Abstract
Wntless (Wls), a gene highly conserved across the animal kingdom, encodes for a transmembrane protein that mediates Wnt ligand secretion. Wls is expressed in developing lung, wherein Wnt signaling is necessary for pulmonary morphogenesis. We hypothesize that Wls plays a critical role in modulating Wnt signaling during lung development and therefore affects processes critical for pulmonary morphogenesis. We generated conditional Wls mutant mice utilizing Shh-Cre and Dermo1-Cre mice to delete Wls in the embryonic respiratory epithelium and mesenchyme, respectively. Epithelial deletion of Wls disrupted lung branching morphogenesis, peripheral lung development and pulmonary endothelial differentiation. Epithelial Wls mutant mice died at birth due to respiratory failure caused by lung hypoplasia and pulmonary hemorrhage. In the lungs of these mice, VEGF and Tie2-angiopoietin signaling pathways, which mediate vascular development, were downregulated from early stages of development. In contrast, deletion of Wls in mesenchymal cells of the developing lung did not alter branching morphogenesis or early mesenchymal differentiation. In vitro assays support the concept that Wls acts in part via Wnt5a to regulate pulmonary vascular development. We conclude that epithelial Wls modulates Wnt ligand activities critical for pulmonary vascular differentiation and peripheral lung morphogenesis. These studies provide a new framework for understanding the molecular mechanisms underlying normal pulmonary vasculature formation and the dysmorphic pulmonary vasculature development associated with congenital lung disease.
Keywords: Wntless, Wnt, lung development, endothelium, epithelium
Introduction
Lung morphogenesis depends on highly organized interactions between epithelium, mesenchyme and endothelium (Shannon and Hyatt, 2004). Signals from the developing pulmonary mesenchyme play critical roles in determining the stereotypical branching pattern of the mammalian lung (Domyan and Sun, 2011; Shannon et al., 1998). In a reciprocal manner, various growth factors secreted by the epithelium promote differentiation of the pulmonary mesenchymal cells (Del Moral et al., 2006; Yin et al., 2008). More specifically, lung morphogenesis relies on the complex interplay of several signaling pathways including Wnt, BMP, Shh and FGF (Cardoso and Whitsett, 2008). Perturbations in these pathways cause congenital malformations that result in perinatal death or chronic lung disease (Morrisey and Hogan, 2010). Wnt ligand reception has been the object of intense scrutiny, but the mechanisms by which Wnt ligands are produced and secreted are relatively less understood.
Wnt ligands are secreted growth factors that mediate a vast array of cellular responses by binding to cell membrane Frizzled (Fzd) receptors, LRP5/6 co-receptors and other receptors, including ROR2. To date, nineteen unique Wnt ligands have been identified in vertebrates. Each triggers specific cell responses by activation of distinct branches of the Wnt signaling pathway (Nusse, 2005). Posttranslational modification of Wnt ligands (acylation, glycosylation, sulfation and lipid modification) is necessary for normal ligand function (Cha et al., 2009; Kadowaki et al., 1996), but reduces Wnt solubility in the aqueous cellular and extracellular space. Recent investigations identify Wntless (Wls), also known as Sprinter, Evi, and Gpr177, as a cargo receptor protein that directs Wnt ligands from the Golgi apparatus to the cell surface by interacting with the lipid-modified domains in the ligands (Banziger et al., 2006; Ching and Nusse, 2006; Coombs et al., 2010; Goodman et al., 2006). With exception of Dorsal, a Drosophila non-acylated Wnt ligand, it is predicted that all Wnt ligands require Wls for secretion to the cell surface (Ching et al., 2008). Furthermore, Wls may be required for function of both canonical and non-canonical branches of the Wnt signaling pathway (Adell et al., 2009).
While the seminal studies in Drosophila demonstrated that ablation of Wls induces abnormalities in wing and epidermis in association with increased intracellular accumulation of Wnt ligands, the role of Wls in vertebrates is only now being elucidated. In the mouse, germline ablation of Wls resulted in embryonic death due to abnormalities in axis specification (Fu et al., 2009). In Xenopus laevis, zygotic depletion of Wls disrupted eye development via a mechanism that involves anomalous secretion of the Wnt4 ligand (Kim et al., 2009). Conditional deletion of Wls revealed roles in pancreas and craniofacial development (Carpenter et al., 2010; Fu et al., 2011), while in vitro and in vivo studies suggested a role for Wls in structural changes in the central nervous system associated with opioid dependence (Jin et al., 2010a; Reyes et al., 2010). These data suggest that Wls serves as a node to control Wnt ligand production in specific biological contexts. Although Wls mRNA and protein have been detected in the mouse lung (Jin et al., 2010b), the role of Wls in lung organogenesis, wherein Wnt signaling is necessary for specification, patterning and growth (Goss et al., 2009; Harris-Johnson et al., 2009; Mucenski et al., 2003; Shu et al., 2005; Shu et al., 2002), remains unknown.
Epithelial-mesenchymal interactions are critical for induction and coordination of vascular development in organs undergoing branching morphogenesis including the lung (Del Moral et al., 2006; van Tuyl et al., 2005). The developing pulmonary vasculature plays an active role in lung formation that goes beyond perfusion. Vascularization of the lung is necessary for normal branching morphogenesis, alveolarization and maintenance of the architecture of the distal airspace (van Tuyl et al., 2007). Abnormal vascular growth during specific stages of lung development may account for lack of alveolar septation, which in turn contributes to the lung hypoplasia characteristic of bronchopulmonary dysplasia (Abman, 2001). Vascular Endothelial Growth Factor (VEGF) (Del Moral et al., 2006; Galambos et al., 2002) and angiopoietin (Ang) (van Tuyl et al., 2007) pathways are critical in pulmonary vascular development and lung growth (Chinoy et al., 2002). While VEGF and Ang are well-established mediators in vascular biology (Breier et al., 1997), recent evidence assigns a role for Wnt signaling in vascular development (Corada et al., 2010; Goodwin and D’Amore, 2002; Ishikawa et al., 2001; Masckauchan et al., 2006; Monkley et al., 1996). For example, Wnt5a induces proliferation and migration of endothelial cells in vitro (Cheng et al., 2008; Masckauchan et al., 2006), and differentiation of embryonic stem cells into endothelial cells (Yang et al., 2009); Wnt7a and Wnt7b induce angiogenesis in the central nervous system (Daneman et al., 2009). While Wnt signaling is necessary for endothelial differentiation in vitro and in the central nervous system, it is unclear whether Wnt signaling interacts with VEGF and Ang to promote pulmonary vascular development. How endothelial cell differentiation is regulated at the level of Wnt secretion and whether Wls promotes endothelial cell differentiation in the developing lung are presently unknown.
By deleting Wls from different compartments of the embryonic lung, we uncovered unexpected roles for Wnt signaling in pulmonary vascular development. Wnt signaling induced differentiation of the endothelial cells by modulating expression of endothelial growth ligands Vegfa and angiopoietin1, and their receptors KDR (Kinase insert domain receptor) and Tie2 (TEK receptor tyrosine kinase). Non-canonical Wnt signaling was found to induce migration of pulmonary endothelial progenitor cells in vitro. In summary, these studies offer insights into the mechanisms by which control of Wnt ligand secretion affects murine lung morphogenesis. This work supports a general model wherein Wls regulates the activity of Wnt signaling pathways in an organ-context-dependent manner.
Materials and Methods
Mouse Breeding and Genotyping
Animals were housed in pathogen-free conditions and handled according to protocols approved by CCHMC Institutional Animal Care and Use Committee (Cincinnati, OH USA). Generation of the Wntless (Wls) conditional knock-out (CKO) mouse has been described previously (Carpenter et al., 2010). WlsShhCre or WlsDermo1Cre mutants were generated by breeding Wlsf/f mice with either ShhCre mice or Dermo1Cre mice and recrossing resultant mice with Wlsf/f. WlsShhCre Tie2 LacZ was obtained by mating Wlsf/wt ShhCre+/− with Tie2LacZ mice. WlsDermo tomato were generated by breeding Wlsf/wt Dermo1Cre+/− with TomatoGFP+ mice. Similar strategy was utilized to generate WlsShhCre Tomato. (Muzumdar et al., 2007). Genotypes of transgenic mice were determined by PCR with genomic DNA isolated from mouse tail or embryonic tissue. Primers utilized for genotyping and efficiency of recombination have been provided as supplementary material (table S1 and fig. S1).
