Abstract
The posttranslational modification of proteins with O-linked β-d-N-acetylglucosamine (O-GlcNAc) on serine and threonine residues occurs in all animals and plants. This modification is dynamic and ubiquitous, and regulates many cellular processes, including transcription, signaling and cytokinesis and is associated with several diseases. Cycling of O-GlcNAc is tightly regulated by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA). Plants have two OGTs, SPINDLY (SPY) and SECRET AGENT (SEC); disruption of both causes embryo lethality. Despite O-GlcNAc modification of proteins being discovered more than 20-years ago, identification and mapping of protein GlcNAcylation is still a challenging task. Here we describe the use of lectin affinity chromatography combined with electron transfer dissociation mass spectrometry to enrich and to detect O-GlcNAc modified peptides from Arabidopsis.
Keywords: O-GlcNAc, Arabidopsis, High-performance liquid chromatography, Mass spectrometry, Electron transfer dissociation, Collision-induced dissociation
1. Introduction
The monosaccharide O-linked β-d-N-acetylglucosamine (O-GlcNAc) modification is a ubiquitous and key modification of nuclear and cytoplasmic proteins (1–3). Perturbation of O-GlcNAc levels is associated with many diseases, such as cancer, diabetes, Alzheimer's, and cardiovascular diseases (4–6). Genetic data has also shown that O-GlcNAcylation is critical for embryonic stem cell viability, as both mice and plants show an embryo-lethal phenotype when O-GlcNAc transferase (OGT) functions are disrupted (7, 8). Different from animals, plants have two distinct OGTs, SPY and SEC (7, 9–12).
There is growing evidence that O-GlcNAc and phosphorylation can play reciprocal roles in regulating protein functions and a “yin-yang” model has been proposed for the possible relationship. For instance, the c-Myc oncoprotein is majorly O-GlcNAcylated at Threonine 58, a known site phosphorylated by the kinase, GSK3β, and a mutational hot spot in lymphomas (13). The two PTMs might compete for the modification of the same or proximal Ser/Thr residues (6).
The understanding of O-GlcNAc regulatory functions has been greatly hampered by a lack of knowledge of the identities of the exact residues to which the sugar is attached for most modified proteins. This situation has been due to lack of effective methods for enrichment, detection, and site assignment. However, recent developments in enrichment of either natively modified (14, 15) or tagged/derivatized peptides combined with either electron capture or transfer dissociation mass spectrometry have provided robust modification site analysis tools (16, 17). Mapping the sites of O-GlcNAcylation is critical not only to elucidate the direct function of the modification (by site-directed mutagenesis and/or antibodies) but also to gain a more mechanistic understanding of potential cross talk between GlcNAcylation and other modifications, including phosphorylation.
2. Materials
2.1. Extraction of Total Proteins from Arabidopsis Tissues
Prepare stocks separately: 1 M Tris–HCl, pH 8.0; 0.5 M ethylene glycol tetraacetic acid (EGTA), pH 8.0; 0.5 M ethylenedinitrilo tetraacetic acid (EDTA), pH 8.0. Autoclave before storage at room temperature. Prepare stocks: 20% (w/v) sodium dodecyl sulfate (SDS), and store at room temperature.
Prepare inhibitor 0.5 mM O-(2-acetamido-2-deoxy-d-glucopyranosylideneamino)N-phenylcarbamate (PUGNAc) in water. Make aliquots and store at –20°C (Sigma).
Protease inhibitor cocktail (Roche). Store at –20°C.
Liquid nitrogen.
Mortar and pestle.
Extraction buffer Y: 100 mM Tris–HCl, pH 8.0, 2% SDS, 1% β-mercaptoethanol, 5 mM EGTA, 10 mM EDTA, 20 μM PUGNAc, and 1× protease inhibitor cocktail. Make freshly each time by mixing aliquots from stocks.
Phenol (Tris buffered, pH 7.5–7.9). Store at 4°C.
