Abstract
Diabetic retinopathy is the leading cause of blindness among individuals of working age in industrialized nations, with most of the vision loss resulting from diabetic macular edema (DME). The formation of DME depends on the action of several growth factors and inflammatory mediators, but vascular endothelial growth factor (VEGF) appears to be critical for breaking down the blood-retinal barrier and promoting the accumulation of macular edema. Laser photocoagulation has been the standard-of-care for three decades, and although it stabilizes vision, significant gains in visual acuity after treatment are unusual. Several VEGF inhibitors (pegaptanib, aflibercept, and ranibizumab) have been initially developed and tested for the treatment of age-related macular degeneration and subsequently for DME. In Phase I, II, and III trials for DME, ranibizumab has been shown to be superior to macular laser photocoagulation and intraocular triamcinolone acetonide injections for improving visual acuity and drying the macula. As a result, ranibizumab is the only anti-VEGF drug that has been approved by the United States Food and Drug Administration for the treatment of DME. Most experts now consider intravitreal anti-VEGF therapy to be standard-of-care for DME involving the fovea.
Keywords: aflibercept, bevacizumab, diabetic macular edema, diabetic retinopathy, ranibizumab, vascular endothelial growth factor
Introduction
Treatment of the most common chorioretinal blinding diseases in industrialized nations has been revolutionized by the recent introduction and rapid acceptance of drugs that inhibit the effects of vascular endothelial growth factor (VEGF).1–5 The rationale for the use of these drugs and the impetus for their development and testing followed the observation that intraocular VEGF concentrations were elevated in these conditions.6,7 Not only do these drugs halt vision loss but they actually improve vision in the majority of patients.1,2,8
Diabetic retinopathy has emerged as the leading cause of blindness among individuals of working age in industrialized nations.9 More than 285 million people worldwide are believed to have diabetes10 and this figure is predicted to double in the next 20 years.11 Severe vision loss is generally caused by proliferative retinopathy-related traction retinal detachment and vitreous hemorrhage, but moderate loss of vision, which is much more common, is usually due to diabetic macular edema (DME).12 The prevalence of DME ranges from 0% to 3% in patients with newly diagnosed diabetes to 28% to 29% in patients with diabetes for over 20 years.13 The Wisconsin Epidemiologic Study of Diabetic Retinopathy also discovered that 98% of type 1 patients and 78% of type 2 patients with diabetes for at least 15 years had some degree of retinopathy.14,15
Adequate control of blood glucose, systemic arterial hypertension, hyperlipidemia, and other risk factors can decrease the development of diabetic retinopathy in type 1 patients by 76% and decrease progression of the disease by 54% over a nine-year period.16 The Early Treatment of Diabetic Retinopathy Trial showed that laser photocoagulation for clinically significant macular edema reduces the risk of moderate vision loss by one half (23% versus 12%) over three years, but gains in vision occur slowly, and only 3% of patients experience a 15-letter improvement.17 Unfortunately, the risks of laser photocoagulation include foveal damage due to inadvertent macular photocoagulation when laser is performed on microaneurysms close to the fovea and postoperative expansion of treatment scars,18 both of which may result in a permanent decrease in visual acuity. Corticosteroid therapy with intraocular injections, inserts, and implants are effective, but all are accompanied by high rates of glaucoma and cataract formation.19
Prospective human trials have demonstrated the efficacy of intravitreal ranibizumab3,20,21 (Lucentis®, Genentech, South San Francisco, CA, USA, and Novartis, Basel, Switzerland), bevacizumab22,23 (Avastin®, Genentech and Novartis), pegaptanib24 (Macugen®, Eyetech, Palm Beach Gardens, FL, USA), and aflibercept25 (Eylea®, Regeneron, Tarrytown, NY, USA) for the treatment of DME. However, of these, only ranibizumab has been approved by the United States Food and Drug Administration for the treatment of DME. This paper justifies the role of intravitreal anti-VEGF therapy for the treatment of this complex vascular disease, with a focus on ranibizumab.
Pathophysiology of diabetic retinopathy
The exact mechanism by which diabetes leads to DME is not known, but substantial evidence indicates that elevated blood glucose levels over time correlate with the onset and progression of diabetic retinopathy.16 High glucose levels activate protein kinase C isoforms, increase flux through the polyol and hexosamine pathways, and increase the synthesis of advanced glycation end-products. Each of these biochemical abnormalities interferes with the efficient functioning of the mitochondrial electron transport chain, resulting in superoxide formation and lowered tissue oxygen tension.26 Intracellular hypoxia stabilizes hypoxia inducible factor (HIF) 1-α, enables it to dimerize with HIF 1-β, migrate to the nucleus, and attach to the promoter region of the VEGF gene.27,28 Hyperglycemia upregulates VEGF even before angiographic evidence of retinal ischemia appears.29 Upregulated VEGF synthesis causes loss of autoregulation of the retinal circulation, venous dilation and beading, breakdown of the blood-retinal barrier, and areas of capillary closure. Histopathologic changes include thickening of the capillary basement membrane30 and apoptosis of vascular endothelial cells and pericytes.31
Several inflammatory mediators, including interleukin-1α, interleukin-6, transforming growth factor-α, transforming growth factor-β, platelet-derived growth factor, and insulin-like growth factor, are upregulated in diabetes.32 These molecules phosphorylate junctional proteins, thereby breaking down the blood-retinal barrier. VEGF acts as both an angiogenesis factor and a chemoattractant, promoting migration of monocytes and macrophages.33,34 Increased synthesis of the intercellular adhesion molecule-1 causes margination of leukocytes, which increases resistance to capillary blood flow and further exacerbates tissue ischemia.35,36 Therefore, ischemia and inflammation create positive feedback that amplifies VEGF production.