Histology, Immunohistochemistry and Immunofluorescence Staining
Embryonic tissue was fixed and embedded in paraffin or frozen using OTC. Sections (6um) were processed for H&E or DAB staining as described (Mucenski et al., 2003). For immunofluorescence, sections were blocked with normal serum and incubated with a mix of primary antibodies overnight at 4C. Fluorochrome-conjugated anti IgG antibodies were applied for one hour at room temperature. Sections were preserved in VECTASHIELD mounting medium with DAPI (Vector) to visualize nuclei. Primary antibodies included Sox2, proSPC, Scgb1a1, Sox17, Foxf1 (Seven Hills Reagents) CD34 (Abcam), α Smooth Muscle Actin (αSMA, Sigma), Sox9, and phosphohistone-H3 (PHH3) (Santa Cruz).
Whole mount X-galactosidase staining
Embryonic lungs were dissected and fixed in 4% paraformaldehyde (PFA) in PBS for 30 minutes, then washed in PBS and stained two to three hours in X-gal staining solution. To stop the reaction, explants were washed in 3% dimethyl sulfoxide-PBS, rinsed in PBS then washed and stored in 70% ethanol. For imaging, explants were dehydrated in methanol and cleared in methyl salicylate. Explants were dehydrated in a graded series of ethanol and subsequently processed for paraffin embedding and sectioning.
Endothelial Cell Isolation and Gene Expression
E 18.5 lungs were minced and disaggregated in a collagenase and trypsin solution for thirty minutes, neutralized with an equal volume of MEM and 10% FBS, strained through 50 μm mesh. The cell suspension was incubated with CD34 (eBioscience) antibody in saponin-containing buffer for thirty minutes and washed three times in PBS. After staining, cells were sorted based on their positivity for CD34 using a FACS Aria flow cytometer. Total RNA was isolated from these cells for gene expression analysis.
Cell Proliferation and Cell Death
Lung sections were labeled with PHH3 antibody to determine mitotic index. Labeled cells and total cells were counted per each field photograph at 40× and ratios of proliferating cells to total cells were calculated. Average mitotic index was determined in three to five randomly selected fields per section. TUNEL assay was performed in sections of E14.5 embryos using a commercially available detection kit (Roche). Endothelial cell proliferation was assessed by flow cytometry using a FacsCallibur flow cytometer (BD) and a commercially available BrdU flow Kit (BD). Pregnant females (gestational day E18.5) were injected intraperitoneally with BrdU (10 ug/ml/Kg) two hours before sacrifice and embryo harvesting. The number of CD31+ and CD34+ cells in single cell suspensions of E18.5 lungs was analyzed. Nucleated cells were gated based on forward and side scatter and were confirmed with Nuclear-ID Red staining (Enzo Life Science). Analysis was performed using FlowJo software (TreeStar). BrdU incorporation and cell-marker positivity were determined based on isotype controls. Endothelial cell apoptosis was determined using an Annexin-V assay kit (Biovision).
Embryonic Whole Lung Explant Culture
Embryonic lungs were harvested at E11.5 and cultured at air-liquid interphase as described (Hyatt et al, 2004). Wnt5a (200ng/ml), or BIO (6-bromoindirubin-3′-oxime) (1uM) were added to the media three hours after initiation of culture. Lungs were harvested and processed for downstream applications after 72 hrs. of culture. Photographs were taken every 24hrs.
RNA Extraction and RT-PCR
Gene expression was determined by quantitative RT-PCR. RNA was isolated from single lungs using a commercially available kit (RNAeasy mini kit or micro kit, Qiagen-Promega). Reverse transcription was performed according to manufacturer instructions (Verso Fisher Sci), and Taqman probes were utilized to detect differential expression using a StepOnePlus RT-PCR System.
Migration Assay
MFLM-4 cells (Akeson et al., 2000) were seeded in fibronectin-coated plates (BD) and incubated in differentiation media (MEM, 1% FBS, bFGF (10 ng/ml) and LIF (1,000 U/ml), Invitrogen). Using a 200ul pipet tip, a wound was induced in the monolayer. Media culture were removed and replaced with fresh differentiation media containing recombinant Wnt ligands Wnt5a or Wnt3a (100 ng/ml) (R&D), Ca2+/calmodulin-dependent protein kinase inhibitor KN93 (10 uM) (Millipore), and JNK inhibitor II (25uM) (Millipore). Images were acquired at 0, 7 and 24 hours post-scratch using bright field microscopy.
Microvasculature imaging
E18.5 embryos were injected with tomato lectin from Lycopersicon esculentum (Vector labs) in umbilical vessels using a method developed by Lang and collaborators (Lang et al., 2011). In brief, after removal from pregnant mice, uterine horns were kept in warmed PBS for 30 minutes. Embryos were isolated from the uterus just before injection with lectin. Embryonic membranes were isolated carefully without disrupting umbilical vessels that remained bound to the placenta. Approximately 500 ul of a 0.5 mg/ml solution of lectin was injected in the umbilical vessel and allowed to circulate in the embryo for at least 20 minutes. During this process, embryos were maintained in paraffin coated dishes partially cover with warmed PBS. Afterwards, embryos were returned to PBS before proceeding to fixation. Embryos were fixed in zinc-formaldehyde overnight, dehydrated in a methanol series then cleared with Murray’s clear (Jahrling et al., 2009; Ott, 2008). Pictures were obtained using a confocal microscope (NikonA1Rsi). Tridimensional reconstruction and movies were generated using ImageJ (NIH) and IMARIS software (BITPLANE Scientific Software).
Statistics
Quantitative data were presented as mean + standard error. Experiments were repeated at least twice with a minimum of three biological replicates for each group. Statistically significant differences were determined by paired T-test or one-way ANOVA followed by post hoc pairwise multiple comparison procedures (Holm-Sidak method). Significance was set at P<0.05.
Results
Deletion of Wls in lung epithelium causes perinatal death
Germline deletion of Wls results in embryonic lethality due to gross anomalies in anterior-posterios axis establishment (Fu et al., 2009). To overcome this limitation, we utilized a conditional Wls knockout mouse (CKO Wls) (Carpenter et al., 2010). Given the importance of Wnt signaling in foregut specification and patterning (Cardoso and Lu, 2006; Li et al., 2008; McLin et al., 2007; Ober et al., 2006), we crossed the CKO Wls mouse with a ShhCre transgenic mouse (Harfe et al., 2004). Endogenous expression of Shh is present in endodermally-derived organs including the epithelium of the developing lung. Animals with genotype Wlsf/wt ShhCre+/wt are phenotypically indistinguishable from wild type. These mice were crossed with Wlsf/f animals to yield Wlsf/f ShhCre+/wt mutants (hereafter WlsShhCre) recovered at expected Mendelian ratios. Deletion of Wls was confirmed in whole lung tissue by PCR (supplementary SFig.1) and by crossing the Wlsf/wt ShhCre+/wt to the Tomato reporter mice (Fig,1D). WlsShhCre mutants die at birth of respiratory failure. Newborn WlsShhCre lungs were hypoplastic and hemorrhagic with partially fused lobes on the right (Fig.1A, panes 1-2). Lung mass of WlsShhCre was approximately one fourth that of control (Fig.1A, pane 3). We also observed a complete lack of tracheal cartilaginous rings without tracheo-esophageal fistula (not shown). Mechanisms underlying the tracheal phenotype are currently under investigation. Gross morphological anomalies were not detected in other organs, including the intestine. These results support the hypothesis that epithelial Wls expression is an essential regulator of respiratory tract morphogenesis.
Figure 1. Wls is necessary for pulmonary growth and branching morphogenesis.
A: Whole mount images of P0 (Postnatal day 0) control and WlsShhCre lungs show hypoplastic lungs, hemorrhage and partially fused lobes in the latter (1). A higher magnification of the WlsShhCre lung is depicted in (2); (RL, right lobe). Pulmonary mass was reduced in WlsShhCre mice (3). B: At E12.5, WlsShhCre lungs have fewer branches. C: H&E staining was performed on sections of pulmonary tissue obtained from several representative stages of lung development (E14.5 to PO). Reduced peripheral lung tissue is observed as development progresses. Images are representative of three samples. D: Efficiency of Cre recombinase activity was confirmed in lungs of WlsShhCre mice using Tomato reporter mice.
