Abstract
Developmental patterning requires the precise interplay of numerous intercellular signaling pathways to ensure that cells are properly specified during tissue formation and organogenesis. The spatiotemporal function of the Notch signaling pathway is strongly influenced by the biosynthesis and intracellular trafficking of signaling components. Receptors and ligands must be trafficked to the cell surface where they interact, and their subsequent endocytic internalization and endosomal trafficking is crucial for both signal propagation and its down-modulation. In a forward genetic screen for mutations that alter intracellular Notch receptor trafficking in Drosophila epithelial tissues, we recovered mutations that disrupt the Catsup gene, which encodes the Drosophila ortholog of the mammalian ZIP7 zinc transporter. Loss of Catsup function causes Notch to accumulate abnormally in the endoplasmic reticulum (ER) and Golgi compartments, resulting in impaired Notch signaling. In addition, Catsup mutant cells exhibit elevated ER stress, suggesting that impaired zinc homeostasis causes increased levels of misfolded proteins within the secretory compartment.
Keywords: Drosophila, Notch, Protein trafficking, Secretory pathway, Zinc transporter
INTRODUCTION
Developmental patterning in metazoans requires the coordinated activity of several intercellular signaling pathways, including the Notch, Wnt, Hedgehog (Hh), Epidermal Growth Factor Receptor (EGFR), Transforming Growth Factor β (TGFβ), Hippo, Fibroblast Growth Factor (FGF) and JAK/STAT pathways. Signaling activities of these pathways are tightly modulated by numerous post-translational processes, many of which directly modify the biochemical properties of the relevant receptors, ligands and other pathway components. For example, lipid modification of Wnt and Hh signaling proteins affects their transport and signaling activities (Steinhauer and Treisman, 2009), and glycosylation of the extracellular domain of the Notch receptor influences its folding and differential affinities for alternative ligands (Rana and Haltiwanger, 2011). In addition, trafficking of signaling pathway components through the secretory pathway to the cell surface, and their subsequent endocytosis and endosomal trafficking, exert profound effects on the strength and duration of developmental signaling. Numerous studies have established that these trafficking processes are crucial for the biosynthesis of appropriate levels of receptors, ligands and other pathway components; the recycling and/or degradation of non-activated receptors; and the efficient propagation of intracellular signals from activated receptor-ligand complexes (Andersson, 2012; Gonnord et al., 2012; Parachoniak and Park, 2012). Although the core mechanisms of the major developmental signaling pathways have now been largely elucidated by genetic and molecular analyses, the more subtle and often pleiotropic effects of membrane trafficking events on these pathways are less well understood.
To identify new genes that are required for trafficking of developmental signaling molecules, we performed a forward genetic screen for mutations that alter the intracellular accumulation of the Notch receptor in developing Drosophila wing tissues. Among the ∼40 new mutants recovered, we obtained two new alleles of the Catecholamines up (Catsup) gene, which has previously been implicated as a negative regulator of tyrosine hydroxylase activity during catecholamine biosynthesis (Stathakis et al., 1999), in synaptic vesicle loading and release of dopamine (Wang et al., 2011), and in the control of sleep behavior (Harbison et al., 2009) in Drosophila. The Catsup gene encodes the Drosophila ortholog of the mammalian ZIP7 protein (also known as SLC39A7), a zinc transporter belonging to the ZIP (Zrt/Irt-like protein) family. Mammalian ZIP7 proteins are present in the endoplasmic reticulum (ER) and Golgi compartments, where they contribute to the release of labile Zn2+ cations into the cytosol from ER/Golgi stores (Taylor et al., 2004; Huang et al., 2005). ZIP7-mediated release of zinc regulates both cell growth and differentiation pathways involving EGFR, HER2, IGF1R and Src signaling, in which cytosolic labile Zn2+ is proposed to inhibit the activity of phosphatases, promoting increased tyrosine kinase activity in these pathways (Murakami and Hirano, 2008; Taylor et al., 2008; Hogstrand et al., 2009). ZIP7 is among the 10% of genes consistently overexpressed in many breast cancers with poor prognosis (Taylor et al., 2007; Hogstrand et al., 2009), and has been shown to contribute to the tamoxifen resistance of EGFR-positive breast cancer cells (Taylor et al., 2008).
Here, we show that loss-of-function mutations in the Drosophila ZIP7 ortholog Catsup results in an abnormal accumulation of membrane proteins, including Notch, EGFR and APPL, in the secretory compartment of wing imaginal disc cells. This defect is accompanied by increased levels of apoptosis, reduced Notch signaling and induction of the ER stress response. Moreover, the aberrant trafficking of Notch is not rescued by reducing the Notch dose or by directed expression of the common chaperone Hsc70 or the Notch-specific chaperone Ofut-1 in mutant cells, suggesting that Catsup plays a more general role in the maintenance of ER/Golgi function. Our findings regarding the relationship of Drosophila ZIP7 to Notch receptor trafficking and signaling emphasize the importance of ion transporters for establishing and maintaining the membrane compartments in which key developmental signaling pathways operate, and suggest a possible link between altered zinc homeostasis and developmental disorders involving aberrant receptor signaling.
MATERIALS AND METHODS
Drosophila genetics
Mutagenesis was performed using standard protocols by administering 35 mM ethylmethanesulfonate to isogenic male flies of genotype y w; P{ry[+t7.2]=neoFRT}40A P{w[+mW.hs]=FRT(whs)}G13, which were used to establish candidate mutant stocks. For screening of 3335 mutagenized second chromosome arms, these stocks were mated to marked 2L and 2R FRT stocks to yield progeny bearing homozygous candidate mutant wing clones using the FLP/FRT method (Xu and Harrison, 1994), using P{Ubi-GFP(S65T)nls}2L FRT40A to mark clones with P{hsFLP}12 as the FLP source. Ten wing discs of each candidate mutant line were harvested and analyzed for abnormal Notch accumulation by immunofluorescence using Notch antibody C17.9C6, as described below. MARCM clone studies (Lee and Luo, 2001) were performed using y w; P{w+mC=tubP-GAL80}LL10 P{ry+t7.2=neoFRT}40A/CyO. Catsup mutant alleles used were Catsup47 and Catsup48 (this study) and the amorphic allele Catsup26 (Stathakis et al., 1999) (kindly provided by J. M. O’Donnell, University of Alabama, Tuscaloosa, AL, USA). Transgenic expression stocks used included the da-GAL4 line w1118; P{da-GAL4.w-}3 (flybase.org/reports/FBrf0182749.html) (kindly provided by J. Jiang, UT Southwestern Medical Center, Dallas, TX, USA), the patched-GAL4 line w; P{w+W.hs=GawB}ptc559.1 (Hinz et al., 1994), the ER marker lines w1118; P{w+mC=PTT-GA}PdiG00198 (Morin et al., 2001) and w; P{w+mC=UAS-GFP.KDEL}11.1 (flybase.org/reports/FBrf0195905.html), the Golgi marker line w; P{w+mC=sqh-EYFP-Golgi}3 (LaJeunesse et al., 2004), the p35 expression line w; P{w+mC=UAS-p35.H}BH2 (flybase.org/reports/FBrf0159879.html) (all from Bloomington Drosophila Stock Center), UAS-Xbp1-EGFP (Ryoo et al., 2007) (kindly provided by H. D. Ryoo, NYU Langone Medical Center, NY, USA), UAS-Hsc70-3.WT B (Elefant and Palter, 1999), UAS-O-fut1.O 11.1 (Okajima and Irvine, 2002), and UAS-Catsup-V5 (this study).
Lethal phase determinations of new Catsup alleles were performed as described previously (Stathakis et al., 1999) using Df(2L)BSC257, a deficiency that uncovers Catsup, and confirmed by scoring survival of non-Tb larval progeny from transheterozygous crosses between Catsup47, Catsup48 and the internal deletion allele Catsup26 (Stathakis et al., 1999) using the compound double balancer SM5::TM6B,Tb.
Sequence and phylogenetic analysis
The Drosophila Catsup and human ZIP7 protein sequences were analyzed using SignalP and TMHMM (DTU, Denmark), aligned using ClustalW (EMBL-EBI, UK), and shaded to denote amino acid identity and similarity using Boxshade (Pasteur Institute, France). Phylogenetic analysis was performed using ZIP protein family sequences from the HomoloGene database (NCBI, Bethesda, USA). A ZIP protein tree was generated using the Neighbor-Joining method with Bootstrap values computed using CLC Sequence Viewer (CLC bio, Denmark). Sequence alignment and phylogenetic analysis figures were assembled using Illustrator CS5 (Adobe).
