Abstract
Purpose
To evaluate the safety and efficacy of ophthalmic MIM-D3, a tyrosine kinase TrkA receptor agonist, in patients with dry eye.
Design
A prospective, two-center, randomized, double-masked, placebo-controlled Phase 2 study.
Methods
A total of 150 dry eye patients were randomized 1:1:1 to study medication (1% MIM-D3, 5% MIM-D3, or placebo) and dosed twice daily (BID) for 28 days. Key eligibility criteria included exacerbation in corneal staining and ocular discomfort in the Controlled Adverse Environment (CAESM) on two visits, separated by 1 week of BID dosing with artificial tears. Safety and efficacy were evaluated at baseline, throughout treatment, and for 2 weeks post-treatment. The pre-specified primary outcome measures were fluorescein corneal staining post-CAE at day 28 and diary worst symptom scores over 28 days. Secondary outcomes included the pre-, post-, and the change from pre- to post-CAE fluorescein and lissamine green staining in both corneal and conjunctival regions, as well as individual diary symptoms.
Results
The prespecified primary endpoints were not met. Compared with placebo, fluorescein corneal staining at day 28 was significantly improved (P < 0.05) in the 1% MIM-D3 group for the assessment of change from pre-CAE to post-CAE. In addition, following CAE exposure, patients in the 1% MIM-D3 group showed significant improvements versus placebo (P < 0.05) in inferior fluorescein and lissamine green staining after 14 and 28 days. Compared with placebo, patients in the 5% MIM-D3 group reported significantly lower daily diary scores for ocular dryness (P < 0.05). In a subgroup defined by higher symptom scores during the run-in period, significant treatment effects (P < 0.05) were observed for diary symptoms for both MIM-D3 doses. Ocular adverse events were mild and not considered to be treatment-related.
Conclusion
Treatment with topical ophthalmic MIM-D3 demonstrated protection against the effects of a CAE challenge on dry eye signs, reduced patient-reported diary symptoms, with a favorable safety profile.
Keywords: nerve growth factor, controlled adverse environment, Mimetogen
Introduction
Dry eye is a multifactorial disease of the tears and ocular surface characterized by dysfunction of the lacrimal functional unit that results in a loss of tear film integrity.1 Patients with tear film instability are likely to experience damage to the ocular surface, disruption of the corneal neural feedback loop, release of proinflammatory mediators in tears and in turn, stimulation of corneal nerves, and episodic symptoms of ocular discomfort and dryness.2,3 This can lead to a further reduction in tear production and impairment in the ability of the ocular surface to respond to environmental challenges, such as wind and low relative humidity, or visual task-oriented challenges, such as video monitor use.4,5 Tear aqueous, lipid, and/or mucin components can be altered or deficient, eventually leading to a chronic disease.6 In order to break this cycle, treatment with a disease-modifying drug with multiple mechanisms of action is needed. To date, the only treatment (cyclosporine A) for dry eye available in the US and Canada targets the inflammatory aspects of the disease.
Nerve growth factor (NGF) belongs to the neurotrophin family of dimeric proteins that regulate the survival and differentiation of neurons in all vertebrate species. Preclinical data support a role for NGF in promoting ocular health, particularly in the realm of physiologic lacrimal function.7–11 The trophic actions of NGF are attributed to the activation of receptor tyrosine kinase TrkA and a common coreceptor p75 neurotrophin receptor (p75 NTR), a member of the tumor necrosis family receptors.12 NGF and TrkA are expressed throughout the ocular surface, including the corneal epithelial cells and sensory neurons, suggesting that they have a physiological role in the homeostasis and regeneration of the corneal stroma and epithelium.13,14 Topically applied NGF has been shown to have therapeutic effects in ocular tissues for neurotrophic keratitis and corneal ulcers; however, its use was associated with ocular and periocular pain.15,17
TrkA has been identified in rat conjunctiva, cultured conjunctival goblet cells, and in human conjunctival epithelial cells.18,19 The conjunctiva is the primary source of both soluble and membrane-associated mucins in the glycocalyx and tear film. These mucins provide a physical and chemical barrier that protects the cornea and conjunctiva from exogenous bacterial and chemical agents, and facilitates maintenance of the smooth, refractive surface that is necessary for clear vision.20 Conjunctival mucin gene expression and secretion may be deficient in several ocular disorders associated with dry eye.21–23 The finding that NGF binding to TrkA in the conjunctiva stimulated mucin release and goblet cell differentiation has suggested the targeting of this receptor to stabilize the tear film and protect its ocular surface barrier function.18,19,24
MIM-D3 is a proteolytically stable, cyclic peptidomimetic that has been shown to be a partial TrkA receptor agonist.25 MIM-D3 demonstrates activities similar to NGF (but does not bind to the p75NTR receptor) and can potentiate the effects of suboptimal concentrations of NGF.25 In vitro studies in cultured primary rat conjunctival cells demonstrated that MIM-D3 stimulated mucin-like secretion and activated mitogen-activated protein kinase (MAPK)1/2 systems involved with mucin production.26 In vivo acute topical treatment of rats resulted in a statistically significant 2.3-fold increase in the concentration of tear fluid mucin-like substances. In a scopolamine-induced dry eye model in rats, topical dosing of 1% MIM-D3 followed by 1 week of no dosing produced a statistically significant 50% reduction in corneal staining, an improvement associated with increased tear mucin-like substances and tear film break-up times.26
We hypothesized that MIM-D3 might show a therapeutic benefit in patients with dry eye disease, due to its multiple mechanisms of action: promoting the survival and differentiation of neuronal cells; stimulating mucin secretion; and improving corneal damage. We prospectively explored the effects of MIM-D3, both in an environmental setting, and in the Controlled Adverse Environment (CAESM) (Ora, Inc, Andover, MA, USA).27 The CAE provides a useful tool for evaluating potential clinically significant protective effects of a drug against the ocular surface stress that occurs during conditions of environmental stress.
