Abstract
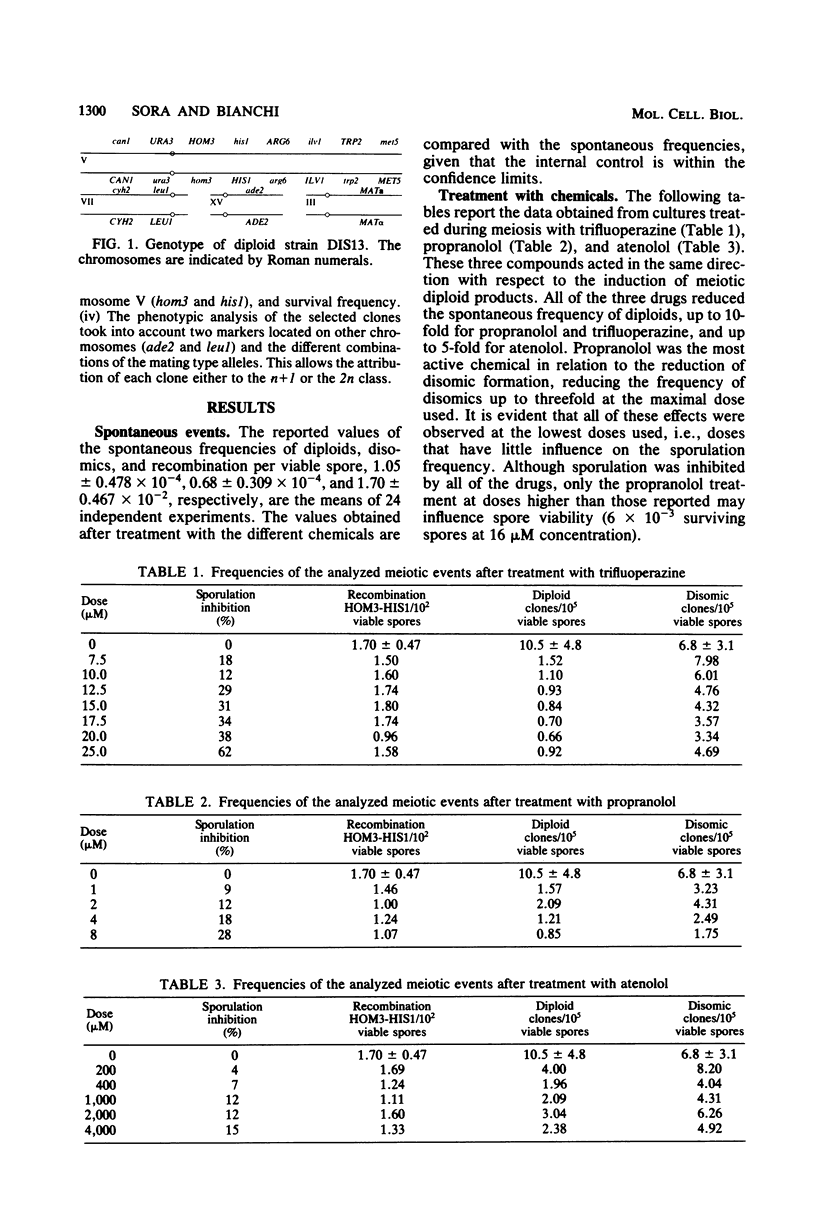
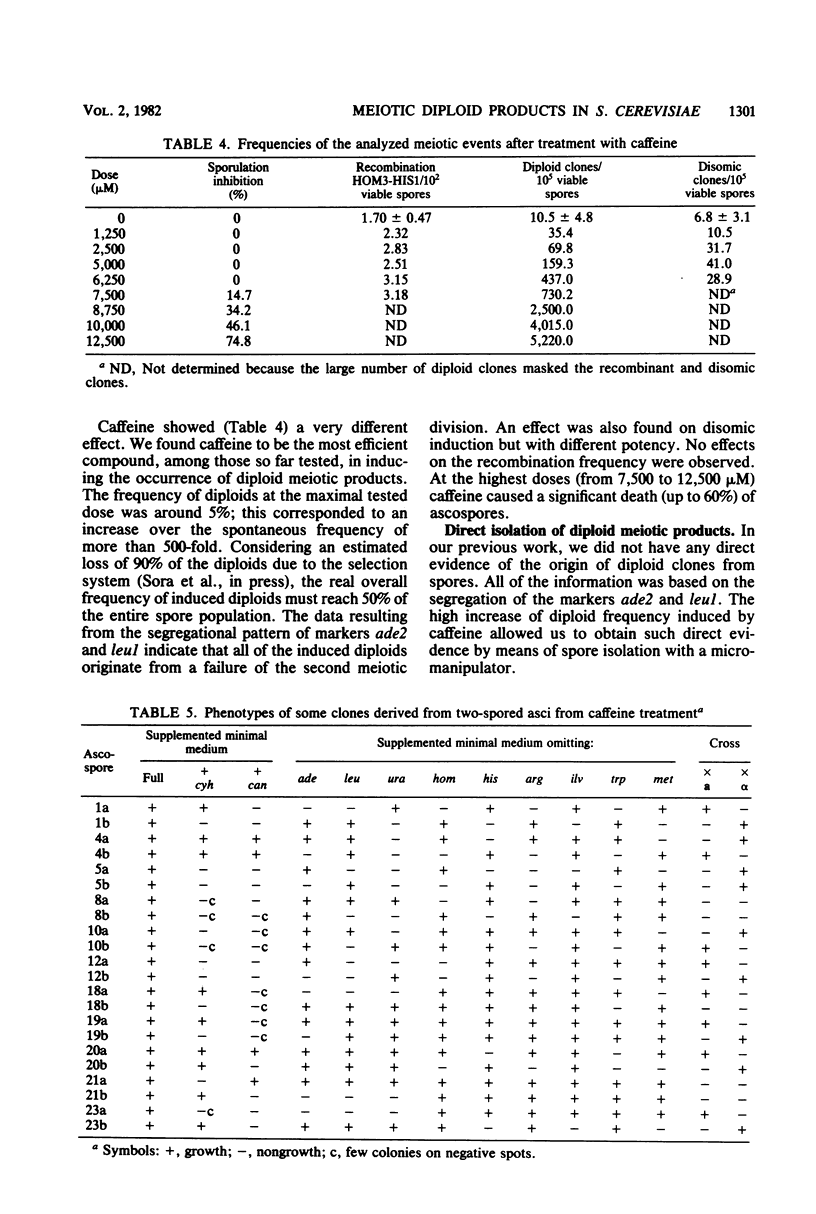
The effect of atenolol, propranolol, trifluoperazine, and caffeine on the occurrence of meiotic diploid and disomic products in Saccharomyces cerevisiae was investigated. We demonstrated that atenolol, propranolol, and trifluoperazine reduce the occurrence of meiotic diploid products and that propranolol also slightly decreases the spontaneous frequency of disomics. On the other hand, caffeine appears to be a powerful inducer of diploid meiotic products, but also shows a lesser effect on disomic induction. Since spontaneous or caffeine-induced diploids arise from a failure of the second meiotic division, it appears that the target of these drugs is at the beginning of the second meiotic division. The only common effect of trifluoperazine and propranolol, mainly investigated in mammals, was an inhibition of calmodulin activity via direct interaction. We tend, therefore, to believe that calmodulin activity must be a crucial point for the second meiotic division to begin. The increased induction of diploids, due to caffeine, may be interpreted as a consequence of an increased cyclic AMP level.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee A. G., Polya G. M. The ligand specificity of the (adenosine 3',5'-monophosphate)-binding site of yeast glyceraldehyde-3-phosphate dehydrogenase. Interaction with adenosine derivatives and pharmacologically-active compounds. Eur J Biochem. 1980 Aug;109(1):51–59. doi: 10.1111/j.1432-1033.1980.tb04766.x. [DOI] [PubMed] [Google Scholar]
