Abstract
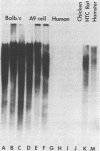
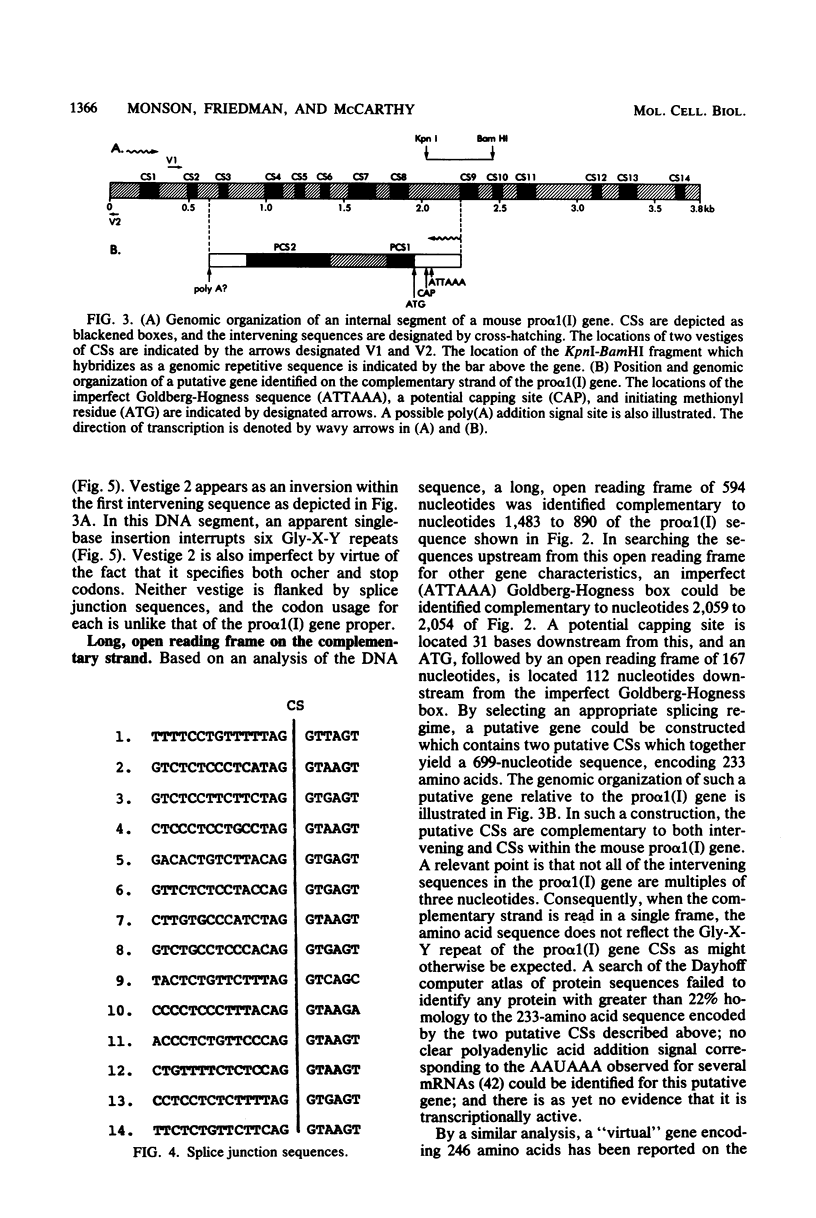
In a 3.8-kilobase mouse DNA sequence encoding amino acid sequences for the pro alpha 1(I) chain of type I procollagen, 14 coding sequences were identified which specify a sequence 95% homologous to amino acid residues 568 to 963 of the bovine alpha 1(I) chain. All of these coding sequences were flanked by appropriate splice junctions following the GT/AG rule. These observations suggest, but do not prove, that this pro alpha 1(I) gene is transcriptionally active. Of the 14 coding sequences, 7 were 54 base pairs in length, whereas the remainder were higher multiples of 54 base pairs. Nonrandom utilization of codons pertained throughout all of the coding sequences showing a preference (56%) for U in the wobble position. Two of the intervening sequences encoded imperfect vestiges of coding sequences which exhibited a codon preference different from that of the pro alpha 1(I) gene proper and were not flanked by splice junctions. One intervening sequence encoded a member of the mouse B1 family of middle repetitive sequences. It was flanked by 8-base-pair direct repeats and had a truncated A-rich region, suggesting that it may be a mobile element. Within this element were sequences which could function as a RNA polymerase III split promoter.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baralle F. E., Shoulders C. C., Proudfoot N. J. The primary structure of the human epsilon-globin gene. Cell. 1980 Oct;21(3):621–626. doi: 10.1016/0092-8674(80)90425-0. [DOI] [PubMed] [Google Scholar]
- Barta A., Richards R. I., Baxter J. D., Shine J. Primary structure and evolution of rat growth hormone gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4867–4871. doi: 10.1073/pnas.78.8.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Pictet R. L., Rutter W. J., Cordell B., Tischer E., Goodman H. M. Sequence of the human insulin gene. Nature. 1980 Mar 6;284(5751):26–32. doi: 10.1038/284026a0. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Brown D. D. Nucleotide sequences in Xenopus 5S DNA required for transcription termination. Cell. 1981 Apr;24(1):261–270. doi: 10.1016/0092-8674(81)90522-5. [DOI] [PubMed] [Google Scholar]
- Bogenhagen D. F., Sakonju S., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: II. The 3' border of the region. Cell. 1980 Jan;19(1):27–35. doi: 10.1016/0092-8674(80)90385-2. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Sage H. Structurally distinct collagen types. Annu Rev Biochem. 1980;49:957–1003. doi: 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Casino A., Cipollaro M., Guerrini A. M., Mastrocinque G., Spena A., Scarlato V. Coding capacity of complementary DNA strands. Nucleic Acids Res. 1981 Mar 25;9(6):1499–1518. doi: 10.1093/nar/9.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Regulation of gene expression: possible role of repetitive sequences. Science. 1979 Jun 8;204(4397):1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Duncan C. H., Jagadeeswaran P., Wang R. R., Weissman S. M. Structural analysis of templates and RNA polymerase III transcripts of Alu family sequences interspersed among the human beta-like globin genes. Gene. 1981 Mar;13(2):185–196. doi: 10.1016/0378-1119(81)90007-x. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Pan J., Duncan C. H., Weissman S. M. Transcriptional analysis of interspersed repetitive polymerase III transcription units in human DNA. Nucleic Acids Res. 1981 Mar 11;9(5):1171–1189. doi: 10.1093/nar/9.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek P. P., Rexrodt F. W., Hopper K. E., Kühn K. The covalent structure of collagen. 2. The amino-acid sequence of alpha1-CB7 from calf-skin collagen. Eur J Biochem. 1973 Oct 5;38(2):396–400. doi: 10.1111/j.1432-1033.1973.tb03072.x. [DOI] [PubMed] [Google Scholar]