The purpose of this study was to evaluate the safety and efficacy of 1% and 5% MIM-D3 ophthalmic solutions compared with placebo, when administered twice daily for 28 days to patients with dry eye disease. This study aimed to determine the optimal dose, patient population, and endpoints.
Methods
Study design
This was a two-center, randomized, double-masked, placebo-controlled study with equal randomization 1:1:1 for 1% MIM-D3, 5% MIM-D3, and placebo in patients with a history of dry eye disease. The study was conducted between November 7, 2010 and May 2, 2011 at Andover Eye Associates in Andover, Massachusetts and at Central Maine Eye Care, Lewiston, ME, USA. The study comprised a 1-week run-in period, followed by a 4-week treatment period and a 2-week follow-up (Figure 1). A total of seven visits were scheduled: two during screening, three during treatment, and two during follow up. The study was conducted in compliance with the International Conference on Harmonisation Good Clinical Practice guidelines, Health Insurance Portability and Accountability Act (HIPAA) and the 1989 version of the Declaration of Helsinki. Institutional review board (IRB), approval was prospective (Alpha IRB, San Clemente, CA, USA), and all patients provided written informed consent. This study has been listed with ClinicalTrials.gov (identifier NCT01257607).
Figure 1.
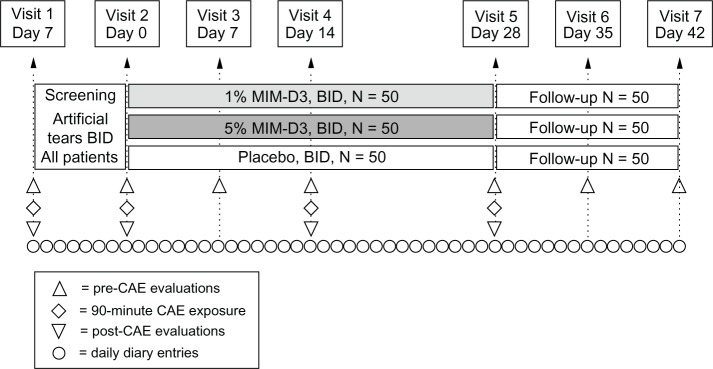
Visit flow chart of a two-center, randomized, double-masked, placebo-controlled study with a novel TrkA agonist (MIM-D3) in patients with a history of dry eye and objective evidence of ongoing dry eye disease.
Notes: The study, which included a 1-week run-in period with artificial tears, comprised two visits of screening/eligibility for patients with modifiable signs and symptoms. randomization and first dose on-site at visit 2 (day 0) were followed with three visits during the treatment period, for endpoint and safety measurements, and two visits of post-treatment follow up for efficacy and safety.
Abbreviations: BID, twice daily; TrkA, receptor tyrosine kinase-A; CAE, controlled Adverse environment.
Patients and selection criteria
Eligible patients were 18 years or older, had a reported history of dry eye for at least 6 months prior to enrollment, and had a history of eye drop use for dry eye symptoms within the previous 6 months. At visit 1, patients had to report a score of ≥2 in at least one of the symptoms on the Ora Calibra™ Four-Symptom Scale (rating burning, dryness, grittiness, and stinging on a six-point [0 to 5] scale, where 0 = none and 5 = most). Patients also had to have a tear film breakup time (TFBUT) ≤ 5 seconds in at least one eye; a sum corneal fluorescein staining score of ≥4, based on the sum of the central, superior, and inferior regions of the cornea with the Ora Calibra™ Fluorescein Staining Scale (reported for each region on a 0–4 scale), and a sum lissamine green conjunctival score of ≥2, based on the sum of the temporal and nasal regions of the conjunctiva with the Ora Calibra™ Lissamine Staining Scale (reported for each region on a 0–4 scale). If initial screening requirements were met, patients were required to demonstrate a ≥1 point increase in fluorescein staining in the inferior region in at least one eye following a 90-minute exposure in the CAE. Additionally, patients had to report an ocular discomfort score (using a five-point [0–4] scale, where 0 = none and 4 = most) of ≥3 at two or more consecutive time points, in at least one eye assessed every 5 minutes during CAE exposure. All patients had to have a corrected visual acuity ≥ logarithm of the minimum angle of resolution (logMAR) +0.7 in both eyes. Patients who met the selection criteria at visit 1 were initiated on self-administered, commercially available artificial tears solution (SensitiveEyes® Bausch & Lomb, Rochester, NY, USA) dosed twice daily (morning and evening) for 7 days until visit 2 (day 0). After this run-in period, at visit 2, eligible patients were required to meet all assessments as described for visit 1 above.
Patients were excluded from the study if they had blepharitis, meibomian gland dysfunction, lid margin inflammation, ocular allergies, ocular inflammation (other than dry eye), ocular rosacea, any viral or bacterial disease of the cornea or conjunctiva within the previous 12 months, worn contact lenses within 30 days of visit 1, or had contact lens-induced dry eye. Patients were excluded if they had Sjögren’s syndrome or lupus erythematosus, a history of lacrimal duct obstruction or laser-assisted in situ keratomileusis (LASIK) surgery within the previous 12 months, any dry eye symptoms associated with LASIK surgery, or any planned ocular and/or lid surgeries during the study period. Patients could not participate if they anticipated using and were not able to discontinue using temporary punctual plugs or any topical ophthalmic prescription or over-the-counter solutions, including Restasis® (cyclosporine ophthalmic emulsion 0.05%) (Allergan, Inc, Irvine, CA, USA) within 60 days of visit 1, as well as artificial tears, gels, or scrubs, or any eye drops within 2 hours of visit 1. Lastly, patients were excluded if they had uncontrolled systemic disease, were nursing or pregnant, enrolled in an investigational drug or device study within 45 days of visit 1, or had been currently using any medication known to cause ocular drying.