Extraction buffer Z: 50 mM Tris–HCl, pH 8.0. Store at 4°C.
Methanol. Store at 4°C.
0.1 M ammonium acetate in methanol. Store at 4°C.
Lysis buffer: 6 M guanidine-HCl. Store at room temperature.
Biorad protein assay kit (Bio-Rad).
2.2. Tryptic Digestion of Protein Samples
Prepare 50 mM NH4HCO3 in water. Store at room temperature.
Reduction reagent: 1 M Tris (2-carboxyethyl) phosphine (TCEP) in 50 mM NH4HCO3. Make aliquots and store in 20°C. Keep in dark.
Alkylation reagent: 550 mM iodoacetamide in 50 mM NH4HCO3. Make it fresh. Keep in dark.
Modified trypsin (Promega).
2.3. Desalting the Peptide Sample by Using C18 Filled Sep-Paks
Formic acid.
Buffer A: 0.1% formic acid.
Buffer B: 70% acetonitrile and 0.1% formic acid.
C18 filled Sep-Pak (Waters).
Syringes (10 ml).
Needles.
Speed vacuum.
Nitrogen.
2.4. Packing a Lectin Weak Affinity Chromatography Column
Resin: WGA-Agarose (Vector Laboratories).
Tubing: Teflon PFA 1/16″ (1.6 mm) OD, 1/25″ (1 mm) ID (Upchurch Scientific).
Packing a lectin weak affinity chromatography (LWAC) buffer: 25 mM Tris, pH 7.8, 200 mM NaCl, 5 mM CaCl2, and 1 mM MgCl2.
LWAC elute buffer: 20 mM GlcNAc (N-acetyl-d-glucosamine) (Sigma).
Large empty column to use as a reservoir for packing resin from: e.g., AP mini-column 10 mm × 120 mm (Waters).
Frit: 2 μm porosity Stainless Steel Frit 0.062″ OD (Upchurch Scientific).
Unions for each end of column: e.g., Stainless Steel ZDV Union 0.02″ Thru-hole (Upchurch Scientific).
2.5. Enriching O-GlcNAc-Modified Peptide by Using LWAC Column
LWAC buffer: 25 mM Tris, pH 7.8, 200 mM NaCl, 5 mM CaCl2, and 1 mM MgCl2.
LWAC elute buffer: 20 mM GlcNAc in LWAC buffer.
AKTA purifier HPLC (GE Healthcare).
2.6. Desalting Peptides by Using C18 Pipette Tip
Buffer A: 0.1% formic acid.
Buffer B: 70% acetonitrile and 0.1% formic acid.
C18 100 μl OMIX pipette tips for micro extraction (Varian).
2.7. Detection by Liquid Chromatography–Tandem Mass Spectrometry
HPLC: Waters Nanoacquity-Ultra Performance LC (Waters).
Linear Ion Trap (LTQ)-Orbitrap XL with Electron Transfer Dissociation (ETD) (Thermo).
HPLC Solvent A: Water/0.1% formic acid.
HPLC Solvent B: Acetonitrile/0.1% formic acid.
Trapping column: 5 μm Symmetry C18, 180 μm inner diameter ×20 mm (Waters).
Analytical column: 1.7 μm BEH130 C18 100 μM inner diameter ×100 mm (Waters).
3. Methods
The lectin wheat germ agglutinin (WGA) has affinity for terminal N-acetylglucosamine (GlcNAc) and sialic acid residues. It has four binding sites, so can bind with high-affinity to branched glycan structures with multiple terminal GlcNAc moieties. However, the affinity for a single GlcNAc residue, as encountered with the regulatory modification of O-GlcNAcylation, found on serines and threonines of nuclear and cytoplasmic proteins, is low. An LWAC protocol has been developed to enrich for O-GlcNAc-modified peptides (14), in which WGA attached to agarose is packed into a long column. Modified peptides can be separated from unmodified peptides through their retardation on these long columns during isocratic HPLC, causing them to elute later than unmodified peptides.
