Abstract
Excessive production of pro-inflammatory cytokines in the senescent brain in response to peripheral immune stimulation is thought to induce behavioral pathology, however, few studies have examined if the increase in pro-inflammatory cytokines is accompanied by an increase in cytokine signaling. Here, we focused on IL-6 as a prototypic pro-inflammatory cytokine and used phosphorylated STAT3 as a marker of IL-6 signaling. In an initial study, IL-6 mRNA and the magnitude and duration of STAT3 activation were increased in the hippocampus of senescent mice compared to adults after i.p. injection of LPS. The LPS-induced increase in STAT3 activity was ablated in aged IL-6−/− mice, suggesting IL-6 is a key driver of STAT3 activity in the aged brain. To determine if IL-6 activated the classical or trans-signaling pathway, before receiving LPS i.p., aged mice were injected ICV with sgp130, an antagonist of the trans-signaling pathway. Importantly, the LPS-induced increases in both IL-6 and STAT3 activity in the hippocampus were inhibited by sgp130. To assess hippocampal function, aged mice were injected ICV with sgp130 and i.p. with LPS immediately after the acquisition phase of contextual fear conditioning, and immobility was assessed in the retention phase 48 h later. LPS reduced immobility in aged mice, indicating immune activation interfered with memory consolidation. However, sgp130 blocked the deficits in contextual fear conditioning caused by LPS. Taken together, the results suggest IL-6 trans-signaling is increased in the senescent brain following peripheral LPS challenge and that sgp130 may protect against infection-related neuroinflammation and cognitive dysfunction in the aged.
Keywords: aging, cognition, hippocampus, interleukin-6, interleukin-6 receptor, lipopolysaccharide, mice, neuroinflammation, soluble gp130, trans-signaling
Introduction
Interleukin-6 (IL-6) has a role in cognitive dysfunction. For example, immunoneutralization of IL-6 lengthened long-term potentiation (LTP) and improved spatial alternation behavior (Balschun et al., 2004) while IL-6-deficient mice were refractory to lipopolysaccharide (LPS)-induced deficits in working memory (Sparkman et al., 2006). IL-6 also disrupts neurogenesis (Vallieres et al., 2002) and is up regulated in an array of neurodegenerative diseases (Gruol and Nelson, 1997; Hofmann et al., 2009; Licastro et al., 2003; Muller et al., 1998). Furthermore, in senescence, constitutive expression of IL-6 by brain microglia is up regulated (Godbout and Johnson, 2004; Ye and Johnson, 1999, 2001) and during infection there is excessive production of pro-inflammatory cytokines including IL-6, leading to prolonged sickness behavior (Abraham and Johnson, 2009; Godbout et al., 2005; Huang et al., 2008), cognitive deficits (Barrientos et al., 2006; Chen et al., 2008; Rosczyk et al., 2008), affective disorders such as increased anxiety and depression (Godbout et al., 2008; Kiecolt-Glaser et al., 2003), and even atrophy of neurons (Richwine et al., 2008). These findings in rodents have led us to speculate that the acute cognitive disorders seen in older adults with an infection are due to excessive production of pro-inflammatory cytokines in the brain, although very little is known regarding the effects of aging on cytokine receptor signaling.
The IL-6 receptor is activated through two separate, but related pathways termed classical and trans-signaling. The receptor consists of two subunits: the IL-6 receptor-alpha chain (IL-6R), which binds IL-6, and the transmembrane signaling subunit, glycoprotein 130 (gp130), which is the intracellular signal transducer and is expressed across all cell types. Classical activation consists of the IL-6 ligand binding to the membrane-bound IL-6R. It is important to note that both receptor subunits (IL-6R and gp130) can be cleaved immediately before the membrane spanning region by alternative splicing or shed by proteolytic enzymes to produce a soluble receptor located in extracellular matrix. The expression of membrane-bound IL-6R is generally limited to a few cells of the immune system, while gp130 is ubiquitously expressed (Heinrich et al., 1998; Kishimoto et al., 1992). Thus, the basis of trans-signaling is the ability of soluble IL-6R (sIL-6R) to bind IL-6 in the extracellular compartment to form an IL-6/sIL-6R complex; this complex has an increased binding affinity to membrane-bound gp130 when compared to IL-6 alone. This mechanism of action confers IL-6 responsiveness in any cell type that expresses gp130 (Jones et al., 2005; Rose-John and Heinrich, 1994).
Upon binding through either the classical or trans-signaling pathway, gp130 dimerizes and autophosphorylates, resulting in the activation of Janus kinase-1 and 2 (Jak1 and Jak2). These tyrosine kinases phosphorylate the cytoplasmic region of gp130 creating recruitment sites for signal transducer and activation of transcription-3 (STAT3), a Src-homology-2 (SH2) domain-containing signaling molecule. Activated STAT3 forms a dimer, autophosphorylates, and translocates to the nucleus where it binds to enhancer elements of the IL-6 promoter region. Thus the main consequence of both classical or trans-signal IL-6 receptor action is to induce gene transcription and subsequent synthesis and secretion of IL-6, although trans-signaling allows this in many more cell types (Heinrich et al., 1998). sIL-6R and soluble gp130 (sgp130) have varying effects on circulating IL-6. sgp130 acts as a partial antagonist, or decoy receptor, by binding the IL-6/sIL-6R complex and preventing it from binding membrane-bound gp130 to initiate signal transduction (Jostock et al., 2001).
Neurons do not seem to express appreciable amounts of IL-6R, however, they do express large amounts of gp130 (Burton et al., 2011; Schobitz et al., 1993), alluding to the importance of IL-6 trans-signaling in the brain. Indeed IL-6 trans-signaling in the brain was recently shown to play a pivotal role in mediating LPS-induced sickness behavior (Burton et al., 2011). Other studies have begun to reveal the anti-versus pro-inflammatory actions of IL-6, as trans-signaling appears to elicit a pro-inflammatory response by causing non-immune cells to produce IL-6 which in turn recruits immune cells to the local site (Barkhausen et al., 2011; Barrientos, 2011; Greenhill et al., 2011; Scheller et al., 2011).
