Abstract
Arsenic is a recognized human carcinogen and there is evidence that arsenic augments the carcinogenicity of DNA damaging agents such as ultraviolet radiation (UVR) thereby acting as a co-carcinogen. Inhibition of DNA repair is one proposed mechanism to account for the co-carcinogenic actions of arsenic. We and others find that arsenite interferes with the function of certain zinc finger DNA repair proteins. Furthermore, we reported that zinc reverses the effects of arsenite in cultured cells and a DNA repair target protein, poly (ADP-ribose) polymerase-1. In order to determine whether zinc ameliorates the effects of arsenite on UVR-induced DNA damage in human keratinocytes and in an in vivo model, normal human epidermal keratinocytes and SKH-1 hairless mice were exposed to arsenite, zinc or both before solar-simulated (ss) UVR exposure. Poly (ADP-ribose) polymerase activity, DNA damage and mutation frequencies at the hprt locus were measured in each treatment group in normal human keratinocytes. DNA damage was assessed in vivo by immunohistochemical staining of skin sections isolated from SKH-1 hairless mice. Cell-based findings demonstrate that ssUVR-induced DNA damage and mutagenesis are enhanced by arsenite, and supplemental zinc partially reverses the arsenite effect. In vivo studies confirm that zinc supplementation decreases arsenite-enhanced DNA damage in response to ssUVR exposure. From these data we can conclude that zinc offsets the impact of arsenic on ssUVR-stimulated DNA damage in cells and in vivo suggesting that zinc supplementation may provide a strategy to improve DNA repair capacity in arsenic exposed human populations.
Keywords: arsenic, DNA damage, skin cancer, ultraviolet radiation, zinc
Introduction
Arsenic is associated with numerous acute and chronic health effects in humans ((ASTDR), 2007; Schuhmacher-Wolz et al., 2009; Smith and Steinmaus, 2009) including increased risk of skin, lung, and urinary tract cancers (Schoen et al., 2004; Yoshida et al., 2004; Rahman et al., 2006; Yu et al., 2006; Schuhmacher-Wolz et al., 2009). Although long recognized as a complete carcinogen with tumor promoting and genotoxic actions, it is becoming more widely appreciated that low and non-cytotoxic concentrations of arsenic greatly enhance the carcinogenicity of other DNA damaging agents (Yamamoto et al., 1995; Germolec et al., 1998; Rossman et al., 2001; Maier et al., 2002; Rossman et al., 2002; Rossman et al., 2004) leading to consideration of arsenic as a co-carcinogen. In one striking example of arsenic co-carcinogenicity, ultraviolet radiation (UVR1)-induced skin carcinogenesis is enhanced in mice receiving arsenic in drinking water with i) nearly 5-fold increase in tumor number per mouse, ii) accelerated time to tumor development, and iii) increase in tumor size and invasiveness compared to UVR alone (Rossman et al., 2001; Rossman et al., 2002; Rossman et al., 2004). One hypothesis to account for the observed co-carcinogenic effects of arsenic is that certain carcinogenic metals inhibit DNA repair leading to an increase in accumulated DNA damage (Durham and Snow, 2006; Witkiewicz-Kucharczyk and Bal, 2006; Beyersmann and Hartwig, 2008; Kitchin and Wallace, 2008; Salnikow and Zhitkovich, 2008).
Two major arms of DNA repair, base excision repair (BER) and nucleotide excision repair (NER), are inhibited by low levels of carcinogenic metals including arsenite. We and others find that sub and low micromolar arsenite levels amplify DNA damage (8-hydroxy-2-deoxyguanosine (8-OHdG), strand break, cyclobutane pyrimidine dimers (CPDs), and adducts) caused by UVR, hydrogen peroxide (H2O2) or benzo[a]pyrene (Hartwig et al., 1997; Tran et al., 2002; Hartwig et al., 2003a; Schwerdtle et al., 2003; Danaee et al., 2004; Beyersmann and Hartwig, 2008; Ding et al., 2008; Qin et al., 2008a; Qin et al., 2008b; Ding et al., 2009; Ebert et al., 2011; Martinez et al., 2011). Certain DNA repair proteins contain a zinc finger motif responsible for DNA binding. Direct zinc finger DNA repair protein targets of arsenic in humans include poly (ADP-ribose) polymerase (PARP)-1 and xeroderma pigmentosum group A (XPA) (Asmuss et al., 2000; Hartwig et al., 2003b; Schwerdtle et al., 2003; Zhou et al., 2011). XPA protein is part of the mammalian NER core incision complex, binds to DNA, and interacts with other NER proteins. In the absence of XPA, no incision complex can form and damaged DNA is not excised (Park and Choi, 2006; Wood, 2010). PARP-1 is activated upon binding to damaged DNA, regulates cellular response to genotoxic insult, and lethality and carcinogenesis in response to DNA damaging agents is greatly enhanced in a PARP-1 null background (Woodhouse and Dianov, 2008; Rouleau et al., 2010). The combined inactivation of PARP-1 and XPA following arsenite exposure is predicted to cause accumulation of UVR-induced DNA lesions and enhanced genotoxicity.
Zinc fingers coordinate zinc through cysteine and histidine residues. We reported arsenite interaction with C3H1 and C4, but not C2H2 zinc finger peptides and proteins (Ding et al., 2009; Zhou et al., 2011). Arsenite-dependent zinc loss from PARP-1 protein was evident at sub-micromolar concentrations and corresponded to decreased enzyme activity and DNA binding capacity. These functions were largely restored upon co-treatment of arsenite and zinc (Ding et al., 2009; Zhou et al., 2011). Both PARP-1 and XPA represent the preferred arsenic binding zinc finger structures (C3H1 and C4, respectively), suggesting the possibility that zinc may offset the effects of arsenic with respect to DNA damage. In this study we investigated the impact of zinc on arsenite-enhancement of solar-simulated (ss) UVR-induced DNA damage in normal human keratinocytes and in mouse epidermis in vivo. From these experiments we conclude that the inhibitory effects of arsenite on DNA repair leading to increased DNA damage can be significantly reversed by concomitant exposure to zinc. This finding suggests that zinc status may be an important determinant in modifying arsenic carcinogenesis or co-carcinogenesis based on inhibition of DNA repair processes. In this study we provide evidence that supplemental zinc can reverse arsenic’s effects on DNA repair inhibition and mutagenesis in cellular and in vivo systems.
