Dear Sirs,
We read with interest in Thrombosis and Haemostasis the study by Hayward et al. on potentiation of platelet aggregation responses by simultaneous measurement of adenosine triphosphate (ATP) release (1). The authors found that addition of the commercial agent Chronolume® (containing 0.2 mg luciferin, 22,000 units d-luciferase plus magnesium sulphate, human serum albumin, stabilizers and buffer) to study ATP release alongside platelet aggregation in a lumi-aggregometer consistently induced a secondary wave of aggregation in response to epinephrine (adrenaline) in platelets obtained from patients with Quebec Platelet Disorder (QPD), whereas assessment of aggregation without Chronolume® showed the expected absence of a secondary wave consistent with the pathology (1). Addition of Chronolume® resulted in increased initial aggregation responses and induction of a secondary wave to epinephrine leading to dense granule release, and was not restricted to patients with QPD (1). This led the authors to conclude that simultaneously measuring ATP release with Chronolume® and light transmission aggregation could alter findings for some human platelet disorders and to advise caution in this practice for investigation of platelet function in patients with suspected platelet defects (1).
Current guidelines recommend the use of lumi-aggregometry to simultaneously assess dense granule secretion with platelet aggregation when investigating platelet disorders (2), as there is evidence that dense granule secretion defects can be misdiagnosed if relying solely on platelet aggregometry (3, 4). We sought to investigate whether potentiation of platelet responses to epinephrine occurred when using Chronolume® to simultaneously assess ATP secretion alongside aggregation in a cohort of patients with clinically diagnosed bleeding disorders.
Participants with clinically diagnosed excessive bleeding and healthy volunteers were recruited to the Genotyping and Phenotyping of Platelets study (GAPP, ISRCTN 77951167) from April 2012 to January 2013 from UK Comprehensive Care Haemophilia Centres. Patients with existing diagnoses of Glanzmann’s thrombasthenia, Bernard-Soulier syndrome, Hermansky Pudlak syndrome or May Hegglin anomaly were excluded. Laboratory testing was deferred in participants exposed within two weeks to drugs known to affect platelet function. This study was approved by the National Research Ethics Service Committee West Midlands – Edgbaston (REC reference: 06/MRE07/36) and all participants gave written informed consent.
Platelet function was assessed using light transmission aggregometry as described previously (5). Platelet-rich plasma (PRP) was prepared by centrifugation of whole blood at 200g for 20 min and autologous platelet-poor plasma (PPP) was obtained by further centrifugation at 1000g for 10 min. Platelet function testing was carried out by light transmission aggregometry alongside measurement of ATP secretion using a dual-channel Chronolog lumi-aggregometer (Model 460 VS). Platelet aggregation measurement without and with Chronolume® (30 μl added to 400 μl of PRP) was carried out simultaneously in response to epinephrine 10 μM with recording left for up to 10 min (Sigma-Aldrich, Poole, UK).
Of the 100 participants studied, 27 were healthy volunteers and 73 were recruited from UK Comprehensive Care Haemophilia Centres based on clinical diagnosis of a platelet function defect. The median age of healthy volunteers and participants with bleeding symptoms was 29 (interquartile range from 28 to 30 years) and 38 years (interquartile range from 12 to 47 years), respectively. The majority of recruited participants were female (75% among participants with suspected platelet function defects and 74% among healthy volunteers).