Vascular endothelial growth factor
The existence of a soluble angiogenesis factor that causes ocular neovascularization and facilitates tumor growth was proposed decades ago.37 A molecule that alters the blood-brain and blood-retinal barriers, known as vascular permeability factor, was discovered in 198338 and vascular endothelial growth factor was discovered by two groups in 1989.39,40 Native VEGF (average molecular weight 35–45 kDa) is actually a mixture of several closely related homodimer glycoproteins that segregate into seven families, ie, VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and placental growth factor.
Most ocular neovascularization and vascular hyperpermeability occurs when isoforms of VEGF-A attach to the extracellular binding site of the transmembrane receptor, VEGFR2.41 Dimerization of the bound receptors induces conformational changes that activate the intracellular tyrosine kinase moieties on the receptors.42 Subsequent phosphorylation of target proteins upregulate several downstream pathways, ie, mas/MAP/MEP/ERK, phosphotidylinositol-3k/AKT, and MAPK, resulting in vascular endothelial cell proliferation and migration, activation of matrix metalloproteinases, breakdown of the blood-retinal barrier, vascular dilation, endothelial cell swelling, attraction of monocytes and macrophages, and attraction of endothelial cell precursors.42 VEGF promotes the survival of vascular endothelial cells and neuroglia.43
Drug development
Scientists at Genentech initially developed a murine antibody that bound all isoforms of human VEGF-A, humanized it, and administered it intravenously for the treatment of advanced colorectal carcinoma and subsequently for lung, renal, and breast carcinomas, and glioblastoma.44 They recognized that antibody-based binding of VEGF-A isoforms held potential for the treatment of chorioretinal vascular diseases but were concerned that the Fc (fragment-crystallizable) fragment of the antibody might incite an adverse immunologic response and prolong its systemic half-life, and that the size of the antibody (149 kDa) might prevent it from penetrating the inner retina. Therefore, instead of using bevacizumab for ophthalmologic conditions, they cleaved the Fab VEGF binding fragment from a full-length antibody, humanized it, and substituted five amino acids into the variable chain to enhance the binding affinity.45 The resulting molecule, ranibizumab, underwent extensive preclinical testing in tissue assays and in rabbit and monkey eyes. On a molar basis, ranibizumab inhibits vascular endothelial cell-induced proliferation and migration of vascular endothelial cells 5–20 times as well as bevacizumab.45 Ranibizumab possesses a strong binding affinity for VEGF165 (KD 46–192 pM) and binds all isoforms of VEGF-A.45,46 After intravitreal injection, ranibizumab exits the vitreous according to a first-order decay profile with a half-life of 2.60–2.88 days in rabbits47,48 and 2.8–3.2 days in monkeys.49,50 The intraocular half-life of ranibizumab in humans appears to be approximately 7.1 days,51 and is considerably shorter than that of bevacizumab (9.8 days).52 Ranibizumab appears to leave the eye without undergoing proteolytic degradation, and since it is eliminated by the kidneys via ultrafiltration, its systemic half-life is quite short (less than six hours).53 Because ranibizumab possesses a short systemic half-life, it does not depress systemic VEGF levels, as has been seen with bevacizumab.54
Early animal and drug trials
Pharmacokinetic studies suggested that ranibizumab concentrations were sufficient to suppress angiogenic activity for one month after intravitreal injection.50 Based on these data and that from early clinical studies, the Phase III registration trials, MARINA (Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration) and ANCHOR (Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration), ranibizumab proved effective for the treatment of exudative age-related macular degeneration when used with monthly dosing intervals.1,2 These regimens produced excellent one-year improvements in vision (+7.6 to +11.3 letters), but attempts to extend the treatment interval to three months resulted in loss of the initial gains,55 and even eyes that were evaluated two months after the loading period already showed losses of 3–4 letters.56 Therefore, monthly ranibizumab injections appeared to be necessary to produce optimal visual improvement in eyes with exudative age-related macular degeneration, and this strategy was carried over to the treatment of DME (Figure 1).
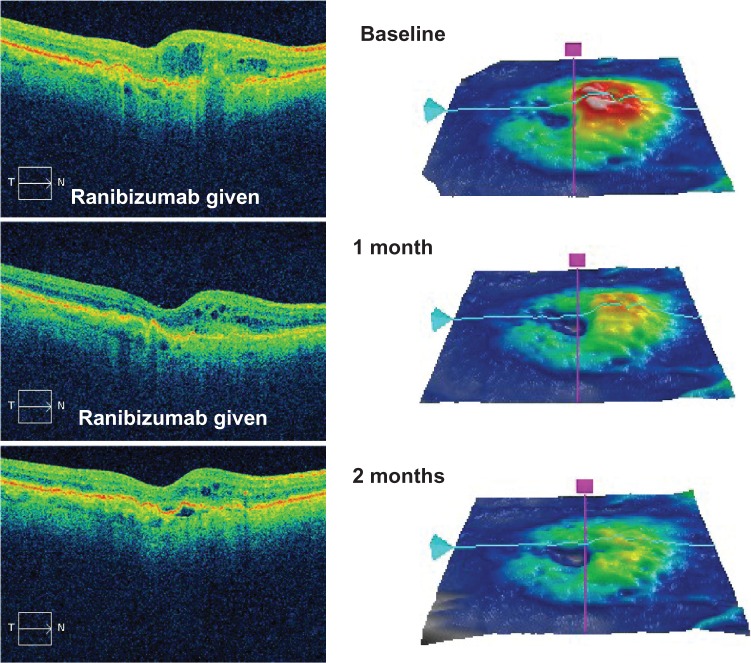
Figure 1.