Methods
Cell culture and treatment
Normal human neonatal epidermal keratinocytes (HEKn) and DermaLife K culture medium with supplements were purchased from Lifeline Cell Technologies (Oceanside, CA). Cells were cultured at 37°C in 95% air/5% CO2-humidified incubators. 10 mM stock solutions of sodium arsenite and zinc chloride (Fluka Chemie, Buchs, Germany) and 100 mM stock solution of hydrogen peroxide (H2O2; Sigma, St. Louis, MO) were prepared in double-distilled water and sterilized using a 0.22-μm syringe filter. Working solutions were prepared by diluting the stock with complete cell growth medium. For experiments involving cell exposures, HEKn cells were rinsed and placed in complete medium containing, arsenite, zinc, or H2O2 as indicated in the Figure legends. Cell viability was determined for all treatment conditions using the colony forming assay. Briefly, cells were treated with arsenite (1 μM), zinc (2 μM), or arsenite plus zinc for 24 h, then exposed to ssUVR (3 kJ/m2). 5000 cells were plated into fresh 60 mm tissue culture plates, incubated for 5 d, stained with Phastgel Blue R (0.2%) and colonies counted to determine percentage of viable cells per treatment group (data not shown).
UV Source
UVR exposures were performed using an Oriel 1000 Watt Solar Ultraviolet Simulator (Oriel Corp., Stratford, CT). This solar simulator produces a high intensity UVR beam in both the UVA (320-400 nm) and UVB (280-320 nm) spectrum with an emission ratio of 14:1 (UVA: UVB). The proportion and intensity of UVA/UVB was measured using a radiospectrometer (Optronics laboratories, Inc.; Orlando, FL) and exposure times were calculated to give the desired doses. Measurements were made with Erythema UV and UVA intensity meter (Solar Light Co., Inc., Philadelphia, PA) in order to estimate minimum erythema dose (MED) for the in vivo portion of this study. Dose MEDs were verified by comparison to a study determining numbers of sunburn cells per exposure level (unpublished data) and visual inspection of the animals.
HPRT Mutation
The HPRT gene mutation assay was conducted as described (Albertini RJ, 1981). HEKn cells were placed in complete medium and cultured at 37°C in 95% air/5% CO2 humidified incubators. Following 24 h incubation, cells were treated with arsenite (1 μM), zinc (2 μM), both or neither and incubated for an additional 24 h. The cells were then exposed to ssUVR (3 kJ/m2) and incubated for an additional 5 days. Cells were trypsinized, counted and 1×105 cells in triplicate from each treatment group were placed in T25 flasks. The medium was subsequently exchanged with medium containing 5 μg/ml 6-thio-guanine and incubated for 6-8 weeks to allow colony growth. One flask was reserved for colony forming assessment of viability at 5 days. All flasks were stained using Phastgel Blue R (0.2%) and colonies enumerated for data analysis. Mutation frequency was determined by dividing mutant colony count by viability colony count for each treatment and normalized to mutants per 106 cells. Data was collected from a minimum of 3 separate experiments and analyzed by one-way ANOVA with Tukey’s multiple comparison tests conducted using Graphpad Prism 5.03 (San Diego, CA).
Animal handling and treatments
SKH-1 mice (21-25 days old) were purchased from Charles River Laboratories (Wilmington, MA). These studies were performed under an approved Institutional Animal Care and Use Committee (IACUC) protocol (#09-100408-HSC). Animals were housed by treatment group and administered arsenite (5 mg/l), zinc (10 mg/l), both or neither in the drinking water for 28 days. Water was freshly prepared and changed every second day, consumption monitored and volumes compared to untreated control animals to ensure equivalent water consumption (data not shown). Controls and treated animals were provided standard mouse chow ad lib. Animals randomly selected from each treatment group were then exposed to a single dose (28 kJ/m2; 1.2 MED of ssUVR, then euthanized at 1 h post exposure. This time point and UV dose was established in preliminary studies and reflects a time point leading to significant, but not maximal, levels of DNA damage with minimal observable surface skin damage (data not shown). The irradiated and UVR naïve dorsal skin was collected, preserved in 10% neutral buffered formalin and paraffin blocks were prepared using standard procedures.
Immunohistochemistry
Paraffin embedded tissue was sectioned using a rotary microtome (Microm HM315) at a thickness of 10 μm. For staining, slides were deparaffinized with three exchanges of xylene (10 min. each) followed by a one min. exchange in absolute ethanol. Sections were rehydrated by sequential 1 min. immersions in 95%, 75%, and 50% ethanol followed by 5 min. in water. Slides were then placed in a 1:10 dilution of H2O2 and methanol for 20 min. A DNA unwinding step was performed using 0.125% trypsin for 10 min. at room temperature, followed by three rinses with 1X phosphate-buffered saline (PBS). Slides were then placed in a 1N HCl solution for 30 min. at room temperature and subsequently rinsed with 1X PBS. For staining of CPDs, slides were blocked in 5% BSA containing 10% goat serum for 10 min., then incubated with anti-CPD antibody (Thymine clone KTM53; Kamiya Biomedical, Seattle, WA) at 1:250 dilution overnight followed by anti-mouse secondary antibody conjugated to Cy3 (Chemicon, Temecula, CA) at 1:200 for 1 h. Slides were mounted with VectaShield plus DAPI (Vector Laboratories, Burlingame, CA) and images were collected on a Zeiss LSM-510 META confocal microscope using a 40x objective. For all other DNA damage markers examined, sections were blocked in 5% horse serum overnight then labeled with primary antibody against phospho-H2Ax (pH2Ax, Cell Signaling, Danvers, MA at 1:75) or pyrimidine-pyrimidone 6,4-photo products (6,4-PP; Kamiya Biomedical, Seattle, WA at 1:1000). Staining was visualized using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) following the manufacturer’s instructions. Images were obtained using a 60X objective on an Olympus IX 70 microscope equipped with a DP72 digital camera and imaging software. The numbers of stained nuclei were counted and divided by the total number of nuclei in each image to give the percentage of stained cells. Data from five different animals were pooled and two-way ANOVA was conducted using Graphpad Prism 5.03 (San Diego, CA).