Although IL-6 is increased in the brain of senescent mice, to our knowledge there have been no studies examining the extent to which peripheral infection influences IL-6 trans-signaling in the brain or its role in cognitive dysfunction. Thus, the present study investigated hippocampal STAT3 activation as a marker for IL-6 signaling and hippocampal-dependent learning and memory in aged mice after peripheral injection of LPS. We further investigated the extent to which inhibition of IL-6 trans-signaling with sgp130 would mitigate STAT3 activation and deficits in learning and memory. The important results suggest that IL-6 trans-signaling is increased in the hippocampus of aged mice after LPS and that IL-6 trans-signaling plays a key role in LPS-induced deficits in hippocampal-dependent learning and memory.
Methods
Animals and surgery
Adult (3–6 months) and aged (22-24 months) male BALB/c, C57BL/6 (IL-6+/+) and IL-6 knockout B6.129S2-Il6tm1 Kopf/J (IL-6−/−) (Kopf et al., 1994) mice were used. All BALB/c mice were obtained from our in-house colony whereas the IL-6+/+ and IL-6−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). The IL-6+/+ and IL-6−/− mice were 2-months old upon receipt. Mice were housed in polypropylene cages and maintained at 21°C under a reverse-phase 12-h light-dark cycle with ad libitum access to water and rodent chow. At the end of each study, mice were examined post mortem for gross signs of disease (e.g., tumors or splenomegaly). Data from mice determined to be unhealthy were excluded from the analysis (<5%).
Surgery
For some experiments intracerebroventricular (ICV) cannulation was performed as described previously (Abraham et al., 2008). Immediately after surgery and again 8-12 h later mice received buprenorphine (0.05 mg/kg s.c.) to aid with any post-operative discomfort. Mice were provided a minimum of 7 days to recover before initiating an experiment. Accurate placement of the cannula was confirmed by allowing 2 μl of sterile saline to flow via gravity into the lateral ventricle. When cannula placement could not be confirmed, mice were excluded from the study. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
Experimental protocols
Mice were handled 1-2 min each day for 7 days before experimentation to acclimate them to handling. To assess the effects of LPS on STAT3 phosphorylation in the hippocampus as well as IL-6 in the hippocampus mice were injected i.p. with sterile saline or 0.33 mg/kg BW (10 μg) LPS (serotype 0127:B8, obtained from Sigma, St. Louis, MO) and killed by CO2 asphyxiation 1, 2, 4, 6, or 8 h later. Blood samples were collected via cardiac puncture into EDTA-coated syringes to obtain plasma, and the brain was rapidly removed and dissected to obtain hippocampal tissue. Plasma and hippocampal tissue were snap frozen in liquid nitrogen and stored at −80° C for later analysis. To assess the role of IL-6 trans-signaling mice were injected ICV with sterile saline containing 0.1% BSA (vehicle) or 100 ng sgp130 ((R&D systems, Minneapolis, MN)) in 2 μl vehicle and then i.p. with sterile saline or 0.33 mg/kg BW (10 μg) LPS. In some cases mice were killed to obtain hippocampal tissue as described above. In other cases, mice were evaluated in a contextual fear conditioning paradigm to assess hippocampal-dependent learning and memory.
Western immunoblotting
To assess IL-6 signaling, hippocampal tissue was unthawed, and lysed in ice cold lysis buffer containing: 100 mM HEPES (7.5 pH), 150 mM NaCl, 1% nonidet P-40 (U.S. Biological, Swampscott, MA), 2 mM EGTA, 2 mM sodium orthovanadate, protease inhibitor cocktail (10 0mM EDTA, 1 μg/mL AEBSF, bestatin, pepstatin A, leupeptin, aprotinin, and E-64), and 1 mM PMSF, and centrifuged at 11,000 × g for 10 min at 4°C to remove all cellular debris. Protein concentration was determined using the BCA Protein Assay according to the manufacturer’s protocol (Bio-Rad, Hercules, CA). Lysate concentration was then normalized and denatured in SDS/PAGE buffer at 95°C and stored at −20°C until use. All lysates were electrophoresed and separated on a 7.5% SDS-PAGE gel, and transferred onto nitrocellulose membranes (GE Healthcare, Minneapolis, MN). The membranes were blocked with 5% non-fat milk and incubated with anti-phosphorylated STAT3 (tyr-705) antibody (Cell Signaling, Danvers, MA) overnight at 4°C. After incubation with an HRP-conjugated secondary antibody, the protein bands were detected with a chemiluminescent substrate (Cell Signaling, Danvers, MA) and Bio-Max film (Eastman Kodak Company, Rochester, NY). For detection of total STAT3 protein, the membranes were stripped with stripping buffer (2% SDS, 6.25 mM Tris.HCL [6.8 pH], 0.704% β-ME), followed by overnight incubation with anti-STAT3 antibody (Cell Signaling, Danvers, MA) at 4°C. The phosphorylated STAT3 protein has alpha and beta isoforms which are expressed in two bands at 86 and 79kDa, respectively. Upon activation, the alpha isoform (86kDa) is the principal isoform that is phosphorylated and the beta isoform (79kDa) represents a point of maximal STAT3 activation. Both bands were quantified using ImageJ 1.41 software (NIH).
Interleukin-6 mRNA measurement by quantitative real-time PCR
Total RNA from hippocampal tissue was isolated using the Tri Reagent protocol (Sigma, St. Louis, MO). A QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA) was used for cDNA synthesis with integrated removal of genomic DNA contamination according to the manufacturer’s protocol. Quantitative real-time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol as previously described (Krzyszton et al., 2008). In brief, cDNA was amplified by PCR where a target cDNA (IL-6, Mm00446190_m1; and a reference cDNA (glucose-3 phosphate dehydrogenase, Mm99999915_g1) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). PCR reactions were performed in triplicate under the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec, and 60°C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as fold difference.