An optical coherence tomography scan (top left) and retinal thickness map (top right) demonstrating macular thickening in an eye with newly diagnosed diabetic macular edema. Two intravitreal injections of ranibizumab (0.3 mg) were administered one month apart, which progressively improved the edema at one month (middle images) and two months (bottom images).
Evidence that anti-VEGF therapy might effectively treat DME emerged from several sources. Animal models of diabetic retinopathy increase the retinal concentrations of both VEGF and VEGFR2.57 Administration of exogenous VEGF to rats produces diabetic retinopathy-like retinal changes with increased capillary permeability58 which can be prevented by administration of the VEGF neutralizing-compound, VEGF Trap A40.59 Intravitreal injection of VEGF into monkey eyes causes neovascularization of the iris.60
Aqueous VEGF levels are higher in patients with DME than in those with central retinal vein occlusions, age-related macular degeneration, or branch retinal vein occlusions,61 and VEGF concentrations are higher in patients with severe as opposed to mild DME.62 Additionally, baseline levels of interleukin (IL)-1α are elevated in all patients with DME, and levels of IL-6 are elevated in most of these patients. Following treatment for DME, aqueous levels of IL-6, IL-1α, and tumor necrosis factor-α were not higher in eyes with residual edema than in those with a dry macula. These findings suggest that although inflammatory mediators probably play a role in the development of DME, their full significance is still unclear.63
Vitreous concentrations of VEGF decrease when human eyes with proliferative diabetic retinopathy are treated with panretinal photocoagulation. Reversing retinal hypoxia, the most potent upregulator of VEGF synthesis, by supplemental administration of oxygen leads to a decrease in DME.64 Patients with DME experienced improved vision (from 20/63 to 20/50) and decreased macular thickness when injected with the VEGF165 inhibitor, pegaptanib.65
Trials in human DME
Two small pilot studies provided early proof that administration of ranibizumab resolves edema and improves vision in patients with DME. In the READ-1 (Ranibizumab for Edema of the mAcula in Diabetes) study, 10 patients with chronic DME received ranibizumab injections at baseline and at months 1, 2, 4, and 6. At the seven-month examination, the mean foveal thickness had improved from 503 μm to 257 μm (an 85% reduction in excess thickness), the macular volume improved from 9.22 mm3 to 7.47 mm3 (a 77% reduction in excess volume), and the mean visual acuity improved by +12.3 letters. A strong correlation between the amount of macular thinning and degree of visual improvement (r2 = 0.78) emerged, and several of the patients had experienced a rapid reduction in macular thickness (median 88 μm; mean 130 μm) by day 7. Capillary nonperfusion remained unchanged throughout the study and patients experienced slight increases in systemic blood pressure, but none developed thromboembolic events.66
The other study also included 10 patients in an open-label, single-center, dose-escalation (0.3 mg, 0.5 mg) manner. Ranibizumab injections were given at baseline and at months 1 and 2. The patients returned for observation at days 3 and 7, and then monthly through six months. The primary endpoints were the incidence and severity of ocular adverse events, and secondary endpoints were changes in visual acuity and appearance of the retina on photography, fluorescein angiography, and optical coherence tomography. At three months, the visual acuities and macular thicknesses in the low-dose and high-dose groups improved by +12 and +7.8 letters and by −45.3 μm and −197.8 μm, respectively. The gains in vision diminished slightly between three and six months, but were better sustained in the low-dose group. Two patients experienced limited sterile inflammation, which was not sufficiently severe to deter further trials.67
The promising results of these pilot studies led to Phase II investigations that produced level II data on the efficacy of ranibizumab for DME (Table 1). READ-2 (Ranibizumab for Edema of the mAcula in Diabetes) was a 14-site, investigator-initiated study that randomized (1:1:1) 126 patients to receive ranibizumab 0.5 mg at baseline, and at 1, 3, and 5 months (group 1), laser at baseline and month 3 if necessary (group 2), or a combination of ranibizumab and laser at baseline and month 3 (group 3).68 Improvement in visual acuity at six months (the primary endpoint) was significantly greater in group 1 than in group 2 (+7.24 letters versus −0.43 letters, respectively, P = 0.01) but group 3 (+3.8 letters) was not significantly different from either of the other two groups. At six months, 22%, 0%, and 8% had average visual acuity improvements of ≥15 letters, and the average excess retinal thickness was reduced by 50%, 33%, and 45%, respectively. In the ranibizumab group, 24% had >90% resolution of excess edema and 54%, 48%, and 32% had ≥50% resolution of excess edema.
Table 1.
Major design characteristics and findings from the pivotal Phase II and III ranibizumab trials for the treatment of diabetic macular edema
| Study name | Study design | Major outcomes |
|---|---|---|
| READ-2 | Three treatment arms: ranibizumab 0.5 mg laser ranibizumab and laser |
Change in VA (letters) at 6 months: +7.24, −0.43, +3.8 ≥15 letter improvement: 22%, 0%, 8% |
| RESOLVE | Three treatment arms: ranibizumab 0.3 mg ranibizumab 0.5 mg sham (rescue laser allowed at 3 months) (dose doubling allowedat 1 month) |
Change in VA (letters) at 1 year: + 10.3 (ranibizumab) +1.4 (sham) CRT improved by: −194.2 μ m (ranibizumab) −48.4 μ m (sham) |
| RESTORE | Three treatment arms: ranibizumab ranibizumab + laser laser |
Change in VA (letters) at 1 year: +6.8, +6.4, +0.9 ≥15 letter improvement: 22.6%, 22.9%, 8.2% Average improvements in CRT: −118 μ m, −128 μ m, −61 μ m |
| DRCR.net Protocol I |
Four treatment arms: ranibizumab + prompt laser ranibizumab + delayed laser triamcinolone + laser sham injection + laser |
Change in VA (letters) at 1 year: +9, +9, +4, +3 Change in VA (letters) at 2 years versus sham +3.7, +5.8, −1.5 Subset analysis: pseudophakic eyes with triamcinolone + laser similar to ranibizumab groups |
| RISE and RIDE | 24-month trials Three treatment arms: sham ranibizumab 0.3 mg or 0.5 mg (rescue laser allowedat 3 months) |
≥15 letter improvement: 18.1%, 44.8%, and 39.2% (RISE) 12.3%, 33.6%, and 33.3% (RIDE) Average vision improvement (letters) +10.9 to +12.5 (ranibizumab) +2.3 and +2.6 (sham) |
Abbreviations: CRT, central retinal thickness; VA, visual acuity; READ-1, Ranibizumab for Edema of the mAcula in Diabetes; RESOLVE, Safety and Efficacy of Ranibizumab in Diabetic Macular Edema with Center involvement; RESTORE, A 12 Month Core Study to Assess the Efficacy and Safety of Ranibizumab Intravitreal injections; RISE, A Study of Ranibizumab injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus; RIDE, A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center involvement Secondary to Diabetes Mellitus; DRCR. net, Diabetic Retinopathy Clinical Research Network.