O-GlcNAcylation site identification using conventional collision-induced dissociation (CID) analysis in a mass spectrometer is usually not possible. CID is a vibrational activation fragmentation process that breaks the weakest bonds in the structure. The O-glycosidic link is significantly more labile than the peptide backbone. Hence, the O-GlcNAc moiety is readily liberated as an oxonium ion before peptide backbone fragmentation, and site assignment cannot be derived from the resulting deglycosylated fragment ions. The recently developed electron capture dissociation and ETD are a radical-based “non-ergodic” fragmentation process that results in mostly peptide backbone cleavage, thus enabling the identification of sites bearing labile posttranslational modifications (14, 15, 18).
3.1. Extraction of Total Proteins from Arabidopsis Tissues
Harvest flower tissues from 45-day-old Arabidopsis plant growing in green house. Freeze in liquid nitrogen.
Grind tissues in a mortar with liquid nitrogen to a fine powder and weigh 2 g tissue powder in 50-ml tube.
Add three volume (6 ml) of buffer Y (see Notes 1 and 2). Vortex for 1 min.
Heat the samples for 10 min at 65°C.
Centrifuge at 20,000 × g for 20 min at 20°C.
Transfer the supernatant to new tubes. Add equal volume of ice-cold phenol (Tris buffer, pH 7.5–7.9), and vortex for 1 min.
Centrifuge at 20,000 × g for 15 min at 4°C to separate phenol and aqueous phases.
Remove the upper aqueous phase to leave the interface intact. Discard the upper aqueous phase.
Re-extract the phenol phase twice with ice-cold Buffer Z as in steps 7 and 8.
Mix with five volumes of cold 0.1 M ammonium acetate in methanol and leave at –20°C overnight to precipitate proteins.
Centrifuge at 20,000 × g for 20 min at 4°C. Keep the pellet. Remove all the supernatant.
Wash the protein pellet twice with 1 ml ice-cold 0.1 M ammonium acetate in methanol and 1 ml cold methanol twice; centrifuge for 5 min and remove the liquid each time (see Note 3).
Resuspend the protein pellet in lysis buffer.
Centrifuge at 20,000 × g for 20 min.
Transfer the supernatant to a new tube, and determine the protein concentration with Bio-Rad protein assay kit using BSA as a standard.
3.2. Tryptic Digestion of Protein Samples
Reduce the disulfide bonds of 2 mg protein sample by adding Tris TCEP to final concentration of 2 mM for 60 min at 56°C.
Alkylate the free cysteines of the protein sample by adding iodoacetamide to final concentration of 10 mM for 60 min at room temperature in the dark.
Dilute the sample with 50 mM NH4HCO3 to make the final guanidine-HCl concentration of 1.5 M (see Notes 4–6).
Add modified trypsin 1:50 w/w overnight at 37°C.
Quench the protease activity by acidification of the reaction mixture with formic acid to final concentration of 1% formic acid.
Centrifuge at 20,000 × g for 10 min to remove insoluble material. Keep the supernatant.
3.3. Desalting the Peptide Sample by Using C18 Sep-Pak
Cut Sep-Pak column ends to reduce dead volume.
Activate the C18 Sep-Pak column: Attach a needle to the syringe and pull buffer B (2 ml); detach the needle, and attach the syringe to the Sep-Paks; and slowly push the buffer through the Sep-Pak. When the buffer has been pushed through the Sep-Pak, remove the syringe from the Sep-Pak and leave Sep-Pak on clean tissue paper.
Equilibrate the C18 Sep-Pak column: Attach another needle to the syringe and pull buffer A (8 ml); detach the needle, and attach the syringe to the Sep-Paks; and slowly push the buffer through the Sep-Pak.
Repeat this equilibration step twice to remove any trace of acetonitrile (see Note 7).