Wls promotes branching morphogenesis
We analyzed the developing WlsShhCre lung and detected abnormalities in branching morphogenesis as early as E12.5 (Fig. 1B). At this stage, WlsShhCre lungs had fewer branches and right lung lobes were partially fused. By E14.5, airways were dilated and extended to pleural edges of the WlsShhCre lung. As development progressed, lung growth was impaired, and extravasated red blood cells were observed in the mesenchyme. By the end of gestation, mutant lungs exhibited generalized hemorrhage. The WlsShhCre phenotype is similar to that observed after deletion of Wnt7b, wherein lung growth, distal development and mesenchymal cell lineage differentiations were impaired (Cohen et al., 2009; Shu et al., 2002). This phenotype is also similar to the impaired branching morphogenesis and growth observed in mouse and chick embryos following Wnt5a over-expression/mis-expression (Li et al., 2005a; Loscertales et al., 2008). In contrast to findings of lung agenesis observed in mice wherein the canonical Wnt-signaling effector β-catenin was deleted in endodermal foregut before lung specification (Goss et al., 2009; Harris-Johnson et al., 2009), the WlsShhCre mice developed lungs. This suggests that deletion of Wls from developing endoderm did not abrogate epithelial canonical Wnt/β-catenin signaling likely triggered by Wnt ligands produced by the mesenchyme. Since Wnt ligands are differentially expressed in lung compartments (De Langhe et al., 2008; Li et al., 2002a; Shu et al., 2002) and Wls is expressed in both mesenchyme and epithelium of developing lung (Fu et al., 2009; Jin et al., 2010b), these data support the concept that epithelial Wls regulates branching morphogenesis likely by controlling the production and secretion of epithelially expressed Wnt ligands.
Wls is necessary for respiratory epithelial cell differentiation
The branching defects observed in WlsShhCre lungs prompted us to analyze whether proximal to distal patterning was disrupted by deletion of Wls. Immunohistochemistry and immunofluorescence staining for airway epithelial cell markers Sox2 and Scgb1a1 and distal progenitor cell markers Sox9 and proSPC were performed. At E14.5 Sox9 was expressed in peripheral epithelial cells, supporting the concept that progenitors of respiratory epithelium were properly specified along the proximal-distal axis. By E18.5, expression of conducting airway markers was expanded to the periphery of the lung while expression of distal epithelial cell marker proSPC was almost absent in WlsShhCre mutants (Fig. 2). Expression of NKX2.1 was detected throughout the respiratory epithelium including distal sacculae of WlsShhCre lungs (data not shown). Remarkably, Sox9, whose expression is downregulated at E18.5 in normal embryos (Okubo et al., 2005; Perl et al., 2005), was strongly expressed in the peripheral cystic sacculae of WlsShhCre mice. Moreover, some Sox9 positive cells were found intermingling with Sox2 expressing cells indicating the disrupted proximal-distal patterning characteristic of the respiratory epithelium in the WlsShhCre mice. This observation also suggests that the peripheral lung hypoplasia observed in WlsShhCre mice may result from decreased proliferation or impaired differentiation of distal epithelial cells rather than from expansion of the proximal compartment of the lung. Thus, epithelial Wls is necessary for differentiation of peripheral respiratory progenitor cells.
Figure 2. Wls is necessary for pulmonary epithelial cell differentiation.
A-D: Immunofluorescence staining was performed for Sox2 and Sox9. While no differences in expression pattern were detected at E14.5 (A, B), Sox9 staining persisted in epithelial cells of the peripheral cysts of the WlsShhCre lungs (arrows, inset D) demonstrating perturbed proximal-distal patterning of the pulmonary epithelium of E18.5 WlsShhCre (compared to controls, arrow head C). Red blood cells were pseudocolored in white. E-H: Immunohistochemistry was performed on sections of E18.5 lungs for Scgb1a1 (E, F), a marker for conducting airway epithelial cells, and proSP-C (G, H), a marker for peripheral respiratory epithelial cells. In WlsShhCre lungs, the domain of Scbg1A1 expression was expanded into the periphery (F), while staining for proSP-C was not detectable (H).
Wls promotes mesenchymal cell proliferation during early stages of lung development
The hypoplastic lung characteristic of WlsShhCre mice was evident from early stages of development. To test whether the lung hypoplasia resulted from abnormal cell proliferation, we stained for pHH3, a mitotic indicator. At E14.5, cell proliferation was reduced in the lungs of WlsShhCre mice, particularly in the mesenchyme where endothelial and smooth muscle cells progenitors are localized (Fig. 3A and B). In TUNEL assays on sections and Annexin V assays on lung cell suspensions, few apoptotic cells were detected and no differences were found between WlsShhCre and control mice (Fig. 3C, 3D, and data not shown). Thus reduced proliferation, especially in lung mesenchyme, is likely to mediate the hypoplastic pulmonary phenotype observed in WlsShhCre mice. These data support the concept that Wls, via epithelially expressed Wnt ligands, promotes mesenchymal proliferation.
Figure 3. Decreased pulmonary cell proliferation in WlsShhCre mice.
A: Immunofluorescence staining for PHH3 was performed on sections from E14.5 mouse lung. B: Mitotic index was determined for mesenchymal and epithelial compartments of developing lung. Proliferation was diminished in the mesenchyme of WlsShhCre lungs (p<0.05 vs Control). C: TUNEL assay revealed few apoptotic cells (arrows) in control and WlsShhCre lungs. D: Quantification of apoptosis in mesenchyme and epithelium did not demonstrate significant differences between control and WlsShhCre lungs. Representative images are shown.
Epithelial Wls is necessary for pulmonary vascular development
Epithelially expressed Wnt ligands induce mesenchymal development, presumably via paracrine signaling (Li et al., 2005a; Rajagopal et al., 2008; Shu et al., 2002). Lung mesenchyme gives rise to various cell types including the fibroblasts, pericytes, smooth muscle cells, and lymphatic and vascular endothelial cells that form the lung vasculature. The extensive pulmonary hemorrhage in WlsShhCre newborns suggested that vascular development was altered in these mice. The expression pattern and levels of several endothelial markers, including the transcription factor Sox17 (which from embryonic morphogenesis exhibits specific vascular expression pattern) (Burtscher et al., 2012; Engert et al., 2009), and the cell surface protein CD34 (Maeda et al., 2002) (Fig.4A,B and 7A) were altered in mutants by E14.5. Endothelial cells in WlsShhCre lungs were clustered and did not form-organized tubules. This pattern became more prominent as development proceeded and is similar to the endothelial cell disorganization observed after Wnt5a misexpression (Loscertales et al., 2008). To further understand how epithelial Wnt signaling regulates endothelial cell differentiation, we analyzed the gene expression profiles of E18.5 CD34+ cells; this cell population is enriched in endothelial cells and largely overlaps (more than 70%) with the population of cells labeled by CD31 (Fig.4C and Supplemental Fig.S2). The expression of endothelial receptors KDR and Tie2 and the transcription factor Sox17 was reduced when compared to CD34+ cells from control lungs suggesting a change in the transcriptional program of these cells. To confirm that these cells have the potential to respond to Wnt signaling, expression of several receptors involved in the reception of Wnt ligands was also assessed.
Figure 4. Pulmonary endothelial development relies on Wnt signaling from the epithelium.
A: Immunohistochemistry for the endothelial cell markers Sox17 and CD34 performed on sections of E14.5 and E17.5 lungs showed diminished expression in WlsShhCre lungs. Images are representative of three or more independent samples. B: FACS histograms of CD34 labeled cells from disaggregated control (black) and mutant (blue) lungs showed fewer endothelial cells in WlsShhCre lungs. C: qRT-PCR performed on CD34+ cells from disaggregated E18.5 lungs demonstrated decreased expression of endothelial markers (KDR, TEK, Flt1, and Sox17) in WlsShhCre CD34+ cells. (n=3,* p<0.05, ** p<0.01 vs. Control). D: SMA mRNA was decreased in lungs of WlsShhCre mice as determined by qRT-PCR; (*p<0.05 vs. Control). Immunohistochemistry on sections of E14.5 lungs showed decreased and discontinuous expression of SMA in airways of WlsShhCre mice (arrows). No differences were detected in vascular SMA expression between control and WlsShhCre lungs; (Ai: Airway, * blood vessel).