PARP-1 Activity and DNA damage Immunocytochemistry
Cells were treated with arsenite (1 μM) and/or zinc (2 μM) then H2O2 (100 μM) was added 10 min. prior to fixation. PARP-1 activity was visualized by indirect immunofluorescence using anti-PAR (Axxora, San Diego, CA) and an anti-mouse cy3-conjugated secondary antibody. PARP-1 activity was assessed by comparing the fluorescent staining intensity of poly(ADP)-ribose (PAR) following DNA damage induced by H2O2 (100 μM) and confirmed using the HT Colorimetric PARP/Apoptosis Assay kit (Trevigen, Inc., Gaithersburg, MD) according to manufacturer’s instructions. For DNA damage assessment, cells were treated as described above with ssUVR (3 kJ/m2) employed as the DNA damaging agent and cells fixed at 1 or 6 h post ssUVR. DNA damage markers were visualized by indirect immunofluorescence using anti-8-OHdG (N45.1, Abcam, Cambridge, MA), anti-phospho-H2Ax (Cell Signaling Technologies, Danvers, MA), anti-CPDs (Thymine clone KTM53) and anti-pyrimidine-pyrimidone 6,4-photo products (6,4-PP, Kamiya Laboratories, Seattle, WA) antibodies in combination with either anti-mouse or anti-rabbit FITC conjugated secondary antibodies (Abcam, Cambridge, MA) diluted in blocking buffer (PBS containing 5% horse serum and 0.05% triton X-100). Cells were mounted with VectaShield +DAPI (Vector Laboratories, Burlingame, CA) and visualized with an Olympus IX70 fluorescence microscope equipped with a DP72 digital camera. A minimum of 10 randomly selected images per slide were obtained per treatment and fluorescence intensity was quantified using Image J image analysis software (http://rsb.info.nih.gov/ij/index.html). Data from a minimum of 3 separate experiments were pooled and statistical significance assessed by one-way ANOVA with Tukey’s multiple comparison tests conducted using Graphpad Prism 5.03 (San Diego, CA).
Results
Arsenite inhibits PARP-1 activity in normal keratinocytes
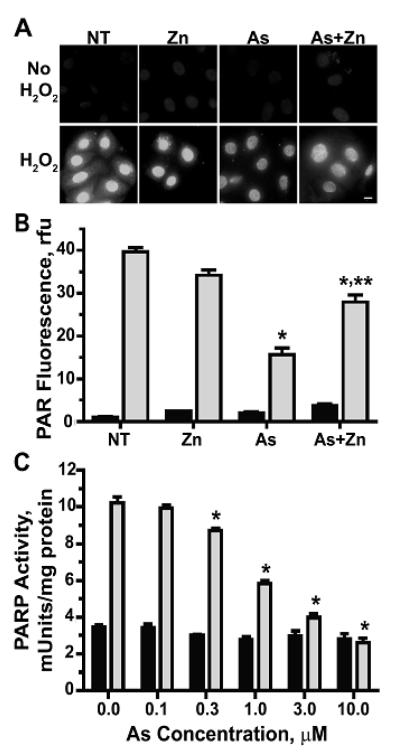
PARP-1 is a sensitive target for inhibition by arsenic (Hartwig et al., 2003b; Qin et al., 2008b; Ding et al., 2009; Zhou et al., 2011) and we demonstrated previously that the concomitant addition of zinc with arsenite partially restores the activity of PARP-1 in HaCat cells (Qin et al., 2008b; Ding et al., 2009). HaCaT cells are an immortalized human keratinocyte line and representative of tumor predisposed cells, so we wanted to establish whether normal human epidermal keratinocytes (HEKn) cells displayed comparable responses. Detection of PARP activity by immunostaining for poly(ADP ribose) (PAR), the product of activated PARP, demonstrates that PARP activity induced by H2O2-mediated DNA damage was significantly decreased by preincubation with arsenite (1 μM) in HEKn cells (Fig. 1). These results were confirmed by a PARP activity assay kit as described in “Materials and Methods” (Fig. 1C). The magnitude of inhibition is similar to that reported for HaCat cells (Qin et al., 2008b) and cell viability as measured by colony forming assay and the CellTiter 96 Non-radioactive cell proliferation assay kit (Promega, Madison, WI) following exposure to arsenite (1 μM) was >85% (data not shown). Cells exposed to arsenite (1 μM) and zinc (2 μM) for 24h, then challenged with H2O2, largely retained PARP activity, suggesting that zinc counteracts the effects of arsenite in a zinc finger DNA repair target in normal human keratinocytes.
Figure 1. Arsenite-induced inhibition of PARP-1 activity is partially restored by zinc.
(A&B) HEKn cells were grown on 4-well chamber slides and treated with zinc (Zn, 2 μM), arsenite (As, 1 μM), both (As+Zn) or left untreated (NT) for 24h. Cells were subsequently exposed to 100 μM H2O2 for 10 min. then fixed and stained for PAR to visualize PARP activity by immunofluorescence microscopy (A). Bar = 10 μm. Panels shown are representative of at least 10 fields per treatment group. (B) Fluorescence intensity measured using Image J. Graph shown is representative of average fluorescent intensity from four independent experiments with intensities measured from at least 10 fields per treatment group per experiment. Bars represent relative intensity ± SEM with no H2O2 (dark bars) to H2O2 treated (light bars). (C) Cells were treated with the indicated concentrations of arsenite (As) and then DNA damage induced via ssUVR (3 kJ/m2) exposure for 1h and PARP-1 activity determined via the HT Colorimetric PARP/Apoptosis Assay kit as described in “Materials and Methods”. Bars represent relative activity ± SEM with no UV (dark bars) to UV treated (light bars). * = significantly different compared with H2O2 or UV only; ** = significantly different compared with As + H2O2 or UV; p<0.05; n=4.