Between months 24 and 36 of the READ-2 trial, all patients were examined monthly and treated with ranibizumab (including patients originally randomized to laser) if the foveal thickness was >250 μm (measured by time domain optical coherence tomography). From months 6 through 24, all patients were evaluated every two months, but because of concerns that patients were being under-treated after month 24 the protocol was amended to allow patients to be treated monthly69 At the end of the second year, patients in the ranibizumab alone group had the thickest foveal measurements (340 μm versus 286 μm versus 258 μm). During the third year, the average visual acuity in the ranibizumab group improved from +7.2 to +10.3 letters and the mean foveal thickness improved from 352 μm to 282 μm, but vision and thickness changes in the laser and ranibizumab plus laser groups (−1.6 versus +2.0 letters and −36 μm versus −24 μm) were not significant. The proportions of patients who improved by ≥15 letters were 32%, 9%, and 21%, and by five letters or more were 71%, 9%, and 71%. The mean number of additional injections in the ranibizumab group (5.4) was significantly more than in the laser group (2.3) but not more than in the ranibizumab + laser group (3.3). Fifty percent of patients receiving ranibizumab required more than six injections while 32% required none. The third year of the study showed that more intensive treatment with ranibizumab (monthly instead of every two months) resulted in improved vision, and that previous laser treatments decreased the need for subsequent ranibizumab injections. It cannot be determined from the data if more laser treatments resulted in poorer visual outcomes or if poorer vision resulted from a delay in ranibizumab therapy.
A much higher dose of ranibizumab (2.0 mg) was studied for both age-related macular degeneration and DME in the HARBOR (A Study of Ranibizumab Administered Monthly or on an As-needed Basis in Patients With Subfoveal Neovascular Age-related Macular Degeneration)70 and READ-371 trials. Patients with DME received either the 2.0 mg or the standard 0.5 mg dose monthly for six months, and then as needed through 12 months. The 0.5 mg dose improved vision better than the 2.0 mg dose (+10.88 letters versus +7.39 letters) and patients receiving the 2.0 mg dose had a higher risk of death due to myocardial infarction (3% versus 0%). Based on the results from READ-3 and HARBOR (in which the 0.5 mg and 2.0 mg doses performed comparably), Genentech elected not to develop the 2.0 mg dose further.
RESOLVE (Safety and Efficacy of Ranibizumab in Diabetic Macular Edema with Center Involvement) was a 12-month, multicenter, sham/control study performed in Europe and Australia.72 One hundred and fifty-one patients with central retinal thickness >300 μm and Early Treatment Diabetic Retinopathy Study vision scores between 73 and 39 were randomized to receive sham treatments or ranibizumab injections of 0.3 mg or 0.5 mg. Previous laser photocoagulation was allowed only if burns were mild and at least 1000 μm from the foveal center. After one month, patients were eligible to have their ranibizumab doses doubled to 0.6 mg or 1 mg if the retinal thickness remained >300 μm or if the thickness improved by <50 μm. Patients received three consecutive monthly ranibizumab (or sham) injections followed by treatment as needed based upon foveal thickness and visual acuity. All eyes were eligible for rescue laser at three months. Patients in the sham arm more commonly had their doses doubled (91.8% versus 68.6%) and rescue laser was performed more commonly in the sham group (34.7% versus 4.9%). At 12 months, best-corrected visual acuity improved by an average +10.3 letters in the ranibizumab groups and +1.4 letters in the sham group (P < 0.001) and central retinal thickness improved by −194.2 μm versus -48.4 μm (P < 0.001). Best corrected visual acuity improved by >10 letters in 60.8% of the ranibizumab-treated patients, compared with only 18.4% of sham-treated patients (P < 0.001). The incidences of hypertension and arterial thromboembolic events were similar between the ranibizumab and sham groups (8.8% versus 10.2% and 2.9% versus 4.1%). There were two cases of endophthalmitis (2%) in the group receiving ranibizumab.
Investigators in the RESOLVE trial allowed dose-doubling in an effort to create the best possible outcomes. However, they discovered that this strategy created heterogeneous groups within each treatment arm and overlapping treatment arms. Further, the RESOLVE trial did not guide physicians as to how to perform laser photocoagulation because it is believed that no satisfactory standard exists for diffuse DME. The RESOLVE authors concluded that “Given the nature of diabetes and variability in patients with DME with regard to disease progression and vision loss, there is a need for an individualized treatment regimen.” Indeed most DME trials featuring ranibizumab, except for RISE (A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus) and RIDE (A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus), have allowed for individualized therapy after a short fixed treatment interval. This approach may have optimized the balance between treatment intensity and outcome for each patient but also created treatment regimens that are considerably more complicated than those featured in the age-related macular degeneration trials.
