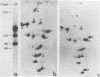
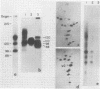
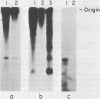
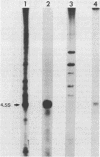
Abstract
A rodent 4.5S RNA molecule with extensive homology to the Alu family of interspersed repetitive DNA sequences has been found physically associated with polyadenylated nuclear and cytoplasmic RNAs (W. Jelinek and L. Leinwand, Cell 15:205-214, 1978; S. Haynes et al., Mol. Cell. Biol. 1:573-583, 1981). In this report, we describe a 4.5S RNA molecule in rat cells whose RNase fingerprints are identical to those of the equivalent mouse molecule. We show that the rat 4.5S RNA is part of a small family of RNA molecules, all sharing sequence homology to the Alu family of DNA sequences. These RNAs are synthesized by RNA polymerase III and are developmentally regulated and short-lived in the cytoplasm. Of this family of small RNAs, only the 4.5S RNA is found associated with polyadenylated RNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Pictet R., Rutter W. J. Analysis of the regions flanking the human insulin gene and sequence of an Alu family member. Nucleic Acids Res. 1980 Sep 25;8(18):4091–4109. doi: 10.1093/nar/8.18.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz E. W., Jr, Wydro R. M., Nadal-Ginard B., Dina D. Moloney murine sarcoma proviral DNA is a transcriptional unit. Nature. 1980 Dec 25;288(5792):665–669. doi: 10.1038/288665a0. [DOI] [PubMed] [Google Scholar]
- Campos R., Jovanovich S., Villarreal L. P. A small RNA complementary to an intervening sequence is produced late in SV40 infection. Nature. 1981 May 28;291(5813):344–346. doi: 10.1038/291344a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Gallo R. C., Wong-Staal F. A human onc gene homologous to the transforming gene (v-sis) of simian sarcoma virus. Nature. 1981 Jul 2;292(5818):31–35. doi: 10.1038/292031a0. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Klein W. H., Britten R. J. Sequence organization in animal DNA and a speculation on hnRNA as a coordinate regulatory transcript. Dev Biol. 1977 Jan;55(1):69–84. doi: 10.1016/0012-1606(77)90320-7. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Jolly D. J., Rubin C. M., Friedmann T., Schmid C. W. Base sequence studies of 300 nucleotide renatured repeated human DNA clones. J Mol Biol. 1981 Sep 5;151(1):17–33. doi: 10.1016/0022-2836(81)90219-9. [DOI] [PubMed] [Google Scholar]
- Deininger P. L., Schmid C. W. An electron microscope study of the DNA sequence organization of the human genome. J Mol Biol. 1976 Sep 25;106(3):773–790. doi: 10.1016/0022-2836(76)90264-3. [DOI] [PubMed] [Google Scholar]
- Dina D., Nadal-Ginard B. Moloney murine sarcoma virions contain subgenome-length mRNA-like molecules that direct the synthesis of sarcoma-specific polypeptides in vitro. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):901–905. doi: 10.1101/sqb.1980.044.01.097. [DOI] [PubMed] [Google Scholar]
- Duncan C. H., Jagadeeswaran P., Wang R. R., Weissman S. M. Structural analysis of templates and RNA polymerase III transcripts of Alu family sequences interspersed among the human beta-like globin genes. Gene. 1981 Mar;13(2):185–196. doi: 10.1016/0378-1119(81)90007-x. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Pan J., Duncan C. H., Weissman S. M. Transcriptional analysis of interspersed repetitive polymerase III transcription units in human DNA. Nucleic Acids Res. 1981 Mar 11;9(5):1171–1189. doi: 10.1093/nar/9.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigwood N. L., Jahn C. L., Hutchison C. A., 3rd, Edgell M. H. Locations of three repetitive sequence families found in BALB/c adult beta-globin clones. Nucleic Acids Res. 1981 Mar 11;9(5):1133–1150. doi: 10.1093/nar/9.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Ikawa Y. A new series of RNAs associated with the genome of spleen focus forming virus (SFFV) and poly(A)-containing RNA from SFFV-infected cells. Nucleic Acids Res. 1979 Oct 25;7(4):895–908. doi: 10.1093/nar/7.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Kato N., Hoshino H. Series of 4.5S RNAs associated with poly(A)-containing RNAs of rodent cells. Nucleic Acids Res. 1979 Oct 25;7(4):909–917. doi: 10.1093/nar/7.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Kato N. Nucleotide sequences of 4.5S RNAs associated with poly(A)-containing RNAs of mouse and hamster cells. Nucleic Acids Res. 1980 Mar 25;8(6):1273–1285. doi: 10.1093/nar/8.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Jelinek W. R. Low molecular weight RNAs transcribed in vitro by RNA polymerase III from Alu-type dispersed repeats in Chinese hamster DNA are also found in vivo. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6130–6134. doi: 10.1073/pnas.78.10.