Arsenite-enhanced UVR-induced mutations are partially reversed by zinc
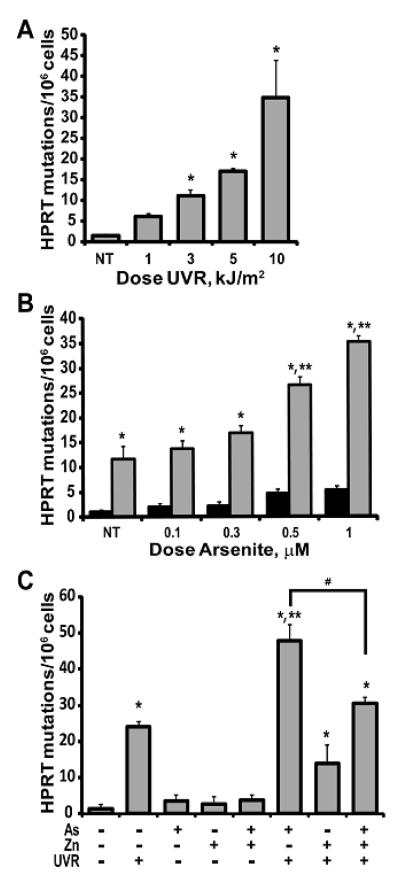
Our previous studies demonstrated that inhibition of PARP activity or silencing of PARP-1 protein increased retention of ssUVR-induced DNA damage in immortalized HaCat cells (Ding et al., 2008; Qin et al., 2008a; Ding et al., 2009). In order to determine whether enhanced DNA damage results in increased mutations, we measured mutation frequencies of HEKn cells at the Hprt locus by selection for growth in 6-thioguanine. The ssUVR exposure of 3 kJ/m2 was chosen based on results from a dose response study (1-10 kJ/m2, Fig. 2A) reflecting significant, but submaximal mutation frequencies at this exposure. HEKn cells were exposed to varying concentrations of arsenite for 24h followed by exposure to 3 kJ/m2 ssUVR (Fig. 2B). A slight, but not statistically significant increase in HPRT mutation frequency was observed with arsenite alone; however, arsenite doses ≥ 0.5 μM significantly increased the number of ssUVR-induced HPRT mutations (Fig. 2B). In contrast, HaCaT cells were less sensitive requiring >1 μM arsenite to significantly increase ssUVR-induced mutations (data not shown). Co-exposure of HEKn cells with arsenite (1 μM) and zinc (2 μM) for 24 h before exposure to ssUVR significantly reduced the number of mutations when compared to arsenite alone (Fig. 2C). These results demonstrate that arsenite amplifies ssUVR-induced mutations and that zinc counteracts the effects of arsenite.
Figure 2. Arsenite increases ssUVR-induced HPRT mutation frequency.
HEKn cells were cultured and treated as indicated for each panel, then plated in triplicate and placed in medium containing 6-thioguanine (5 μg/ml) for mutant selection as described in “Materials and Methods”. (A) Dose response for UVR exposure. (B) Arsenite dose response (24 h treatment) without (dark bars) and with (light bars) UVR (3 kJ/m2) exposure. (C) Zinc (Zn, 2 μM; 24 h treatment) reduction of UVR-induced (UVR, 3 kJ/m2), arsenite-enhanced (As, 1 μM) mutations. Graphs represent mutations/million cells ± SEM for each treatment group. * = significantly different from untreated control (NT); ** = significantly different from UV alone; # = significantly different from As+UVR; p<0.05; n ≥ 3.
DNA damage persists in cells pre-incubated with arsenite
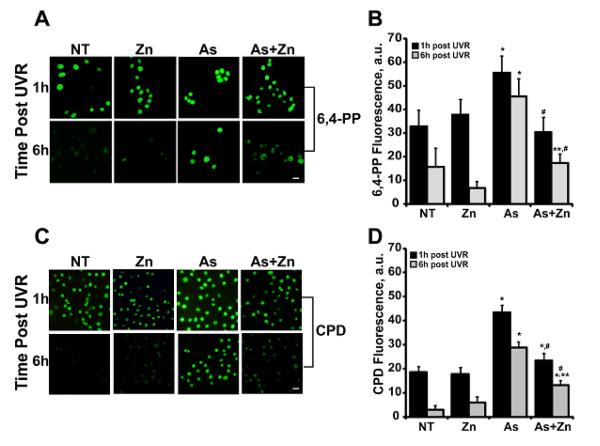
While mutations in Hprt indicate that arsenite promotes persistent UVR-induced DNA lesions, this approach does not define whether specific types of UVR-induced DNA damage are preferentially increased by arsenite. Pyrimidine-pyrimidone 6,4-photo products (6,4-PP) and cyclobutane pyrimidine dimers (CPDs) are examples of direct UVR photodamage (Black et al., 1997; Pfeifer and Besaratinia, 2012). Exposure of HEKn cells to 3 kJ/m2 ssUVR increased 6,4-PP and CPD formation as detected by immunostaining for the specific lesions. This increase is evident at 1 h post-exposure with some repair evident at 6 h post-ssUVR exposure (Fig. 3; NT represents ssUVR only). Pre-incubation with arsenite (1 μM) significantly increased 6,4-PPs and CPDs at 1 and 6 h post ssUVR exposure compared to cells exposed to ssUVR only (Fig. 3). Co-exposure of arsenite with zinc significantly decreased the arsenite augmentation of ssUVR-induced 6,4-PPs and CPDs at both time points (Fig. 3).
Figure 3. Effects of zinc on arsenite-dependent increase of ssUVR-induced direct DNA damage.