Immunohistology
Wing imaginal discs were dissected, fixed for 30 minutes in PLP fixative (Gaul et al., 1992) and immunostained (Hu and Fortini, 2003) using the following primary antibodies: mouse Notch intracellular domain antibody C17.9C6 (1:500) (Fehon et al., 1990) (DSHB); mouse Notch extracellular domain antibody C458.2H (1:500) (Diederich et al., 1994) (DSHB); mouse Delta antibody C594.9B (1:500) (Qi et al., 1999) (DSHB); mouse Cut 2B10 (1:1000) (Blochlinger et al., 1990) (DSHB); rat anti-DE-Cad DCAD2 (1:20) (Oda et al., 1994) (DSHB); mouse anti-Lamin ADL67.10 (1:50) (Riemer et al., 1995) (DSHB); mouse anti-α-Spectrin 3A9 (1:10) (Dubreuil et al., 1987) (DSHB); goat anti-EGFR dC-20 and dL-20 (1:500 each; Santa Cruz Biotechnology); rabbit anti-cleaved Caspase 3 9661S (1:200; Cell Signaling); rabbit anti-baculovirus p35 IMG-5740 (1:1000; Imgenex); rabbit anti-Hsc70 NBP1-55105 (1:400; Novus Biologicals); mouse anti-β-galactosidase Z37BA (1:1000; Promega); mouse anti-V5 mAb (1:1000; Invitrogen); rabbit anti-V5 NB600-381 (1:500; Novus Biologicals); rabbit anti-GFP 598 (1:1000; MBL); and rat anti-GFP GF090R (1:500; NacalaiTesque).
For live tissue labeling, wing discs were dissected on ice in Schneider cell medium (Gibco) and incubated with antibody for 40 minutes at room temperature, using mouse anti-Notch antibody C458.2H (1:500) directed against the extracellular domain of Notch or mouse anti-Delta antibody C594.9B (1:500) directed against the extracellular domain of Delta. Unbound antibody was removed by rinsing three times followed by two 10-minute washes in Schneider cell medium on ice. The discs were then fixed in PLP for 30 minutes and processed further with secondary antibodies as above.
For nuclear staining, immunostained wing discs were incubated for 15 minutes at room temperature in a 1:1000 dilution of Hoechst 33258 (Invitrogen) (Latt et al., 1975) in PBS-T (PBS with 0.3% Triton X-100), followed by two 10-minute washes in PBS-T prior to mounting under coverslips.
For zinc probe studies, third instar whole brain-imaginal disc complexes were dissected and incubated for 2 hours in S2 cell medium (Gibco BRL), followed by three 20-minute washes in PBS, fixation in PLP for 30 minutes and Notch C17.9C6 antibody staining as above. The following zinc probes and concentrations were used: 5 μM FluoZin-3 (Invitrogen), 25 μM Newport Green (Invitrogen) and 100 μM Zinquin (Enzo Life Sciences).
Adult wing analysis
Adult wings were removed, mounted in DPX mounting medium (Electron Microscopy Sciences) under coverslips and examined by bright-field microscopy.
RESULTS
Abnormal subcellular accumulation of the Notch receptor in Drosophila Catsup mutants
To identify new genes required for proper Notch trafficking, we designed a forward genetic screen in which homozygous mutant tissue is directly examined for aberrant Notch accumulation using antibody immunoanalysis. Because important trafficking genes would likely encode products essential for organismal viability, we created clones of homozygous mutant tissue in developing imaginal wing discs of otherwise heterozygous Drosophila using the FLP-FRT mosaic method (Xu and Harrison, 1994) (see Materials and methods). This approach also allows mutant tissues to be compared directly with adjacent heterozygous tissue in each sample, eliminating variability in fixation time, antibody penetration and other parameters. Following screening of 3335 mutagenized second chromosome arms, we recovered over 40 genes that, when mutated, alter the pattern of Notch trafficking, as visualized using an antibody directed against the Notch intracellular domain (mAb C17.9C6) (Fehon et al., 1990). Because the immunoscreening protocol detects total Notch protein using an antibody directed against the Notch intracellular domain in fixed permeabilized tissue, new mutations were recovered that alter secretory and/or endocytic trafficking of Notch.
Two new mutant alleles of the Drosophila Catsup gene (Stathakis et al., 1999; Harbison et al., 2009; Wang et al., 2011) were recovered that display an abnormal pattern of Notch accumulation in homozygous mutant clones. In both mutants, Notch accumulates within the cytoplasm of wing disc mutant clone cells, leading to abnormally high levels of Notch through the entire apicobasal profile of the polarized columnar epithelium (Fig. 1A-C′). Although most of the abnormal Notch accumulation is detected in basal cell regions, aberrant Notch localization is also observed in the apical cell regions near the septate junctions of the wing disc columnar epithelium (Fig. 1A,A′). To determine whether Notch accumulates predominantly in secretory or endocytic membrane compartments, we employed a live-cell antibody binding method on Catsup clone-bearing wing discs. When live, non-permeabilized discs are incubated with antibodies that bind to the extracellular domain of Notch (mAb C458.2H; Diederich et al., 1994), antibody access requires exposure of the Notch epitope at the cell surface, so the antibody fails to label the secretory pool of newly synthesized Notch that has not yet reached the surface (Periz and Fortini, 1999). Incubating Catsup mosaic mutant discs with anti-Notch extracellular antibody C458.2H, followed by tissue fixation and imaging revealed that no abnormal Notch accumulation was observed for the non-permeabilized mutant cells, implying that the aberrant Notch trafficking detected in the earlier total Notch immunostaining analysis reflected an accumulation of Notch in the secretory pathway (Fig. 1D-E′). We did not detect any apparent loss of apical membrane-localized Notch in this live-cell staining approach (Fig. 1D,D′), implying that although reduced Catsup activity causes over-accumulation of Notch in the secretory pathway, it does not completely abrogate transport of Notch to the cell surface, the site of ligand-induced Notch activation.
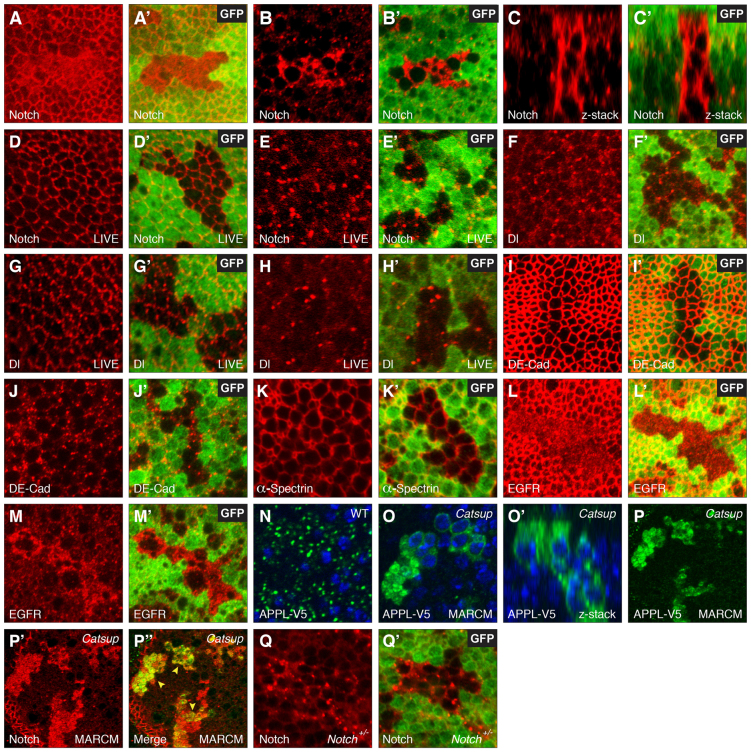
Fig. 1.