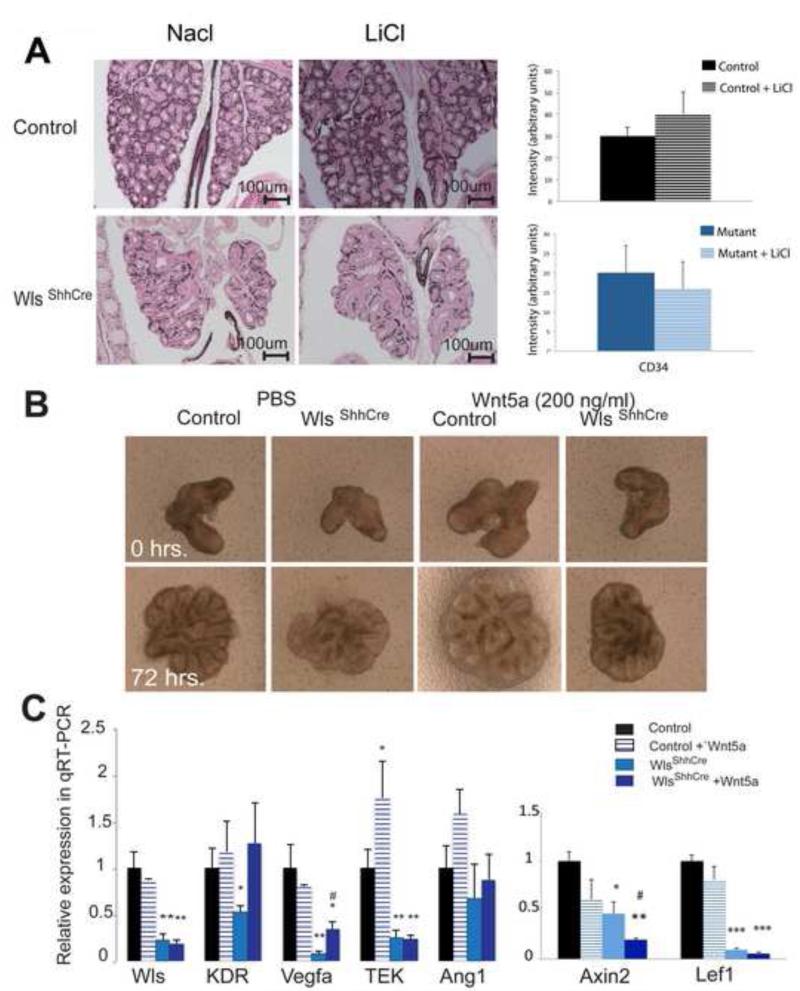
Figure 7. Wnt5a modulates endothelial gene expression in vitro in lungs of WlsShhCre mice.
A: Pregnant mice were injected with LiCl or NaCl (200mg/kg body weight) once daily from E10.5 to E12.5. No changes in embryonic growth or pulmonary branching morphogenesis were detected after treatment with LiCl. Expression of the endothelial marker CD34 was not improved by treatment with LiCl, as shown by immunohistochemistry in sections from lungs of WlsShhCre and control mice at E14.5. Intensity of the CD34 staining was not increased by LiCl treatment. B: Incubation of embryonic lung explants with rWnt5a (200ng/ml) for 72 hours did not rescue the impaired branching and growth characteristics of WlsShhcre lungs. C: Wnt5a induced expression of some endothelial markers in fetal lung explants as determined by qRT-PCR performed on whole explant; (results are average of 4 to 6 samples, *p<0.05 vs. Control, **p<0.01 vs. Control, #p<0.05 vs. Mutant).
Expression of Fzd receptors (FZD1, FZD4) in mutant and control cells was similar; in fact, we detected a modest increase in the expression of FZD7 and Ror2 receptor in mutants. The Ror2 receptor has been shown to mediate Wnt5a signaling in several contexts, including chick lung organogenesis (Loscertales et al., 2008). Since Wnt7b is required for vascular muscle cell development, (Cohen et al., 2009; Shu et al., 2002) we analyzed αSMA mRNA and the expression pattern. At E14.5 αSMA mRNA was decreased in whole lung homogenate from WlsShhCre mice (Fig.4D). Immunohistochemistry analysis showed that vascular αSMA staining was similar in WlsShhCre and control lungs. In contrast, abnormalities in airway αSMA staining were observed in WlsShhCre lungs being decreased and discontinuous along the airways (Fig.4D). Taken together these data support the concept that epithelial Wls is required for development of endothelial cells in the pulmonary microvasculature.
Mesenchymal deletion of Wls does not alter early lung morphogenesis
We tested if mesenchymal deletion of Wls disturbed epithelial and endothelial development by deleting Wls in mesenchyme utilizing a Dermo1-Cre mouse (Yu et al., 2003). Efficient deletion of Wls was demonstrated by PCR (Supplemental Fig.S1B) and RT-PCR (Fig. 5C). Dermo1 Cre activity was observed in foregut mesenchyme as early as E9.5 using the reporter mice TomatoGFP+ (Supplemental Fig.S1C). Specific and efficient Dermo1Cre mediated recombination was detected in mesenchyme of WlsDermo1CreTomato lungs (Fig.5A). WlsDermo1Cre mutants died in utero at approximately E14.5. Despite a complex set of anomalies observed in several organs and skeletal system, lung abnormalities were not seen in WlsDermo1Cre mice. Expression of the signaling molecule Shh, and endothelial markers including Sox17 and CD34, was unaltered (Fig. 5B and 5C and not shown). A modest increase in Ang-1 mRNA was observed (Fig. 5C). Airway smooth muscle differentiation was unaltered in WlsDermo1 Cre as determined by staining of αSMA (Supplemental Fig.S3). Because these embryos died at mid-gestation due to cardiac anomalies, analysis of lung morphogenesis beyond E12.5 was not possible. To overcome this limitation, embryonic lung explant cultures were used to determine the developmental potential of WlsDermo1Cre lungs. At E12.5, a subtle alteration in branching pattern (elongated branches) was observed in WlsDermo1Cre lungs, but mutant lungs developed comparably to control in in vitro explant culture (Fig. 5D). The subtly altered branching pattern in WlsDermo1Cre may be secondary to the skeletal defects observed in these embryos. These results and data from epithelial deletion of Wntless suggested that mesenchymal Wnt ligands regulated by Wls are not essential for lung morphogenesis.
Figure 5. Mesenchymal deletion of Wntless does not affect pulmonary morphogenesis.
Wntless was conditionally deleted in developing mesenchyme using Dermo1Cre mice. A: Genetic labeling using Tomato reporter mice showed efficient and specific recombination in pulmonary mesenchyme mediated by Dermo1Cre in WlsDermo1Cre lungs at E12.5. B: Immunohistochemical staining for Sox2 (proximal airway) and Sox17 (endothelium) was unaltered in lungs of WlsDermo1Cre mice. C: Quantitative RT-PCR analysis of whole lung homogenates from WlsDermo1Cre embryos at E12.5 revealed no changes in expression of most endothelial genes; (n=4, *p<0.05 vs. Control, **p<0.01 vs. Control, ***p<0.001 vs. Control). D: Lungs from WlsDermo1Cre mice branched normally in fetal lung explant culture.