HEKn cells were grown on 4-well chamber slides and treated with zinc (Zn, 2 μM), arsenite (As, 1 μM), both (As+Zn) or left untreated (NT) for 24h. Cells were subsequently exposed to ssUVR (UVR, 3 kJ/m2), fixed at the indicated times and stained for 6,4-PP (A) or CPDs (C). Quantification of relative fluorescence intensity was performed via Image J analysis for 6,4-PP (B) or CPDs (D). Panels and graphs shown are representative of three independent experiments with average fluorescent intensities measured from at least 10 fields per treatment group per experiment. Bar = 10 μm. * = significantly different from time matched untreated, ssUVR exposed controls (NT); ** = significantly different between treatment matched times; # = significantly different from time matched arsenite; p <0.05; n = 3.
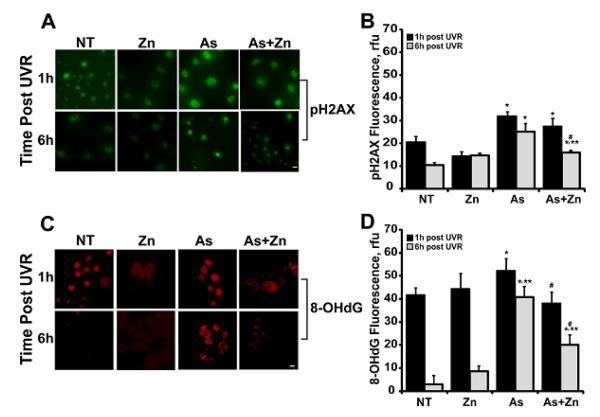
In addition to direct photolesions, ssUVR also causes DNA strand breaks and oxidative damage (Pfeifer and Besaratinia, 2012; Sage et al., 2012). Immunostaining for pH2AX and 8-OHdG was performed to detect the potential impact of arsenite and zinc on these forms of DNA damage.
Arsenite pretreatment increased pH2AX staining induced by ssUVR and damage was largely retained at 6 h post-exposure (Fig. 4A, B; NT represents ssUVR only). Although 8-OHdG is only slightly increased by arsenite at 1 h post-ssUVR exposure, the damage was persistent with little decline after 6 h (Fig. 4C, D). Less damage was retained in cells co-treated with arsenite and zinc (Fig. 4D). Taken together, the findings for all four markers of DNA damage illustrate a broad contribution of arsenite to the damaging effects of ssUVR and a positive impact of zinc.
Figure 4. Effects of zinc on arsenite-dependent increase of ssUVR-induced oxidative DNA damage.
HEKn cells were grown on 4-well chamber slides and treated with zinc (Zn, 2 μM), arsenite (As, 1 μM), both (As+Zn) or left untreated (NT) for 24 h. Cells were subsequently exposed to ssUVR (UVR, 3 kJ/m2), fixed at the indicated times and stained for pH2AX (A) or 8-OHdG (C). Quantification of relative fluorescence intensity was performed via Image J analysis for pH2AX (B) or 8-OHdG (D). Panels and graphs shown are representative of three independent experiments with average fluorescent intensities from at least 10 fields per treatment group per experiment. Bar = 10 mm. * = significantly different from time matched untreated, UVR exposed controls (NT); ** = significantly different between treatment matched times; # = significantly different from time matched arsenite; p <0.05; n = 3.
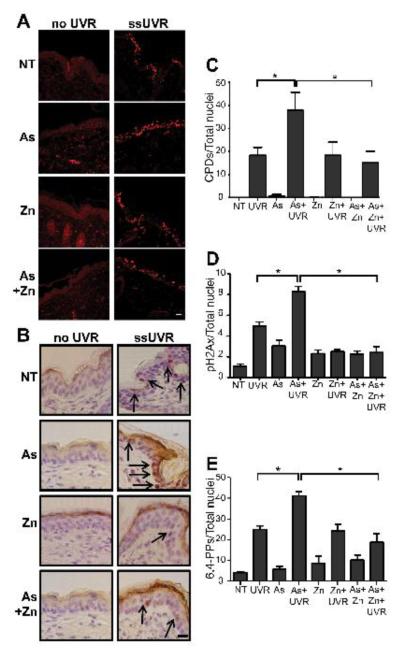
Effects of arsenite on DNA damage in vivo
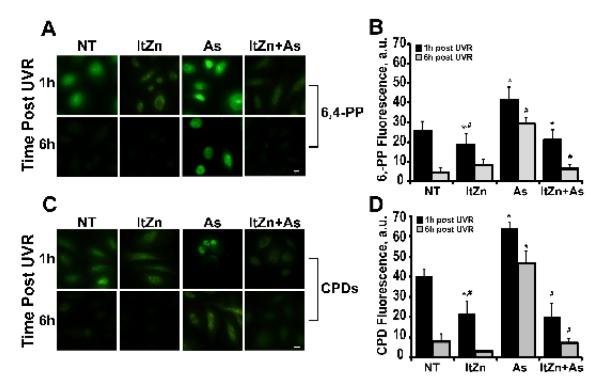
The preceding findings illustrate functional interaction between arsenite, zinc and ssUVR with regard to DNA damage in normal human keratinocytes, and further studies were performed to investigate these interactions in an in vivo system. For these studies, SHK-1 hairless mice were administered arsenite, zinc, both or neither in drinking water for 28 days prior to ssUVR exposure as described under “Materials and Methods”. Fixed, paraffin-embedded dorsal skin sections were stained for DNA damage markers (CPDs, pH2AX and 6,4-PP) and staining intensity was quantified. Exposure to 28 kJ/m2 ssUVR increased the fraction of nuclei positive for CPDs, pH2AX and 6,4-PP in the epidermis (Fig. 5). A significant increase of all three DNA damage markers was observed in the ssUVR-exposed epidermis isolated from arsenite exposed mice compared to ssUVR alone. ssUVR-induced DNA lesions were significantly decreased in mice receiving arsenite and zinc when compared to arsenite alone (Fig. 5). Sections stained for 8-OHdG showed comparable results (data not shown). Because the response to zinc was more pronounced in vivo compared to the findings in cultured normal human keratinocytes, we tested whether the extended duration of zinc exposure in vivo might account for the difference. Normal keratinocytes were cultured for 2-3 weeks in medium supplemented with zinc (2 μM) before exposure to arsenite and measurement of ssUVR stimulated DNA damage. Extended zinc incubation decreased induction of 6,4-PP (1 h post ssUVR) by 53%, and a greater reduction of the arsenite response (compare Fig. 3B and 6B). Extended zinc exposure also decreased arsenite enhancement of ssUVR CPD formation (compare Fig. 3D and 6D). These data indicate that extended exposure to zinc results in greater resistance to and recovery from at least a sub-set of UVR-induced lesions, suggesting a possible protective role for zinc.