Notch accumulates abnormally in pre-endocytic compartments in Catsup mutant tissues. (A-M′) Confocal optical sections through Drosophila wing imaginal discs bearing homozygous mutant clones of Catsup, immunostained for specific proteins (red) together with the corresponding GFP signal (green) to identify clone locations in each image pair (absence of green signal in A′,B′,C′,D′,E′,F′,G′,H′,I′,J′,K′,L′,M′). (A-C′) Notch accumulation in apical membranes (A,A′) and basal cell regions (B,B′) of fixed tissue clones, and in a confocal z-series (C,C′) encompassing the apicobasal extent of the disc monolayer epithelium from apical (top) to basal (bottom). (D-E′) Distribution of newly endocytosed Notch in live tissue that was labeled with antibodies directed against the Notch extracellular domain prior to fixation, showing apical membranes (D,D′) and basal cell regions (E,E′). (F-H′) Delta (Dl) protein distribution in basal cell regions of fixed tissue (F,F′), apical membranes of live-stained tissue (G,G′) and basal cell regions (H,H′) of live-stained tissue. (I-J′) DE-Cadherin (DE-Cad; apical region in I,I′; basal region in J,J′). (K,K′) α-Spectrin distribution in fixed tissue clones. (L-M′) EGFR distribution in apical (L,L′) and basal (M,M′) regions of fixed tissue clones. (N) Distribution of epitope-tagged Amyloid Precursor-like Protein (APPL-V5) expressed under control of daughterless gene regulatory elements in wild-type control tissue. (O,O′) Accumulation of APPL-V5 (green) in Catsup mutant clones, shown in an apical view (O) and in an apicobasal confocal z-series (O′). Catsup clones were produced in wing discs using the MARCM system (Lee and Luo, 2001) to express APPL-V5 in clone cells only. Nuclei are counterstained with Hoechst 33258 (blue) in panels N-O′. (P-P′) Colocalization of transgenically expressed APPL-V5 (P; green signal) and endogenous Notch (P′; red signal) in fixed Catsup clone tissue; merged overlay of the confocal signals in P and P′ is shown in P′; yellow arrowheads indicate colocalized Notch and APPL-V5 overaccumulation. (Q,Q′) Notch accumulation in a basal cell region of a Catsup mutant clone in a N54l9/+ genetic background (Notch signal in red; GFP signal in green as in A-M′); heterozygous Notch larvae were identified by their reduced Cut expression at the DV boundary (see Materials and methods).
We also examined the distribution of several other membrane-bound proteins in Catsup mutant clones with both the fixed-tissue and live-cell methods to gauge the specificity of the protein trafficking phenotype. No abnormal accumulation was detected for the Notch ligand Delta (Fig. 1F-H′), DE-Cadherin (Fig. 1I-J′) or α-Spectrin (Fig. 1K,K′) when monitored using antibodies directed against these proteins (Dubreuil et al., 1987; Oda et al., 1994; Qi et al., 1999). By contrast, cells mutant for Catsup exhibited increased accumulation of EGFR in basal cell regions and reduced levels of EGFR at the apical plasma membrane (Fig. 1L-M′).
We also investigated whether loss of Catsup activity alters the subcellular distribution of the Drosophila Amyloid Precursor-Like Protein (APPL), which, like the Notch receptor, is a Type I single-pass transmembrane protein that undergoes intramembrane proteolysis mediated by the γ-secretase complex (Groth et al., 2010). Transgenic Drosophila were produced in which an epitope-tagged APPL-V5 protein was expressed in wing imaginal discs under the control of daughterless-GAL4 (da-GAL4) (flybase.org/reports/FBrf0182749.html), and in which expression of APPL-V5 is directed exclusively to homozygous mutant clonal tissue using the MARCM system (Lee and Luo, 2001) (see Materials and methods). In contrast to the above clone studies, the MARCM system generates Catsup mutant clones that are positively marked by expression of the UAS-driven APPL-V5, which is indicated in green in Fig. 1N-P′. Comparison of APPL-V5 protein distribution in wild-type control tissue and homozygous Catsup mutant clones revealed that APPL accumulates abnormally at high levels in Catsup-deficient cells relative to wild-type control cells (Fig. 1N-O′). In addition, APPL-V5 displays a high degree of colocalization with abnormal Notch accumulation within these Catsup mutant clones (Fig. 1P-P′).
To address whether the abnormal Notch accumulation seen in Catsup mutant clones can be prevented by reducing the amount of Notch that is trafficked through the secretory pathway, we examined Notch localization in Catsup clones of heterozygous N54l9/+ flies, which bear a protein-null small deficiency for the Notch locus. Reducing the Notch dose fails to suppress the basic trafficking phenotype (Fig. 1Q,Q′), although Notch accumulation seems less prominent than in Catsup clones with a wild-type Notch gene dose (compare Fig. 1Q with 1B).
Molecular lesions in new Catsup alleles
Genetic mapping and complementation analysis was used to establish that the two new mutations are alleles of the Drosophila Catsup gene. To confirm this identification, we generated a UAS-Catsup cDNA construct, and demonstrated that ubiquitous expression of this wild-type Catsup cDNA under the regulatory control of a daughterless-GAL4 driver line was able to fully rescue the lethality associated with either of the new Catsup alleles when transheterozygous with the previously isolated allele Catsup26 (supplementary material Table S1). In accordance with the established mutant allele nomenclature guidelines for Drosophila (flybase.org/static_pages/docs/nomenclature/nomenclature3.html), we refer to our two new Catsup alleles as Catsup47 and Catsup48. Sequencing of these alleles showed that Catsup47 results in a G178D amino acid substitution in the highly conserved second transmembrane segment of the Catsup protein, and that Catsup48 causes a I288T amino acid substitution in the large cytosolic loop domain between the third transmembrane segment and the HELP domain (Suzuki and Endo, 2002) (Fig. 2; supplementary material Fig. S1B). Phylogenetic analysis indicates that the Drosophila Catsup protein is most similar to the mammalian ZIP7 and related ZIP13 classes of zinc transporters, and only distantly related to other mammalian ZIP family members (supplementary material Fig. S1A). With respect to amino acid sequence conservation, Catsup shares 53% identity and 62% similarity with human ZIP7 (supplementary material Fig. S1B).
Fig. 2.
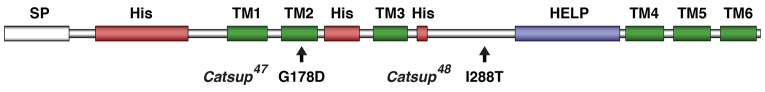
Structure of Drosophila Catsup protein and locations of the Catsup47 and Catsup48 mutations. Catsup protein showing the predicted signal peptide (SP), histidine-rich regions (His), conserved HELP domain (Suzuki and Endo, 2002) and six transmembrane domains (TM1-6). Locations of amino acid alterations in the newly isolated mutants Catsup47 and Catsup48 are indicated.
To gauge the phenotypic severity of our newly recovered Catsup alleles, we determined their lethal phase (see Materials and methods) and compared it with those of a well-characterized allelic series of previously isolated Catsup mutations (Stathakis et al., 1999). Both Catsup47 and Catsup48 cause lethality at the first instar larval stage: virtually all embryos hatch, but the larvae remain developmentally arrested at the first instar stage for 3-4 days, are akinetic and eventually die before progressing to the second instar. These phenotypes are identical to those described for group V (GV) Catsup alleles, according to the classification scheme of Stathakis et al. (Stathakis et al., 1999), which ranges from G1 (least severe) to GV (most severe). Although Catsup47 and Catsup48 are strong loss-of-function alleles, they are unlikely to represent completely null mutants, as other GV alleles have been reported to show significant levels of embryonic lethality (up to 26%) (Stathakis et al., 1999). To confirm our findings with our new Catsup alleles, which were induced on an FRT40A chromosome for clonal analysis, we attempted to recombine the previously characterized molecular null allele Catsup26 (Stathakis et al., 1999) onto the FRT40A chromosome. Unfortunately, Catsup is located ∼2 cM from the FRT40A insertion site, and despite establishing and screening 267 candidate recombinant lines, we were unable to recover a single recombinant chromosome bearing both FRT40A and the Catsup26 mutation.
Notch accumulates abnormally in intracellular compartments of mutant cells
To investigate the intracellular compartment(s) in which Notch accumulates in the Catsup mutants, we performed double-labeling studies with Notch and organelle markers for the ER and Golgi compartments. For both Catsup47 and Catsup48 mutant clones, the elevated Notch accumulation was detected in a perinuclear compartment that partially overlapped with the ER-specific marker PDI-GFP (Morin et al., 2001) (Fig. 3A-A′) and to a lesser extent with the Golgi-specific marker sqh-Golgi-YFP (LaJeunesse et al., 2004) (Fig. 3B-B′). However, PDI-GFP expression itself was much higher in the Catsup mutant clone cells compared with surrounding wild-type tissues (Fig. 3A), suggesting that the ER is greatly expanded and/or that ER function is altered in a manner that leads to strong upregulation of PDI-GFP.