Interleukin-6 detection in hippocampus and plasma
Hippocampal tissue was lysed in ice cold lysis buffer and protein concentrations were determined using the BCA protein assay according to manufacturer’s protocol. The antibodies and standards for the IL-6 ELISA were used according to the description by the manufacturer (eBiosciences San Diego, CA). Plasma samples were assayed for IL-6 using a bead-based immunoassay kit combined with a Cytokine Reagent kit, as described by the manufacturer (Bio-Rad, Hercules, CA).
Contextual fear conditioning
A contextual fear conditioning paradigm was used as described previously with few modifications (Peleg et al., 2010). The fear conditioning apparatus consisted of an opaque conditioning cage (30 × 30 × 30 cm) with a transparent ceiling to permit video recording by an overhead camera. The floor consisted of a series of stainless steel rods wired to a shock generator and scrambler (ENV-414S, MED Associates, St. Albans, VT). On acquisition day, mice were placed in the cage for 120 s, followed by a 2-s foot-shock (0.75 mA); this time interval was repeated and 30 s after the delivery of the second shock mice were co-administered saline or sgp130 ICV and saline or LPS i.p. To test the hippocampal-dependent contextual fear conditioning, 48 h after acquisition mice were placed in the original conditioning cage for the time equal to that in acquisition (4 min and 30 s). A trained observer who was blind to experimental treatments scored the fearful experience by a continuous measurement of freezing (complete immobility), the dominant fear behavioral response (Fanselow, 2000). In interpreting data from the fear conditioning paradigm, an increase in freezing in the retention phase (conducted 48 h after acquisition) indicates an improvement in learning.
Statistical analysis
All data were analyzed using Statview and Statistical Analysis System software (SAS Inst., Cary, NC). Data were subjected to a univariate analysis to ensure normality. In the time course and knockout study, STAT3 phosphorylation, IL-6 mRNA and protein were subjected to a two-way ANOVA in which age (adult or aged) and LPS (sterile saline or 10 μg) were between subject measures. Percent freezing on retention day, IL-6 protein levels, and STAT3 phosphorylation were subjected to a two-way ANOVA in which sgp130 (vehicle or 100 ng), and LPS (sterile saline or 10 μg) were between subject measures. When appropriate, data from individual time points were subjected to ANOVA to determine treatment effects. Post hoc Student’s t test of least square means was used to determine if treatment means were significantly different from one another (p<0.05). All data are presented as mean ± SEM.