Interventions
Eligible patients were randomized at day 0 in a 1:1:1 ratio into one of three treatment groups: MIM-D3 1%, MIM-D3 5%, or placebo (the vehicle of MIM-D3). The clinical dosage form and packaging of MIM-D3 and the placebo ophthalmic solutions were identical sterile, low-density polyethylene unit-dose nonpreserved 1 mL bottles. They were packaged in foil-wrap pouches to prevent light exposure, each containing two single-use bottles. Throughout the study, between day 0 and day 28, patients were instructed to instill one drop of study medication in each eye two times daily, once in the morning and once in the evening before bed. Patients were not permitted to use artificial tears (except for Bausch & Lomb Sensitive Eyes® during the run-in period) or any concomitant topical ocular treatment during the course of the study. Patients were assigned randomization kit numbers in strict numerical sequence, using a code generated by an independent biostatistician. All investigators, study and site personnel, and patients were masked to the treatment assignments. When medically necessary, the investigator was entitled to open a sealed envelope of the patient randomization to determine which treatment was assigned. At the end of the study, all (opened and unopened) envelopes were returned with the study products. Patients were dispensed kits with sufficient study treatment for 2 weeks; they were instructed not to dose on the morning of scheduled visit days.
Patients were evaluated on days 7 (visit 3), 14 (visit 4), and 28 (visit 5) during the dosing period. Days 35 (visit 6) and 42 (visit 7) were post-treatment follow-up evaluations. Exposure to the CAE occurred on days 14 and 28. At each study visit, a panel of dry eye signs and symptoms and safety measures were evaluated (including both before [pre-CAE] and after CAE [post-CAE] exposure). Each day, patients used a study diary to record the severity of their dry eye symptoms prior to the morning and evening dosing, grading overall ocular discomfort, ocular dryness, grittiness, burning, and stinging using a six-point (0 to 5) scale where 0 = least and 5 = worst. The diary was used throughout the study: during the run-in, study treatment, and follow-up periods.
Outcome measures
Efficacy
The primary efficacy objective endpoint (sign) was total corneal fluorescein staining (sum of central, inferior and superior regions) evaluated post-CAE at day 28, as measured by the Ora Calibra scale. The primary subjective endpoint (symptom) was subject diary data (worst symptom) over the 4-week treatment period. For diary data, the worst symptom for each patient was identified as the symptom with the highest mean score recorded in the diary during the run-in period. If there was a tie for the highest mean score, then the most severe symptom was chosen.
Secondary sign endpoints assessed at each visit, both pre- and post-CAE, included Ora Calibra™ Fluorescein and Lissamine Staining (both graded in five regions: inferior, superior, central cornea, and nasal and temporal conjunctiva, with scores provided in single regions, summed by corneal and conjunctival regions, and by sum total of all regions), TFBUT, conjunctival and lid margin redness, blink rate, and unanesthetized Schirmer’s test (measured only post-CAE). Corneal sensitivity (assessed with the Cochet-Bonnet esthesiometer) and tear osmolarity (TearLab® Osmolarity System, TearLab, San Diego, CA, USA) were measured pre-CAE only on days 0, 14, 28, and 42.
Secondary symptom endpoints assessed at each visit (both pre- and post-CAE) were ocular discomfort and the four-symptom questionnaire (each symptom). Ocular discomfort was also graded every 5 minutes during the CAE, and the Ocular Surface Disease Index (OSDI©; Allergan, Inc) was assessed pre-CAE. For this, the investigator asked the subject a series of 12 questions related to the frequency of occurrence of dry eye symptoms during the previous week. Patients rated each eye using a five-point scale where 0 = none of the time and 4 = all of the time. Secondary patient diary endpoints included the data for individual symptoms.
Safety
Adverse events (AEs), slit-lamp biomicroscopy (both pre-and post-CAE), and visual acuity pre-CAE were monitored at every visit. Dilated fundoscopy and intraocular pressure were assessed at visits 1 and 7. Blood draws for hematology and blood chemistry were performed prior to and 30 minutes following first and last dose.
Statistical analysis
For the primary sign endpoint analysis, a two-sample t-test was used to compare 1% MIM-D3 ophthalmic solution with placebo. For the primary symptom endpoint analysis, the worst symptom mean daily score was analyzed across the 4-week treatment period, using a generalized linear model that accounted for repeated measures with an unstructured correlation structure, including terms for diary day and the treatment by day interaction. For both the primary sign and symptom, if the difference was significant at the 0.05 level (two-sided), 5% MIM-D3 ophthalmic solution was compared with placebo as well. The primary analysis was performed on the intent to treat (ITT) population with the last-observation carried-forward method for missing values. For the prespecified primary endpoint to be achieved, statistical significance was required for both the primary sign and symptom, hence no multiplicity of adjustment was necessary.
Secondary efficacy variables were analyzed as above on the ITT and Per-Protocol populations with observed data only. The Per-Protocol population, defined prior to unmasking of the database, excluded patients with significant protocol deviations and any patient who did not complete the study. All data collected on CAE visit days were analyzed at each time point (pre- and post-CAE), as well as on the derived variable of post-CAE minus pre-CAE (termed herein as CAE-induced). The mean change from baseline (visit 2, pre-CAE), Wilcoxon rank-sum tests, and analysis of covariance (ANCOVA) models, adjusting for baseline scores, were the prespecified sensitivity analyses. For patient diary data, individual symptoms and worst symptom were analyzed, as above, across the 4-week treatment and 2-week follow-up periods. Additionally, each diary day and time point (morning, evening, daily average) was analyzed separately using t-test, Wilcoxon rank-sum-tests, and ANCOVA models. All secondary endpoint analyses were prescribed in the statistical analysis plan of the study, and all tests were performed at the 0.05 significance level (two-sided).
The study protocol was designed to enroll 50 patients per group. Assuming a 10% drop out rate and a common standard deviation of 1.8, this study was designed a priori to have 90% power to detect a 10.4% difference in corneal fluorescein staining scores after the CAE challenge (ie, 1.25 units on a 0–12 scale). For objective efficacy endpoints, the unit of analysis was the “worse eye,” defined as the eye that met all protocol-specified inclusion criteria. When both eyes were eligible, the worse eye was the eye with worse inferior corneal staining after CAE exposure at baseline, or if both eyes had the same score, the worse eye had the earliest onset of symptomatic reaction during CAE exposure. If both eyes had the same response time, the right eye was used.