Fig. 3.
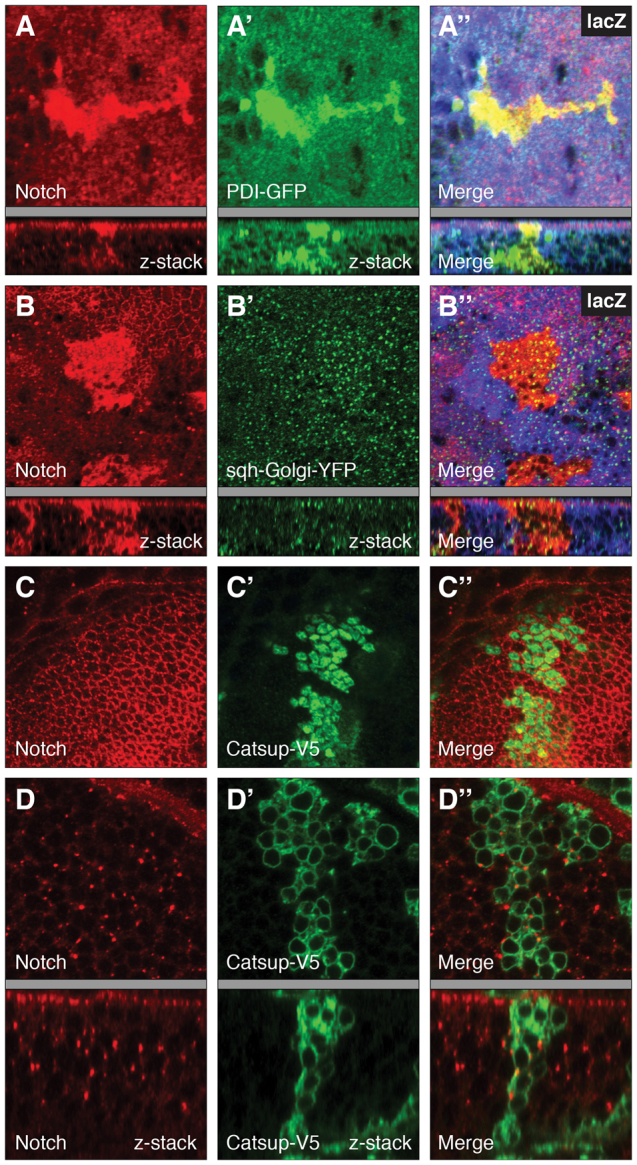
Accumulation of Notch in the biosynthetic and secretory compartments in Catsup mutant clones. (A-B′) Catsup mutant clones genetically marked by absence of lacZ expression were generated in wing imaginal discs and examined for Notch accumulation (red; A,B) together with either the ER chaperone marker PDI-GFP (green; A′) or the Golgi marker sqh-Golgi-YFP (green; Golgi-YFP; B′), with corresponding merged images of Notch and the relevant organelle marker (A′,B′). (C-D′) Functional rescue of Notch trafficking defects in Catsup mutant clones expressing transgenic wild-type Catsup-V5. Homozygous Catsup mutant clones were produced in wing discs using the MARCM system (Lee and Luo, 2001) to express wild-type Catsup-V5 in the mutant clone cells, then immunostained for endogenous Notch (red; C,D), the V5 epitope tag, which marks mutant cells only (green; C′,D′), and examined for apical (C,C′) and basal (D,D′) accumulation of Notch and Catsup-V5. C′ and D′ show the corresponding merged Notch and Catsup-V5 signals. For each sample in A-D′, the corresponding confocal z-series showing the apicobasal distribution of signal(s) is included beneath the gray bar.
Expression of a wild-type Catsup cDNA rescue transgene in Catsup mutant clones restored the normal pattern of Notch protein expression in the secretory pathway, confirming that the observed Notch trafficking defects in the new mutants are specifically attributable to loss of Catsup protein function (Fig. 3C-D′).
We also used this epitope-tagged, wild-type Catsup cDNA transgene to investigate the subcellular localization of the Catsup protein in Drosophila wing disc peripodial cells and wing imaginal discs. In both tissues, Catsup-V5 accumulates most strongly in a perinuclear network that displays a highly polarized orientation with respect to the nuclei in the columnar epithelium of the wing imaginal disc (Fig. 4A-B′). Analysis of the Catsup-V5 protein distribution using secretory organelle markers revealed that both Catsup-V5 and the ER-specific marker KDEL-GFP (flybase.org/reports/FBrf0195905.html) are detected in a partially overlapping pattern in the perinuclear ER region. However, Catsup-V5 displays a very diffuse distribution, whereas KDEL-GFP exhibits highly localized intense punctate accumulations, in addition to the more diffuse pattern (Fig. 4C-F′). By contrast, the Golgi-specific marker GM130 shows a very pronounced punctate distribution that is spatially very close to the perinuclear region of high Catsup-V5 accumulation, but appears to label subcellular positions that are distinct from those positive for Catsup-V5 (Fig. 4G-J′). In wing disc cells, for example, GM130-positive puncta appear to be nestled in areas that are adjacent to but not identical to Catsup-V5 accumulation (compare Fig. 4J with 4J′). These findings are generally consistent with the reported subcellular distribution of ZIP7 in the ER and Golgi in mammalian cells (Taylor et al., 2004; Huang et al., 2005), as well as our finding of increased Notch accumulation in the secretory compartment in Catsup-deficient Drosophila cells as noted above.
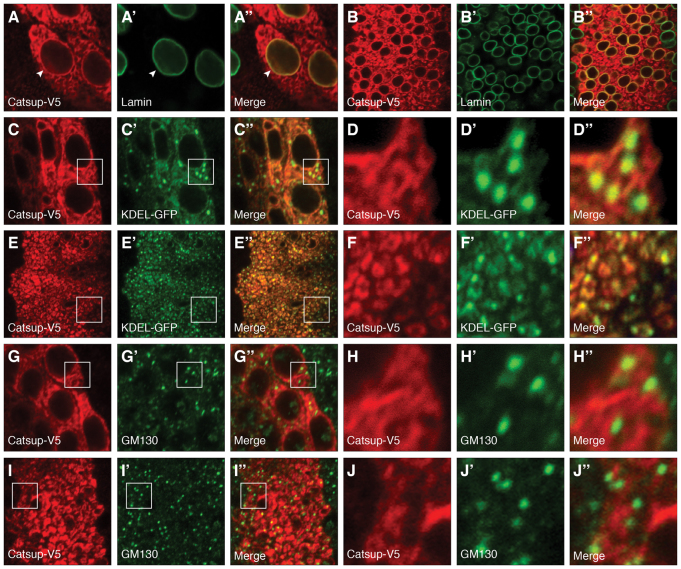
Fig. 4.
Localization of Catsup in the biosynthetic and secretory pathway. (A-J′) Localization of transgenically expressed, epitope-tagged Catsup-V5 (red) expressed under regulatory control of patched-GAL4 (Hinz et al., 1994) together with various organelle markers (green) in wing disc peripodial cells (A-A′,C-D′,G-H′) or in wing imaginal disc proper cells (B-B′,E-F′,I-J′). For each tissue sample, the set of three images shows Catsup-V5 protein (A-J), a specific organelle marker (nuclear envelope Lamin in A′,B′; ER-specific KDEL signal in C′,D′,E′,F′; and Golgi-specific GM130 signal in G′,H′,I′,J′), with merged images of the Catsup and the relevant organelle marker in the corresponding panels A′,B′,C′,D′,E′,F′,G′,H′,I′,J′. For each of the low-magnification wing disc images, boxed regions are shown at higher magnification in corresponding panels D-D′,F-F′,H-H′,J-J′.
To investigate whether loss of Catsup function leads to observable changes in zinc levels in cells, we analyzed Catsup mutant clones with the zinc probes FluoZin-3, Zinquin or Newport Green. For all three probes, we noted a very weak, barely detectable, increase in zinc probe signal in Catsup mutant cells compared with surrounding non-mutant cells (supplementary material Fig. S2). However, given the resolution of the confocal microscopy imaging used for these studies, we were unable to determine whether these subtle increases in zinc probe signals represent a specific elevation in zinc ion levels or whether it reflects an expansion of the ER compartment, leading to more zinc probe binding in the Catsup-deficient cells.