Load the C18 Sep-Pak column with the peptide sample: Attach another needle to the syringe and pull peptide samples; detach the needle and attach the syringe to the Sep-Paks; slowly push the buffer through the Sep-Pak, collecting the flow through back into the original peptide sample tube.
Repeat the loading step four more times to ensure maximal binding of the peptides to the column.
Wash the Sep-Pak column to remove salts: Attach another needle to a new syringe and pull buffer A 8 ml; detach the needle and attach the syringe to the Sep-Paks; and slowly push the buffer through the Sep-Pak, discard the flow through.
Repeat the washing step four more times to completely wash away salts and other contaminants.
Elute the peptides from the Sep-Pak: Attach another needle to a new syringe and pull buffer B 1 ml; detach the needle and attach the syringe to the Sep-Paks; and slowly push the buffer through the Sep-Pak, and collect the eluate.
Repeat the elution step and collect the eluate in the same tube.
Dry the tube in Speedvac to complete dryness.
Store the peptides in –80°C freezer before use.
3.4. Packing a Lectin Weak Affinity Chromatography Column
Insert Frit into union and attach to one end of Teflon tubing (see Note 8).
Attach reservoir column to the other end of Teflon tubing (see Note 9).
Partially fill reservoir column with WGA-agarose resin.
Pack resin into Teflon tubing using an HPLC pump delivering LWAC buffer at a flow rate of 50–200 μl/min, making sure that the back pressure never exceeds 2 MPa (20 mbar) (see Notes 10 and 11).
It may be necessary to replenish the reservoir column with more resin on several occasions (see Note 12). When doing this, stop the HPLC and wait for pressure to drop to zero before disconnecting.
When the column is packed to a long enough length (see Note 13), stop the packing, and then cut the back end of the column at the point at which it is packed up to in order to remove dead volume.
Attach union to the back end of the column, and then cap each end to prevent column from drying out.
Store the LWAC column at 4°C.
3.5. Enriching O-GlcNAc-Modified Peptides by Using LWAC Column
Resuspend the peptides in 200 μl LWAC buffer.
Install the LWAC column and flush lines with LWAC buffer for 5 min at a flow rate of 100 μl/min.
Load sample and wait for the main peak to elute.
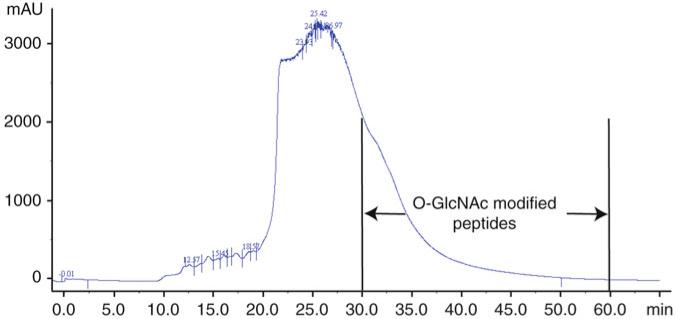
Manually collect fractions at 1-min intervals during the elution of the main UV-visible peak and subsequent tail of the peak (see Note 14). An example of the HPLC results produced is shown in Fig. 1. A total of more than ten fractions are analyzed by mass spectrometry to identify GlcNAc-modified peptides.
At the end of the run, inject 200 μl of LWAC buffer containing 20 mM GlcNAc to elute any complex glycans (see Note 15).
Store the column at 4°C.
Store the fractions at –80°C freezer before further use.
Fig. 1.
Lectin weak affinity chromatography enrichment of Arabidopsis O-linked β-d-N-acetylglucosamine (O-GlcNAc)-modified peptides using a WGA-Agarose column with LWAC buffer at a flow rate of 100 μl/min. Peptide elution is monitored at 205 nm. The region containing (O-GlcNAc) modified peptides is labeled.
3.6. Desalting Peptides by Using C18 Pipette Tip
Sample treatment: Adjust sample to a 1% formic acid concentration using a 10% formic acid solution.
Activate tip: Aspirate 100 μl buffer B and discard solvent.