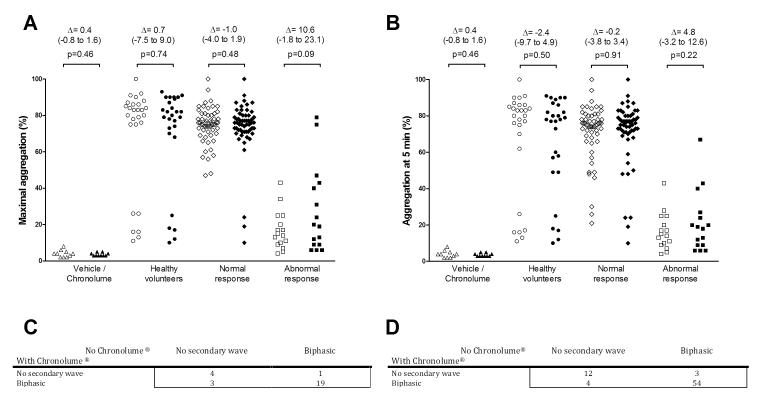
A platelet defect was found on platelet function testing in 38% of participants with bleeding symptoms (62% had no demonstrable platelet defect, 16% had a Gi-like defect, 11% had a dense granule secretion defect, 4% had a defect in the thromboxane pathway and 7% had a complex phenotype – for description of classification definitions, please refer to (5)). As shown in the Figure, addition of the vehicle (PBS) or Chronolume® alone did not induce aggregation in 10 healthy volunteers. Moreover, addition of Chronolume® did not result in a significant increase in maximal platelet aggregation or platelet aggregation 5 minutes after addition of epinephrine in healthy volunteers (n=27) or in participants with bleeding symptoms (n=57, 78%) who had a sustained and biphasic response to epinephrine 10 μM. In 16 (22%) participants with bleeding symptoms, there was no secondary wave in response to epinephrine 10 μM. Addition of Chronolume® in this group had no significant effect on maximal platelet aggregation in response to epinephrine 10 μM (mean difference between aggregation levels with vs. without Chronolume® = 10.6%, 95%CI -1.8% to 23.1%, p=0.09). Addition of Chronolume® induced a secondary wave of aggregation in 4 out of 16 participants who had no secondary wave in response to epinephrine in the absence of Chronolume®. However, addition of Chronolume® resulted in abrogation of a secondary wave in 3 out of 57 participants with biphasic aggregation. Overall, the McNemar test showed no impact of adding Chronolume® on induction of a secondary wave in response to epinephrine 10 μM (Figure, p=0.625 in healthy volunteers and p=1.0 in patients with bleeding symptoms).
Figure.
Effect of adding Chronolume® on platelet responses to epinephrine 10 μM in healthy volunteers (n=27), in participants with bleeding symptoms who had a sustained and biphasic response to epinephrine 10 μM (n=57, “normal response”), and in participants with bleeding symptoms who displayed no secondary wave in response to epinephrine 10 μM (n=16, “abnormal response”).
A. Maximal aggregation. Open symbols without Chronolume®, closed symbols with Chronolume®. Δ (95% CI) represents the mean difference between aggregation levels with vs. without Chronolume®.
B. Aggregation at 5 minutes after addition of epinephrine 10 μM. Open symbols without Chronolume®, closed symbols with Chronolume®. Δ (95% CI) represents the mean difference between aggregation levels with vs. without Chronolume®.
C. Cross-comparison of the presence of a secondary wave in response to epinephrine 10 μM in healthy volunteers. McNemar test, p=0.625
D. Cross-comparison of the presence of a secondary wave in response to epinephrine 10 μM in participants with bleeding symptoms. McNemar test, p=1.0
Our findings in patients with bleeding disorders suggestive of a platelet defect do not support the finding that Chronolume® consistently induces a secondary wave of aggregation in response to epinephrine 10 μM in patients with an aberrant response to this agonist in the absence of Chronolume®. This is in agreement with findings by Callan et al. who have found no effect of Chronolume® on human platelets, whereas it was shown to potentiate aggregation in response to ADP and collagen in canine, feline, bovine and equine platelets (6). Potentiation of platelet aggregation by Chronolume® had been suggested by previous studies which used a slightly different formulation of the reagent (7, 8); however, absence of effect and even the opposite effect (i.e. inhibition of platelet responses when luciferin:luciferase-containing reagents (including Chronolume®) were used) have also been reported (9, 10). Closer examination of the patients whose results were discrepant in the presence of Chronolume® in our study revealed that the final diagnosis of the presence of a platelet function defect would not have been affected by using Chronolume® to simultaneously assess ATP secretion alongside aggregation to epinephrine 10μM. In the cases where a secondary wave of adrenaline was induced in the presence of Chronolume®, it was either significantly delayed (>300 sec which represents the 95th percentile of locally-derived normal ranges) or was accompanied by abnormalities in ADP-induced aggregation (also dependent on appropriate activity of the Gi-coupled P2Y12 receptor) (5). The opposite effect, i.e. abrogation of a secondary wave when Chronolume® was added, was seen in one participant labelled as having a Gi-like defect. The interpretation was not affected as abnormalities in ADP-induced aggregation were also noted in the same participant, alongside a shift in dose-response to epinephrine. It is noteworthy that similar differences in the presence of Chronolume® were seen in healthy volunteers (Figure), with either induction or abrogation of a secondary wave in response to epinephrine 10 μM, which suggests that the variation seen in the patients with bleeding symptoms is within the variability of the assay in the normal population, consistent with previous literature (2). It is also important to note that the induction of a secondary wave of aggregation in response to epinephrine can sometimes require more than 5 minutes after agonist addition to develop (Figure), thus strengthening the recommendation to monitor response to epinephrine over a longer period (8 to 10 min) (11). Overall our results suggest that the presence of Chronolume® may occasionally induce or abrogate the secondary wave of aggregation in response to epinephrine 10μM, but that this effect does not have an impact on either diagnosis of an underlying platelet function defect, or classification of such a defect in participants with a history of excessive bleeding. We would therefore recommend using epinephrine in the presence of Chronolume® as part of a rationalised panel of agonists and agonist concentrations, as proposed previously (5).