Loss of Catsup activity enhances Notch mutant wing phenotypes and affects Notch signaling at the dorsal/ventral wing boundary
To investigate the functional consequences of reduced Catsup activity on Notch signaling, we asked whether loss of Catsup leads to adult Notch-related phenotypes in clones, interacts genetically with Notch pathway genes or affects the expression of Notch-regulated transcriptional target genes. Homozygous mutant clones for both Catsup47 and Catsup48 lead to wing notching at low frequency (<5%), whereas wild-type clones produced in parallel experiments using the same isogenic chromosome II used for the EMS mutagenesis and FRT-based genetic screening did not show any detectable wing notching (Fig. 5A-B). However, the penetrance of these wing notching phenotypes was difficult to assess quantitatively, owing to the spatial and temporal variables affecting mutant clone production. We also found that Catsup47, Catsup48 and the previously described Catsup26 mutant allele all significantly enhanced the incidence of wing notching in the strong Notch mutant allele Notch264-107; and Catsup47 and Catsup26 similarly enhanced the wing notching caused by the Notch deficiency Notch54l9, although Catsup48 did not enhance the Notch54l9 wing phenotype to a statistically significant degree (Fig. 5C-G).
Fig. 5.
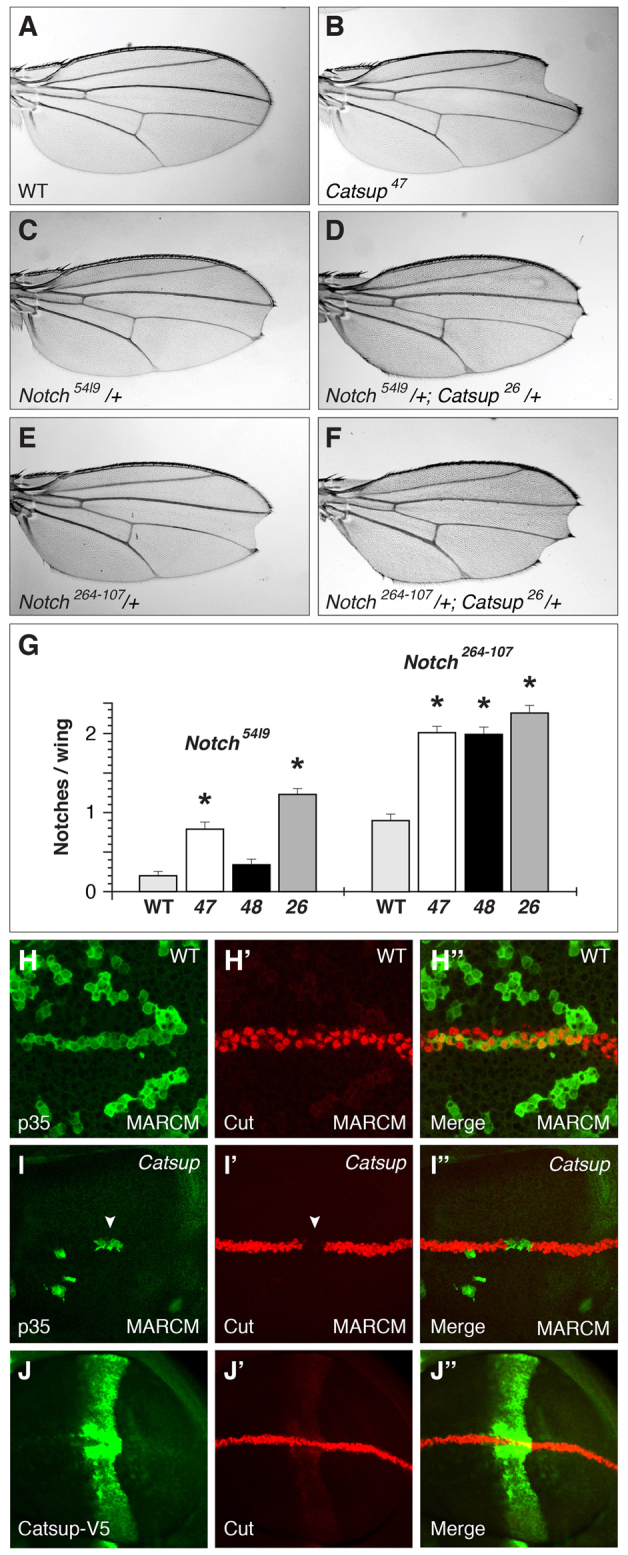
Notch-related phenotypes in Catsup-deficient tissues and cells. (A) Wild-type adult Drosophila wing blade. (B) Adult wing bearing a Catsup47 mutant clone, showing wing notching and missing wing margin material at tip. (C-F) Representative adult wing notching phenotypes of Notch54l9/+ heterozygotes (C), Notch54l9/+; Catsup26/+ double heterozygotes (D), Notch264-107/+ heterozygotes (E) and Notch264-107/+; Catsup26/+ double heterozygotes (F). (G) Average number of notches per wing blade in Notch54l9/+ and Notch264-107/+ heterozygotes in combination with either a Catsup wild-type genotype (WT), Catsup47/+ (47), Catsup48/+ (48) and Catsup26/+ (26), as denoted at the bottom; asterisks indicate Catsup heterozygous mutant genotypes that exhibit statistically significant (P<0.05) increases in wing notching incidence relative to Catsup+ (n=50 flies/100 wings per genotype. Notch54l9 genotypes: wild type=0.20±0.049; Catsup47=0.79±0.081, P=2.6×10-9; Catsup48=0.34±0.064, P=0.084; Catsup26=1.23±0.066, P=2.1×10-20. Notch264-107 genotypes: wild type=0.90±0.075; Catsup47=2.01±0.073 P=5.9×10-23; Catsup48=1.99±0.086, P=4.0×10-18; Catsup26=2.26±0.090, P=2.6×10-24). The Catsup wild-type genotype used for these crosses was the parental stock y w; P{ry[+t7.2]=neoFRT}40A P{w[+mW.hs]=FRT(whs)}G13 employed in our initial mutagenesis screen. Data are mean numbers of notches per wing ± s.e.m. (H-I′) Expression of the Notch target Cut (red in H′,I′) in p35-expressing wild-type control clones (H-H′) and Catsup mutant clones (I-I′), in which the clone cells are positively marked by p35 expression (green in H and I). Merged images of the Cut and p35 signals are shown on the right in H′ and I′; white arrowhead in I and I′ indicates Catsup mutant cells at the DV boundary that fail to express Cut. (J-J′) Overexpression of Catsup-V5 (green) under the control of patched-GAL4 (J) does not affect Cut expression (J′; red) where Catsup-V5 overexpression intersects with the DV boundary; merged signals for Catsup-V5 and Cut expression are shown in J′.
In the developing wing primordium, Notch signaling is responsible for patterning of the dorsal-ventral (DV) boundary that ultimately gives rise to the specialized sensory bristles along the adult wing margin (Micchelli and Blair, 1999; Rauskolb et al., 1999). We produced Catsup mutant clones that encompass the DV boundary using the MARCM method (Lee and Luo, 2001) to express the survival factor p35 in the clonal tissue, facilitating production of larger clones that span the DV boundary (see Materials and methods). Catsup mutant clones that span the DV boundary show reduced expression of the Notch target gene cut (Blochlinger et al., 1990) at the DV boundary, whereas wild-type control clones produced in an identical manner do not show any reduction in cut expression at the boundary (Fig. 5H-I′). Because DV boundary formation requires fringe-dependent amplification of Notch signaling along the presumptive boundary, our observation that this process is selectively impaired in Catsup mutant cells suggests that loss of Catsup has differential effects on Notch signaling that are most pronounced in patterning events with a particularly stringent requirement for highly active Notch signaling.
Consistent with our finding that forced expression of Catsup-V5 using MARCM did not result in any obvious changes in Notch subcellular localization (Fig. 3C-D′), we found that overexpression of Catsup-V5 using the patched-GAL4 driver had no apparent effect on Cut expression at the DV boundary (Fig. 5J-J′).
Induction of ER stress and apoptosis in Catsup-deficient cells
Given the abnormal accumulation of proteins within the secretory pathway in Catsup mutant cells, we examined whether this membrane protein mislocalization was associated with ER stress and induction of the unfolded protein response (UPR). In wild-type wing discs, cells within the disc proper exhibit low or undetectable levels of ER stress, as indicated by expression of the spliced form of Xbp1, an ER stress sensor (Ryoo et al., 2007), although high Xbp1 expression was detected in a subset of peripodial membrane cells of the wing disc (Fig. 6A-C). By contrast, cells in Catsup homozygous mutant clones display strong induction of Xbp1 splicing in addition to aberrant Notch protein accumulation, consistent with elevated levels of ER stress compared with adjacent wild-type tissue in the clone-bearing wing discs (Fig. 6D-D′).