- Cherksey B. D., Zadunaisky J. A., Murphy R. B. Cytoskeletal constraint of the beta-adrenergic receptor in frog erythrocyte membranes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6401–6405. doi: 10.1073/pnas.77.11.6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Jameson L., Caplow M. Modification of microtubule steady-state dynamics by phosphorylation of the microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3413–3417. doi: 10.1073/pnas.78.6.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Esposito R. E., Esposito M. S. Aberrant nuclear behavior at meiosis and anucleate spore formation by sporulation-deficient (SPO) mutants of Saccharomyces cerevisiae. Exp Cell Res. 1974 Jan;83(1):166–174. doi: 10.1016/0014-4827(74)90700-9. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Adenosine 3',5'-phosphate in fungi. Microbiol Rev. 1981 Sep;45(3):462–480. doi: 10.1128/mr.45.3.462-480.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasenick M. M., Stein P. J., Bitensky M. W. The regulatory subunit of adenylate cyclase interacts with cytoskeletal components. Nature. 1981 Dec 10;294(5841):560–562. doi: 10.1038/294560a0. [DOI] [PubMed] [Google Scholar]
- Schild D., Byers B. Diploid spore formation and other meiotic effects of two cell-division-cycle mutations of Saccharomyces cerevisiae. Genetics. 1980 Dec;96(4):859–876. doi: 10.1093/genetics/96.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora S., Lucchini G., Magni G. E. Meiotic Diploid Progeny and Meiotic Nondisjunction in SACCHAROMYCES CEREVISIAE. Genetics. 1982 May;101(1):17–33. doi: 10.1093/genetics/101.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]


