Abstract
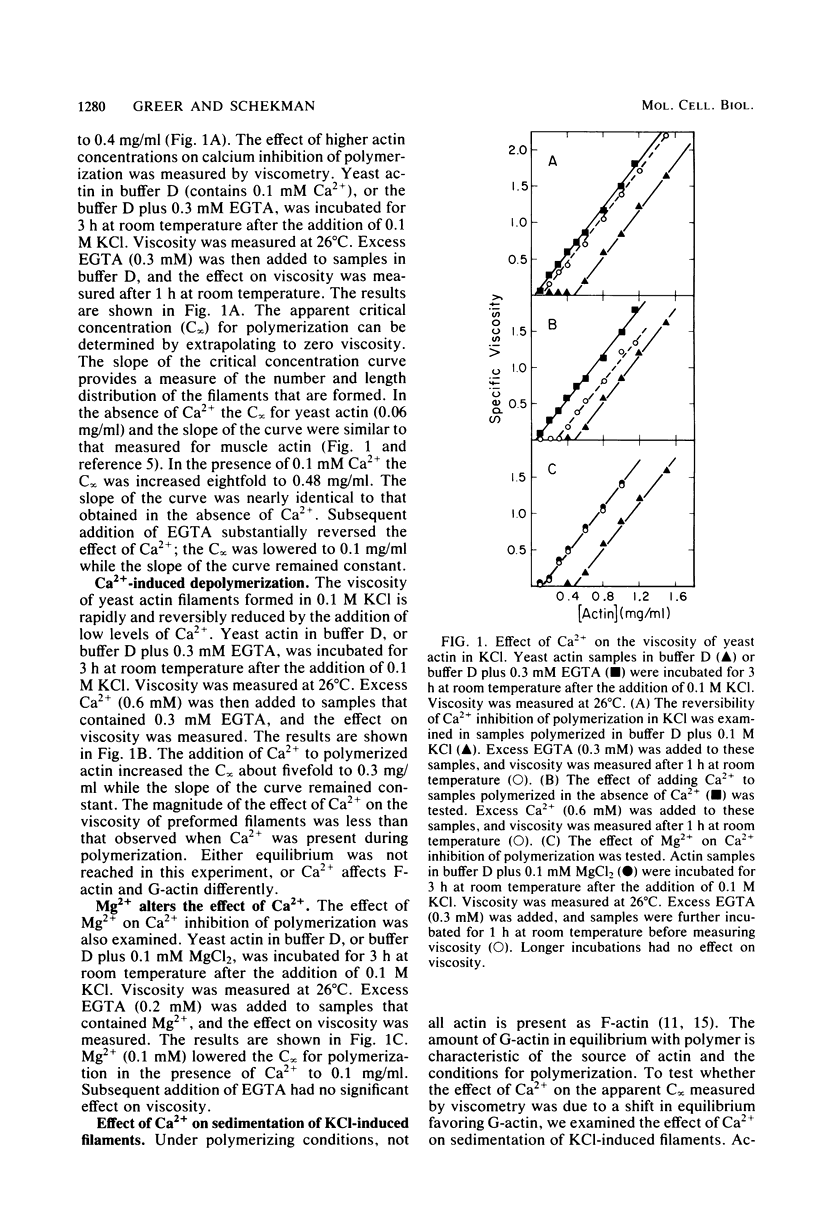
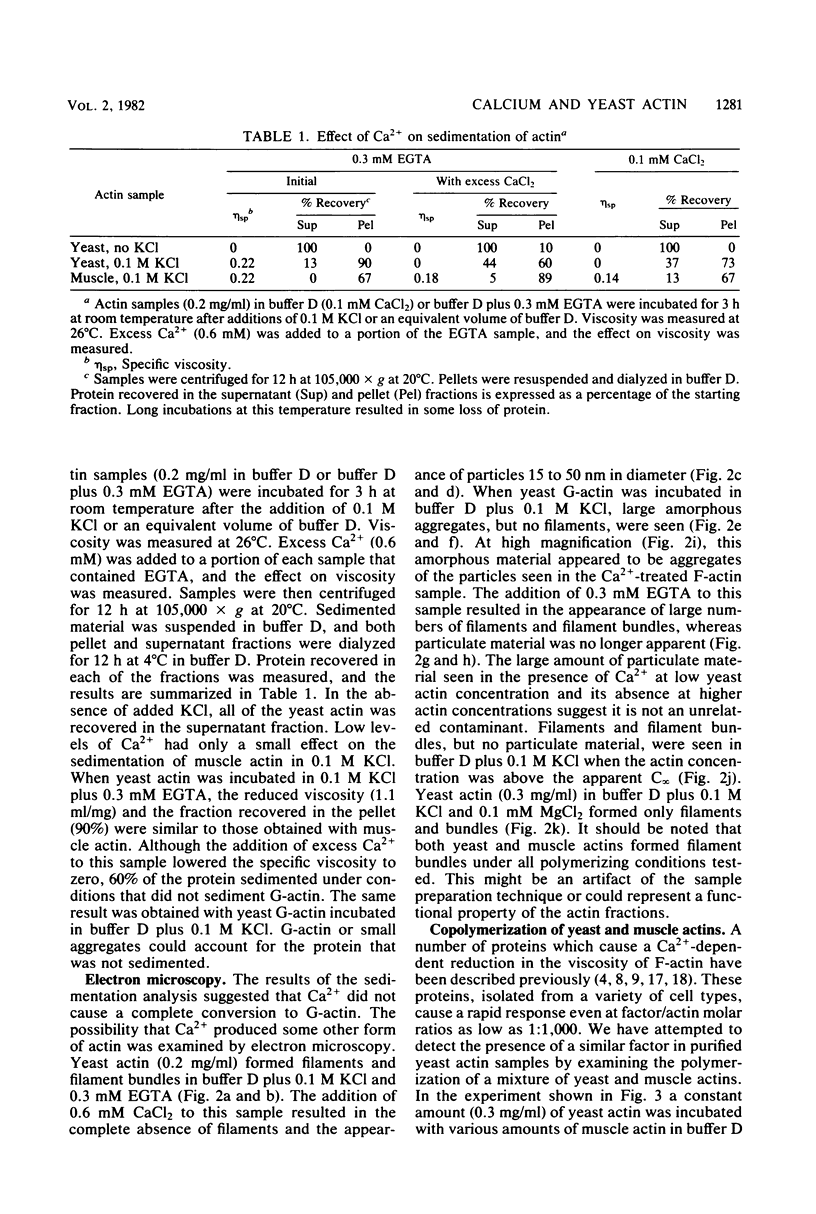
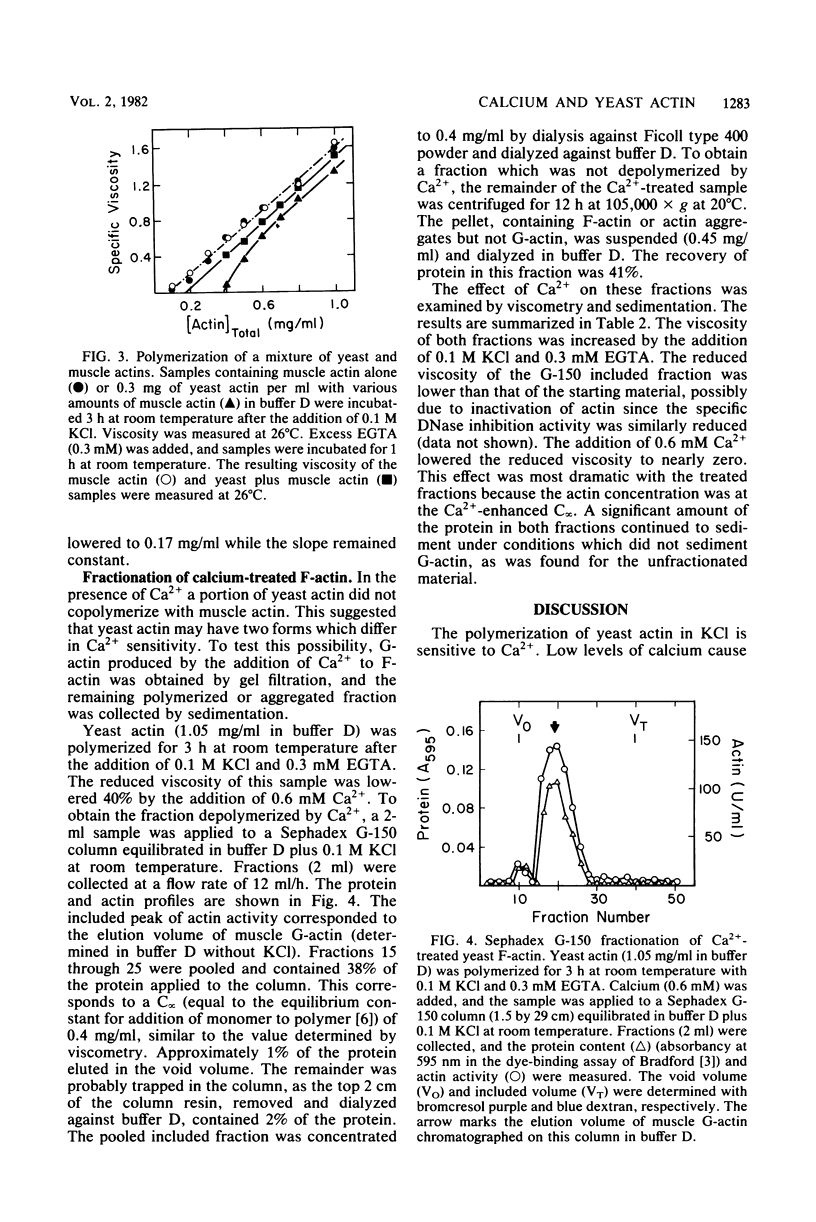
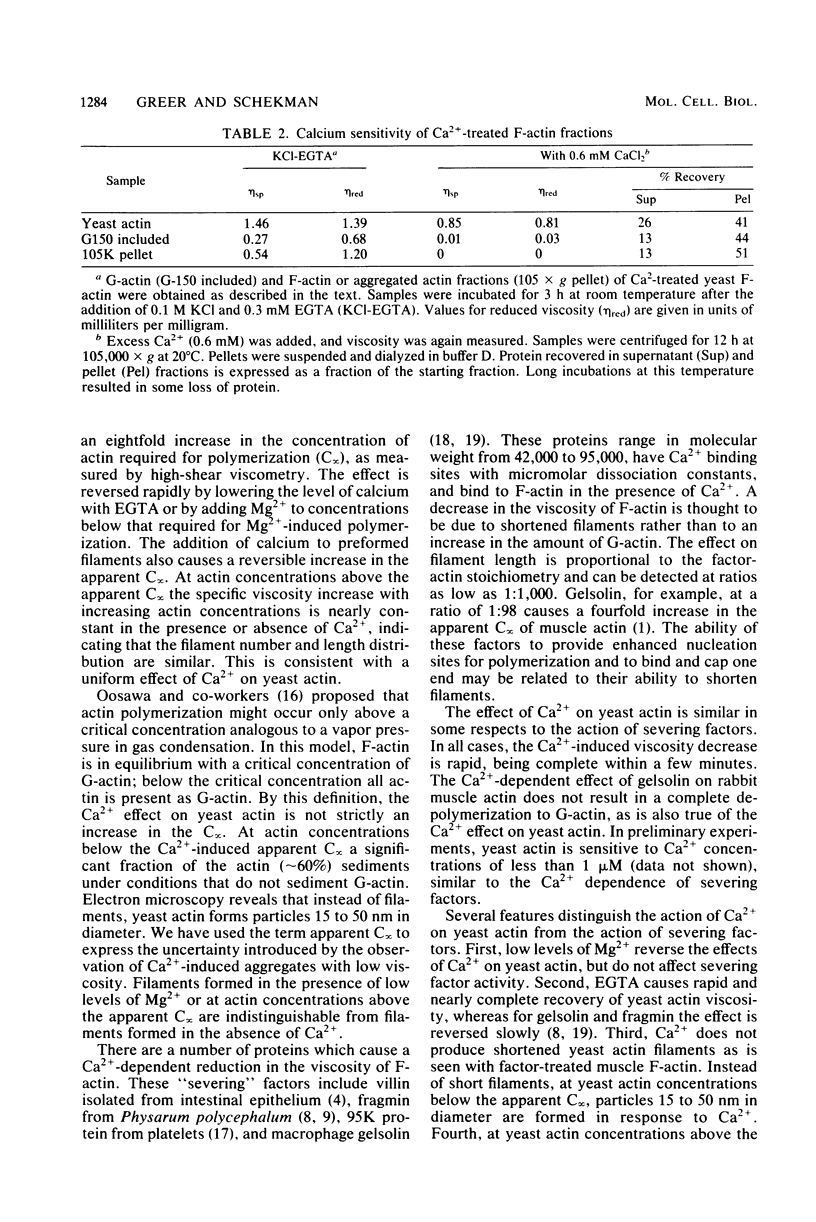
Low levels of Ca2+ dramatically influence the polymerization of Saccharomyces cerevisiae actin in KCl. The apparent critical concentration for polymerization (C infinity) increases eightfold in the presence of 0.1 mM Ca2+. This effect is rapidly reversed by the addition of ethylene glycol bis(beta-aminoethyl ether)-N,N'-tetraacetic acid or of 0.1 mM Mg2+. Furthermore, the addition of Ca2+ to polymerized actin causes a reversible increase in the apparent C infinity. In the presence of Ca2+, at actin concentrations below the apparent C infinity, particles of 15 to 50 nm in diameter are seen instead of filaments. These particles are separated from soluble actin when Ca2+-treated