Safety endpoints analyzed for both eyes included treatment comparisons for visual acuity and intraocular pressure, using t-tests and Wilcoxon rank-sum tests, and for slit-lamp biomicroscopy and dilated fundoscopy, using Fisher’s exact tests. All AEs and medical history were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 13.1. Statistical programming and analysis was performed using SAS® Version 9.2 (SAS Institute, Cary, NC, USA).
A post hoc subgroup analysis was performed in which patients were divided into two populations based on the initial severity of a particular diary symptom, defined as the mean value of diary data recorded between day 7 and day 0. This analysis classified the subgroups with daily scores ≤ median or >median score for a given symptom during the run-in period. The median scores were determined as 2.70 for worst symptom, 2.50 for ocular dryness, 2.44 for ocular discomfort, 1.72 for grittiness, 1.13 for burning, and 0.85 for stinging.
Results
Patient characteristics
A total of 301 patients were screened. Of these, 150 were enrolled in the study, with fifty patients assigned to each of three groups: 1% MIM-D3, 5% MIM-D3, and placebo. Six patients did not complete the study, and two had major protocol deviations (Table 1). All patients received their first dose of assigned study medication on site at day 0. There were no statistically significant differences in gender or age among the treatment groups (Table 1).
Table 1.
Patient disposition and demographics in a study of a novel TrkA agonist (MIM-D3) for dry eye disease
| Characteristic | 1% MIM-D3 (N = 50) | 5% MIM-D3 (N = 50) | Placebo (N = 50) | All patients (N = 150) |
|---|---|---|---|---|
| Mean age (SD) | 58.0 (13.4) | 58.2 (15.1) | 58.4 (15.7) | 58.2 (14.6) |
| Sex, N (%) | ||||
| Male | 16 (32) | 11 (22) | 10 (20) | 37 (24.6) |
| Female | 34 (68) | 39 (78) | 40 (80) | 113 (75.3) |
| Caucasian, N (%) | 45 (90.0) | 45 (90.0) | 45 (90.0) | 135 (90.0) |
| Patients who completed the study | 48 (96%) | 49 (98%) | 47 (94%) | 144 (96%) |
| Discontinued | ||||
| Adverse events | 1 (2%) | 0 | 1 (2%) | 2 (4%) |
| Noncompliance | 1 (2%) | 1 (2%) | 0 | 2 (4%) |
| Administrative | 0 | 0 | 2 (4%) | 2 (4%) |
| Protocol deviation | 0 | 2 (4%) | 0 | 2 (4%) |
Abbreviations: SD, standard deviation; TrkA, receptor tyrosine kinase-A.
Objective endpoints: signs
At baseline, the mean total corneal fluorescein staining scores ranged between 5.16 and 5.35 in the pre-CAE assessment and between 7.59 and 7.85 in the post-CAE assessment, with no significant differences across treatment groups. For the primary sign efficacy endpoint at day 28, the total corneal staining scores post-CAE for the last observation carried forward in the MIM-D3 treated groups were numerically lower than the scores in the placebo group. These differences (−0.47 in the 1% MIM-D3 group and −0.40 in the 5% MIM-D3 group) were not statistically significant compared with placebo (P = 0.199 and P = 0.265, respectively). However, assessment of CAE-induced staining, defined as the change from pre- to post-CAE staining scores revealed improvements compared with placebo, particularly with the 1% MIM-D3 dose, in the total corneal scores (Table 2). Furthermore, at this assessment, MIM-D3-treated groups had consistently lower fluorescein staining scores in various corneal and conjunctival regions compared with placebo (Figure 2).
Table 2.
Total corneal fluorescein staining in a study of a novel TrkA agonist (MIM-D3) for dry eye disease
| Assessmenta,b | 1% MIM-D3 | 5% MIM-D3 | Placebo |
|---|---|---|---|
| Pre-CAE | |||
| Mean ± SD | 4.83 ± 1.63 | 4.80 ± 1.72 | 4.55 ± 1.77 |
| P-valuec | 0.429 | 0.484 | – |
| Post-CAE | |||
| Mean ± SD | 6.27 ± 2.03 | 6.44 ± 1.97 | 6.73 ± 1.63 |
| P-value | 0.217 | 0.432 | – |
| Change from pre- to post-CAE | |||
| Mean ± SD | 1.44 ± 1.67 | 1.66 ± 1.51 | 2.18 ± 1.59 |
| P-value | 0.028d | 0.107 | – |
Notes:
Ora Calibra™ Scale, 0–12;
at day 28, ITT population with observed data only;
P-values calculated using a two-sample t-test comparing active treatments with placebo;
significance was also achieved with an ANCOVA model, with treatment and baseline scores as covariates, comparing active treatments with placebo.
Abbreviations: ANCOVA, analysis of covariance; ITT, intent to treat; SD, standard deviation, TrkA, receptor tyrosine kinase-A; CAE, Controlled Adverse Environment.
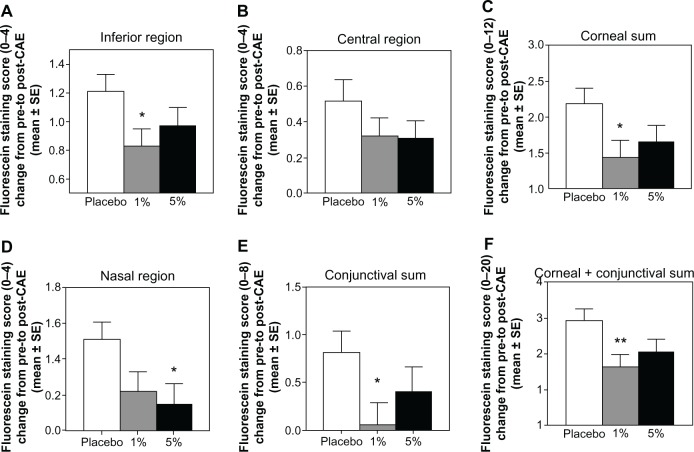
Figure 2.