Epithelial Wls is required for pulmonary capillary bed formation
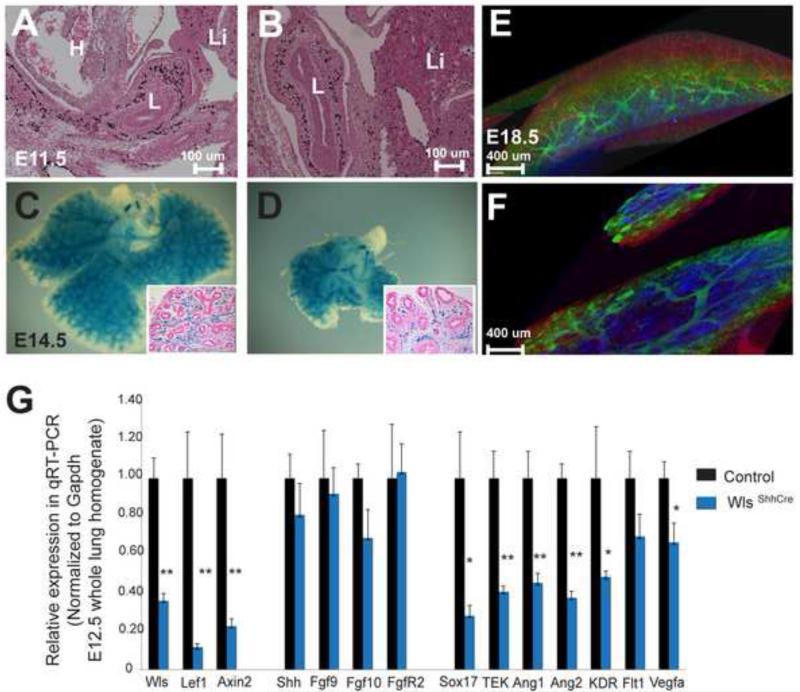
Using FACS we determined that the number of endothelial cells at E18.5 was reduced in WlsShhCre lungs (Fig. 4b), while CD31+ and CD34+ cells proliferated at similar rates in WlsShhCre and control lungs (data not shown). In light of these findings, we studied earlier stages of pulmonary endothelial development to test the hypothesis that endothelial cell progenitors are diminished in WlsShhCre mutants. At E11.5, no differences in endothelial marker staining were observed in the WlsShhCre lung (Fig.6A). This finding suggests that the diminished number of endothelial cells found at the end of gestation was not due to anomalous specification of endothelial precursors. Since mesenchymal cell proliferation at E14.5 was reduced in WlsShhCre lungs, we reasoned that this may account for the reduced number of endothelial cells seen later in development. To test this hypothesis, we crossed the Wlsf/wt ShhCre/wt with Tie2lacZ mice. In these mice, a fragment of the murine Tie2 promoter selectively drives the expression of the LacZ reporter gene in endothelial cells (Schlaeger et al., 1997; van Tuyl et al., 2005). The LacZ staining was reduced in WlsShhCre as early as E14.5 indicating decreased vascularization (Fig. 6C and D). Confocal images using fluorescein labeled Lycopersicon esculentum (tomato) lectin to stain endothelial cells of WlsShhCre mice at E18.5 demonstrated decreased formation of the pulmonary vasculature at E18.5. Dysmorphic peripheral blood vessels with a paucity of capillaries and enlarged cystic vessels or “lacunae” similar to those seen in hemangiomas were observed in WlsShhCre mice (Fig. 6F and Supplemental Movies 1 & 2).
Figure 6. Epithelial Wnt signaling directs vascular development.
A, B: Immunohistochemistry for endothelial marker Sox17 was performed on sections of E11.5 lungs. The staining pattern was similar in control (A) and WlsShhCre lungs (B). Endothelial cells were genetically labeled by crossing the WlsShhCre mice with Tie2LacZ mice; (L=lung, Li= liver, H= heart). C, D: Whole mount images of E14.5 control (C) and WlsShhCre (D) lungs are shown. Insets show cross sections of whole mount. The peripheral vascular network was reduced in WlsShhCre mice. E, F: The pulmonary vasculature was labeled with tomato lectin injected into umbilical vessels of E18.5 mice. Confocal-aided z stacking of images and 3D reconstruction was performed, and then monochromatic data were converted into RGB spectrum using depth color-coding. The anomalous distal vasculature in WlsShhCre consisted of dilated vessels (F), as opposed to the stereotypical microvasculature present in control embryos (E). G: Gene expression analysis of E12.5 whole lung homogenates by qRT-PCR showed decreased expression of endothelial markers in WlsShhCre mice; (n=4, * p<0.05 vs. Control, **p< 0.01 vs. Control).
Epithelial Wls acts upstream of VEGF and Tie2-angiopoietin and independently of Shh and FGF signaling
The hypoplastic lung and vascular abnormalities seen in WlsShhCre mice suggest that Wls acts upstream of signaling pathways controlling branching morphogenesis, proliferation and vascular development; the vascular defects in WlsShhCre lungs (Fig. 6) were similar to those seen after VEGF perturbation and angiopoietin misexpression (Del Moral et al., 2006; Galambos et al., 2002). Since Shh and FGFs are involved in development of the pulmonary vasculature, we sought to test if Wls acts upstream of these signaling pathways to mediate proper vascular development. At E12.5, an early stage of pulmonary vascular development characterized by proliferation of endothelial cells and formation of immature vascular lumina (Yamamoto et al, 2007), neither Shh, FGF9 nor FGF10 mRNAs were altered in WlsShhCre lungs. In contrast, expression of endothelial growth factors Vegfa and Ang-1, and their receptors VEGFR2 (KDR) and Tie2 (TEK) were decreased (Fig.6E). These data suggest that epithelial Wls signaling plays a key role in vascular development and that epithelial Wls modulates VEGF and angiopoietin pathways independently of Shh and FGF signaling pathways.
Activation of canonical Wnt signaling is insufficient to rescue the loss-of-function phenotype in WlsShhCre
Wntless mediates secretion of Wnt ligands that trigger either canonical, non-canonical or both types of responses and thus controls the Wnt signaling response. To assess whether activation of canonical Wnt/β-catenin signaling rescues epithelial Wls deletion, pregnant mice were injected from E10.5 to E12.5 with LiCl. This salt inhibits GSK3β thus stabilizing β-catenin. While previous studies demonstrated that LiCl treatment rescued the pulmonary phenotype induced by deletion of individual canonical Wnt ligands in vivo (Cohen et al., 2009; Goss et al., 2009), we were unable to restore abnormalities in branching morphogenesis induced by the epithelial deletion of Wls even at higher LiCl concentrations than previously used (Fig 7A). While staining for the endothelial marker CD34 was not altered by LiCL treatment (Fig 7a), LiCl induced expression of cyclin D1 a canonical Wnt target thereby, establishing the effectiveness of the LiCl treatment (Supplemental Fig.S4). Pharmacological activation of canonical Wnt signaling using BIO (a GSK3β inhibitor) (Sato et al., 2004) did not rescue the branching morphogenesis or growth abnormalities observed in WlsShhCre lungs in vitro (Supplemental Fig.S5A). Moreover, BIO treatment did not induce the expression of endothelial genes Vegfa, Ang1, KDR or TEK in WlsShhCre lungs (Supplemental Fig. S5B). Taken together these data indicate that restoration of canonical Wnt signaling was insufficient to rescue the abnormalities in pulmonary growth and branching observed in WlsShhCre mice.
Wnt5a modulates endothelial gene expression in WlsShhCre lungs
We tested the extent to which loss of non-canonical Wnt signaling accounts for the phenotype observed after epithelial deletion of Wntless. WlsShhCre embryonic lung explants were treated with recombinant Wnt5a ligand, a ligand that mediates non-canonical signaling in several contexts including developing lung (Loscertales et al., 2008). While growth and branching defects in WlsShhCre explants were not restored by Wnt5a, (Fig. 7B), TEK mRNA was induced by Wnt5a in control lung explants. In WlsShhCre lung explants KDR and Vegfa mRNAs were only partially restored by Wnt5a. However, the expression of canonical Wnt signaling targets Lef1 and Axin2 was not induced by addition of Wnt5a in culture media (Fig. 7C). Together, these data suggest that epithelial Wnt5a influences pulmonary endothelial differentiation, via a mechanism that may involve non-canonical Wnt signaling.
Wnt5a induces endothelial cell migration via non-canonical Wnt signaling
To determine the extent to which canonical or non-canonical Wnt ligands expressed in the embryonic lung epithelium mediate migration and tubulogenesis of pulmonary endothelial cells we performed in vitro studies using the MFLM-4 cell line. These cells were immortalized from wild type mouse pulmonary mesenchyme at E14.5, which express endothelial cell markers, and form tubules when grown on Matrigel (Akeson et al, 2000). We performed “scratch” migration assays and studied the effects of recombinant Wnt3a (a prototypical canonical Wnt ligand) and Wnt5a (a prototypical non-canonical ligand) on cell migration (Fig. 8). While Wnt3a treated cells migrated normally, addition of Wnt5a to the media enhanced the ability of the cells to migrate and close the wound. To further test whether Wnt5a influences cell migration via non-canonical Wnt signaling, MFLM-4 cells were treated with KN93 or JNK inhibitor II (non-canonical Ca2+ or JNK inhibitors, respectively). Incubation with JNK inhibitor II impaired cell migration. Moreover, JNK inhibitor II abrogated Wnt5a-induced migratory effects. Non-canonical Wnt/Ca2+ inhibitor KN93 had little effect on cell migration; however, co-incubation of KN93 with Wnt5a ligand prevented the ability of the cells to migrate in response to Wnt5a (Fig. 8A).