Fig. 6.
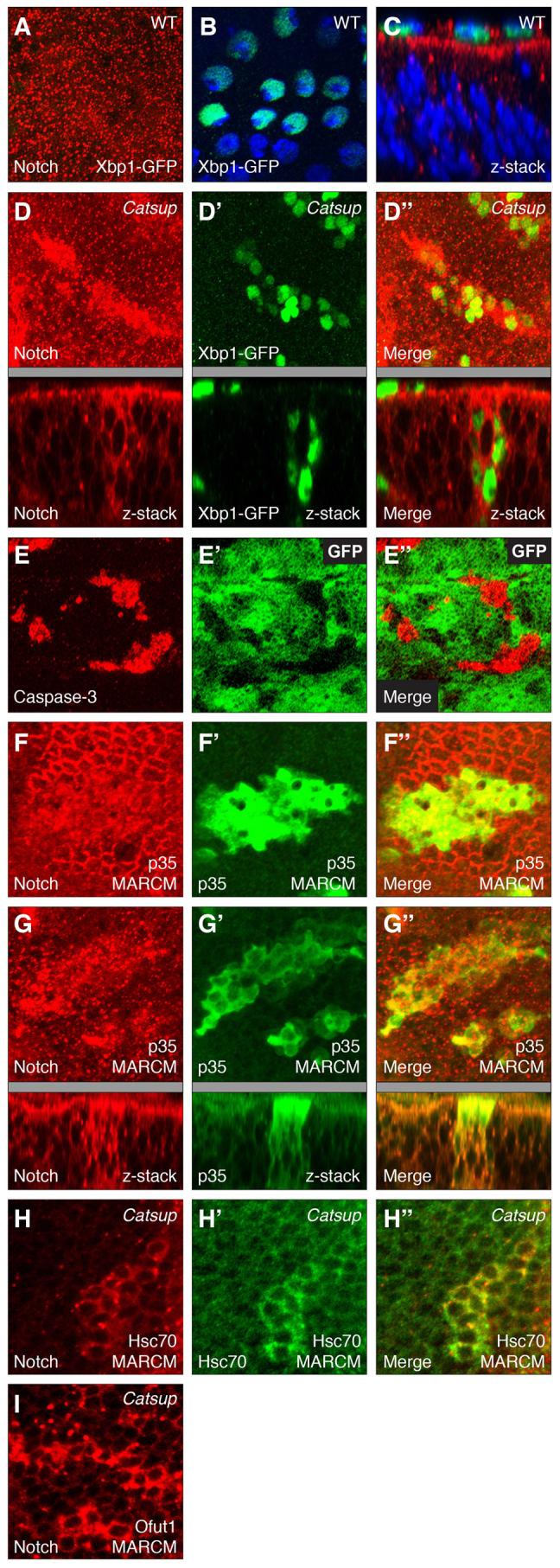
Induction of ER stress and apoptosis in Catsup mutant cells. (A) Notch distribution (red) and lack of induction of Xbp1-GFP (green) in wild-type Drosophila wing disc cells; the image represents a compilation of ∼40 separate confocal scans extending from the apical surface to basement membrane. (B) Induction of Xbp1-GFP (green) in wing disc peripodial membrane cells; nuclei counterstained with Hoechst 33258 (blue). (C) Notch accumulation (red), Xbp1-GFP expression (green) and nuclear Hoechst 33258 (blue) in a confocal z-series showing an apicolateral region of the wing disc and overlying apical peripodial membrane (top). (D-D′) Notch distribution (red in D), induction of Xbp1-GFP (green), and merged Notch and Xbp1-GFP signals (D′) in Catsup-deficient clones marked by elevated Notch accumulation; images represent compilations of ∼40 separate confocal scans extending from the apical surface to basement membrane. For each xy confocal image in D-D′, a corresponding z-series displaying the apicobasal axis of the disc is shown beneath the gray bar. (E-E′) Expression of activated Caspase 3 (red in E) in Catsup mutant wing disc clones, showing clone locations (areas devoid of green GFP signal in E′), and merged image of both signals in E′. (F-G′) MARCM-directed expression of baculovirus survival factor p35 (green in F′,G′; see Materials and methods) permits survival of larger Catsup mutant clones, which exhibit reduced apical Notch accumulation (red in F), elevated basal Notch accumulation (red in G) and pronounced vesicular Notch accumulation (compare mutant clone cells with non-mutant cells within F and G); F-F′ and G-G′ depict apical and basal cell regions, respectively, merged Notch and p35 signals are shown in F′ and G′, and corresponding apicobasal confocal z-series are shown beneath the gray bars for G-G′. (H-H′) Elevated Notch accumulation (red) in basal cell regions of a Catsup mutant clone (H) that overexpresses Hsc70 (green) using MARCM (H′); merged Notch and Hsc70 signals are depicted in H′. (I) Abnormal Notch accumulation (red) in multiple unmarked Catsup mutant clones that overexpress O-fut1 using MARCM.
In addition, Catsup mutant clones display strong expression of activated Caspase 3, indicating that loss of the Catsup zinc transporter results in higher levels of apoptosis in wing disc cells (Fig. 6E-E′). This finding is consistent with our observation that Catsup mutant clones are typically smaller than control clones of wild-type cells produced using identical clone induction and growth conditions, suggesting that Catsup mutant cells do not survive and proliferate as well as wild-type cells. To counteract this effect, we generated Catsup mutant clones that simultaneously expressed the baculoviral survival factor p35 using the MARCM system (Lee and Luo, 2001) as described above, and observed that although larger mutant clones were obtained with this approach, these clones nevertheless exhibit high levels of intracellular Notch accumulation, indistinguishable from that of clones produced in the absence of p35 (Fig. 6F-G′). Moreover, neither expression of the general chaperone Hsc70 nor expression of the Notch-specific chaperone O-fut1 using the MARCM system were able to suppress the Notch trafficking abnormalities in Catsup mutant clones (Fig. 6H-I). Taken together, these results indicate that the protein trafficking defects, ER stress induction and apoptosis observed in Catsup-deficient cells are not solely due to misfolding of secreted membrane proteins and more likely reflect a more fundamental impairment of secretory compartment function.
DISCUSSION
Catsup is a member of the ZIP7 protein family of zinc transporters (Hogstrand et al., 2009). In Drosophila, Catsup has been shown to act as a negative regulator of catecholamine biosynthesis (Stathakis et al., 1999), and in synaptic transport and release of dopamine in dopaminergic neurons (Wang et al., 2011). Molecular polymorphisms in the Catsup locus are associated with genetic variation in Drosophila sleep patterns, linking ZIP7 function to neurophysiological processes that regulate behavior in flies (Harbison et al., 2009). A second Drosophila ZIP family member is encoded by the fear of intimacy gene, which is required for developmental cell migrations during gonad morphogenesis (Van Doren et al., 2003; Mathews et al., 2005; Mathews et al., 2006) and embryonic glial cell patterning (Pielage et al., 2004). The human ZIP7 zinc transporter, which is the human ZIP protein family member most closely related to Drosophila Catsup, mediates the release of zinc from the ER and Golgi (Taylor et al., 2004; Huang et al., 2005), an event that triggers activation of downstream pathways that promote cell proliferation (Taylor et al., 2008). These findings suggest that ZIP7 orthologs might act as multifunctional proteins involved in the regulation of a wide range of cellular processes during development and adult tissue homeostasis.
In this study, we identify an unanticipated role of Catsup in regulating the trafficking of membrane proteins within the secretory pathway. We find that Notch, EGFR and APPL accumulate abnormally in the ER and Golgi compartments in wing disc cells lacking Catsup gene function. With respect to its effects on signaling, we determined that loss of Catsup activity leads to disruption of Notch activation at the presumptive wing margin in Drosophila, causing reduced expression of the Notch target gene cut at the margin and occasional loss of wing margin material at the adult wing margin. It should be noted that loss of Catsup function does not completely abrogate Notch signaling, as expression of other Notch targets in non-margin regions of the wing anlagen was not obviously perturbed in Catsup mutant cells. As formation of the Drosophila wing margin depends upon a Fringe- and Notch-dependent amplification mechanism to generate high levels of Notch signaling in a two-cell wide stripe at the margin, we favor the idea that this Notch-dependent signaling mechanism is especially sensitive to loss of Catsup and associated impairment of Notch secretory trafficking because it requires optimal levels of Notch activity.