Results
LPS-induced STAT3 phosphorylation is increased in hippocampus of aged mice
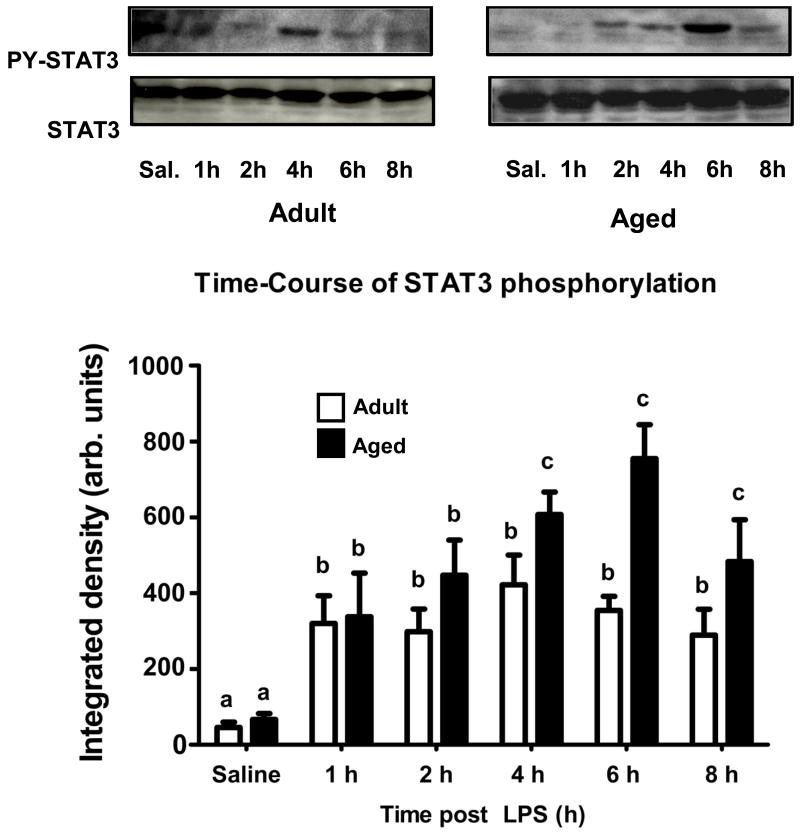
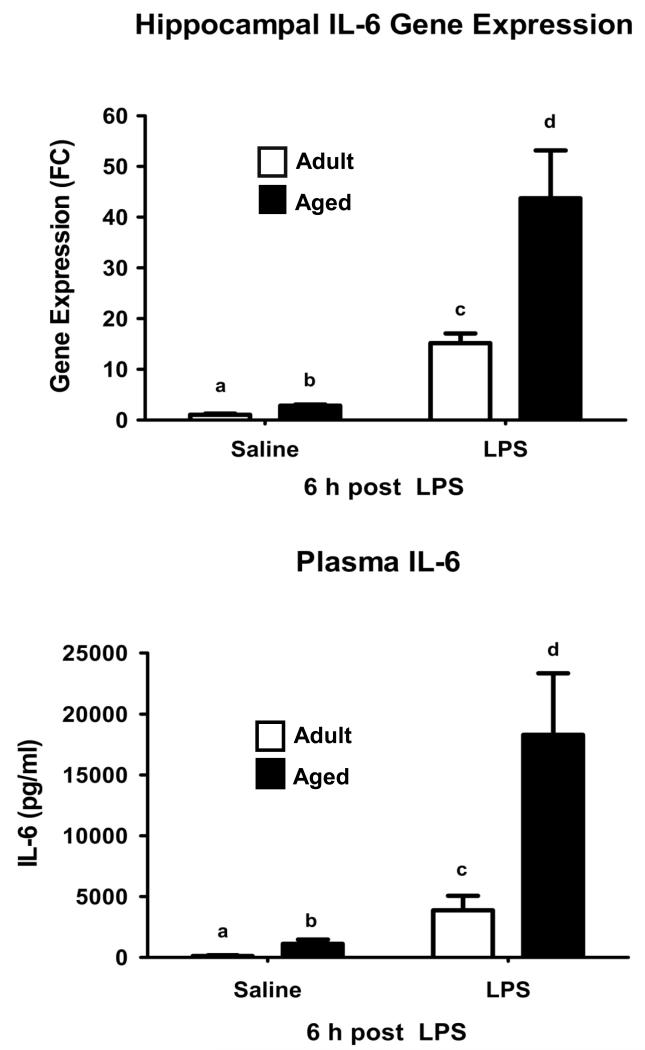
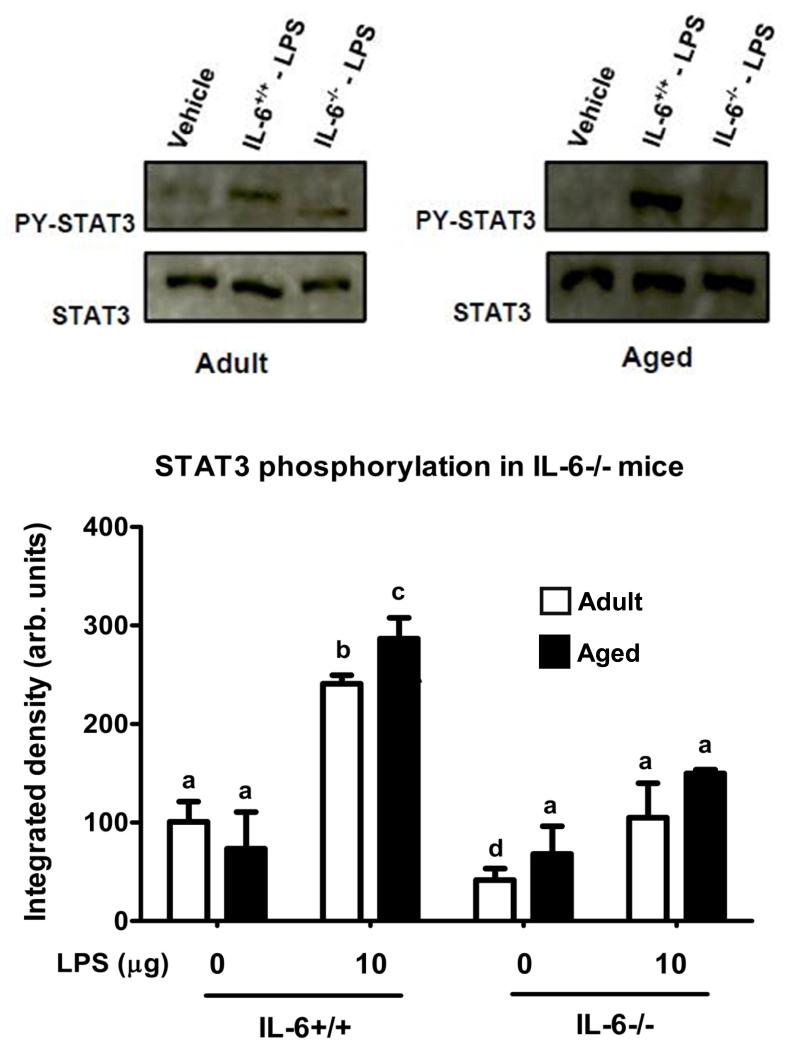
As a read out for IL-6 signaling, STAT3 phosphorylation was determined in the hippocampus of adult and aged mice after i.p. injection of LPS. Figure 1 shows representative western blots revealing phosphorylated STAT3 in hippocampi collected from adult and aged mice at various times after injection of LPS and the mean STAT3 phosphorylation from 8 or 9 treatment replicates at each time point for each age. As expected STAT3 activity in the hippocampus was increased 1 h after LPS in both adult and aged animals. However, whereas the level of STAT3 phosphorylation reached a plateau by 1 h in adults, it continued to increase in the aged. An age × LPS interaction at 4 h [F (1,34) = 4.319, P< 0.05] and at 6 h [F (1,34) =7.857, P< 0.01] indicated a marked increase in STAT3 activity in the hippocampus of aged mice. As we surmised that increased IL-6 was responsible for the increased STAT3 activity, plasma IL-6 and IL-6 mRNA in the hippocampus were measured in adult and aged mice 6 h after LPS injection when STAT3 phosphorylation was maximal in the aged. As evident in Figure 2, after LPS injection, plasma IL-6 and hippocampal IL-6 mRNA were markedly higher in aged mice compared to adults [F (1,24) = 15.731, P< 0.001], and [F (1,24) = 8.132, P< 0.01], respectively. Collectively, these data show an association between increased levels of IL-6 and IL-6 signaling in the hippocampus of senescent mice during times of peak sickness. To confirm that IL-6 was responsible for the increased STAT3 activity, a subsequent study was conducted using adult and aged IL-6+/+ and IL-6−/− mice. Figure 3 shows representative western blots revealing phosphorylated STAT3 in hippocampi collected from adult and aged IL-6+/+ and IL-6−/− mice 6 h after injection of LPS and the mean STAT3 phosphorylation from 6 or 7 treatment replicates. Despite employing a different genetic background than in the first study (C57BL/6 vs. BALB/c), once again hippocampal STAT3 activity was increased in aged IL-6+/+ mice compared to adult IL-6+/+ mice [F (1,26) = 4.671, P< 0.05] after LPS injection (Figure 3). Importantly, the LPS-induced increase in STAT3 activity was ablated in IL-6−/− mice (Figure 3), suggesting IL-6 is a key driver of STAT3 activity in the aged brain after peripheral immune stimulation.
Figure 1. Activated STAT3 in the hippocampus of adult and aged mice after peripheral injection of LPS.