Figure 5. Effects of zinc on arsenite-dependent increase of ssUVR-induced DNA damage in vivo.
SHK-1 mice were treated with arsenite (As, 5 mg/l), zinc (10, mg/l), both (As+Zn) or nothing (NT) in their drinking water for 28 days. Animals were then exposed to a single dose of ssUVR (UVR, 28 kJ/m2, 1.2 MED). Irradiated dorsal skin was collected after 1 h, formalin fixed, processed and sections stained as described in “Materials and Methods”. (A) Confocal microscopy images representative of CPD staining from animals treated as indicated with ratio of stained nuclei per total nuclei graphically represented in (C). (B) Images representative of sections peroxidase stained for pH2AX from animals treated as indicated. Arrows indicate examples of positive nuclei. Graphical representation of ratio of pH2AX (D) or 6,4-PP (E) stained nuclei per total nuclei. Bar = 10 mm. * = significantly different between indicated groups; p <0.05; n ≥ 5.
Figure 6. Extended incubation with zinc reduces arsenite-dependent increase of ssUVR-induced DNA damage.
HEKn cells were grown in medium supplemented with zinc (2 μM) for 2-3 weeks (long term [lt]Zn) and then plated on 4-well chamber slides. Cells maintained in un-supplemented medium were plated as controls. Cells were treated with arsenite (As, 1 μM), or left untreated (NT) for 24 h, then exposed to ssUVR (3 kJ/m2), fixed at the indicated times and stained for 6,4-PP (A) or CPDs (C). Quantification of relative fluorescence intensity was performed via Image J analysis for 6,4-PP (B) or CPDs (D). Panels and graphs shown are representative of three independent experiments with average fluorescent intensities from at least 10 fields per treatment group per experiment. Bar = 10 μm. * = significantly different from time matched controls (NT); # = significantly different from time matched arsenite; p <0.05; n = 3.
Discussion
Certain key DNA repair proteins (e.g., XPA and PARP-1) contain zinc finger domains that are essential for DNA binding and protein function. Several lines of evidence demonstrate that zinc finger domains are sites of arsenic interaction. Arsenite can occupy zinc finger peptides and displace zinc from a PARP-1 peptide (Kitchin and Wallace, 2008; Piatek et al., 2008; Ding et al., 2009; Zhou et al., 2011). Furthermore, arsenite decreased zinc content of PARP-1 and XPA isolated from arsenite exposed cells (Zhou et al., 2011). Using an immortalized keratinocyte line, we established that increasing zinc concentrations reversed arsenite-dependent PARP-1 inhibition and reduced the arsenite enhancement of ssUVR-induced 8-OHdG formation (Ding et al., 2009) and strand break (Qin et al., 2008b). These findings indicated the potential for zinc to offset the effects of arsenic in the enhancement of UVR-stimulated oxidative DNA damage.
The current study demonstrates that zinc reduces the augmentation of numerous forms of DNA damage by arsenite in normal human keratinocytes. Arsenite also increased ssUVR-induced mutations and this response was decreased by co-exposure of arsenic and zinc. Using the mouse model of arsenic co-carcinogenesis established by Rossman and colleagues (Rossman et al., 2002), we found that exposure to arsenite in drinking water significantly increased the ssUVR-induced CPDs, 6,4-PP and pH2AX. Furthermore, co-administration of zinc essentially eliminated the effect of arsenite illustrating that an interaction between arsenic and zinc is retained in vivo.
Inhibition of DNA repair related to disruption of zinc finger function by arsenite is consistent with the published literature regarding zinc deficiency and decreased DNA repair capacity. Zinc adequacy is important for the maintenance of genomic integrity (Ho, 2004; Song et al., 2009a; Sharif et al., 2012). In an experimental rat model, even marginal zinc deficiency led to impairment of DNA repair (Song et al., 2009b). Furthermore, a study in humans demonstrated a positive correlation between PARP activity and zinc status in human cells and zinc supplementation improved PARP activity as measured by cellular poly(ADP-ribosyl)ation capacity indicating that the effects of zinc deficiency can be measured in specific DNA repair proteins (Kunzmann et al., 2008). Here we show that zinc supplementation can protect against arsenite enhancement of DNA damage in response to an insult such as UVR. These findings suggest that the reported arsenic-induced zinc depletion in PARP-1 and XPA protein is an important mechanism for the observed increased susceptibility to DNA-damaging agents such as UVR and account for the co-carcinogenic activities of arsenic.
Our recent publications demonstrate the interaction of arsenite with the zinc finger motif of a zinc finger DNA repair protein, resulting in the loss of zinc, decreased protein function and consequent accumulation of DNA damage. The current study provides direct evidence that supplemental zinc can reverse the DNA damage augmentation and mutagenesis by arsenite in human cells and in vivo. These findings suggest that zinc deficiency or adequacy may be an important determinant in the co-carcinogenic potential of arsenic.
Highlights.
Low levels of arsenite enhance UV-induced DNA damage in human keratinocytes
UV-initiated HPRT mutation frequency is enhanced by arsenite
Zinc supplementation offsets DNA damage and mutation frequency enhanced by arsenite
Zinc-dependent reduction of arsenite enhanced DNA damage is confirmed in vivo
Acknowledgements
The authors would like to acknowledge support from the National Institutes of Health R01 ES015826 and 3R01ES015826-03S1 (to KJL and LGH), NRSA F31 1F31ES019823-01 (to BK) and support to MMS from GM-060201. The work was also supported by UNM Cancer Research and Treatment Center (5P30CA118100) core facilities. Images in this paper were generated in the University of New Mexico & Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed on: http://hsc.unm.edu/crtc/microscopy/Facility.html. We also acknowledge Sarah Liu and Kelsey Thompson for assistance with tissue preparation and data collection and thank Dr. Donna F. Kusewitt (MD Anderson) for consultation on the project.