Equilibration: Aspirate 100 μl buffer A and discard solvent. Repeat four more times to remove any trace of acetonitrile (see Note 7).
Apply sample: Aspirate and dispense pretreated sample into the tip three to five times (see Notes 16 and 17).
Wash tip: Aspirate buffer A and discard solvent. Repeat.
Elute peptides: Aspirate 100 μl buffer B and pipette the tip five times.
Reduce the sample volume and remove the acetonitrile by vacuum concentration.
Store the peptides at –80°C freezer or directly resuspend the peptides in buffer A for further mass spectrometry analysis.
3.7. Detection by Liquid Chromatography–Tandem Mass Spectrometry
The peptide mixtures are analyzed by online nanoflow liquid chromatography–tandem mass spectrometry (LC–MS/MS) on a Nanoacquity ultraperformance LC system connected to an LTQ-XL Orbitrap with ETD source.
Peptides are first loaded onto a trapping column packed with C18, and then washed with 0.1% formic acid. The trapping column is connected to an analytical column.
The reverse-phase liquid chromatography is performed at a flow rate of 400 nl/min.
A 90-min gradient is used; peptides are eluted by a gradient from 2 to 30% solvent B over 65 min followed by a short wash at 50% solvent B, before returning to starting conditions. Peptide components elute over a period of ~65 min during these runs.
The effluent from the HPLC column is directly interfaced with the mass spectrometer.
The LTQ Orbitrap XL instrument is operated in data-dependent mode to automatically switch between full-scan MS and MS/MS acquisition.
After a precursor scan of intact peptides is measured in the orbitrap by scanning from m/z 350–1,400, the three most intense multiply charged precursors are selected for both CID and ETD analysis in the linear ion trap.
Activation times are 30 and 100 ms for CID and ETD fragmentation, respectively. Automatic gain control (AGC) targets are 100,000 ions for orbitrap scans and 10,000 for MS/MS scans, and the AGC for the fluoranthene ions used for ETD is 100,000. Supplemental activation of the charge-reduced species is used in the ETD analysis to improve fragmentation. Dynamic exclusion for 60 s is used to prevent repeated analysis of the same components.
3.8. Data Analysis
Separate ETD and CID peak lists are generated from raw data files using an in-house script PAVA, and then CID and ETD data are searched separately using Protein Prospector version 5.7.1 (19, 20) against a database that consists of the Uni-ProtKB protein database downloaded on 1 November 2011, to which a randomized version has been concatenated. Only Arabidopsis entries are searched, meaning a total of 101,922 entries are queried. ETD peptide results are reported using a peptide false discovery rate level of 0.5% according to concatenated database search results.
For both CID and ETD data, a precursor mass tolerance of 20 ppm and a fragment mass error tolerance of 0.6 Da are allowed.
Modification parameters: Carbamidomethylcysteine is searched as a constant modification. Variable modifications include protein N-terminal acetylation, peptide N-terminal glutamine conversion to pyroglutamate, and methionine oxidation. For ETD data, HexNAc modification of Serine or Threonine and asparagine residues is considered. For CID data, HexNAc modification of serine or threonine and asparagines residues is considered, but in addition, a modification observed as a neutral loss of 203.08 Da (so all fragments are assumed not to have GlcNAc attached) is considered from serines and threonines (see Note 18).