Catsup and ER function
We also determined that loss of Catsup function leads to the activation of ER stress and apoptotic pathways, suggesting that abnormal ER function and associated pleiotropic effects on protein biosynthesis are likely to underlie the developmental defects seen in tissues arising from Catsup-deficient cell clones. Cellular homeostasis and survival depend crucially upon proper ER function, and ER mechanisms are responsible for monitoring the quality of proteins that are being synthesized, folded and assembled to ensure that proteins are functionally sound before they are delivered to their intracellular target compartments or secreted into the extracellular milieu. If these physiological protein synthesis and secretion mechanisms within the ER are impaired, unfolded proteins and/or protein aggregates accumulate in the ER, which are detected by quality control mechanisms that initiate the unfolded protein response (UPR) to prevent accumulation of damaged proteins. Misfolded proteins are retained in the ER and are subsequently transported into the cytosol for proteosomal degradation. If ER stress is protracted, or if UPR mechanisms are unable to restore ER homeostasis, the cell will typically undergo apoptotic cell death (Walter and Ron, 2011).
The activation of the ER stress sensor Xbp1 and the ER chaperone PDI in Catsup mutant wing disc cells indicate that in these cells, the ER is overloaded with misfolded membrane proteins and responds to this homeostatic imbalance by activating the protein quality control machinery. The fact that Catsup mutant clones also exhibited Caspase 3 activation, a key feature of ER stress-induced apoptosis (Gorman et al., 2012), implies that chronic ER stress might be a crucial physiological feature of Catsup mutant cells that contributes to their reduced viability. Indeed, we observed that Catsup mutant clones are usually rather small in size compared with nearby genetically wild-type twin spot clones or control clones of wild-type cells, suggesting that processes affecting cell proliferation and/or viability are affected in the Catsup-deficient cells.
These effects on mutant clone growth are reminiscent of the small clone phenotype observed in flies lacking sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) activity, where Notch and other proteins are similarly mislocalized in the secretory pathway (Periz and Fortini, 1999). Intriguingly, small molecule inhibitors and cDNA enhancers of human SERCA have recently been recovered in convergent high-throughput screens targeting a leukemia-associated Notch1 allele, where SERCA inhibition was found to impair maturation and activity of oncogenic mutant Notch1 receptors and to induce G0/G1 arrest in Notch1-mutated human leukemia cells (Roti et al., 2013). The striking parallels between the modulatory effects of SERCA and Catsup/ZIP7 on Drosophila Notch secretory trafficking and signaling suggest that it might be worthwhile to test whether human ZIP7 inhibition likewise might have potentially therapeutic effects on overactive Notch signaling in human cancers.
Concluding remarks
Our finding that loss of the Drosophila ZIP7 zinc transporter encoded by the Catsup locus leads to defective secretory trafficking of membrane proteins, effects on Notch-dependent tissue patterning, increased ER stress and apoptosis extends the known physiological functions of the ZIP7 family of zinc transporters. As noted above, previous studies of ZIP7 activity have implicated this family of zinc transporters in rather disparate physiological processes, ranging from catecholamine biosynthesis, synaptic vesicle loading and release of dopamine, and sleep behavior in Drosophila (Stathakis et al., 1999; Harbison et al., 2009; Wang et al., 2011) to inhibition of tyrosine kinase-mediated cell proliferation in mammalian cells (Murakami and Hirano, 2008; Taylor et al., 2008; Hogstrand et al., 2009). The requirement for ZIP7 activity for normal trafficking of Notch, EGFR and the fly APP ortholog in Drosophila tissues suggests that ZIP7 might have pleiotropic roles in other cellular processes that depend upon proper Zn2+ homeostasis in the ER and Golgi compartments, as well as regulation of cytosolic Zn2+ by release of ER/Golgi zinc ion stores. Dysregulated ZIP7 function might thus be a contributing factor to other human diseases in addition to its already documented role in breast cancer (Taylor et al., 2008). Many human cancers are associated with abnormal Notch signaling (Groth and Fortini, 2012; South et al., 2012), and Alzheimer′s disease is characterized by changes in zinc homeostasis, high levels of Zn2+ in amyloid plaques and disturbances in sleep patterns (Cuajungco et al., 2005; Frederickson et al., 2005). Further underscoring the clinical importance of ZIP transporters and zinc homeostasis, loss-of-function mutations in the human Golgi-localized zinc transporter ZIP13 cause a disorder resembling Ehlers-Danlos syndrome, which is characterized by hyperelastic skin, loose joints, muscular atrophy and skeletal dysplasia (Fukada et al., 2008; Giunta et al., 2008). Finally, the requirement for Drosophila ZIP7 zinc transporter function in normal secretory trafficking of Notch and EGFR, together with the previously documented role of the SERCA class Ca2+-ATPase in trafficking of Notch and the Sevenless receptor tyrosine kinase in the ER/Golgi compartments (Periz and Fortini, 1999), emphasize the importance of ion homeostasis for maintaining the functional integrity of secretory pathway organelles during developmental signal transduction in Drosophila.
Supplementary Material
Acknowledgments
We thank lab members for comments on the manuscript; M. Y. Covarrubias for confocal microscopy assistance; J. Jiang, J. M. O’Donnell, H. D. Ryoo, G. Thomas, the University of Iowa Developmental Biology Hybridoma Bank and the Bloomington Drosophila Stock Center for antibodies and fly stocks.
Footnotes
Funding
This research was supported by the National Institutes of Health (NIH) [R01 GM087650 from the National Institute of General Medical Sciences (NIGMS)]; by funding from Thomas Jefferson University Department of Biochemistry; by the National Cancer Institute (NCI) Intramural Research Program; and by the Kimmel Cancer Center Confocal Core Facility, which is supported in part by NCI Cancer Center Support Grant P30 CA56036. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Author contributions
M.E.F. and T.S. designed the genetic screen for Notch trafficking mutants, T.S. performed the screen and the initial characterization of the new Catsup mutants, C.G. performed the detailed characterization of the Catsup mutant phenotypes with assistance from M.R.K., M.W. and M.E.F., and C.G. and M.E.F. wrote the manuscript with contributions from T.S., M.R.K. and M.W.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.088336/-/DC1
References
- Andersson E. R. (2012). The role of endocytosis in activating and regulating signal transduction. Cell. Mol. Life Sci. 69, 1755–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blochlinger K., Bodmer R., Jan L. Y., Jan Y. N. (1990). Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 4, 1322–1331 [DOI] [PubMed] [Google Scholar]
- Cuajungco M. P., Frederickson C. J., Bush A. I. (2005). Amyloid-β metal interaction and metal chelation. Subcell. Biochem. 38, 235–254 [DOI] [PubMed] [Google Scholar]
- Diederich R. J., Matsuno K., Hing H., Artavanis-Tsakonas S. (1994). Cytosolic interaction between deltex and Notch ankyrin repeats implicates deltex in the Notch signaling pathway. Development 120, 473–481 [DOI] [PubMed] [Google Scholar]
- Dubreuil R., Byers T. J., Branton D., Goldstein L. S., Kiehart D. P. (1987). Drosophilia spectrin. I. Characterization of the purified protein. J. Cell Biol. 105, 2095–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefant F., Palter K. B. (1999). Tissue-specific expression of dominant negative mutant Drosophila HSC70 causes developmental defects and lethality. Mol. Biol. Cell 10, 2101–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon R. G., Kooh P. J., Rebay I., Regan C. L., Xu T., Muskavitch M. A. T., Artavanis-Tsakonas S. (1990). Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell 61, 523–534 [DOI] [PubMed] [Google Scholar]
- Frederickson C. J., Koh J. Y., Bush A. I. (2005). The neurobiology of zinc in health and disease. Nat. Rev. Neurosci. 6, 449–462 [DOI] [PubMed] [Google Scholar]
