The brain is unique among virtually all somatic organs in its lack of a conventional lymphatic vasculature1–3. In the periphery, the lymphatic circulation facilitates the clearance of extracellular proteins and excess fluid from the interstitium, a role critical to tissue homeostasis and function4, 5. Yet within the brain, despite its complex architecture and high metabolic activity, and neural cells’ sensitivity to changes in the extracellular environment, no specialized organ-wide anatomic structure has yet been identified that facilitates the efficient ‘lymphatic’ clearance of extracellular solutes and fluid from the brain parenchyma.
Current understanding of brain insterstitial solute clearance
For small molecules and hydrophobic compounds, efflux across the blood brain barrier (BBB) is relatively unrestricted. Molecules that are substrates for specific BBB transporters are also readily cleared from the brain6, 7. Other compounds must be cleared from the brain interstitium to the cerebrospinal fluid (CSF) compartment, where they are ultimately eliminated to the blood stream via arachnoid granulations or to peripheral lymphatics along cranial nerves1, 8, 9. However, the distances between much of the brain tissue and the CSF compartments are too great for efficient clearance by simple diffusion, particularly for large molecules (such as peptides and proteins) with low diffusion coefficients6. Rather, the clearance of these interstitial solutes from the brain is attributed to bulk flow, by which convective currents of interstitial fluid (ISF) ‘sweep’ solutes along at a high rate that is largely independent of molecular size1, 2, 6, 7.
In a controversial series of studies, Grady and colleagues10, 11 suggested that brain ISF may exchange with CSF along ‘paravascular’ routes surrounding cerebral blood vessels. As these findings appeared to be subsequently refuted by Cserr and colleagues12, 13, such ‘retrograde’ movement of CSF into the brain parenchyma is now thought to be of comparatively minor physiological importance1. However, if a substantial amount of CSF moves through the brain interstitium, and if this flux occurs along defined anatomical pathways, this would fundamentally alter our understanding of how CSF facilitates the clearance of interstitial solutes and metabolic wastes from the brain.
The glymphatic pathway: a paravascular pathway for interstitial solute clearance
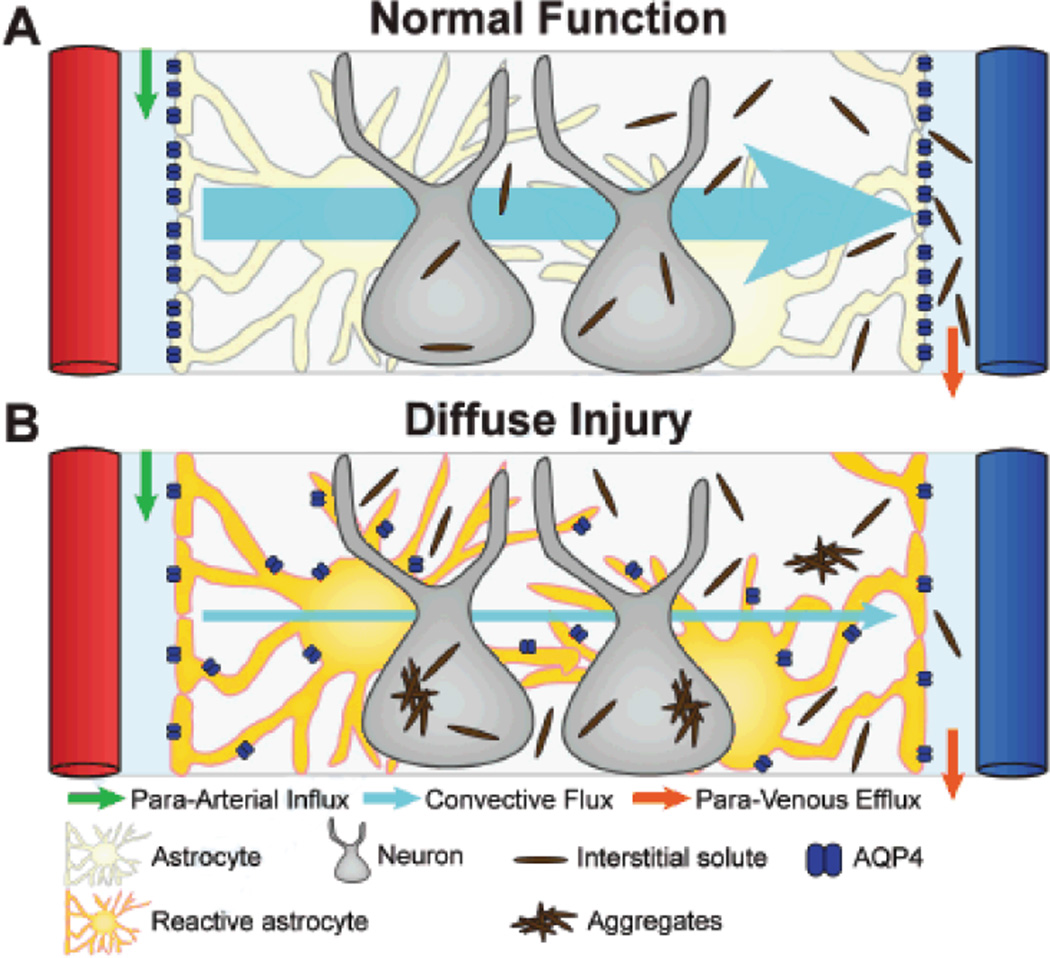
In a recent study14 we define for the first time a brain-wide anatomical pathway that facilitates the exchange of CSF and ISF and the clearance of interstitial solutes from the brain. This pathway consists of 3 elements: a para-arterial CSF influx route, a para-venous ISF clearance route, and a trans-parenchymal pathway that is dependent upon astroglial water transport via the astrocytic aquaporin-4 (AQP4) water channel (represented in schematic form in Figure 1A).