The Diabetic Retinopathy Clinical Research Network (DRCR.net) protocol I compared ranibizumab + either prompt or deferred laser photocoagulation with triamcinolone + laser and sham injections + laser.73 Eight hundred and fifty-four patients were enrolled and treated according to the 4:2:7 rule, ie, four monthly injections followed by additional injections as required at the next two visits, followed by seven visits in which the drug was administered at the investigator’s discretion if there was no improvement. At one year, the average improvements in visual acuity were +9, +9, +4, and +3 letters. Changes in macular thickness were similar in the ranibizumab and triamcinolone groups but greater than in patients receiving laser. Three eyes (0.8%) treated with ranibizumab developed endophthalmitis, and cataracts and elevated intraocular pressure were more common in the triamcinolone group. The two-year outcomes were similar to those at one year, ie, 50% of ranibizumab treated eyes improved by >10 letters and 33% improved by >15 letters, but only 5% lost 10 letters. On average, the initial improvements with triamcinolone generally diminished after six months, but the improvements in vision rivaled those with ranibizumab in pseudophakic eyes. Steroid-induced cataracts cause most of the vision loss in these patients and elevation of intraocular pressure remains a frequent complication.
It had been thought that first thinning the retina with pharmacotherapy might enable better penetration of light to the retinal pigment epithelium and improve the efficacy of laser. At two years, the average change in visual acuity compared with sham + laser was +3.7 letters in the ranibizumab + prompt laser group, +5.8 letters in the ranibizumab + deferred laser group, and −1.5 letters in the triamcinolone + laser group. Because of these differences in visual improvement, eyes that were originally assigned to receive sham + laser or triamcinolone + laser were given the opportunity to receive ranibizumab. The average number of lasers through one year for the sham/laser, ranibizumab + prompt laser, and triamcinolone + laser groups were three, two, and two, respectively. In the ranibizumab + deferred laser group, 72% of patients received no additional laser after the 24-week laser visit. Between years 1 and 2, 47% of sham + laser, 57% of ranibizumab + deferred laser, 46% of triamcinolone + laser, and 72% of ranibizumab + deferred laser patients required no further laser treatment. Between years 1 and 2, the median number of injections in the ranibizumab + prompt laser and ranibizumab + deferred laser groups were two and three, respectively. In pseudophakic eyes, visual improvements with triamcinolone + laser were similar to those receiving ranibizumab. Improvements measured by optical coherence tomography paralleled the improvements in vision, with the ranibizumab groups experiencing the most thinning. During year 2, the optical coherence tomography and visual acuity results were stable for the ranibizumab groups, whereas the optical coherence tomography findings improved but without associated improvements in visual acuity in the laser group. For the triamcinolone and two ranibizumab groups, the proportions of patients who underwent cataract surgery were 54%, 14%, and 14%. Compared with those in the sham + laser group, patients in the ranibizumab and triamcinolone groups were less likely to receive panretinal photocoagulation. Based upon the two-year findings, the study has been extended from three to five years.3
In RESTORE (A 12 Month Core Study to Assess the Efficacy and Safety of Ranibizumab [Intravitreal Injections] in Patients With Visual Impairment Due to Diabetic Macular Edema and a 24 Month Open-label Extension Study), patients were treated with ranibizumab, ranibizumab + laser, or laser alone.20 Patients received three monthly treatments of ranibizumab and subsequent injections as needed. Mean improvements in vision at one year were +6.8, +6.4, and +0.9 letters. At least 15 letters of vision were gained by 22.6%, 22.9%, and 8.2% of patients in each group, and average improvements in central retinal thickness were −118 μm, −128 μm, and −61 μm. The mean number of ranibizumab injections was seven in the ranibizumab alone group and 6.8 in the ranibizumab + laser group. The most common ocular adverse events were eye pain, and the incidence of systemic hypertension was equal between all groups. Pooled incidence rates of endophthalmitis from both the RESOLVE and RESTORE trials totaled 1.4%.
RISE and RIDE were parallel Phase III trials designed to garner sufficient evidence to obtain regulatory approval.21 Patients in these identical multicenter 24-month trials were randomized to receive monthly 0.3 mg or 0.5 mg ranibizumab or sham injections. Rescue laser treatment was allowed at three months for all groups, and crossover to ranibizumab was allowed at 24 months. Patients receiving ranibizumab consistently achieved superior gains in vision compared with those randomized to sham, a finding that applied to all subtypes of diabetic patients, and to those who were previously treated or treatment-naïve. Improvement of ≥15 letters in vision (the primary efficacy endpoint) in patients receiving sham injections, 0.3 mg ranibizumab, or 0.5 mg ranibizumab, was achieved by 18.1%, 44.8%, and 39.2% of patients in RISE, and 12.3%, 33.6%, and 33.3% of patients in RIDE. Mean best corrected visual acuity in ranibizumab-treated patients improved from +10.9 to +12.5 letters compared with +2.3 and +2.6 letters in the sham groups. Ranibizumab improved vision rapidly, with patients experiencing statistically significant changes of +4 to +5 letters by seven days after the first injection. Ranibizumab prevented the loss of ≥15 letters of vision in 96.1%–98.4% of patients, compared with 89.8%–91.5% in sham patients. As was seen in the age-related macular degeneration trials, patients with the worst baseline visual acuity experienced the largest gains. Resolution of macular edema on optical coherence tomography and cessation of leakage on fluorescein angiography were both more commonly seen in patients receiving ranibizumab. Average improvements in central retinal thickness of −250.6 μm to −270.7 μm for patients receiving ranibizumab compare favorably with −125.8 μm to −133.4 μm for sham-treated patients.