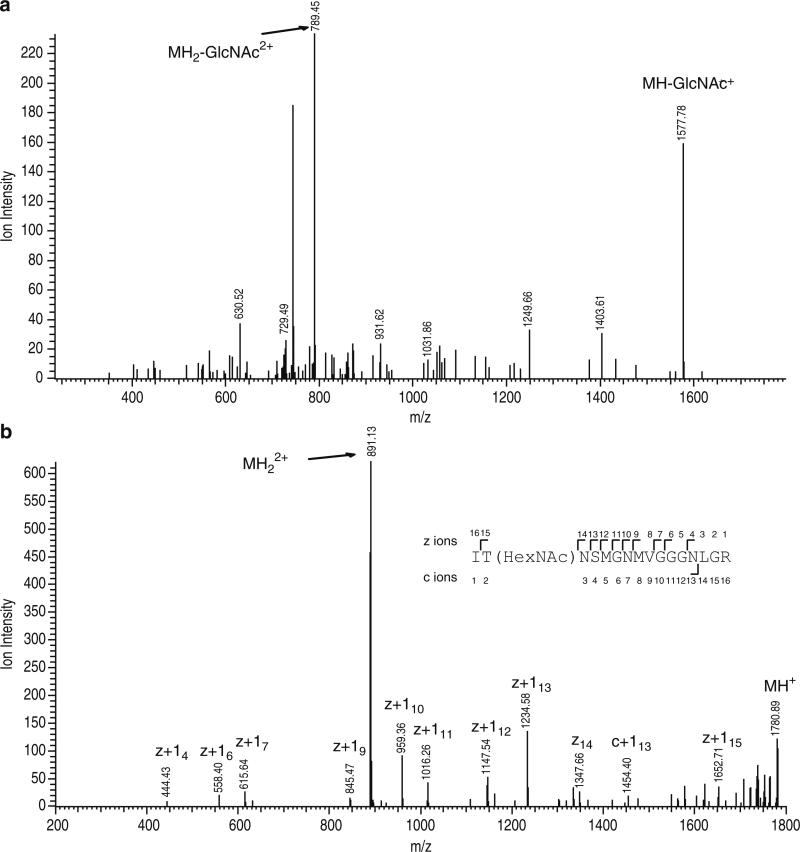
Assignments of all modified peptides are checked manually; in every case, the CID results are consistent with the ETD assignment (see Note 18). An example result is shown in Fig. 2.
Fig. 2.
Collision-induced dissociation (CID) and electron transfer dissociation (ETD) spectra of an m/z 890.9212 2+ precursor (theoretical m/z = 890.9195, +2.0 ppm). (a) The molecular weight of this peptide is shifted to higher mass of 203 Da and the CID spectrum displays loss of sugar [MH2-GlcNAc2+] and [MH-GlcNAc+], showing this peptide to be O-linked β-d-N-acetylglucosamine (O-GlcNAc) modified. (b) Interpretation of the ETD spectrum of the species of m/z 890.9212 establishes a doubly charged peptide ITNSMGNMVGGGNLGR with Threonine 2 being modified by O-GlcNAc.
Acknowledgments
This work was financially supported by the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the US Department of Energy through Grant DEFG02-08ER15973, and by NIH (R01GM066258) and NSF (IOS0724688). The UCSF Mass Spectrometry Facility (A.L.B., Director) is supported by the Biomedical Research Technology Program of the National Centre for Research Resources, NIH NCRR RR01614.
Footnotes
This extraction method is adapted from the protocol for preparation of proteins for 2-D DIGE (21). Alternative approaches to extract proteins would be acceptable as long as O-GlcNAc inhibitors are included in the extraction buffer.
The O-GlcNAc modification can be easily removed by hydrolases, such as O-GlcNAcase or hexosaminidases during cell lysis. Hence, it is important to include inhibitors during the extraction and purification processes that preserve the levels of O-GlcNAc on proteins. PUGNAc is a potent competitive inhibitor of hexosaminidases that inhibits all three known human hexosamindases [HEXA/B and O-GlcNAcase (OGA)] (22).
It is better not to disturb the pellets at this stage. Remove all the liquid and let the pellet dry for 1 min.
Maximum allowable concentration of guanidine-HCl for trypsin is less than 1.5 M.
To achieve the highest digestion efficiency, optimize the solution close to pH 7.5–9.0.