- Fukada T., Civic N., Furuichi T., Shimoda S., Mishima K., Higashiyama H., Idaira Y., Asada Y., Kitamura H., Yamasaki S., et al. (2008). The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-β signaling pathways. PLoS ONE 3, e3642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaul U., Mardon G., Rubin G. M. (1992). A putative Ras GTPase activating protein acts as a negative regulator of signaling by the Sevenless receptor tyrosine kinase. Cell 68, 1007–1019 [DOI] [PubMed] [Google Scholar]
- Giunta C., Elçioglu N. H., Albrecht B., Eich G., Chambaz C., Janecke A. R., Yeowell H., Weis M., Eyre D. R., Kraenzlin M., et al. (2008). Spondylocheiro dysplastic form of the Ehlers-Danlos syndrome - an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am. J. Hum. Genet. 82, 1290–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnord P., Blouin C. M., Lamaze C. (2012). Membrane trafficking and signaling: two sides of the same coin. Semin. Cell Dev. Biol. 23, 154–164 [DOI] [PubMed] [Google Scholar]
- Gorman A. M., Healy S. J., Jäger R., Samali A. (2012). Stress management at the ER: regulators of ER stress-induced apoptosis. Pharmacol. Ther. 134, 306–316 [DOI] [PubMed] [Google Scholar]
- Groth C., Fortini M. E. (2012). Therapeutic approaches to modulating Notch signaling: current challenges and future prospects. Semin. Cell Dev. Biol. 23, 465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth C., Alvord W. G., Quiñones O. A., Fortini M. E. (2010). Pharmacological analysis of Drosophila melanogaster γ-secretase with respect to differential proteolysis of Notch and APP. Mol. Pharmacol. 77, 567–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison S. T., Carbone M. A., Ayroles J. F., Stone E. A., Lyman R. F., Mackay T. F. (2009). Co-regulated transcriptional networks contribute to natural genetic variation in Drosophila sleep. Nat. Genet. 41, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz U., Giebel B., Campos-Ortega J. A. (1994). The basic-helix-loop-helix domain of Drosophila lethal of scute protein is sufficient for proneural function and activates neurogenic genes. Cell 76, 77–87 [DOI] [PubMed] [Google Scholar]
- Hogstrand C., Kille P., Nicholson R. I., Taylor K. M. (2009). Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 15, 101–111 [DOI] [PubMed] [Google Scholar]
- Hu Y., Fortini M. E. (2003). Different cofactor activities in γ-secretase assembly: evidence for a nicastrin-Aph-1 subcomplex. J. Cell Biol. 161, 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Kirschke C. P., Zhang Y., Yu Y. Y. (2005). The ZIP7 gene (Slc39a7) encodes a zinc transporter involved in zinc homeostasis of the Golgi apparatus. J. Biol. Chem. 280, 15456–15463 [DOI] [PubMed] [Google Scholar]
- LaJeunesse D. R., Buckner S. M., Lake J., Na C., Pirt A., Fromson K. (2004). Three new Drosophila markers of intracellular membranes. Biotechniques 36, 784-788, 790 [DOI] [PubMed] [Google Scholar]
- Latt S. A., Stetten G., Juergens L. A., Willard H. F., Scher C. D. (1975). Recent developments in the detection of deoxyribonucleic acid synthesis by 33258 Hoechst fluorescence. J. Histochem. Cytochem. 23, 493–505 [DOI] [PubMed] [Google Scholar]
- Lee T., Luo L. (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251–254 [DOI] [PubMed] [Google Scholar]
- Mathews W. R., Wang F., Eide D. J., Van Doren M. (2005). Drosophila fear of intimacy encodes a Zrt/IRT-like protein (ZIP) family zinc transporter functionally related to mammalian ZIP proteins. J. Biol. Chem. 280, 787–795 [DOI] [PubMed] [Google Scholar]
- Mathews W. R., Ong D., Milutinovich A. B., Van Doren M. (2006). Zinc transport activity of Fear of Intimacy is essential for proper gonad morphogenesis and DE-cadherin expression. Development 133, 1143–1153 [DOI] [PubMed] [Google Scholar]
- Micchelli C. A., Blair S. S. (1999). Dorsoventral lineage restriction in wing imaginal discs requires Notch. Nature 401, 473–476 [DOI] [PubMed] [Google Scholar]
- Morin X., Daneman R., Zavortink M., Chia W. (2001). A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc. Natl. Acad. Sci. USA 98, 15050–15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M., Hirano T. (2008). Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 99, 1515–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda H., Uemura T., Harada Y., Iwai Y., Takeichi M. (1994). A Drosophila homolog of cadherin associated with armadillo and essential for embryonic cell-cell adhesion. Dev. Biol. 165, 716–726 [DOI] [PubMed] [Google Scholar]
- Okajima T., Irvine K. D. (2002). Regulation of Notch signaling by O-linked fucose. Cell 111, 893–904 [DOI] [PubMed] [Google Scholar]
- Parachoniak C. A., Park M. (2012). Dynamics of receptor trafficking in tumorigenicity. Trends Cell Biol. 22, 231–240 [DOI] [PubMed] [Google Scholar]
- Periz G., Fortini M. E. (1999). Ca(2+)-ATPase function is required for intracellular trafficking of the Notch receptor in Drosophila. EMBO J. 18, 5983–5993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J., Kippert A., Zhu M., Klämbt C. (2004). The Drosophila transmembrane protein Fear-of-intimacy controls glial cell migration. Dev. Biol. 275, 245–257 [DOI] [PubMed] [Google Scholar]
- Qi H., Rand M. D., Wu X., Sestan N., Wang W., Rakic P., Xu T., Artavanis-Tsakonas S. (1999). Processing of the Notch ligand Delta by the metalloprotease Kuzbanian. Science 283, 91–94 [DOI] [PubMed] [Google Scholar]
- Rana N. A., Haltiwanger R. S. (2011). Fringe benefits: functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr. Opin. Struct. Biol. 21, 583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C., Correia T., Irvine K. D. (1999). Fringe-dependent separation of dorsal and ventral cells in the Drosophila wing. Nature 401, 476–480 [DOI] [PubMed] [Google Scholar]
- Riemer D., Stuurman N., Berrios M., Hunter C., Fisher P. A., Weber K. (1995). Expression of Drosophila lamin C is developmentally regulated: analogies with vertebrate A-type lamins. J. Cell Sci. 108, 3189–3198 [DOI] [PubMed] [Google Scholar]
- Roti G., Carlton A., Ross K. N., Markstein M., Pajcini K., Su A. H., Perrimon N., Pear W. S., Kung A. L., Blacklow S. C., et al. (2013). Complementary genomic screens identify SERCA as a therapeutic target in NOTCH1 mutated cancer. Cancer Cell 23, 390–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo H. D., Domingos P. M., Kang M. J., Steller H. (2007). Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 26, 242–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- South A. P., Cho R. J., Aster J. C. (2012). The double-edged sword of Notch signaling in cancer. Semin. Cell Dev. Biol. 23, 458–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathakis D. G., Burton D. Y., McIvor W. E., Krishnakumar S., Wright T. R. F., O’Donnell J. M. (1999). The catecholamines up (Catsup) protein of Drosophila melanogaster functions as a negative regulator of tyrosine hydroxylase activity. Genetics 153, 361–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer J., Treisman J. E. (2009). Lipid-modified morphogens: functions of fats. Curr. Opin. Genet. Dev. 19, 308–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Endo T. (2002). Ermelin, an endoplasmic reticulum transmembrane protein, contains the novel HELP domain conserved in eukaryotes. Gene 284, 31–40 [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Morgan H. E., Johnson A., Nicholson R. I. (2004). Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem. J. 377, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. M., Morgan H. E., Smart K., Zahari N. M., Pumford S., Ellis I. O., Robertson J. F., Nicholson R. I. (2007). The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol. Med. 13, 396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. M., Vichova P., Jordan N., Hiscox S., Hendley R., Nicholson R. I. (2008). ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer cells. Endocrinology 149, 4912–4920 [DOI] [PubMed] [Google Scholar]
- Van Doren M., Mathews W. R., Samuels M., Moore L. A., Broihier H. T., Lehmann R. (2003). fear of intimacy encodes a novel transmembrane protein required for gonad morphogenesis in Drosophila. Development 130, 2355–2364 [DOI] [PubMed] [Google Scholar]
- Walter P., Ron D. (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 [DOI] [PubMed] [Google Scholar]
- Wang Z., Ferdousy F., Lawal H., Huang Z., Daigle J. G., Izevbaye I., Doherty O., Thomas J., Stathakis D. G., O’Donnell J. M. (2011). Catecholamines up integrates dopamine synthesis and synaptic trafficking. J. Neurochem. 119, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Harrison S. D. (1994). Mosaic analysis using FLP recombinase. Methods Cell Biol. 44, 655–681 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.