filamentous actin is sedimented at high speed; both the soluble and particulate fractions retain Ca2+-sensitive polymerization. The Ca2+ effect is S. cerevisiae actin-specific: the C infinity for rabbit muscle actin is not affected by the presence of Ca2+ and S. cerevisiae actin. Ca2+ may act directly on S. cerevisiae actin to control the assembly state in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar N., Kaminsky E., Leibovich S. J., Oplatka A. Rabbit skeletal muscle F-actin can be stable at low ionic strength, provided trace amounts of Ca2+ are absent. Biochim Biophys Acta. 1979 Apr 25;577(2):267–272. doi: 10.1016/0005-2795(79)90030-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Seidel R. Molecular cloning of the actin gene from yeast Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Mar 11;8(5):1043–1059. doi: 10.1093/nar/8.5.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J., Boyer J. L., Korn E. D. Comparative biochemistry of non-muscle actins. J Biol Chem. 1977 Nov 25;252(22):8300–8309. [PubMed] [Google Scholar]
- Greer C., Schekman R. Actin from Saccharomyces cerevisiae. Mol Cell Biol. 1982 Oct;2(10):1270–1278. doi: 10.1128/mcb.2.10.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T., Takahashi S., Hayashi H., Hatano S. Fragmin: a calcium ion sensitive regulatory factor on the formation of actin filaments. Biochemistry. 1980 Jun 10;19(12):2677–2683. doi: 10.1021/bi00553a021. [DOI] [PubMed] [Google Scholar]
- Hinssen H. An actin-modulating protein from Physarum polycephalum. II. Ca++-dependence and other properties. Eur J Cell Biol. 1981 Feb;23(2):234–240. [PubMed] [Google Scholar]
- Hitchcock-DeGregori S. E. Actin assembly. Nature. 1980 Dec 4;288(5790):437–438. doi: 10.1038/288437a0. [DOI] [PubMed] [Google Scholar]
- KASAI M., ASAKURA S., OOSAWA F. The G-F equilibrium in actin solutions under various conditions. Biochim Biophys Acta. 1962 Feb 12;57:13–21. doi: 10.1016/0006-3002(62)91072-7. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Abelson J. Isolation and sequence of the gene for actin in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3912–3916. doi: 10.1073/pnas.77.7.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Field C., Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980 Aug;21(1):205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Zaner K. S., Stossel T. P. Ca2+ control of actin gelation. Interaction of gelsolin with actin filaments and regulation of actin gelation. J Biol Chem. 1980 Oct 10;255(19):9494–9500. [PubMed] [Google Scholar]