In conclusion, our data support the use of Chronolume® to simultaneously assess dense granule secretion with platelet aggregation when investigating patients with excessive bleeding, and suggest that this practice does not mask platelet defects including those that reduce platelet aggregation response to epinephrine. In general, we would advise that caution should be exercised in labelling a patient with a platelet defect based on an isolated abnormal finding with epinephrine. Aggregation findings with epinephrine should be taken in context with results obtained with a panel of other agonists to aid in the diagnosis of a platelet defect, including a secretion defect (5).
Acknowledgements
The GAPP programme is chaired by Steve P. Watson and has principal investigators in Bristol (Andrew Mumford and Stuart Mundell), London (Paul Gissen) and Sheffield (Martina Daly). We would like to acknowledge the support of all of the GAPP team and Consultant Haematologists at our collaborating UK Haemophilia Comprehensive Care Centres.
Funding: This work was supported by the British Heart Foundation and Wellcome Trust. SPW holds a BHF chair (CH/03/003). ML is supported by the Canadian Institute of Health Research (MFE-107592) and the British Heart Foundation (PG/11/31/28835). GL is supported by the Wellcome Trust (093994).
Footnotes
Declaration of interest No conflicts of interest to declare.
References
- 1.Hayward CP, Moffat KA, Castilloux JF, et al. Simultaneous measurement of adenosine triphosphate release and aggregation potentiates human platelet aggregation responses for some subjects, including persons with Quebec platelet disorder. Thromb Haemost. 2012;107:726–34. doi: 10.1160/TH11-10-0740. [DOI] [PubMed] [Google Scholar]
- 2.Harrison P, Mackie I, Mumford A, et al. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol. 2011;155:30–44. doi: 10.1111/j.1365-2141.2011.08793.x. [DOI] [PubMed] [Google Scholar]
- 3.Pai M, Wang G, Moffat KA, et al. Diagnostic usefulness of a lumi-aggregometer adenosine triphosphate release assay for the assessment of platelet function disorders. Am J Clin Pathol. 2011;136:350–8. doi: 10.1309/AJCP9IPR1TFLUAGM. [DOI] [PubMed] [Google Scholar]
- 4.Nieuwenhuis HK, Akkerman JW, Sixma JJ. Patients with a prolonged bleeding time and normal aggregation tests may have storage pool deficiency: studies on one hundred six patients. Blood. 1987;70:620–3. [PubMed] [Google Scholar]
- 5.Dawood BB, Lowe GC, Lordkipanidzé M, et al. Evaluation of participants with suspected heritable platelet function disorders including recommendation and validation of a streamlined agonist panel. Blood. 2012;120:5041–9. doi: 10.1182/blood-2012-07-444281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callan MB, Shofer FS, Wojenski C, et al. Chrono-lume and magnesium potentiate aggregation of canine but not human platelets in citrated platelet-rich plasma. Thromb Haemost. 1998;80:176–80. [PubMed] [Google Scholar]
- 7.Mehta P, Mehta J, Ostrowski N, et al. Potentiation of platelet aggregation by Chronolume. Thromb Res. 1983;32:509–12. doi: 10.1016/0049-3848(83)90260-8. [DOI] [PubMed] [Google Scholar]
- 8.Zwierzina WD, Kunz F. A method of testing platelet aggregation in native whole blood. Thromb Res. 1985;38:91–100. doi: 10.1016/0049-3848(85)90010-6. [DOI] [PubMed] [Google Scholar]
- 9.Pfueller SL, Broadway M. Inhibition of platelet aggregation and [14C] serotonin release by luciferin: luciferase preparations. Thromb Res. 1986;44:539–42. doi: 10.1016/0049-3848(86)90331-2. [DOI] [PubMed] [Google Scholar]
- 10.Thompson NT, Scrutton MC. Inhibition by luciferin-luciferase reagents of aggregatory responses to excitatory agonists in washed platelet suspensions. Thromb Res. 1985;38:109–19. doi: 10.1016/0049-3848(85)90053-2. [DOI] [PubMed] [Google Scholar]
- 11.Nash CA, Severin S, Dawood BB, et al. Src family kinases are essential for primary aggregation by G(i) -coupled receptors. J Thromb Haemost. 2010;8:2273–82. doi: 10.1111/j.1538-7836.2010.03992.x. [DOI] [PubMed] [Google Scholar]