Footnotes
Conflict of Interest Statement The authors declare there are no conflicts interests.
List of abbreviations: 6,4-PP, 6,4-photo products; 8-OHdG, 8-hydroxy-2-deoxyguanosine; BER, base excision repair; BSA, bovine serum albumin; CPDs, cyclobutane pyrimidine dimers; HEKn, normal neonatal human epidermal keratinocytes; HPRT, hypoxanthine-guanine phosphoribosyltransferase ; kJ, kilojoules; MED, minimum erythemal dose; NER, nucleotide excision repair; PAR, poly (ADP-ribose); PARP, poly (ADP-ribose) polymerase; PBS, phosphate-buffered saline; pH2AX, phospho-histone H2AX; UVR, ultraviolet radiation; ssUVR, solar simulated ultraviolet radiation; XPA , xeroderma pigmentosum group A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ASTDR EPA Report Toxicological Profile for Arsenic. Registry, P.H.S.A.f.T.S.a.D., Services, U.S.D.o.H.a.H. 2007.
- Albertini RJ, A E, Quinn AS, Albertini RR, Porter E.B.H.a.I.H., editors. Population and Biological Aspects of Human Mutation. Academic Press; New York: 1981. Human somatic cell mutation: in vivo variant lymphocyte frequencies as determined by 6-thioguanine resistance; pp. 235–263. [Google Scholar]
- Asmuss M, Mullenders LH, Eker A, Hartwig A. Differential effects of toxic metal compounds on the activities of Fpg and XPA, two zinc finger proteins involved in DNA repair. Carcinogenesis. 2000;21:2097–2104. doi: 10.1093/carcin/21.11.2097. [DOI] [PubMed] [Google Scholar]
- Beyersmann D, Hartwig A. Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch Toxicol. 2008;82:493–512. doi: 10.1007/s00204-008-0313-y. [DOI] [PubMed] [Google Scholar]
- Black HS, deGruijl FR, Forbes PD, Cleaver JE, Ananthaswamy HN, deFabo EC, Ullrich SE, Tyrrell RM. Photocarcinogenesis: an overview. J Photochem Photobiol B. 1997;40:29–47. doi: 10.1016/s1011-1344(97)00021-3. [DOI] [PubMed] [Google Scholar]
- Danaee H, Nelson HH, Liber H, Little JB, Kelsey KT. Low dose exposure to sodium arsenite synergistically interacts with UV radiation to induce mutations and alter DNA repair in human cells. Mutagenesis. 2004;19:143–148. doi: 10.1093/mutage/geh010. [DOI] [PubMed] [Google Scholar]
- Ding W, Hudson LG, Sun X, Feng C, Liu KJ. As(III) inhibits ultraviolet radiationinduced cyclobutane pyrimidine dimer repair via generation of nitric oxide in human keratinocytes. Free Radic Biol Med. 2008;45:1065–1072. doi: 10.1016/j.freeradbiomed.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Liu W, Cooper KL, Qin XJ, de Souza Bergo PL, Hudson LG, Liu KJ. Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage. J Biol Chem. 2009;284:6809–6817. doi: 10.1074/jbc.M805566200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham TR, Snow ET. Metal ions and carcinogenesis. EXS. 2006:97–130. doi: 10.1007/3-7643-7378-4_5. [DOI] [PubMed] [Google Scholar]
- Ebert F, Weiss A, Bultemeyer M, Hamann I, Hartwig A, Schwerdtle T. Arsenicals affect base excision repair by several mechanisms. Mutat Res. 2011;715:32–41. doi: 10.1016/j.mrfmmm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Spalding J, Yu HS, Chen GS, Simeonova PP, Humble MC, Bruccoleri A, Boorman GA, Foley JF, Yoshida T, Luster MI. Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors. Am J Pathol. 1998;153:1775–1785. doi: 10.1016/S0002-9440(10)65692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig A, Blessing H, Schwerdtle T, Walter I. Modulation of DNA repair processes by arsenic and selenium compounds. Toxicology. 2003a;193:161–169. doi: 10.1016/j.tox.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Hartwig A, Groblinghoff UD, Beyersmann D, Natarajan AT, Filon R, Mullenders LH. Interaction of arsenic(III) with nucleotide excision repair in UV-irradiated human fibroblasts. Carcinogenesis. 1997;18:399–405. doi: 10.1093/carcin/18.2.399. [DOI] [PubMed] [Google Scholar]
- Hartwig A, Pelzer A, Asmuss M, Burkle A. Very low concentrations of arsenite suppress poly(ADP-ribosyl)ation in mammalian cells. Int J Cancer. 2003b;104:1–6. doi: 10.1002/ijc.10911. [DOI] [PubMed] [Google Scholar]
- Ho E. Zinc deficiency, DNA damage and cancer risk. J Nutr Biochem. 2004;15:572–578. doi: 10.1016/j.jnutbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Kitchin KT, Wallace K. The role of protein binding of trivalent arsenicals in arsenic carcinogenesis and toxicity. J Inorg Biochem. 2008;102:532–539. doi: 10.1016/j.jinorgbio.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Kunzmann A, Dedoussis G, Jajte J, Malavolta M, Mocchegiani E, Burkle A. Effect of zinc on cellular poly(ADP-ribosyl)ation capacity. Exp Gerontol. 2008;43:409–414. doi: 10.1016/j.exger.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Maier A, Schumann BL, Chang X, Talaska G, Puga A. Arsenic co-exposure potentiates benzo[a]pyrene genotoxicity. Mutat Res. 2002;517:101–111. doi: 10.1016/s1383-5718(02)00057-8. [DOI] [PubMed] [Google Scholar]
- Martinez VD, Vucic EA, Adonis M, Gil L, Lam WL. Arsenic biotransformation as a cancer promoting factor by inducing DNA damage and disruption of repair mechanisms. Mol Biol Int. 2011;2011:718974. doi: 10.4061/2011/718974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CJ, Choi BS. The protein shuffle. Sequential interactions among components of the human nucleotide excision repair pathway. FEBS J. 2006;273:1600–1608. doi: 10.1111/j.1742-4658.2006.05189.x. [DOI] [PubMed] [Google Scholar]