Adult and aged BALB/c mice were injected i.p. with LPS and hippocampal tissue was collected at various time points after injection to measure phosphorylated STAT3. The upper panel shows representative western blots and the bar graph shows mean STAT3 phosphorylation (± SEM) from 8-9 treatment replicates at each time point for each age (because STAT3 activity was not affected in saline controls over time, data from saline treated mice at different time points was pooled. Means with different letters are significantly different (P<0.05).
Figure 2. Plasma IL-6 and IL-6 mRNA in the hippocampus in aged and adult mice 6 h after LPS injection.
Adult and aged BALB/c mice were injected i.p. with LPS and 6 h later, blood and brain tissue were collected for analysis. Bars represent the mean ± SEM (n = 7-8). Means with different letters are significantly different (P<0.05).
Figure 3. Phosphorylated STAT3 in hippocampus of adult and aged IL6+/+ and IL-6−/− mice after injection of LPS.
Adult and aged IL-6+/+ and IL-6−/− mice were injected i.p. with LPS and hippocampal tissue was collected to measure phosphorylated STAT3. The upper panel shows representative western blots and the bar graph shows mean STAT3 phosphorylation (± SEM) from 6-7 treatment replicates. Means with different letters are significantly different (P<0.05).
IL-6 trans-signaling induces STAT3 activity in hippocampus of aged mice
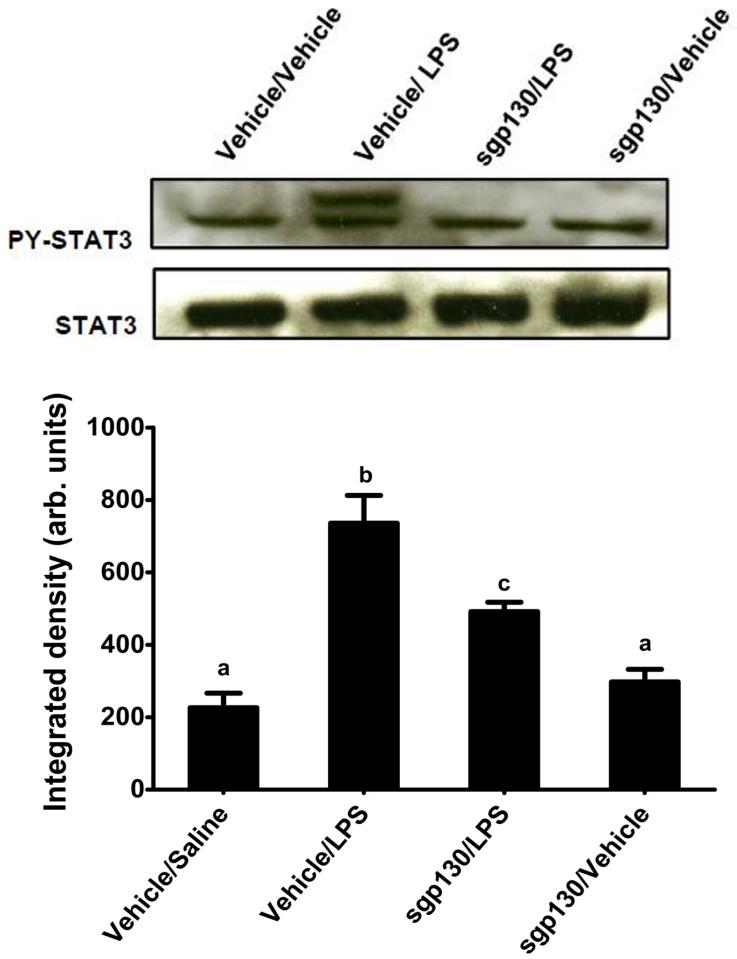
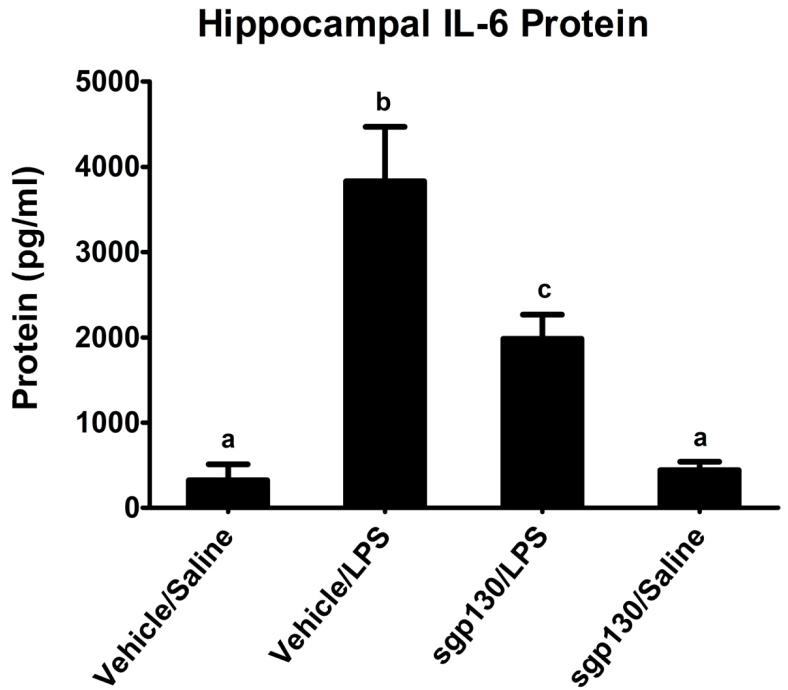
To determine if the age-related increase in hippocampal STAT3 activity after LPS injection was mediated by IL-6 via the classical or trans-signaling pathway, aged BALB/c mice were surgically fit with an indwelling ICV cannula. Following recovery, mice were injected ICV with sgp130 and i.p. with LPS. Similar to earlier results, LPS up regulated STAT3 phospho-protein and IL-6 in the hippocampus (Figure 4 and 5, respectively). Furthermore, there was a sgp130 × LPS interaction where both LPS-induced IL-6 [F (1,35) = 4.578, P< 0.04] and STAT3 activity [F (1,35) = 4.131, P< 0.05] in the hippocampus, was markedly reduced by sgp130. As sgp130 binds IL-6 that is coupled with sIL-6R preventing it from activating membrane bound gp130 (Barkhausen et al., 2011), these data suggest a key role for IL-6 trans-signaling in the hippocampus.
