and discectomy: a randomised, double-blinded, placebo-controlled study. Singapore Med J. 2011;52(12):883–889. [PubMed] [Google Scholar]
- 38.Ménigaux C, Adam F, Guignard B, Sessler DI, Chauvin M. Preoperative gabapentin decreases anxiety and improves early functional recovery from knee surgery. Anesth Analg. 2005;100(5):1394–1399. doi: 10.1213/01.ANE.0000152010.74739.B8. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon MH, Yakash TL. The effect of intrathecal gabapentin on pain behavior and hemodynamics on the formalin test in the rat. Anesth Analg. 1999;89(2):434–439. doi: 10.1097/00000539-199908000-00034. [DOI] [PubMed] [Google Scholar]
- 40.van den Berg AA, Halliday EM, Soomro EM, Rasheed A, Baloch M. Reducing cardiovascular responses to laryngoscopy and tracheal intubation: a comparison of equipotent doses of tramadol, nalbuphine and pethidine, with placebo. Middle East J Anesthesiol. 2004;17(6):1023–1036. [PubMed] [Google Scholar]
- 41.Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta-analysis of single-dose oral tramadol plus acetaminophen in acute postoperative pain. J Pain Symptom Manage. 2002;23(2):121–130. doi: 10.1016/s0885-3924(01)00404-3. [DOI] [PubMed] [Google Scholar]
- 42.Moore RA, McQuay HJ. Single-patient data meta-analysis of 3453 postoperative patients: oral tramadol versus placebo, codeine and combination analgesics. Pain. 1997;69(3):287–294. doi: 10.1016/S0304-3959(96)03291-5. [DOI] [PubMed] [Google Scholar]