- Pfeifer GP, Besaratinia A. UV wavelength-dependent DNA damage and human nonmelanoma and melanoma skin cancer. Photochem Photobiol Sci. 2012;11:90–97. doi: 10.1039/c1pp05144j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatek K, Schwerdtle T, Hartwig A, Bal W. Monomethylarsonous acid destroys a tetrathiolate zinc finger much more efficiently than inorganic arsenite: mechanistic considerations and consequences for DNA repair inhibition. Chem Res Toxicol. 2008;21:600–606. doi: 10.1021/tx7003135. [DOI] [PubMed] [Google Scholar]
- Qin XJ, Hudson LG, Liu W, Ding W, Cooper KL, Liu KJ. Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol. 2008a;21:1806–1813. doi: 10.1021/tx8001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XJ, Hudson LG, Liu W, Timmins GS, Liu KJ. Low concentration of arsenite exacerbates UVR-induced DNA strand breaks by inhibiting PARP-1 activity. Toxicol Appl Pharmacol. 2008b;232:41–50. doi: 10.1016/j.taap.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Vahter M, Wahed MA, Sohel N, Yunus M, Streatfield PK, El Arifeen S, Bhuiya A, Zaman K, Chowdhury AM, Ekstrom EC, Persson LA. Prevalence of arsenic exposure and skin lesions. A population based survey in Matlab, Bangladesh. J Epidemiol Community Health. 2006;60:242–248. doi: 10.1136/jech.2005.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol Appl Pharmacol. 2004;198:394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis. Toxicol Appl Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite cocarcinogenesis: an animal model derived from genetic toxicology studies. Environ Health Perspect. 2002;110(Suppl 5):749–752. doi: 10.1289/ehp.02110s5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293–301. doi: 10.1038/nrc2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage E, Girard PM, Francesconi S. Unravelling UVA-induced mutagenesis. Photochem Photobiol Sci. 2012;11:74–80. doi: 10.1039/c1pp05219e. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen A, Beck B, Sharma R, Dube E. Arsenic toxicity at low doses: epidemiological and mode of action considerations. Toxicol Appl Pharmacol. 2004;198:253–267. doi: 10.1016/j.taap.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Schuhmacher-Wolz U, Dieter HH, Klein D, Schneider K. Oral exposure to inorganic arsenic: evaluation of its carcinogenic and non-carcinogenic effects. Crit Rev Toxicol. 2009;39:271–298. doi: 10.1080/10408440802291505. [DOI] [PubMed] [Google Scholar]
- Schwerdtle T, Walter I, Hartwig A. Arsenite and its biomethylated metabolites interfere with the formation and repair of stable BPDE-induced DNA adducts in human cells and impair XPAzf and Fpg. DNA Repair (Amst) 2003;2:1449–1463. doi: 10.1016/j.dnarep.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Sharif R, Thomas P, Zalewski P, Fenech M. The role of zinc in genomic stability. Mutat Res. 2012;733:111–121. doi: 10.1016/j.mrfmmm.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Smith AH, Steinmaus CM. Health effects of arsenic and chromium in drinking water: recent human findings. Annu Rev Public Health. 2009;30:107–122. doi: 10.1146/annurev.publhealth.031308.100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Chung CS, Bruno RS, Traber MG, Brown KH, King JC, Ho E. Dietary zinc restriction and repletion affects DNA integrity in healthy men. Am J Clin Nutr. 2009a;90:321–328. doi: 10.3945/ajcn.2008.27300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Leonard SW, Traber MG, Ho E. Zinc deficiency affects DNA damage, oxidative stress, antioxidant defenses, and DNA repair in rats. J Nutr. 2009b;139:1626–1631. doi: 10.3945/jn.109.106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HP, Prakash AS, Barnard R, Chiswell B, Ng JC. Arsenic inhibits the repair of DNA damage induced by benzo(a)pyrene. Toxicol Lett. 2002;133:59–67. doi: 10.1016/s0378-4274(02)00088-7. [DOI] [PubMed] [Google Scholar]
- Witkiewicz-Kucharczyk A, Bal W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol Lett. 2006;162:29–42. doi: 10.1016/j.toxlet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Wood RD. Mammalian nucleotide excision repair proteins and interstrand crosslink repair. Environ Mol Mutagen. 2010;51:520–526. doi: 10.1002/em.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse BC, Dianov GL. Poly ADP-ribose polymerase-1: an international molecule of mystery. DNA Repair (Amst) 2008;7:1077–1086. doi: 10.1016/j.dnarep.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Konishi Y, Matsuda T, Murai T, Shibata MA, Matsui-Yuasa I, Otani S, Kuroda K, Endo G, Fukushima S. Cancer induction by an organic arsenic compound, dimethylarsinic acid (cacodylic acid), in F344/DuCrj rats after pretreatment with five carcinogens. Cancer Res. 1995;55:1271–1276. [PubMed] [Google Scholar]
- Yoshida T, Yamauchi H, Fan Sun G. Chronic health effects in people exposed to arsenic via the drinking water: dose-response relationships in review. Toxicol Appl Pharmacol. 2004;198:243–252. doi: 10.1016/j.taap.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Yu HS, Liao WT, Chai CY. Arsenic carcinogenesis in the skin. J Biomed Sci. 2006;13:657–666. doi: 10.1007/s11373-006-9092-8. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, Hudson LG. Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J Biol Chem. 2011;286:22855–22863. doi: 10.1074/jbc.M111.232926. [DOI] [PMC free article] [PubMed] [Google Scholar]