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Toomey T. P., Leinwand L., Jelinek W. R. The Chinese hamster Alu-equivalent sequence: a conserved highly repetitious, interspersed deoxyribonucleic acid sequence in mammals has a structure suggestive of a transposable element. Mol Cell Biol. 1981 Jul;1(7):573–583. doi: 10.1128/mcb.1.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossenlopp P., Wells D., Chambon P. Animal DNA-dependent RNA polymerases. Partial purification and properties of three classes of RNA polymerases from uninfected and adenovirus-infected HeLa cells. Eur J Biochem. 1975 Oct 1;58(1):237–251. doi: 10.1111/j.1432-1033.1975.tb02369.x. [DOI] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R. Inverted repeated DNA from Chinese hamster ovary cells studied with cloned DNA fragments. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2679–2683. doi: 10.1073/pnas.75.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Evans R., Wilson M., Salditt-Georgieff M., Darnell J. E. Oligonucleotides in heterogeneous nuclear RNA: similarity of inverted repeats and RNA from repetitious DNA sites. Biochemistry. 1978 Jul 11;17(14):2776–2783. doi: 10.1021/bi00607a012. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Leinwand L. Low molecular weight RNAs hydrogen-bonded to nuclear and cytoplasmic poly(A)-terminated RNA from cultured Chinese hamster ovary cells. Cell. 1978 Sep;15(1):205–214. doi: 10.1016/0092-8674(78)90095-8. [DOI] [PubMed] [Google Scholar]
- Krauter K. S., Soeiro R., Nadal-Ginard B. Transcriptional regulation of ribosomal RNA accumulation during L6E9 myoblast differentiation. J Mol Biol. 1979 Nov 15;134(4):727–741. doi: 10.1016/0022-2836(79)90482-0. [DOI] [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Clarkson S. G. Nucleotide sequence of genes coding for tRNAPhe and tRNATyr from a repeating unit of X. laevis DNA. Cell. 1980 Feb;19(2):345–353. doi: 10.1016/0092-8674(80)90509-7. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Itoh M., Okada N., Miyata T. Novel models for RNA splicing that involve a small nuclear RNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4471–4474. doi: 10.1073/pnas.78.7.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page G. S., Smith S., Goodman H. M. DNA sequence of the rat growth hormone gene: location of the 5' terminus of the growth hormone mRNA and identification of an internal transposon-like element. Nucleic Acids Res. 1981 May 11;9(9):2087–2104. doi: 10.1093/nar/9.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J., Elder J. T., Duncan C. H., Weissman S. M. Structural analysis of interspersed repetitive polymerase III transcription units in human DNA. Nucleic Acids Res. 1981 Mar 11;9(5):1151–1170. [PMC free article] [PubMed] [Google Scholar]
- Penman S., Rosbash M., Penman M. Messenger and heterogeneous nuclear RNA in HeLa cells: differential inhibition by cordycepin. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1878–1885. doi: 10.1073/pnas.67.4.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA-protein complexes and newly synthesized ribosomal subunits: analysis of free particles and components of polyribosomes. J Mol Biol. 1968 Jul 14;35(1):37–59. doi: 10.1016/s0022-2836(68)80035-x. [DOI] [PubMed] [Google Scholar]
- Peters G., Harada F., Dahlberg J. E., Panet A., Haseltine W. A., Baltimore D. Low-molecular-weight RNAs of Moloney murine leukemia virus: identification of the primer for RNA-directed DNA synthesis. J Virol. 1977 Mar;21(3):1031–1041. doi: 10.1128/jvi.21.3.1031-1041.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Ro-Choi T. S., Henning D., Busch H. Primary sequence of U-1 nuclear ribonucleic acid of Novikoff hepatoma ascites cells. J Biol Chem. 1974 Oct 25;249(20):6486–6494. [PubMed] [Google Scholar]
- Ro-Choi T. S., Redy R., Henning D., Takano T., Taylor C. W., Busch H. Nucleotide sequence of 4.5 S ribonucleic acid of Novikoff hepatoma cell nuclei. J Biol Chem. 1972 May 25;247(10):3205–3222. [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid C. W., Deininger P. L. Sequence organization of the human genome. Cell. 1975 Nov;6(3):345–358. doi: 10.1016/0092-8674(75)90184-1. [DOI] [PubMed] [Google Scholar]
- Shibata H., Ro-Choi T. S., Reddy R., Choi Y. C., Henning D., Busch H. The primary nucleotide sequence of nuclear U-2 ribonucleic acid. The 5'-terminal portion of the molecule. J Biol Chem. 1975 May 25;250(10):3909–3920. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weiner A. M. An abundant cytoplasmic 7S RNA is complementary to the dominant interspersed middle repetitive DNA sequence family in the human genome. Cell. 1980 Nov;22(1 Pt 1):209–218. doi: 10.1016/0092-8674(80)90169-5. [DOI] [PubMed] [Google Scholar]
- Zieve G. W. Two groups of small stable RNAs. Cell. 1981 Aug;25(2):296–297. doi: 10.1016/0092-8674(81)90046-5. [DOI] [PubMed] [Google Scholar]