Because laser treatment was delayed by three months, even in the sham groups, the RISE and RIDE trials did not provide a direct comparison of ranibizumab with laser, as was done in the RESTORE and DRCR.net trials. Fewer ranibizumab-treated patients than sham patients required pan retinal photocoagulation (0%–1.6% versus 11%) and retinopathy was less likely to worsen and more likely to improve in the ranibizumab groups. Overall, patients receiving ranibizumab required fewer lasers than did the sham groups (0.3–0.8 versus 1.6–1.8).
Safety profiles were acceptable and similar to those seen in other ranibizumab trials. Endophthalmitis occurred in four of 500 patients (0.8%) and there were three cases of traumatic cataracts. The incidence rates of nonfatal myocardial infarction, cerebrovascular accident, and death from vascular or unknown causes were 4.9%–5.5% in the sham groups and 2.2%–8.8%% in the ranibizumab groups.
Concern that anti-VEGF therapy may worsen diabetic retinopathy-induced macular ischemia has been raised. VEGF dilates retinal vessels, promotes fenestration of the choriocapillaris, and promotes the survival of retinal vascular endothelial cells, so VEGF blockade could further occlude diseased macular vessels and decrease retinal oxygenation. In a literature review on this subject, Manousaridis and Talks74 list case reports that suggest worsening of macular ischemia as a result of anti-VEGF therapy, but they ultimately conclude that definitive evidence does not exist. Furthermore, they recommend that eyes with coexisting DME and macular ischemia receive anti-VEGF therapy.
Analysis
Five trials, ie, two DRCR.net trials, RESTORE, RISE, and RIDE, have provided level I evidence supporting use of ranibizumab for the treatment of DME, and two additional trials have provided level II evidence. These trials demonstrate that ranibizumab therapy for DME is safe and effective for two years. Level II evidence also supports the use of pegaptanib (one trial),24 bevacizumab (five trials),23,75–78 and aflibercept (one trial). Further studies with pegaptanib are unlikely, but two Phase III registration trials for aflibercept are ongoing. Data that directly compare different anti-VEGF drugs in the treatment of DME are not yet available, but the recently commissioned DRCR.net protocol T is randomizing patients to receive bevacizumab, ranibizumab, or aflibercept.79
The various DME trials that included ranibizumab provide a wealth of clinical data but they fail to define an agreed upon “best approach” for the treatment of DME. The author’s treatment algorithm, which incorporates many of the following concepts, is shown in Figure 2. The ranibizumab trials support the use of ranibizumab as monotherapy for center-involving DME. The anatomic response rates are generally good and central retinal thickness improves in the majority of treated eyes. However, despite the wealth of data from these trials, best use of laser photocoagulation in the era of anti-VEGF therapy has not been determined. DRCR.net suggested that ranibizumab combined with laser photocoagulation, either prompt or delayed, is superior to laser alone,73 whereas READ-2 showed that ranibizumab monotherapy was superior to combination therapy or laser monotherapy.
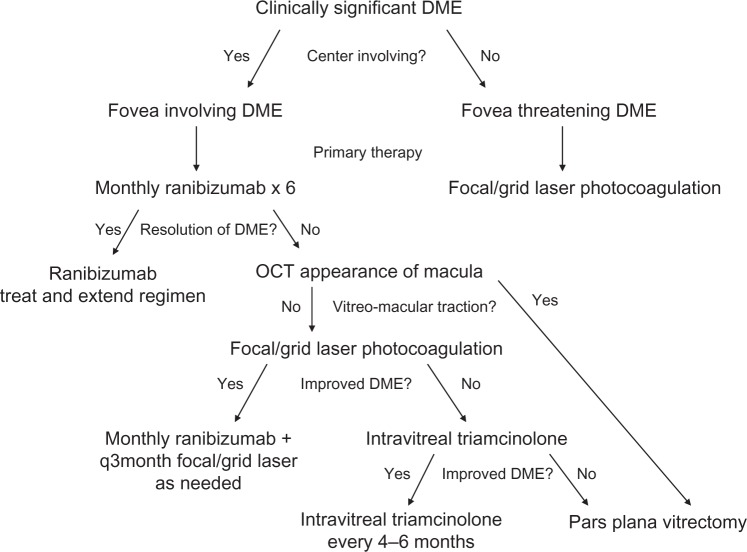
Figure 2.
Treatment algorithm for clinically significant diabetic macular edema featuring use of intravitreal ranibizumab for edema involving the center and the use of laser photocoagulation as adjuvant therapy for incomplete responders. Intravitreal triamcinolone injections and pars plana vitrectomy are generally secondary and tertiary treatments.
Abbreviations: DME, diabetic macular edema; q3month, every three months; OCT, optical coherence tomography.
Ranibizumab therapy for DME has been shown to be both effective and safe. For patients with age-related macular degeneration who are treated with ranibizumab, the combined rates of myocardial infarction and cerebrovascular accident in MARINA and ANCHOR were higher at one year but not at two years.80 In SAILOR (A Study to Evaluate Ranibizumab in Subjects With Choroidal Neovascularization Secondary to Age-Related Macular Degeneration), the incidence rates of cerebrovascular accidents in patients with previous strokes were 10% higher in ranibizumab-treated patients. However, most safety analyses regarding the use of ranibizumab fail to identify significant concerns in patients with DME, age-related macular degeneration or vein occlusions.81 Ocular adverse events from anti-VEGF injections, including pain, irritation, conjunctival hemorrhage, and chemical keratitis, occur commonly but are transient, and rarely limit the continued delivery of treatment. Injection-related cataracts and retinal detachments have been reported but are rarely seen. Infectious endophthalmitis is the most feared complication of therapy, and with continued injections appears to occur in approximately 1% of patients.