Figure 4. Effects of sgp130 on LPS-induced STAT3 activity in the hippocampus of aged mice.
Aged BALB/c mice were injected ICV with sgp130 and i.p. with LPS and hippocampal tissue was collected 6 h later. The upper panel shows representative western blots and the bar graph shows mean STAT3 phosphorylation (± SEM) from 8-9 treatment replicates. Means with different letters are significantly different (P<0.05).
Figure 5. Effects of sgp130 on LPS-induced IL-6 in the hippocampus of aged mice.
Aged BALB/c mice were injected ICV with sgp130 and i.p. with LPS and hippocampal tissue was collected 6 h later for determination of IL-6. The bar graph shows mean IL-6 concentration (± SEM) from 8-9 treatment replicates. Means with different letters are significantly different (P<0.05).
sgp130 inhibits LPS-induced deficits in contextual fear conditioning
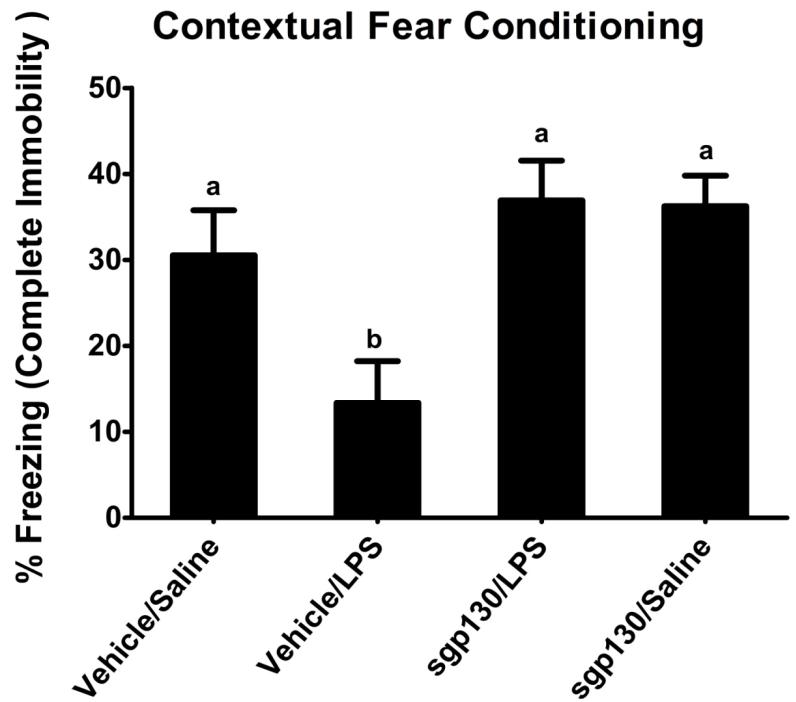
Circulating IL-6 and cognitive dysfunction are positively correlated (Bellinger et al., 1995; Gruol and Nelson, 1997; Maggio et al., 2006; Weaver et al., 2002), and acute cognitive disorders are common in elderly patients during peripheral infection. Thus, we were eager to know if inhibiting IL-6 trans-signaling would protect aged mice from LPS-induced deficits in cognition. Aged BALB/c mice were injected ICV with sgp130 and i.p. with LPS immediately after the acquisition phase of contextual fear conditioning. Forty-eight hours later their freezing response in the retention phase was assessed. As evident in Figure 6, LPS reduced immobility in aged mice, indicating immune activation interfered with memory consolidation. However, ICV injection of sgp130 which reduced STAT3 activation and IL-6 in the hippocampus, completely blocked the deficit in hippocampal-based contextual fear conditioning caused by LPS [F (1,58) = 5.639, P< 0.03].
Figure 6. Effects of sgp130 on the LPS-induced deficit in contextual fear conditioning in aged mice.
Aged BALB/c mice were injected ICV with sgp130 and i.p. with LPS immediately after the acquisition phase of contextual fear conditioning. Forty-eight hours later immobility in the retention phase was assessed. Bars represent the mean ± SEM (n = 12-15). Means with different letters are significantly different (P < 0.05).
Discussion
Excessive production of pro-inflammatory cytokines in the senescent brain in response to peripheral immune stimulation (Godbout and Johnson, 2009) is thought to induce behavioral pathology and be a forerunner to neurodegenerative disease. However, few studies have examined if the increase in pro-inflammatory cytokines is accompanied by an increase in cytokine signaling, which would be a prerequisite if the behavioral changes are driven by the pro-inflammatory cytokine response. By focusing on IL-6 as a prototypical pro-inflammatory cytokine, the present results show that the magnitude and duration of IL-6 signaling are increased in the hippocampus of senescent mice compared to adults after peripheral injection of LPS. They further suggest a key role for IL-6 trans-signaling in hippocampal dysfunction in the senescent and highlight the potential value of sgp130 for preventing infection-related behavioral pathology.