CAESM-induced fluorescein staining results in multiple regions of the eye, in patients with a history of dry eye and objective evidence of ongoing dry eye disease, after 28 days of treatment with a novel TrkA agonist (MIM-D3).
Notes: The data are reported as the change from pre- to post-CAE. Treatment with 1% MIM-D3 showed significant (*P < 0.05 or **P < 0.01 from a two-sample t-test) improvement compared with placebo in multiple regions, at day 28. Corneal sum refers to the sum of scores of the inferior, central, and superior regions. Conjunctival sum refers to the sum of scores of the nasal and temporal regions.
Abbreviations: CAE, Controlled Adverse Environment; SE, standard error; TrkA, receptor tyrosine kinase-A.
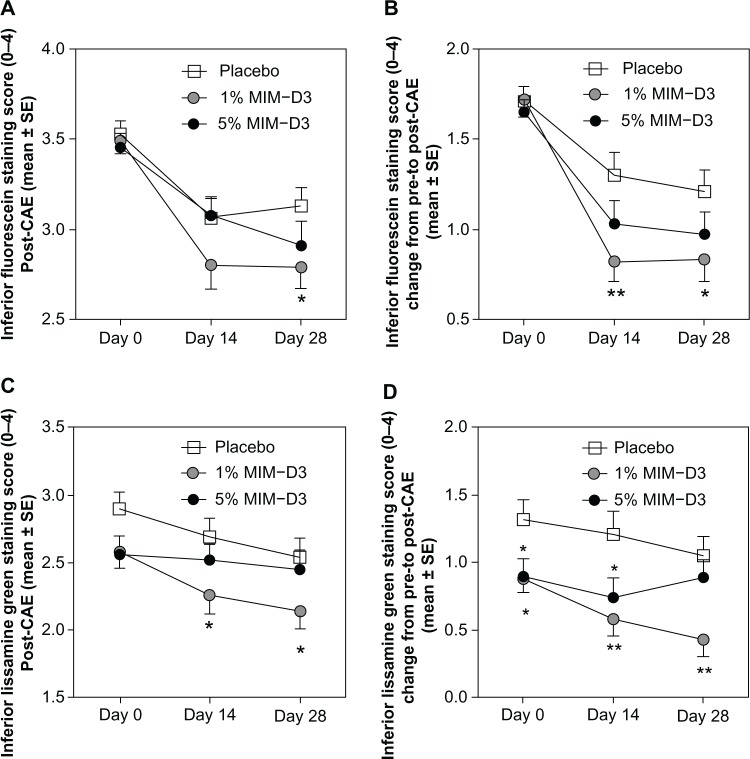
Individual staining regions revealed improvements, particularly with the 1% MIM-D3 dose in the inferior region at days 14 and 28. Fluorescein staining scores in the inferior corneal region were improved in the MIM-D3 treated groups compared with placebo, in the post-CAE (Figure 3A) and CAE-induced staining (Figure 3B) assessments. The results for lissamine green staining were consistent with those for fluorescein staining. At days 14 and 28, lissamine green staining scores in the inferior region were lower in the 1% MIM-D3 group than in the placebo group, in post-CAE (Figure 3C) and CAE-induced staining (Figure 3D) assessments. Additionally, CAE-induced lissamine staining scores were lower in the 1% MIM-D3 group compared with placebo, in multiple regions at day 14, particularly in corneal sum (−0.92) (P = 0.011 t-test; P = 0.008 ANCOVA), conjunctival sum (−0.43) (P = 0.037 t-test; P = 0.049 ANCOVA), and corneal plus conjunctival sum (−1.34) (P = 0.003 t-test; P = 0.003 ANCOVA).
Figure 3.
Mean fluorescein and lissamine staining scores in the inferior cornea after challenge in the CAESM, in patients with a history of dry eye and objective evidence of ongoing dry eye disease, after 28 days of treatment with a novel TrkA agonist (MIM-D3). Treatment with 1% MIM-D3 showed significant (*P < 0.05 or **P < 0.01 from a two-sample t-test) improvement compared with placebo as early as day 14. (A) Mean fluorescein staining scores measured post-CAE; (B) mean CAE-induced fluorescein staining reported as the change from pre- to post-CAE; (C) mean lissamine green staining scores measured post-CAE; and (D) mean CAE-induced lissamine green staining reported as the change from pre- to post-CAE.
Abbreviations: CAE, controlled Adverse Environment; SE, standard error; TrkA, receptor tyrosine kinase-A.
There were no improvements in TFBUT, conjunctival and lid margin redness, blink rate, corneal sensitivity, tear osmolarity, and unanesthetized Schirmer’s test among the treatment groups in the ITT population throughout the study.