Figure 8. Non-canonical Wnt signaling promotes migration in MFLM-4 cells.
Scratch assays were performed using MFLM-4 cells (embryonic mouse pulmonary mesenchymal cells) in the presence of Wnt3a (100ng/ml), Wnt5a (100ng/ml), JNK inhibitor II (25 uM), or KN93 (10uM). Wnt5a induced cell migration while incubation in presence of Wnt3a did not alter cell migration. Wnt5a induced cell migration via JNK signaling and to lesser degree via Ca2+, as determined by assays performed in the presence of Wnt5a and JNK inhibitor II or KN93. Representative images are shown; (results are average of 3 to 6 samples, **p< 0.01 vs. Control, ***p < 0.001 vs. Control, ##p< 0.01 vs. Wnt5a, ###p< 0.001 vs. Wnt5a).
Neither Wnt3a nor Wnt5a altered tube formation in MFLM-4 cells. Inhibition of non-canonical Wnt signaling induced by incubation with JNK inhibitor II or KN93 impaired the ability of MFLM-4 cells to form tubules, while DKK1 (a canonical Wnt signaling inhibitor) had no effect on tubulogenesis (Supplemental Fig. S6). Taken together, these in vitro data support the concept that non-canonical Wnt signaling via JNK and to a lesser extent Wnt/Ca2+ pathway, promotes migration of embryonic pulmonary endothelial cells.
Discussion
Present findings demonstrated that deletion of Wls from the embryonic pulmonary epithelium inhibited growth and differentiation of peripheral lung, and disrupted both branching morphogenesis and formation of the pulmonary vasculature. In contrast, deletion of Wls from the mesenchymal compartment of the embryonic lung had little effect on epithelial and microvascular development. Epithelial Wls was required for appropriate expression of essential regulators of vascular development: KDR, Vegfa, TEK, and Ang-1. Non-canonical Wnt signaling promoted migration of pulmonary endothelial cells in vitro. Taken together, these studies support the concept that Wls serves as a modulator of Wnt signaling activity in epithelial cells of the fetal lung by controlling unique aspects of pulmonary morphogenesis and vascular development.
Differentiation of pulmonary endothelium is dependent upon epithelial Wnt signaling
VEGF and Tie-angiopoietin are important signaling pathways mediating pulmonary vascular development (Del Moral et al., 2006; Hato et al., 2009; Hiragun et al., 2000; Maniscalco et al., 2002; van Tuyl et al., 2007; Yamamoto et al., 2007). As early as E12.5, mRNA encoding receptors for Vegfa (KDR, FLT1) and angiopoietin1 (TEK) were decreased in the WlsShhCre lung before detection of vascular abnormalities. The ligands Vegfa and Ang1, both expressed by pulmonary epithelial cells were decreased in WlsShhCre mice as well. Thus, epithelial Wls is required for expression of endothelial growth factors that have important paracrine roles in the differentiation and proliferation of endothelial cell precursors in the lung mesenchyme. The finding that TEK and KDR mRNA were decreased in WlsShhCre lungs suggests that epithelial Wnt ligands may signal directly to endothelial cells to induce endothelial gene expression, a finding in part supported by embryonic lung explant culture wherein the addition of Wnt5a modestly induced KDR and TEK mRNA. These data are consistent with previous studies wherein Wnt5a induced endothelial TEK, Flt1, and Flk-1 expression in vitro (Masckauchan et al., 2006; Yang et al., 2009).
Deletion of Wls in embryonic epithelium caused defects in pulmonary vascular architecture, suggesting that Wls induces endothelial cell differentiation via a paracrine mechanism that requires Wnt ligand secretion from the epithelial compartment. At E14.5, the density of the pulmonary microvasculature in WlsShhCre lungs was decreased. At later stages (E18.5), the proximal regions of the pulmonary vasculature were misshapen and peripheral regions consisted of enlarged dilated vessels with few capillaries. These abnormalities in vascular architecture were not observed after deletion of Wnt7b (Cohen et al., 2009; Shu et al., 2002) or missexpression of Wnt5a (Li et al., 2005b) and seem to be unique to the epithelial Wls deletion. The disrupted architecture of the vasculature observed in WlsShhCre lungs is likely caused by abnormal endothelial cell migration and tubulogenesis. The role of Wnt signaling in endothelial migration was supported by in vitro studies, wherein non-canonical Wnt signaling via JNK -and to lesser extent Wnt/Ca2+ signaling-promoted migration of MFLM-4 pulmonary endothelial cells. Present data are supported by previous in vitro studies demonstrating a role for Wnt5a in endothelial cell migration and tubulogenesis (Cheng et al., 2008; Masckauchan et al., 2006).
Although canonical Wnt signaling has been linked to endothelial development—at least in the central nervous system (Daneman et al., 2009; Liebner et al., 2008; Stenman et al., 2008) the in vitro studies support a role for Wls-mediated Wnt secretion in endothelial cell differentiation and migration independent of canonical Wnt signaling. Previous studies demonstrated abnormalities in pulmonary vascular development after deletion of the canonical Wnt7b ligand. In contrast to the present study, these abnormalities were linked to abnormal vascular smooth muscle cell proliferation without the effects on endothelial cell differentiation or on the architecture of the peripheral microvasculature seen in WlsShhCre mice (Cohen et al., 2009; Shu et al., 2002; Wang et al., 2005).
In vitro studies presented here suggest that non-canonical Wnt signaling plays a critical role for pulmonary vascular development, much as they do in other systems such as the retinal myeloid cells (Stefater et al, 2011). In WlsShhCre mice, the identity of the non-canonical ligands mediating pulmonary vascular differentiation in embryonic lung remains unclear. Because Wnt5a, a prototypical non-canonical Wnt ligand, is expressed in the developing lung epithelium, it is likely that this ligand mediates pulmonary vascular development. However the ex vivo data, shows that Wnt5a only partially rescues the vascular abnormalities in WlsShhCre, which suggests that other Wnt ligands and other growth factors may synergize with Wnt5a to promote vascular development. While our in vitro data support a role for Wnt5a on pulmonary endothelial development mediated in part via non-canonical Wnt signaling, we cannot rule out that in vivo Wnt5a may trigger canonical Wnt signaling during pulmonary vascular development.
Epithelial Wnt signaling is necessary for proximal-distal patterning and differentiation of the respiratory epithelium
The importance of Wnt signaling for proper patterning of the embryonic lung epithelium is well established (Li et al., 2002b; Mucenski et al., 2003; Shu et al., 2002). As deletion of Wls likely prevents the secretion of Wnt ligands from the epithelial compartment, the severe defects in branching morphogenesis and peripheral lung differentiation observed in WlsShhCre mice support the concept that these processes require Wnt ligand secretion from the epithelium.
Anomalous expression patterns of Scgb1a1 and Sox2, (markers of conducting airway epithelial cells), and the near-absent expression of proSP-C, (a marker of peripheral epithelial respiratory cells) in the WlsShhCre mice, support the concept that epithelial Wls is required for proper proximal-distal patterning of the lung. This anomalous patterning of the respiratory epithelium was not due to lack of early specification of the peripheral lung. However, at E18.5, Sox9, a marker for peripheral pulmonary progenitor cells, was strongly expressed in distal regions of cystic lungs in the WlsShhCre mice. This finding and the lack of expression of proSP-C, suggests that these progenitor cells remain undifferentiated and that Wls is critical for epithelial cell differentiation. These data are also consistent with previous studies where the lack of differentiation of distal respiratory cells correlated with upregulation of Sox9 expression (Okubo et al., 2005). Deletion of the epithelially expressed Wnt7b or Wnt5a ligands did not affect distal respiratory cell differentiation in prior studies (Li et al., 2002b; Rajagopal et al., 2008; Shu et al., 2002). In contrast, present data suggest that epithelial Wnt signaling is required for differentiation of progenitor cells that will form alveolar regions later in development.