In the present study, STAT3 phosphorylation was used as an indicator of IL-6 signaling. Both classical and trans-signaling pathways cause gp130 to dimerize, resulting in an intracellular cascade that recruits and phosphorylates STAT3. Although other cytokines that were previously detected in the CNS also activate STAT3 (e.g., IL-10, leukemia inhibitory factor, and oncostatin M), the fact that STAT3 activation was not increased after LPS injection in aged IL-6−/− mice strongly suggests the increased STAT3 phosphorylation in aged wild type mice was due to IL-6 (Figure 3). This is further supported by the fact that IL-6 mRNA and protein were markedly higher in the hippocampus of aged mice compared to adults at the time corresponding to maximal STAT3 phosphorylation. However, it should be noted that other cytokines that can activate STAT3 were not measured in this study. Due to the fact that sgp130 binds the IL-6-sIL-6R complex in the extracellular matrix and prevents it from activating membrane bound gp130, it is a naturally occurring antagonist of the IL-6 trans-signaling pathway (Jostock et al., 2001). Since it has a low affinity for IL-6 that is unbound to sIL-6R, sgp130 does not interfere with IL-6R signaling (Jones et al., 2005; Muller-Newen et al., 1998). Therefore, sgp130 can be used to elucidate the contribution of the IL-6 trans-signaling pathway to biologically relevant outcomes. For example, sgp130 was recently reported to reduce LPS-induced sickness behavior in mice (Burton et al., 2011). The finding that LPS-induced sickness was mediated by the trans-signaling pathway and not the classical pathway was consistent with the finding that Neuro.2A cells (a neuronal cell line) expressed abundant levels of gp130 but very little IL-6R (Burton et al., 2011). If neurons express few IL-6R but are laden with gp130 and mostly influenced by IL-6 through the trans-signaling pathway, this would explain earlier work that found recombinant IL-6 had little effect on behavior when injected ICV but induced profound sickness when co-administered with sIL-6R (Schobitz et al., 1995). In the present study sgp130 given ICV reduced LPS-induced STAT3 activity in the hippocampus of aged mice, further highlighting the importance of IL-6 trans-signaling in the brain. These findings regarding IL-6 trans-signaling in the brain are consistent with a recent study of IL-6 trans-signaling and TLR4-driven inflammatory responses in the periphery (Greenhill et al., 2011). Cross-talk between JAK/STAT and TLR4 pathways is now viewed as a broad-based mechanism that regulates the severity of inflammation. Other studies suggest inhibiting IL-6 trans-signaling with sgp130 may be helpful in treating a number of inflammatory conditions including arthritis, peritonitis, and colitis (Coles et al., 2007; McLoughlin et al., 2005; Richards et al., 2006).
Activated STAT3 translocates to the nucleus where it binds to enhancer elements of the IL-6 gene to induce transcription. Thus, a primary effect of IL-6 is to induce expression of more IL-6 (Aaronson and Horvath, 2002). Although our goal was to investigate IL-6 signaling in the senescent brain as opposed to mechanisms responsible for the exaggerated production of the cytokine, the present study suggests the involvement of a positive feedback loop initiated through trans-signaling where IL-6 begets IL-6. This is best exemplified in Figure 5 where sgp130 given ICV is shown to reduce LPS-induced IL-6 in the hippocampus by about half.
Due to the fact that sgp130 reduced STAT3 activity and IL-6 levels in the hippocampus, it was reasonable to postulate that it would also inhibit LPS-induced deficits in hippocampal-dependent learning and memory. We chose to focus on hippocampal-dependent contextual fear conditioning. This is important because immune stimuli are known to inhibit consolidation of memories in hippocampal-dependent tasks (Pugh, 2001; Pugh et al., 1998), and hippocampal-dependent tasks are more easily disrupted in aged animals compared to adults (Adams et al., 2001; Barrientos et al., 2006; Bruunsgaard et al., 2001; Chen et al., 2008; Cortese et al., 2011). In the present study, aged mice given LPS after the acquisition phase showed less immobility when reintroduced to the environment where they experienced mild shock, meaning they were less effective at establishing an association between the environment and noxious stimulus, which could be dangerous in a natural setting. However, treatment with sgp130 completely blocked this effect of LPS, suggesting IL-6 trans-signaling is involved in infection-related hippocampal dysfunction. Consistent with previous studies, results with adult animals showed contextual fear conditioning was impaired by post-training LPS, although the age × treatment interaction did not reach significance (data not shown). This result is probably due to the fact that behavior was assessed at a single time point, and a number of studies display a protraction in LPS-induced behavioral deficits and not necessarily a difference in magnitude.
Ongoing studies are aimed at identifying the cell type(s) in the brain that is responsible, for the IL-6 trans-signaling-mediated response to LPS during aging; proposed mechanisms include modulation of the blood-brain barrier, chemokine activation, and oxidative stress. It is interesting that blocking trans-signaling was sufficient to protect contextual fear conditioning since other pro-inflammatory cytokines such as IL-1β are known to inhibit hippocampal-dependent learning and memory during infection (Yirmiya et al., 2002), but signal through different pathways. However, we previously showed that mice deficient in IL-6 (i.e., IL-6−/− mice) also had reduced levels of IL-1β and TNFα in the hippocampus after peripheral LPS challenge, suggesting IL-6 plays a permissive role in the production and/or activities of other pro-inflammatory cytokines (Sparkman et al., 2006). This is consistent with a recent study by Greenhill et al. (2011) that suggested IL-6 trans-signaling is an important determinant in TLR4-driven inflammation. Taken together, the present study suggests that sgp130 may be useful for protecting against infection-related neuroinflammation and cognitive dysfunction in the aged.
Acknowledgements
This research was supported by NIH grant R01-AG16710 to R.W.J. M.D.B. is supported by NIH diversity supplement SR01-AG16710.
Footnotes
Competing interests
The authors of this manuscript declare that they have no actual or potential competing interests.
Authors’ contributions
MDB was involved in research experimentation, statistical analysis, and writing of the manuscript. RWJ directed all aspects of this research project including experimental design, research experimentation, statistical analysis, and writing of the manuscript.