Subjective endpoints: symptoms
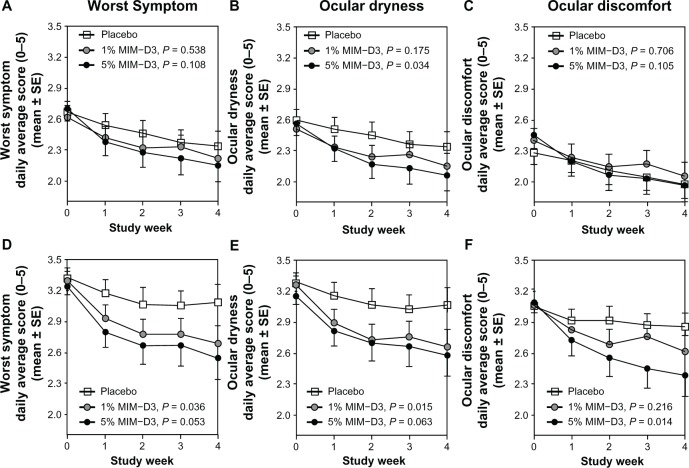
At baseline, the mean diary worst symptom scores ranged between 2.60 and 2.97, with no significant differences across treatment groups. For the primary symptom efficacy end-point, the diary data of worst symptom scores over the 4-week treatment period showed statistically nonsignificant improvements for the MIM-D3-treated groups relative to the placebo group, with greater differences observed in the 5% group (least squares [LS] mean = 2.23 vs placebo LS mean = 2.41) (P = 0.108). At baseline, the most common worst symptom, reported by 71 patients (47%), was ocular dryness, followed by ocular discomfort reported in 60 patients (40%). Together, grittiness, burning, and stinging were reported as the worst symptom in only 19 (13%) patients. In the analyses of individual symptoms, the 4-week treatment with either MIM-D3 dose resulted in a greater alleviation of the symptom ocular dryness compared with placebo, particularly in the 5% MIM-D3 group (LS mean = 2.16 compared with placebo, LS mean = 2.40) (P = 0.034) (Figure 4 A–C).
Figure 4.
Key symptom diary results in patients with a history of dry eye and objective evidence of ongoing dry eye disease, after 28 days of treatment with a novel TrkA agonist (MIM-D3). (A–C) The mean daily diary scores for worst symptom, ocular dryness, and ocular discomfort are represented by mean weekly averages reported in the ITT population: 1% MIM-D3 (n = 48), 5% MIM-D3 (n = 49), and placebo (n = 47); (D–F). The mean daily diary scores for worst symptom, ocular dryness, and ocular discomfort are represented by mean weekly averages reported in the more symptomatic subgroup of the population (based on severity of symptoms at baseline): 1% MIM-D3 (n = 23–25), 5% MIM-D3 (n = 28–30), and placebo (n = 21–23) groups.
Note: Both 1% and 5% MIM-D3-treated groups showed significant improvement (P-values are from a generalized linear model with repeated measures and an exchangeable correlation structure over the 4-week treatment period) in reducing symptoms compared with placebo.
Abbreviations: ITT, intent to treat; SE, standard error; TrkA, receptor tyrosine kinase-A.
In the on-site visit assessments of symptoms, patients treated with 5% MIM-D3 had a lower mean change in the CAE-induced symptom stinging compared with the placebo group at day 14 (−0.40) (P = 0.029 t-test; P = 0.075 ANCOVA) and day 28 (−0.50) (P = 0.013 t-test; P = 0.047 ANCOVA). There were no improvements in any other of the on-site visit assessments of symptoms, including the OSDI, in the ITT population throughout the study.
Diary subgroup post hoc analysis
A post hoc subgroup analysis was performed on the diary data to determine whether initial symptom severity correlated with the treatment outcome. For each dry eye symptom recorded in the diary, we used the median score during the 1-week run-in period as the basis for classification. Thus, each treatment group was divided into two subgroups: patients whose mean score over the run-in period was ≤ to the ITT population median score, and patients whose mean run-in period score was > the ITT population median score. The latter subgroup of more symptomatic patients in both 1% and 5% MIM-D3 groups showed improvements in symptoms relative to that in the placebo group, for several symptoms (Figure 4 D–F). Specifically, patients in the 1% MIM-D3 subgroup experienced improvements in ocular dryness (P = 0.015) and in worst symptom scores (P = 0.036), and patients in the 5% MIM-D3 subgroup experienced improvement in ocular discomfort (P = 0.014). The treatment effect observed in the 1% MIM-D3 group compared with that in the placebo group at day 28 in the ITT population, with respect to CAE-induced inferior staining scores (−0.38), was still evident in these subsets of more symptomatic patients (−0.37, −0.42, and −0.33 for the groups, classified by worst symptom, ocular dryness, and ocular discomfort, respectively).
Safety evaluations
A total of 87 AEs occurred in 56 (37.3%) patients during the study; these were distributed fairly evenly among the treatment groups (Table 3). There were no serious ocular AEs. A total of 26 ocular AEs, the majority of which was mild and not considered to be treatment related, occurred in 21 (14%) patients. One subject in the placebo group withdrew from the study because of moderate eye irritation not associated with instillation. Treatment-related ocular events were considered for two patients (4%) in the 1% MIM-D3 group (mild instillation site pain and reaction), four patients (8%) in the 5% MIM-D3 group (mild blepharitis, increased lacrimation, instillation site pain, and increased intraocular pressure), and five patients (10%) in the placebo group (mild conjunctival edema, moderate eye irritation, moderate dellen, severe eye irritation, and severe pain).
Table 3.
Summary of treatment-emergent AEs reported in a study of a novel TrkA agonist (MIM-D3) for dry eye disease
| Placebo (N = 50) | 1% MIM-D3 (N = 50) | 5% MIM-D3 (N = 50) | |
|---|---|---|---|
| Number of adverse events | 30 | 22 | 35 |
| Patients with any adverse event (%) | 18 (36.0%) | 15 (30.0%) | 23 (46.0%) |
| Ocular events | 8 | 10 | 8 |
| Patients with any ocular event (%) | 6 (12%) | 8 (16%) | 7 (14%) |
| All serious adverse events | 1 | 1 | 1 |
| Serious ocular events | 0 | 0 | 0 |
| All adverse events causing discontinuation | 1 | 1 | 0 |
| Ocular adverse events causing discontinuation | 1 | 0 | 0 |
Abbreviations: AEs, adverse events; TrkA, receptor tyrosine kinase-A.
Systemic AEs occurred least frequently in the 1% MIM-D3 treatment group (1% MIM-D3 = 12 AEs, 5% MIM-D3 = 27 AEs, placebo = 22 AEs). None of the three serious AEs (one per treatment group) or the one AE in the 1% MIM-D3 group that resulted in subject withdrawal (septic knee joint) were considered related to the study medication. There were no concerns raised by any of the ophthalmic examinations at any study visit.