It is acceptable to use urea with or without thiourea to dissolve the protein sample; however, long incubation in the presence of urea may lead to carbamylation of the available amino terminus (43 Da mass increase) of the proteins as well as the side chain (ε-amino groups) of lysine residues (43 Da mass increase; resulting in a protein that is unsuitable for many enzymatic digests including tryptic cleavage). Maximum allowable concentration of urea and thiourea for trypsin is less than 1 M total.
Binding of peptides to the C18 (this is an 18 carbon hydrocarbon chain that is bonded to the silicate) matrix is a result of hydrophobic interactions. For efficient binding, sample solutions must be free from any organic solvent because even a small proportion of organic solvent can prevent adsorption of some peptides.
A frit is used in the in-line filter to remove unwanted particulate from the solution.
To get a good separation of modified peptides from the unmodified peptides, we recommend packing a column of at least 3 meters in length (we have used columns up to 10 meters in length).
An issue with these columns is that the agarose can compress. Hence, we recommend keeping the column pressure below 2 MPa (20 mbar). This means operating at a flow rate between 50–150 μl/min (longer columns require lower flow rates).
On occasions, the column may stop packing. If this happens, a possible solution may be to agitate the reservoir column. If this still does not remedy the problem, then stop the HPLC (wait for the pressure to drop to zero). It is then possible to reverse the direction of the column by moving the frit to the other end of the column.
Packing the tubing requires 0.8 ml of resin per meter of column.
Packing these columns is slow—expect it to take all day to make a column.
Depending on the length of your column, the O-GlcNAc-modified peptides elute in the tail of the main peak or a little afterwards. Do not worry if you do not see a distinct peak for modified peptides, and collect fractions even beyond the point at which there is any obvious UV absorbance (there will probably still be peptides there).
Inject LWAC Elute Buffer to elute any complex glycans that may be bound to the column. The GlcNAc in this buffer absorbs at 214 nm, so do not be concerned if you observe a large UV peak.
To achieve maximum sample binding, the whole sample must be passed several times through the C18 matrix.
For steps 4–6, to achieve optimum flow and peptide recovery, do not introduce air through the tip.
Modified peptides spectra need to be manually inspected, particularly for site assignment reliability. One characteristic of O-linked GlcNAc-modified peptides is the loss of O-GlcNAc in CID spectra. N-linked GlcNAc-modified peptides have been detected in Arabidopsis O-GlcNAc-enriched samples, similar as reported in ref 15. However, N-linked GlcNAc is stable in CID mass spectrometry, so loss of GlcNAc will not be detected from N-linked GlcNAc-modified peptides.
References
- 1.Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. Cross talk between O-GlcNAcylation and phosphorylation: roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 2011;80:6.1–6.34. doi: 10.1146/annurev-biochem-060608-102511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart G, Housley M, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 3.Olszewski NE, West CM, Sassi SO, Hartweck LM. O-GlcNAc protein modification in plants: evolution and function. Biochim Biophys Acta. 2009;1800:49–56. doi: 10.1016/j.bbagen.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transfer-ase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 5.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozcan S, Andrali SS, Cantrell JE. Modulation of transcription factor function by O-GlcNAc modification. Biochim Biophys Acta. 2010;1799:353–364. doi: 10.1016/j.bbagrm.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartweck LM, Scott CL, Olszewski NE. Two O-linked N-acetylglucosamine transferase genes of Arabidopsis thaliana L. Heynh. have overlapping functions necessary for gamete and seed development. Genetics. 2002;161:1279–1291. doi: 10.1093/genetics/161.