References
- Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiology of Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Central inhibition of interleukin-1 beta ameliorates sickness behavior in aged mice. Brain Behavior and Immunity. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, Rapp PR, Morrison JH. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J. Comp. Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. Faseb Journal. 2004;18:1788–+. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Barkhausen T, Tschernig T, Rosenstiel P, van Griensven M, Vonberg RP, Dorsch M, Mueller-Heine A, Chalaris A, Scheller J, Rose-John S, Seegert D, Krettek C, Waetzig GH. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Critical Care Medicine. 2011;39:1407–1413. doi: 10.1097/CCM.0b013e318211ff56. [DOI] [PubMed] [Google Scholar]
- Barrientos RM. Voluntary exercise as an anti-neuroinflammatory therapeutic. Brain Behavior and Immunity. 2011;25:1061–1062. doi: 10.1016/j.bbi.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiology of Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Bellinger FP, Madamba SG, Campbell IL, Siggins GR. Reduced long-term potentiation in the dentate gyrus of transgenic mice with cerebral overexpression of interleukin-6. Neuroscience Letters. 1995;198:95–98. doi: 10.1016/0304-3940(95)11976-4. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Michaud B, Poli V, Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiol. Behav. 2000;70:367–373. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Curr Opin Hematol. 2001;131:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Burton MD, Sparkman NL, Johnson RW. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. J Neuroinflammation. 2011;8:54. doi: 10.1186/1742-2094-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behavior and Immunity. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles B, Fielding CA, Rose-John S, Scheller J, Jones SA, O’Donnell VB. Classic interleukin-6 receptor signaling and interleukin-6 trans-signaling differentially control angiotensin II-dependent hypertension, cardiac signal transducer and activator of transcription-3 activation, and vascular hypertrophy in vivo. American Journal of Pathology. 2007;171:315–325. doi: 10.2353/ajpath.2007.061078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese GP, Barrientos RM, Maier SF, Patterson SL. Aging and a peripheral immune challenge interact to reduce mature brain-derived neurotrophic factor and activation of TrkB, PLCgamma1, and ERK in hippocampal synaptoneurosomes. J Neurosci. 2011;31:4274–4279. doi: 10.1523/JNEUROSCI.5818-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Contextual fear, gestalt memories, and the hippocampus. Behav Brain Res. 2000;110:73–81. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice after activation of the peripheral innate immune system. Faseb Journal. 2005;19:1329–+. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Interleukin-6 in the aging brain. Journal of Neuroimmunology. 2004;147:141–144. doi: 10.1016/j.jneuroim.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Johnson RW. Age and Neuroinflammation: A Lifetime of Psychoneuroimmune Consequences. Immunology and Allergy Clinics of North America. 2009;29:321–+. doi: 10.1016/j.iac.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, Connor JO, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill CJ, Rose-John S, Lissilaa R, Ferlin W, Ernst M, Hertzog PJ, Mansell A, Jenkins BJ. IL-6 Trans-Signaling Modulates TLR4-Dependent Inflammatory Responses via STAT3. Journal of Immunology. 2011;186:1199–1208. doi: 10.4049/jimmunol.1002971. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Nelson TE. Physiological and pathological roles of interleukin-6 in the central nervous system. Molecular Neurobiology. 1997;15:307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochemical Journal. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann KW, Schuh AFS, Saute J, Townsend R, Fricke D, Leke R, Souza DO, Portela LV, Chaves MLF, Rieder CRM. Interleukin-6 Serum Levels in Patients with Parkinson’s Disease. Neurochemical Research. 2009;34:1401–1404. doi: 10.1007/s11064-009-9921-z. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiology of Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: The in vivo consequences. Journal of Interferon and Cytokine Research. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- Jostock T, Mullberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. European Journal of Biochemistry. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Akira S, Taga T. IL-6 receptor and mechanism of signal transduction. International Journal of Immunopharmacology. 1992;14:431–438. doi: 10.1016/0192-0561(92)90173-i. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Imparied immune and acute-phase responses in interleukin-6 deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2008;295:R1109–R1114. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F, Grimaldi LM, Bonafe M, Martina C, Olivieri F, Cavallone L, Giovanietti S, Masliah E, Franceschi C. Interleukin-6 gene alleles affect the risk of Alzheimer’s disease and levels of the cytokine in blood and brain. Neurobiol Aging. 2003;24:921–926. doi: 10.1016/s0197-4580(03)00013-7. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: A magnificent pathway. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin RM, Jenkins BJ, Grail D, Williams AS, Fielding CA, Parker CR, Ernst M, Topley N, Jones SA. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9589–9594. doi: 10.1073/pnas.0501794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Newen G, Kuster A, Hemmann U, Keul R, Horsten U, Martens A, Graeve L, Wijdenes J, Heinrich PC. Soluble IL-6 receptor potentiates the antagonistic activity of soluble gp130 on IL-6 responses. Journal of Immunology. 1998;161:6347–6355. [PubMed] [Google Scholar]
- Muller T, Blum-Degen D, Przuntek H, Kuhn W. Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta Neurologica Scandinavica. 1998;98:142–144. doi: 10.1111/j.1600-0404.1998.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Pugh CR. The immune system and memory consolidation: a role for the cytokine IL-1 beta. Neuroscience and Biobehavioral Reviews. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behavior and Immunity. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Richards PJ, Nowell MA, Horiuchi S, McLoughlin RM, Fielding CA, Grau S, Yamamoto N, Ehrmann M, Rose-John S, Williams AS, Topley N, Jones SA. Functional characterization of a soluble gp130 isoform and its therapeutic capacity in an experimental model of inflammatory arthritis. Arthritis and Rheumatism. 2006;54:1662–1672. doi: 10.1002/art.21818. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33:1369–1377. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Experimental Gerontology. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochemical Journal. 1994;300:281–290. doi: 10.1042/bj3000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica Et Biophysica Acta-Molecular Cell Research. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- Schobitz B, Dekloet ER, Sutanto W, Holsboer F. Cellular localization of the interleukin-6 mRNA and interleukin-6 receptor mRNA in rat brain. European Journal of Neuroscience. 1993;5:1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Schobitz B, Pezeshki G, Pohl T, Hemmann U, Heinrich PC, Holsboer F, Reul J. Soluble interleukin-6 (IL-6) receptor augments central effects of IL-6 in-vivo. Faseb Journal. 1995;9:659–664. doi: 10.1096/fasebj.9.8.7768358. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JRR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. Journal of Neuroscience. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallieres L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. Journal of Neuroscience. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline - MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. Journal of Neuroimmunology. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Regulation of interleukin-6 gene expression in brain of aged mice by nuclear factor kappa B. Journal of Neuroimmunology. 2001;117:87–96. doi: 10.1016/s0165-5728(01)00316-2. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Winocur G, Goshen I. Brain interleukin-1 is involved in spatial memory and passive avoidance conditioning. Neurobiol Learn Mem. 2002;78:379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]