Discussion
The present study demonstrated improvements in ocular surface staining and patient-reported symptoms with MIM-D3, beginning as early as after 2 weeks of treatment. Specifically, improvements were demonstrated with the 1% MIM-D3 dose, in post-CAE staining scores and with the 5% MIM-D3 dose, in symptom scores. Furthermore, in a subgroup analysis of more symptomatic patients at baseline, both concentrations of MIM-D3 demonstrated a reduction of patient-reported symptoms and signs.
Considerable difficulties exist in assessing a drug effect in dry eye, due to the daily and diurnal variability of signs and symptoms resulting from the confounding variables of environment and lifestyle and from compensatory mechanisms that may mask the underlying disease.28,29 The CAE model was used, in this study, for induction of a dry eye state, of a magnitude that allowed for identifying clinically significant treatment effects. The primary efficacy sign endpoint was improvement in total fluorescein corneal staining post-CAE, at day 28. While this endpoint was not achieved after 4 weeks of treatment, the 1% MIM-D3 dose demonstrated a consistent trend for protection against CAE-exacerbated fluorescein and lissamine green staining of the cornea (5.5% to 6.2% treatment effect), particularly the more exposed inferior cornea (9.5% to 15.5% treatment effect) and the conjunctiva (5.4% to 6.8% treatment effect).
With regard to symptoms, patients in both MIM-D3-treated groups reported continual improvement; however, only the 5% MIM-D3 group reported a 4.8% treatment effect in dryness scores over 28 days (P = 0.034) in the environment. In a subgroup analysis of more symptomatic patients, there was a greater improvement with MIM-D3 compared with placebo for diary-reported ocular dryness (6.8% treatment effect) and ocular discomfort (8% treatment effect). The greater efficacy of MIM-D3 in highly symptomatic patients might suggest a separation of therapeutic drug effect from the lubricating effect of placebo. Additionally, improvement in stinging was observed after CAE challenge in patients treated with 5% MIM-D3. Burning and stinging sensations are consistent with the sensory response to hyperosmolarity and tear film instability.30
The CAE model has previously been used to select dry eye patients for a clinical trial.31,32 MIM-D3 improved CAE-induced corneal staining by 5%–6%, a range comparable with that achieved with other treatments in environmental assessments of CAE-responsive patient populations.31,32 The MIM-D3 ophthalmic solutions were also shown to improve commonly reported dry eye symptoms reported daily in the diary in the environmental phase of the study.
The limitations of this study involve the multiple comparisons and the post hoc timing of some analyses. Although, all endpoints were prescribed in the statistical analyses plan, no adjustment for multiplicity was made. However, the consistency of these results, ie, MIM-D3 nearly always showed a numerically greater treatment effect than its vehicle, suggests that there was a clinically meaningful improvement in signs and symptoms of dry eye. These protective effects against adverse environmental exposure and symptomatic relief in the environment in dry eye patients are thought to be due to MIM-D3’s multiple pharmacological targets, including activation of the TrkA receptor and stimulation of mucin secretion.
Both concentrations of MIM-D3 ophthalmic solutions (1% and 5%) were well tolerated with twice daily dosing. There were no issues of note in biomicroscopic or ophthalmoscopic examination. The number of treatment-emergent AEs was very low and, in general, the majority was mild to moderate. The only ocular treatment-emergent AEs rated as severe were reported in the placebo group.
In summary, the results of this study show that treatment with MIM-D3 Ophthalmic Solutions resulted in ocular surface protection, defined as a reduction in the signs and symptoms of dry eye exacerbated by a CAE and as symptomatic relief of dry eye in the environment.
Acknowledgments
The authors wish to acknowledge the contributions of Kim Brazzell, Debra A Schaumberg, Stephen C Pflugfelder, Dale Usner, and Lisa Smith for statistical, clinical, and scientific interpretations.
Contribution of authors
KM, HG, GC, and GWO were involved in the design of the study; GT and JL conducted the study; GT, JL, HG, and GWO participated in data collection and management; KM, HG, TL, GC, and GWO performed analysis and interpretation of the data; KM, GT, JL, HG, TL, GC, and GWO participated in preparation and review of the manuscript.
Disclosure
Mimetogen Pharmaceuticals Inc, Montreal, QC, Canada, sponsored the study. Part of the material in this manuscript has been previously presented at the Association for Research in Vision and Ophthalmology Annual Meeting, May, 2012.
KM, TL, and GC are employees of Mimetogen Pharmaceuticals Inc, have stock options and stock (GC) in Mimetogen Pharmaceuticals, and hold patents related to this work; GT is a consultant to Ora, Inc; HG is an employee of Statistics and Data Corporation, which received fees for data monitoring and statistical analysis. GWO is an employee of Ora, Inc, which received fees for conducting the study and has stock in Mimetogen Pharmaceuticals, Inc. The authors report no other conflicts of interest.