The high cost of ocular pharmacotherapy has become a recent concern for physicians, patients, insurance companies, and governments. In the United States, ranibizumab costs $390 per 0.1 mg, or $1170 per injection for the treatment of DME (approved dose 0.3 mg) and $1950 per injection for the treatment of age-related macular degeneration (approved dose 0.5 mg). Although ranibizumab appears to be the most effective treatment for DME approved by the Food and Drug Administration, the high cost of the drug also makes it the most expensive. The yearly cost per line of vision gained is $5099 for laser, $1329–$2246 for bevacizumab, $3749 for triamcinolone, and $11,372–$11,609 for ranibizumab, and the costs per quality-adjusted life-year are $5862 for laser, $2013–$4160 for bevacizumab, $6246 for triamcinolone, and $19,251–$23,119 for ranibizumab.82
Conclusion
Intravitreal ranibizumab injections are effective for the treatment of DME, either as monotherapy or in combination with prompt or delayed laser. Ranibizumab improves vision more than either intraocular triamcinolone acetonide or laser photocoagulation, and appears to have a favorable safety profile. Because of these impressive results, the standard-of-care for DME with macular edema involving the fovea now includes the use of an anti-VEGF drug.
Footnotes
Disclosure
The author is on the advisory boards of Allergan and Regeneron, and is a consultant to Boehringer-Ingelheim.
References
- 1.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;334:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 3.Diabetic Retinopathy Clinical Research Network; Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–614. doi: 10.1016/j.ophtha.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown DM, Campochiaro PA, Singh RP, CRUISE investigators Ranibizumab for macular edema following central retinal vein occlusion. Six month primary end point results of a phase III study. Ophthalmology. 2010;117:1124–1133. doi: 10.1016/j.ophtha.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Brown DM, Campochiaro PA, Bhisitful RB, et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118:1594–1602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Aiello LP, Avery RI, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 7.Funatsu H, Yamashita H, Ikeda T, Nakanishi Y, Kitano S, Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol. 2002;133:537–543. doi: 10.1016/s0002-9394(02)01323-5. [DOI] [PubMed] [Google Scholar]
- 8.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 9.American Academy of Ophthalmology Retina/Vitreous Panel Preferred Practice Pattern Guidelines. Diabetic Retinopathy San Francisco, CA: American Academy of Ophthalmology; 20084Available at: http//one.aao.org/CE/PracticeGuidelines/PPP.aspxAccessed April 9, 2012 [Google Scholar]
- 10.International Diabetes Federation IDF Diabetes Atlas 4th edBrussels, Belgium: International Diabetes Federation Executive Office; 2009Available from: http://www.diabetesatlas.org/Accessed April 20, 2011 [Google Scholar]
- 11.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen QD, Shah SM, Heier JS, et al. Primary end point (six months) results of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2009;116:2175–2181. doi: 10.1016/j.ophtha.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91:1464–1474. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic study of diabetic retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102:520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 16.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 17.Photocoagulation for diabetic macular edema Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- 18.Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109:1549–1551. doi: 10.1001/archopht.1991.01080110085041. [DOI] [PubMed] [Google Scholar]
- 19.Stewart MW. Corticosteroid use for diabetic macular edema: old fad or new trend? Curr Diab Rep. 2012;12:364–375. doi: 10.1007/s11892-012-0281-8. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study. Ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen QD, Brown DM, Marcus DM, et al; on behalf of the RISE, RIDE Research Group Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Arevalo JF, Sanchez JG, Wu L, et al. Primary intravitreal bevacizumab for diffuse diabetic macular edema. The Pan-American Collaborative Retina Study Group at 24 months. Ophthalmology. 2009;116:1488–1497. doi: 10.1016/j.ophtha.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) Ophthalmology. 2010;117:1078–1086. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Sultan MB, Zhou D, Loftus J, et al. Macugen 1013 Study Group A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011;118:1107–1118. doi: 10.1016/j.ophtha.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 25.Do DV, Schmidt-Erfurth U, Gonzalez VH, et al. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118:1819–1826. doi: 10.1016/j.ophtha.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 27.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13:29–37. doi: 10.1006/scdb.2001.0287. [DOI] [PubMed] [Google Scholar]
- 29.Vinores SA, Youssri AI, Luna JD, et al. Upregulation of vascular endothelial growth factor in ischemic and non-ischemic human and experimental retinal disease. Histol Histopathol. 1997;12:99–109. [PubMed] [Google Scholar]
- 30.Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev. 2003;19:442–455. doi: 10.1002/dmrr.415. [DOI] [PubMed] [Google Scholar]
- 31.Ejaz S, Chekarova I, Ejaz A, et al. Importance of pericytes and mechanisms of pericyte loss during diabetes retinopathy. Diabetes Obes Metab. 2008;10:53–63. doi: 10.1111/j.1463-1326.2007.00795.x. [DOI] [PubMed] [Google Scholar]
- 32.Stewart MW. The expanding role of vascular endothelial growth factor inhibitors in ophthalmology. Mayo Clin Proc. 2012;87:77–88. doi: 10.1016/j.mayocp.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu M, Perez VL, Ma N, et al. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999;40:1808–1812. [PubMed] [Google Scholar]
- 34.Barleon B, Sozzani S, Zhou D, et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 35.Miyamoto K, Khosrof S, Bursell, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joussen AM, Murata T, Tsujikawa A, et al. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michaelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. Trans Ophthalmol Soc UK. 1948;68:1625–1710. [Google Scholar]