The lung hypoplasia and branching morphogenesis defects seen in WlsShhCre mice are similar in some ways to those caused by the deletion of Shh (Miller et al., 2004), FGF9 and FGF10 (Ramasamy et al., 2007; Sekine et al., 1999) in developing lung. In several organs including the lung, Wnt ligands can regulate expression of FGF ligands or Shh (Cohen et al., 2007; Goss et al., 2011; Li et al., 2005b; Li et al., 2002b); however, Shh, FGF9, and FGF10 mRNAs were unchanged in WlsShhCre lungs. In our in vitro studies, activation of canonical Wnt signaling or Wnt5a alone was not sufficient to promote growth or restore branching morphogenesis in the WlsShhCre lung. These results support a model wherein Wls promotes branching morphogenesis independently of Shh or FGF signaling and that several growth factors may be necessary to ensure proliferation and pulmonary branching morphogenesis.
Mesenchymal Wnt signaling is not essential for epithelial and endothelial differentiation
Previous studies demonstrated the importance of mesenchyme for lung specification, branching and differentiation (Goss et al., 2009; Hyatt et al., 2004). Epithelial deletion of Wls impaired endothelial development; but it is unknown if the primary target of the epithelial signal is the endothelium itself or the mesenchyme, which in turn may signal to the endothelium. The present study demonstrated that deletion of Wls from the pulmonary mesenchyme has little effect on early stages of lung morphogenesis. This finding was supported by the fact that expression of epithelial and endothelial markers were unaltered in the embryonic lung after mesenchymal deletion of Wls. In contrast, severe developmental abnormalities were detected in other organ systems and future studies will address this phenotype.
Wnt2, expressed in the developing pulmonary mesenchyme, is necessary and sufficient to induce airway smooth muscle cell differentiation (Goss et al, 2011). In WlsDermo1Cre mutants, we did not detect abnormalities in airway smooth muscle cells despite mesenchymal deletion of Wls. This contradictory finding may be explained by differences related to cell types in which Cre-mediated recombination takes place or by compensatory epithelial Wnt ligand secretion that in WlsDermo1Cre lungs are capable of inducing smooth muscle cell differentiation. Analysis of the WlsDermo1CreTomato reporter mice showed efficient and widespread mesenchymal deletion of Wls, supporting a compensatory mechanism. It is also possible that in the developing lung, Wnt2 secretion may be independent of Wls. Analysis of the phenotypically normal WlsDermo1Cre lungs show that Wnt signals from the mesenchyme that rely on Wls for secretion are not necessary for lung morphogenesis and are not required for pulmonary vascular development. In contrast, Wnt ligands expressed by the pulmonary mesenchyme are unable to prevent the abnormal morphogenesis caused by deletion of Wls in the lung epithelium of WlsShhCre mice. In summary, epithelial Wls is critical for lung development while mesenchymal Wls is not required during early lung morphogenesis.
Conclusions
Wls is a modulator of Wnt signaling pathways during lung development that is required for growth, differentiation and patterning of the respiratory epithelium and endothelium. While Wnt ligands are expressed in both epithelial and mesenchymal compartments, the respiratory phenotype produced by conditional knockout of Wls in the epithelium demonstrated that Wnt ligand secretion from the epithelium is critical for pulmonary morphogenesis. We propose a model whereby Wls promotes mesenchymal and endothelial cell differentiation via a mechanism that requires secretion of Wnt ligands expressed by the respiratory epithelium, includingWnt5a. (Fig. 9). Proper endothelial development in turn is required for growth and differentiation of respiratory epithelial progenitors.
Figure 9. Model.
Deletion of Wntless in embryonic lung epithelium prevents the secretion of Wnt ligands that regulate the Wnt signaling activity in developing lung. Epithelial Wnt ligands are required for differentiation of distal respiratory epithelial cells. Epithelially expressed Wnt ligands induce expression of endothelial growth factors Vegfa and Ang1. These factors in turn promote formation of the pulmonary microvasculature. Epithelial non-canonical Wnt signaling may directly signal to the endothelium to promote pulmonary microvascular development. We propose that non-canonical Wnt signaling influences cell migration and that Wnt5a modulates expression of endothelial cell growth factors mediating pulmonary vascular development.
Supplementary Material
HIGHLIGHTS.
Wls modulates Wnt signaling activity in embryonic lung.
Epithelial Wls activity is necessary for respiratory epithelial cell differentiation.
Mesenchymal Wls signaling is not essential for early lung morphogenesis.
Wls-mediated secretion of epithelial Wnts promotes pulmonary vascular development.
Acknowledgments
We thank Dr. John Shannon’s lab for assistance with embryonic lung culture and Ms. Stephanie Lang for umbilical vessel injections. We also acknowledge the assistance of Dr. Matt Kofron with confocal microscopy and Mr. Chuck Crimmel with graphics. D.S. would also like to thank Dr. Aaron Zorn for useful comments on the manuscript. This work was partially supported by research grant No. 5-FY12-59 from March Of Dimes to D.S. and NIH LRCC, 110964 and HL-108907 to J.W. Flow cytometric data were acquired using equipment maintained by the Research Flow Cytometry Core in the Division of Rheumatology at Cincinnati Children’s Hospital Medical Center, supported in part by NIH AR-47363, NIH DK78392 and NIH DK90971.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001;164:1755–1756. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- Adell T, Salo E, Boutros M, Bartscherer K. Smed-Evi/Wntless is required for beta-catenin-dependent and -independent processes during planarian regeneration. Development. 2009;136:905–910. doi: 10.1242/dev.033761. [DOI] [PubMed] [Google Scholar]
- Akeson AL, Wetzel B, Thompson FY, Brooks SK, Paradis H, Gendron RL, Greenberg JM. Embryonic vasculogenesis by endothelial precursor cells derived from lung mesenchyme. Dev Dyn. 2000;217:11–23. doi: 10.1002/(SICI)1097-0177(200001)217:1<11::AID-DVDY2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Breier G, Damert A, Plate KH, Risau W. Angiogenesis in embryos and ischemic diseases. Thromb Haemost. 1997;78:678–683. [PubMed] [Google Scholar]
- Burtscher I, Barkey W, Schwarzfischer M, Theis FJ, Lickert H. The Sox17-mCherry fusion mouse line allows visualization of endoderm and vascular endothelial development. Genesis. 2012;50:496–505. doi: 10.1002/dvg.20829. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Whitsett JA. Resident cellular components of the lung: developmental aspects. Proc Am Thorac Soc. 2008;5:767–771. doi: 10.1513/pats.200803-026HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter AC, Rao S, Wells JM, Campbell K, Lang RA. Generation of mice with a conditional null allele for Wntless. Genesis. 2010:1–5. doi: 10.1002/dvg.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha SW, Tadjuidje E, White J, Wells J, Mayhew C, Wylie C, Heasman J. Wnt11/5a complex formation caused by tyrosine sulfation increases canonical signaling activity. Curr Biol. 2009;19:1573–1580. doi: 10.1016/j.cub.2009.07.062. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2008;365:285–290. doi: 10.1016/j.bbrc.2007.10.166. [DOI] [PubMed] [Google Scholar]
- Ching W, Hang HC, Nusse R. Lipid-independent secretion of a Drosophila Wnt protein. J Biol Chem. 2008;283:17092–17098. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Nusse R. A dedicated Wnt secretion factor. Cell. 2006;125:432–433. doi: 10.1016/j.cell.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Chinoy MR, Graybill MM, Miller SA, Lang CM, Kauffman GL. Angiopoietin-1 and VEGF in vascular development and angiogenesis in hypoplastic lungs. Am J Physiol Lung Cell Mol Physiol. 2002;283:L60–66. doi: 10.1152/ajplung.00317.2001. [DOI] [PubMed] [Google Scholar]
- Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009;119:2538–2549. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, Utomo V, Banerjee N, Zhang ZH, Jadulco RC, Concepcion GP, Bugni TS, Harper MK, Mihalek I, Jones CM, Ireland CM, Virshup DM. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corada M, Nyqvist D, Orsenigo F, Caprini A, Giampietro C, Taketo MM, Iruela-Arispe ML, Adams RH, Dejana E. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell. 2010;18:938–949. doi: 10.1016/j.devcel.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe SP, Carraro G, Tefft D, Li C, Xu X, Chai Y, Minoo P, Hajihosseini MK, Drouin J, Kaartinen V, Bellusci S. Formation and differentiation of multiple mesenchymal lineages during lung development is regulated by beta-catenin signaling. PLoS One. 2008;3:e1516. doi: 10.1371/journal.pone.0001516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, Warburton D. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 2006;290:177–188. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Domyan ET, Sun X. Patterning and plasticity in development of the respiratory lineage. Dev Dyn. 2011;240:477–485. doi: 10.1002/dvdy.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert S, Liao WP, Burtscher I, Lickert H. Sox17-2A-iCre: a knock-in mouse line expressing Cre recombinase in endoderm and vascular endothelial cells. Genesis. 2009;47:603–610. doi: 10.1002/dvg.20540. [DOI] [PubMed] [Google Scholar]
- Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 2011;240:365–371. doi: 10.1002/dvdy.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci U S A. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D’Amore PA, DeMello DE. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol. 2002;27:194–203. doi: 10.1165/ajrcmb.27.2.4703. [DOI] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, Spana EP, Selva EM. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Goodwin AM, D’Amore PA. Wnt signaling in the vasculature. Angiogenesis. 2002;5:1–9. doi: 10.1023/a:1021563510866. [DOI] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Cheng L, Yang J, Zhou D, Cohen ED, Morrisey EE. Wnt2 signaling is necessary and sufficient to activate the airway smooth muscle program in the lung by regulating myocardin/Mrtf-B and Fgf10 expression. Dev Biol. 2011;356:541–552. doi: 10.1016/j.ydbio.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, Tian H, McMahon AP, Tabin CJ. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. beta-Catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hato T, Kimura Y, Morisada T, Koh GY, Miyata K, Tabata M, Kadomatsu T, Endo M, Urano T, Arai F, Araki K, Suda T, Kobayashi K, Oike Y. Angiopoietins contribute to lung development by regulating pulmonary vascular network formation. Biochem Biophys Res Commun. 2009;381:218–223. doi: 10.1016/j.bbrc.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Hiragun T, Morita E, Shindo H, Tanaka T, Kameyoshi Y, Okabe T, Kanno M, Yamamoto S. Altered in vitro apoptosis of cultured mast cells prepared from an inbred strain of mice, NC/Kuj. Clin Exp Allergy. 2000;30:433–438. doi: 10.1046/j.1365-2222.2000.00726.x. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Shangguan X, Shannon JM. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1116–1126. doi: 10.1152/ajplung.00033.2004. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128:25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Jahrling N, Becker K, Dodt HU. 3D-reconstruction of blood vessels by ultramicroscopy. Organogenesis. 2009;5:145–148. doi: 10.4161/org.5.4.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Kittanakom S, Wong V, Reyes BA, Van Bockstaele EJ, Stagljar I, Berrettini W, Levenson R. Interaction of the mu-opioid receptor with GPR177 (Wntless) inhibits Wnt secretion: potential implications for opioid dependence. BMC Neurosci. 2010a;11:33. doi: 10.1186/1471-2202-11-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Morse M, Frey C, Petko J, Levenson R. Expression of GPR177 (Wntless/Evi/Sprinter), a highly conserved Wnt-transport protein, in rat tissues, zebrafish embryos, and cultured human cells. Dev Dyn. 2010b doi: 10.1002/dvdy.22369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki T, Wilder E, Klingensmith J, Zachary K, Perrimon N. The segment polarity gene porcupine encodes a putative multitransmembrane protein involved in Wingless processing. Genes Dev. 1996;10:3116–3128. doi: 10.1101/gad.10.24.3116. [DOI] [PubMed] [Google Scholar]
- Kim H, Cheong SM, Ryu J, Jung HJ, Jho EH, Han JK. Xenopus Wntless and the retromer complex cooperate to regulate XWnt4 secretion. Mol Cell Biol. 2009;29:2118–2128. doi: 10.1128/MCB.01503-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Jones H, Omar K, Lim FY, Keswani S, Crombleholme T, Habli M. 3D Confocal imaging of mouse placental vasculature after Lycopersicum Esculentum (Tomato) lectin injections into maternal and fetal circulations. Placenta. 2011:32. [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005a;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005b;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002a;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002b;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscertales M, Mikels AJ, Hu JK, Donahoe PK, Roberts DJ. Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development. 2008;135:1365–1376. doi: 10.1242/dev.010504. [DOI] [PubMed] [Google Scholar]
- Maeda S, Suzuki S, Suzuki T, Endo M, Moriya T, Chida M, Kondo T, Sasano H. Analysis of intrapulmonary vessels and epithelial-endothelial interactions in the human developing lung. Lab Invest. 2002;82:293–301. doi: 10.1038/labinvest.3780423. [DOI] [PubMed] [Google Scholar]
- Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282:L811–823. doi: 10.1152/ajplung.00325.2001. [DOI] [PubMed] [Google Scholar]
- Masckauchan TN, Agalliu D, Vorontchikhina M, Ahn A, Parmalee NL, Li CM, Khoo A, Tycko B, Brown AM, Kitajewski J. Wnt5a signaling induces proliferation and survival of endothelial cells in vitro and expression of MMP-1 and Tie-2. Mol Biol Cell. 2006;17:5163–5172. doi: 10.1091/mbc.E06-04-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- Monkley SJ, Delaney SJ, Pennisi DJ, Christiansen JH, Wainwright BJ. Targeted disruption of the Wnt2 gene results in placentation defects. Development. 1996;122:3343–3353. doi: 10.1242/dev.122.11.3343. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15:28–32. doi: 10.1038/sj.cr.7290260. [DOI] [PubMed] [Google Scholar]
- Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- Ott SR. Confocal microscopy in large insect brains: zinc-formaldehyde fixation improves synapsin immunostaining and preservation of morphology in whole-mounts. J Neurosci Methods. 2008;172:220–230. doi: 10.1016/j.jneumeth.2008.04.031. [DOI] [PubMed] [Google Scholar]
- Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. 2005;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- Rajagopal J, Carroll TJ, Guseh JS, Bores SA, Blank LJ, Anderson WJ, Yu J, Zhou Q, McMahon AP, Melton DA. Wnt7b stimulates embryonic lung growth by coordinately increasing the replication of epithelium and mesenchyme. Development. 2008;135:1625–1634. doi: 10.1242/dev.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy SK, Mailleux AA, Gupte VV, Mata F, Sala FG, Veltmaat JM, Del Moral PM, De Langhe S, Parsa S, Kelly LK, Kelly R, Shia W, Keshet E, Minoo P, Warburton D, Bellusci S. Fgf10 dosage is critical for the amplification of epithelial cell progenitors and for the formation of multiple mesenchymal lineages during lung development. Dev Biol. 2007;307:237–247. doi: 10.1016/j.ydbio.2007.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes AR, Levenson R, Berrettini W, Van Bockstaele EJ. Ultrastructural relationship between the mu opioid receptor and its interacting protein, GPR177, in striatal neurons. Brain Res. 2010 doi: 10.1016/j.brainres.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci U S A. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- van Tuyl M, Groenman F, Wang J, Kuliszewski M, Liu J, Tibboel D, Post M. Angiogenic factors stimulate tubular branching morphogenesis of sonic hedgehog-deficient lungs. Dev Biol. 2007;303:514–526. doi: 10.1016/j.ydbio.2006.11.029. [DOI] [PubMed] [Google Scholar]
- van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol. 2005;288:L167–178. doi: 10.1152/ajplung.00185.2004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25:5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Shiraishi I, Dai P, Hamaoka K, Takamatsu T. Regulation of embryonic lung vascular development by vascular endothelial growth factor receptors, Flk-1 and Flt-1. Anat Rec (Hoboken) 2007;290:958–973. doi: 10.1002/ar.20564. [DOI] [PubMed] [Google Scholar]
- Yang DH, Yoon JY, Lee SH, Bryja V, Andersson ER, Arenas E, Kwon YG, Choi KY. Wnt5a is required for endothelial differentiation of embryonic stem cells and vascularization via pathways involving both Wnt/beta-catenin and protein kinase Calpha. Circ Res. 2009;104:372–379. doi: 10.1161/CIRCRESAHA.108.185405. [DOI] [PubMed] [Google Scholar]
- Yin Y, White AC, Huh SH, Hilton MJ, Kanazawa H, Long F, Ornitz DM. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol. 2008;319:426–436. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, Towler DA, Ornitz DM. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.