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA. 2000;97:5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobsen SE, Olszewski NE. Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell. 1993;5:887–896. doi: 10.1105/tpc.5.8.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobsen SE, Binkowski KA, Olszewski NE. SPINDLY, a tetratricopeptide repeat protein involved in gibberellin signal transduction in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:9292–9296. doi: 10.1073/pnas.93.17.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izhaki A, Swain SM, Tseng TS, Borochov A, Olszewski NE, Weiss D. The role of SPY and its TPR domain in the regulation of gibberellin action throughout the life cycle of Petunia hybrida plants. Plant J. 2001;28:181–190. doi: 10.1046/j.1365-313x.2001.01144.x. [DOI] [PubMed] [Google Scholar]
- 12.Hartweck LM, Genger RK, Grey WM, Olszewski NE. SECRET AGENT and SPINDLY have overlapping roles in the development of Arabidopsis thaliana L. Heyn. J Exp Bot. 2006;57:865–875. doi: 10.1093/jxb/erj071. [DOI] [PubMed] [Google Scholar]
- 13.Chou TY, Hart GW, Dang CV. c-Myc is glycosylated at threonine 58, a known phosphorylation site and a mutational hot spot in lymphomas. J Biol Chem. 1995;270:18961–18965. doi: 10.1074/jbc.270.32.18961. [DOI] [PubMed] [Google Scholar]
- 14.Vosseller K, Trinidad JC, Chalkley RJ, Specht CG, Thalhammer A, Lynn AJ, Snedecor JO, Guan S, Medzihradszky KF, Maltby DA, Schoepfer R, Burlingame AL. O-linked N-acetylglucosamine proteomics of postsynaptic density preparations using lectin weak affinity chromatography and mass spectrometry. Mol Cell Proteomics. 2006;5:923–934. doi: 10.1074/mcp.T500040-MCP200. [DOI] [PubMed] [Google Scholar]
- 15.Chalkley RJ, Thalhammer A, Schoepfer R, Burlingame AL. Identification of protein O-GlcNAcylation sites using electron transfer dissociation mass spectrometry on native peptides. Proc Natl Acad Sci USA. 2009;106:8894–8899. doi: 10.1073/pnas.0900288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khidekel N, Ficarro SB, Peters EC, Hsieh-Wilson LC. Exploring the O-GlcNAc proteome: direct identification of O-GlcNAc-modified proteins from the brain. Proc Natl Acad Sci USA. 2004;101:13132–13137. doi: 10.1073/pnas.0403471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Udeshi ND, O'Malley M, Shabanowitz J, Hunt DF, Hart GW. Enrichment and site mapping of O-linked N-acetylglucosa-mine by a combination of chemical/enzymatic tagging, photochemical cleavage, and electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2009;9:153–160. doi: 10.1074/mcp.M900268-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelleher NL, Zubarev RA, Bush K, Furie B, Furie BC, McLafferty FW, Walsh CT. Localization of labile posttranslational modifications by electron capture dissociation: the case of gamma-carboxyglutamic acid. Anal Chem. 1999;71:4250–4253. doi: 10.1021/ac990684x. [DOI] [PubMed] [Google Scholar]
- 19.Chalkley RJ, Baker PR, Hansen KC, Medzihradszky KF, Allen NP, Rexach M, Burlingame AL. Comprehensive analysis of a multidimensional liquid chromatography mass spectrometry dataset acquired on a quadrupole selecting, quadrupole collision cell, time-of-flight mass spectrometer: I. How much of the data is theoretically interpretable by search engines? Mol Cell Proteomics. 2005;4:1189–1193. doi: 10.1074/mcp.D500001-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Chalkley RJ, Baker PR, Huang L, Hansen KC, Allen NP, Rexach M, Burlingame AL. Comprehensive analysis of a multidimensional liquid chromatography mass spectrometry dataset acquired on a quadrupole selecting, quadrupole collision cell, time-of-flight mass spectrometer: II. New developments in protein prospector allow for reliable and comprehensive automatic analysis of large datasets. Mol Cell Proteomics. 2005;4:1194–1204. doi: 10.1074/mcp.D500002-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Deng Z, Zhang X, Tang W, Oses-Prieto JA, Suzuki N, Gendron JM, Chen H, Guan S, Chalkley RJ, Peterman TK, Burlingame AL, Wang ZY. A proteomics study of brassinosteroid response in Arabidopsis. Mol Cell Proteomics. 2007;6:2058–2071. doi: 10.1074/mcp.M700123-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene) amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]