References
- 1.International Dry Eye Workshop Research in dry eye: report of the Research Subcommittee of the International Dry Eye Work Shop (2007) Ocul Surf. 2007;5(2):179–193. doi: 10.1016/s1542-0124(12)70086-1. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012;10(1):2–14. doi: 10.1016/j.jtos.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Begley CG, Chalmers RL, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Invest Ophthalmol Vis Sci. 2003;44(11):4753–4761. doi: 10.1167/iovs.03-0270. [DOI] [PubMed] [Google Scholar]
- 4.McCulley JP, Uchiyama E, Aronowicz JD, Butovich IA. Impact of evaporation on aqueous tear loss. Trans Am Ophthalmol Soc. 2006;104:121–128. [PMC free article] [PubMed] [Google Scholar]
- 5.Cadona G, Garcia C, Serés C, Vilesca M, Gispets J. Blink rate, blink amplitude, and tear film integrity during dynamic visual display terminal tasks. Curr Eye Res. 2011;36(3):190–197. doi: 10.3109/02713683.2010.544442. [DOI] [PubMed] [Google Scholar]
- 6.International Dry Eye Workshop The definition and classification of dry eye disease, report of the Definition and Classification Subcommittee of the International Dry Eye Workshop 2007. Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. [DOI] [PubMed] [Google Scholar]
- 7.Kruse FE, Tseng SC. Growth factors modulate clonal growth and differentiation of cultured rabbit limbal and corneal epithelium. Invest Ophthalmol Vis Sci. 1993;34(6):1963–1976. [PubMed] [Google Scholar]
- 8.Lambiase A, Manni L, Bonini S, Rama P, Micera A, Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. 2000;41(5):1063–1069. [PubMed] [Google Scholar]
- 9.Joo MJ, Yuhan KR, Hyon JY, et al. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. 2004;122(9):1338–1341. doi: 10.1001/archopht.122.9.1338. [DOI] [PubMed] [Google Scholar]
- 10.Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2005;46(9):3121–3127. doi: 10.1167/iovs.05-0241. [DOI] [PubMed] [Google Scholar]
- 11.Coassin M, Lambiase A, Costa N, et al. Efficacy of topical nerve growth factor treatment in dogs affected by dry eye. Graefes Arch Clin Exp Ophthalmol. 2005;243(2):151–155. doi: 10.1007/s00417-004-0955-2. [DOI] [PubMed] [Google Scholar]
- 12.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11(3):272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 13.de Castro F, Silos-Santiago I, López de Armentia M, Barbacid M, Belmonte C. Corneal innervation and sensitivity to noxious stimuli in trkA knockout mice. Eur J Neurosci. 1998;10(1):146–152. doi: 10.1046/j.1460-9568.1998.00037.x. [DOI] [PubMed] [Google Scholar]
- 14.Qi H, Li DQ, Shine HD, et al. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res. 2008;86(1):34–40. doi: 10.1016/j.exer.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. 1998;338(17):1174–1180. doi: 10.1056/NEJM199804233381702. [DOI] [PubMed] [Google Scholar]
- 16.Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107(7):1347–1351. doi: 10.1016/s0161-6420(00)00163-9. [DOI] [PubMed] [Google Scholar]
- 17.Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. 2006;25(3):352–355. doi: 10.1097/01.ico.0000176609.42838.df. [DOI] [PubMed] [Google Scholar]
- 18.Rios JD, Ghinelli E, Gu J, Hodges RR, Dartt DA. Role of neurotrophins and neurotrophin receptors in rat conjunctival goblet cell secretion and proliferation. Invest Ophthalmol Vis Sci. 2007;48(4):1543–1551. doi: 10.1167/iovs.06-1226. [DOI] [PubMed] [Google Scholar]
- 19.Lambiase A, Micera A, Pellegrini G, et al. In vitro evidence of nerve growth factor effects on human conjunctival epithelial cell differentiation and mucin gene expression. Invest Ophthalmol Vis Sci. 2009;50(10):4622–4630. doi: 10.1167/iovs.08-2716. [DOI] [PubMed] [Google Scholar]
- 20.Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78(3):379–388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 21.Argüeso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2002;43(4):1004–1011. [PubMed] [Google Scholar]
- 22.Nakamura Y, Yokoi N, Tokushige H, Kinoshita S. Sialic acid in human tear fluid decreases in dry eye. Jpn J Ophthalmol. 2004;48(6):519–523. doi: 10.1007/s10384-004-0111-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao H, Jumblatt JE, Wood TO, Jumblatt MM. Quantification of MUC5AC protein in human tears. Cornea. 2001;20(8):873–877. doi: 10.1097/00003226-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Sun X, Wang Z, Li R, Li L. The effect of nerve growth factor on differentiation of corneal limbal epithelial cells to conjunctival goblet cells in vitro. Mol Vis. 2010;16:2739–2744. [PMC free article] [PubMed] [Google Scholar]
- 25.Maliartchouk S, Feng Y, Ivanisevic L, et al. A designed peptidomimetic agonistic ligand of TrkA nerve growth factor receptors. Mol Pharmacol. 2000;57(2):385–391. [PubMed] [Google Scholar]
- 26.Jain P, Li R, Saragovi HU, Cumberlidge G, Meerovitch K. An NGF mimetic, MIM-D3, stimulates conjunctival cell glycoconjugate secretion and demonstrates therapeutic efficacy in a rate model in dry eye. Exp Eye Res. 2011;93(4):503–512. doi: 10.1016/j.exer.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 27.Ousler GW, Gomes PJ, Welch D, Abelson MB. Methodologies for the study of ocular surface disease. Ocul Surf. 2005;3(3):143–154. doi: 10.1016/s1542-0124(12)70196-9. [DOI] [PubMed] [Google Scholar]
- 28.Foulks GN. Challenges and pitfalls in clinical trials of treatments for dry eye. Ocul Surf. 2003;1(1):20–30. doi: 10.1016/s1542-0124(12)70004-6. [DOI] [PubMed] [Google Scholar]
- 29.Walker PM, Lane KJ, Ousler GW, 3rd, Abelson MB. Diurnal variation of visual function and the signs and symptoms of dry eye. Cornea. 2010;29(6):607–612. doi: 10.1097/ICO.0b013e3181c11e45. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Begley C, Chen M, et al. A link between tear instability and hyperosmolarity in dry eye. Invest Ophthalmol Vis Sci. 2009;50(8):3671–3679. doi: 10.1167/iovs.08-2689. [DOI] [PubMed] [Google Scholar]
- 31.Semba CP, Torkildsen GL, Lonsdale JD, et al. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am J Ophthalmol. 2012;153(6):1050–1060. doi: 10.1016/j.ajo.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Patane MA, Cohen A, From S, Torkildsen G, Welch D, Ousler GW., 3rd Ocular iontophoresis of EGP-437 (dexamethasone phosphate) in dry eye patients: results of a randomized clinical trial. Clin Ophthalmol. 2011;5:633–643. doi: 10.2147/OPTH.S19349. [DOI] [PMC free article] [PubMed] [Google Scholar]