- Fietzek P. P., Rexrodt F. W., Wendt P., Stark M., Kühn K. The covalent structure of collagen. Amino-acid sequence of peptide 1-CB6-C2. Eur J Biochem. 1972 Oct 17;30(1):163–168. doi: 10.1111/j.1432-1033.1972.tb02083.x. [DOI] [PubMed] [Google Scholar]
- Fowlkes D. M., Shenk T. Transcriptional control regions of the adenovirus VAI RNA gene. Cell. 1980 Nov;22(2 Pt 2):405–413. doi: 10.1016/0092-8674(80)90351-7. [DOI] [PubMed] [Google Scholar]
- Franchini G., Even J., Sherr C. J., Wong-Staal F. onc sequences (v-fes) of Snyder-Theilen feline sarcoma virus are derived from noncontiguous regions of a cat cellular gene (c-fes). Nature. 1981 Mar 12;290(5802):154–157. doi: 10.1038/290154a0. [DOI] [PubMed] [Google Scholar]
- Fuller F., Boedtker H. Sequence determination and analysis of the 3' region of chicken pro-alpha 1(I) and pro-alpha 2(I) collagen messenger ribonucleic acids including the carboxy-terminal propeptide sequences. Biochemistry. 1981 Feb 17;20(4):996–1006. doi: 10.1021/bi00507a054. [DOI] [PubMed] [Google Scholar]
- Galli G., Hofstetter H., Birnstiel M. L. Two conserved sequence blocks within eukaryotic tRNA genes are major promoter elements. Nature. 1981 Dec 17;294(5842):626–631. doi: 10.1038/294626a0. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Harada F., Kato N. Nucleotide sequences of 4.5S RNAs associated with poly(A)-containing RNAs of mouse and hamster cells. Nucleic Acids Res. 1980 Mar 25;8(6):1273–1285. doi: 10.1093/nar/8.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Jelinek W. R. Low molecular weight RNAs transcribed in vitro by RNA polymerase III from Alu-type dispersed repeats in Chinese hamster DNA are also found in vivo. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6130–6134. doi: 10.1073/pnas.78.10.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Toomey T. P., Leinwand L., Jelinek W. R. The Chinese hamster Alu-equivalent sequence: a conserved highly repetitious, interspersed deoxyribonucleic acid sequence in mammals has a structure suggestive of a transposable element. Mol Cell Biol. 1981 Jul;1(7):573–583. doi: 10.1128/mcb.1.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter H., Kressman A., Birnstiel M. L. A split promoter for a eucaryotic tRNA gene. Cell. 1981 May;24(2):573–585. doi: 10.1016/0092-8674(81)90348-2. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Hieter P. A., McBride O. W., Swan D., Leder P. Processed genes: a dispersed human immunoglobulin gene bearing evidence of RNA-type processing. Nature. 1982 Mar 25;296(5855):321–325. doi: 10.1038/296321a0. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P., Forget B. G., Weissman S. M. Short interspersed repetitive DNA elements in eucaryotes: transposable DNA elements generated by reverse transcription of RNA pol III transcripts? Cell. 1981 Oct;26(2 Pt 2):141–142. doi: 10.1016/0092-8674(81)90296-8. [DOI] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Martin G. R., Klebe R. J., Fietzek P. P., Woolley D. E. Localization of the binding site for cell attachment in the alpha1(I) chain of collagen. J Biol Chem. 1978 Aug 25;253(16):5642–5646. [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazowska J., Jacq C., Slonimski P. P. Sequence of introns and flanking exons in wild-type and box3 mutants of cytochrome b reveals an interlaced splicing protein coded by an intron. Cell. 1980 Nov;22(2 Pt 2):333–348. doi: 10.1016/0092-8674(80)90344-x. [DOI] [PubMed] [Google Scholar]