- 38.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;12:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 39.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 40.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart MW. Aflibercept (VEGF-TRAP): the next anti-VEGF drug. Inflamm Allergy Drug Targets. 2011;10:497–508. doi: 10.2174/187152811798104872. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 43.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphotidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 46.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bakri SJ, Snyder MR, Reid JM, Pulido JS, Ezzat MK, Singh RJ. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114:2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Christofordis JB, Carlton MM, Knopp MV, Hinkle GH. Pet/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Invest Ophthalmol Vis Sci. 2011;52:5899–5903. doi: 10.1167/iovs.10-6862. [DOI] [PubMed] [Google Scholar]
- 49.Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999;27:536–544. doi: 10.1177/019262339902700507. [DOI] [PubMed] [Google Scholar]
- 50.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 51.Krohne TU, Liu Z, Holz FG, Meyer CH. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am J Ophthalmol. 2012;154:682–686. doi: 10.1016/j.ajo.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 52.Meyer CH, Krohne TU, Holz FG. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina. 2011;31:1877–1884. doi: 10.1097/IAE.0b013e318217373c. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Lu T, Tuomi L, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013;54:1616–1624. doi: 10.1167/iovs.12-10260. [DOI] [PubMed] [Google Scholar]
- 54.Chakravarthy U, Harding SP, Rogers CA, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 55.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 1. Am J Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt-Erfurth U, Eldem B, Guymer R, et al. EXCITE Study Group Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118:831–839. doi: 10.1016/j.ophtha.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Gilbert RE, Vranes D, Berka JL, et al. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1998;145:574–584. [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 59.Qaum T, Xu Q, Joussen AM, et al. VEGF-initiated blood-retinal barrier breakdown in early diabetes. Invest Ophthalmol Vis Sci. 2001;42:2408–2413. [PubMed] [Google Scholar]
- 60.Tolentino MJ, McLeod DS, Taomoto M, Otsuji T, Adamis AP, Lutty GA. Pathologic features of vascular endothelial growth factor-induced retinopathy in the nonhuman primate. Am J Ophthalmol. 2002;133:373–385. doi: 10.1016/s0002-9394(01)01381-2. [DOI] [PubMed] [Google Scholar]
- 61.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133:70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 62.Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690–1696. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- 63.Campochiaro PA, Choy DF, Do DV, et al. Monitoring ocular drug therapy by analysis of aqueous samples. Ophthalmology. 2009;116:2158–2164. doi: 10.1016/j.ophtha.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 64.Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, Campochiaro PA. Supplemental oxygen improves diabetic macular edema: a pilot study. Invest Ophthalmol Vis Sci. 2004;45:617–624. doi: 10.1167/iovs.03-0557. [DOI] [PubMed] [Google Scholar]
- 65.Macugen Diabetic Retinopathy Study Group A phase II randomized double-masked trial of pegaptanib, an anti-vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology. 2005;112:1747–1757. doi: 10.1016/j.ophtha.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is critical stimulus for diabetic macular edema. Am J Ophthalmol. 2006;142:961–969. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 67.Chun DW, Heier JS, Topping TM, Duker JS, Bankert JM. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology. 2006;113:1706–1712. doi: 10.1016/j.ophtha.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–2151. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 69.Do DV, Nguyen QD, Khwaja AA, et al. READ-2 Study Group Ranibizumab for edema of the macula in diabetes study; 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol. 2013;131:139–145. doi: 10.1001/2013.jamaophthalmol.91. [DOI] [PubMed] [Google Scholar]
- 70.Busbee BG, Ho AC, Brown DM, et al. HARBOR Study Group Twelve-month efficacy and safety of 0.5 mg or 2 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013 Jan 23; doi: 10.1016/j.ophtha.2012.10.014. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 71.Do DV, Campochiaro PA, Boyer DS, et al. Read 3 Research Group Six-month and one-year interim results of the READ 3 study: ranibizumab for edema of the macula in diabetes; Abstract presented at the 2012 annual meeting of the Association for Research in Vision and Ophthalmology; May 6–9, 2012; Fort Lauderdale, FL. [Google Scholar]
- 72.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manousaridis K, Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? Br J Ophthalmol. 2012;96:179–184. doi: 10.1136/bjophthalmol-2011-301087. [DOI] [PubMed] [Google Scholar]
- 75.Soheilian M, Ramezani A, Obudi A, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116:1142–1150. doi: 10.1016/j.ophtha.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 76.Diabetic Retinopathy Clinical Research Network A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–1867. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Solaiman KA, Diab MM, Abo-Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina. 2011;30:1638–1645. doi: 10.1097/IAE.0b013e3181e1ed07. [DOI] [PubMed] [Google Scholar]
- 78.Lam DS, Lai TY, Lee VY, et al. Efficacy of 1.25 mg versus 2.5 mg intravitreal bevacizumab for diabetic macular edema: six-month results of a randomized controlled trial. Retina. 2009;29:292–299. doi: 10.1097/IAE.0b013e31819a2d61. [DOI] [PubMed] [Google Scholar]
- 79.Clinicaltrials.gov. Diabetic Retinopathy Clinical Research Network Comparative effectiveness study of intravitreal aflibercept, bevacizumab, and ranibizumab for DME ClinicalTrial.gov Identifier NCT01627249. Available from: clinicaltrials.gov/ct2/show/NCT01627249Accessed October 15, 2012
- 80.Brown DM, Michels M, Kaiser PK, et al. ANCHOR Study Group. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR Study. Ophthalmology. 2009;116:57–65. doi: 10.1016/j.ophtha.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 81.Schmidt-Erfurth U. Clinical safety of ranibizumab in age-related macular degeneration. Expert Opin Drug Saf. 2010;9:149–165. doi: 10.1517/14740330903418422. [DOI] [PubMed] [Google Scholar]
- 82.Smiddy WE. Economic considerations of macular edema therapies. Ophthalmology. 2011;118:1827–1833. doi: 10.1016/j.ophtha.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]