- Leder A., Swan D., Ruddle F., D'Eustachio P., Leder P. Dispersion of alpha-like globin genes of the mouse to three different chromosomes. Nature. 1981 Sep 17;293(5829):196–200. doi: 10.1038/293196a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Monson J. M., McCarthy B. J. Identification of a Balb/c mouse pro alpha 1(I) procollagen gene: evidence for insertions or deletions in gene coding sequences. DNA. 1981;1(1):59–69. doi: 10.1089/dna.1.1981.1.59. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder A., Leder P. Unusual alpha-globin-like gene that has cleanly lost both globin intervening sequences. Proc Natl Acad Sci U S A. 1980 May;77(5):2806–2809. doi: 10.1073/pnas.77.5.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page G. S., Smith S., Goodman H. M. DNA sequence of the rat growth hormone gene: location of the 5' terminus of the growth hormone mRNA and identification of an internal transposon-like element. Nucleic Acids Res. 1981 May 11;9(9):2087–2104. doi: 10.1093/nar/9.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M. P., Boyd C. D., Tolstoshev P., Crystal R. G. Structural organization of a 17 KB segment of the alpha 2 collagen gene: evaluation by R loop mapping. Nucleic Acids Res. 1980 May 24;8(10):2241–2253. doi: 10.1093/nar/8.10.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Leder A., Nau M., Norman B., Leder P. Antibody diversity. Science. 1978 Oct 6;202(4363):11–17. doi: 10.1126/science.99815. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sánchez F., Natzle J. E., Cleveland D. W., Kirschner M. W., McCarthy B. J. A dispersed multigene family encoding tubulin in Drosophila melanogaster. Cell. 1980 Dec;22(3):845–854. doi: 10.1016/0092-8674(80)90561-9. [DOI] [PubMed] [Google Scholar]
- Temin H. M. Origin of retroviruses from cellular moveable genetic elements. Cell. 1980 Oct;21(3):599–600. doi: 10.1016/0092-8674(80)90420-1. [DOI] [PubMed] [Google Scholar]
- Van Arsdell S. W., Denison R. A., Bernstein L. B., Weiner A. M., Manser T., Gesteland R. F. Direct repeats flank three small nuclear RNA pseudogenes in the human genome. Cell. 1981 Oct;26(1 Pt 1):11–17. doi: 10.1016/0092-8674(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Vanin E. F., Goldberg G. I., Tucker P. W., Smithies O. A mouse alpha-globin-related pseudogene lacking intervening sequences. Nature. 1980 Jul 17;286(5770):222–226. doi: 10.1038/286222a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt P., von der Mark K., Rexrodt F., Kühn K. The covalent structure of collagen. The amino-acid sequence of the 112-residues. Amino-terminal part of peptide 1-CB6 from calf-skin collagen. Eur J Biochem. 1972 Oct 17;30(1):169–183. doi: 10.1111/j.1432-1033.1972.tb02084.x. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cripe T. P., Gwo-Shu Lee M., Cowan N. J. Evidence that a human beta-tubulin pseudogene is derived from its corresponding mRNA. Nature. 1982 May 6;297(5861):83–84. doi: 10.1038/297083a0. [DOI] [PubMed] [Google Scholar]
- Wozney J., Hanahan D., Tate V., Boedtker H., Doty P. Structure of the pro alpha 2 (I) collagen gene. Nature. 1981 Nov 12;294(5837):129–135. doi: 10.1038/294129a0. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Avvedimento V. E., Mudryj M., Ohkubo H., Vogeli G., Irani M., Pastan I., de Crombrugghe B. The collagen gene: evidence for its evolutinary assembly by amplification of a DNA segment containing an exon of 54 bp. Cell. 1980 Dec;22(3):887–892. doi: 10.1016/0092-8674(80)90565-6. [DOI] [PubMed] [Google Scholar]