Figure 1. Schematic of glymphatic pathway function in normal and diseased brain.
Conceptual framework for the failure of glymphatic interstitial solute clearance after diffuse injury. (A) In the healthy brain, CSF from the subarachnoid space rapidly enters the brain along paravascular channels surrounding penetrating arteries (green arrow) and exchanges with brain ISF. ISF and solutes are cleared to paravascular spaces surrounding large caliber draining veins (orange arrows). Convective bulk fluid flux between the paravascular CSF influx and ISF efflux pathways is facilitated by astroglial water transport through AQP4 expressed exclusively along perivascular astrocytic endfeet. This convective bulk flow facilitates the clearance of interstitial solutes from the brain. (B) Reactive astrogliosis that occurs after diffuse injury such as microinfarction or mild traumatic brain injury causes in the mis-localization of AQP4 from the perivascular endfeet to the rest of the astrocytic soma. This results in the loss of efficient interstitial bulk flow, and the failure of glymphatic interstitial solute clearance and may contribute to the deposition of extracellular and intracellular protein aggregates (such as amyloid β or tau) after diffuse injury.
Using in vivo 2-photon and ex vivo confocal imaging of small molecular weight fluorescent CSF tracers, we found that a large proportion (>40%14) of subarachnoid CSF rapidly enters the brain parenchyma along paravascular spaces surrounding penetrating arteries throughout the brain. CSF tracer entered the brain initially through the Virchow-Robin space, then followed arterial vascular smooth muscle basement membrane to reach the basal lamina of the brain capillary bed. At all levels of this paravascular route, CSF tracer entered into the interstitial space, reflecting the exchange of CSF and ISF14. Para-arterial CSF influx extended throughout the brain, and appeared to occur along virtually all penetrating arteries. ISF clearance pathways, in contrast, were restricted to a specific group of large caliber draining veins. Fluorescent tracer injected directly into the interstitium of the cortex, striatum or thalamus was cleared medially to the internal cerebral veins and great vein of Galen and ventro-laterally to the caudal rhinal vein14.
The astroglial AQP4 water channel is expressed in a highly polarized manner in perivascular astrocytic endfeet that immediately bound these paravascular CSF influx and ISF clearance pathways (Figure 1A, 2A)15, 16. We proposed that these perivascular water channels may facilitate the convective bulk flow of fluid from the para-arterial CSF influx pathway through the interstitium, and along the para-venous clearance route. To test this, we evaluated paravascular CSF influx in global Aqp4 knockout mice by both in vivo 2-photon and ex vivo fluorescence imaging. Compared to wild type controls, CSF influx into and through the parenchyma of Aqp4-null mice was dramatically reduced14. Similarly, when we evaluated the rate of interstitial solute clearance from the brain using a radio-tracer clearance assay, we found that interstitial solute clearance was reduced by ~70% in Aqp4-null mice. As detailed in our recent study14, these findings demonstrate that AQP4-dependent bulk flow couples CSF influx along the para-arterial pathway to ISF clearance along the para-venous route, forming an organ-wide system that facilitates the clearance of interstitial solutes from the brain parenchyma. Based upon this glial dependence and the functional and structural homology to the peripheral lymphatic system, we have termed this glio-vascular pathway the ‘glymphatic’ system (Figure 1A).
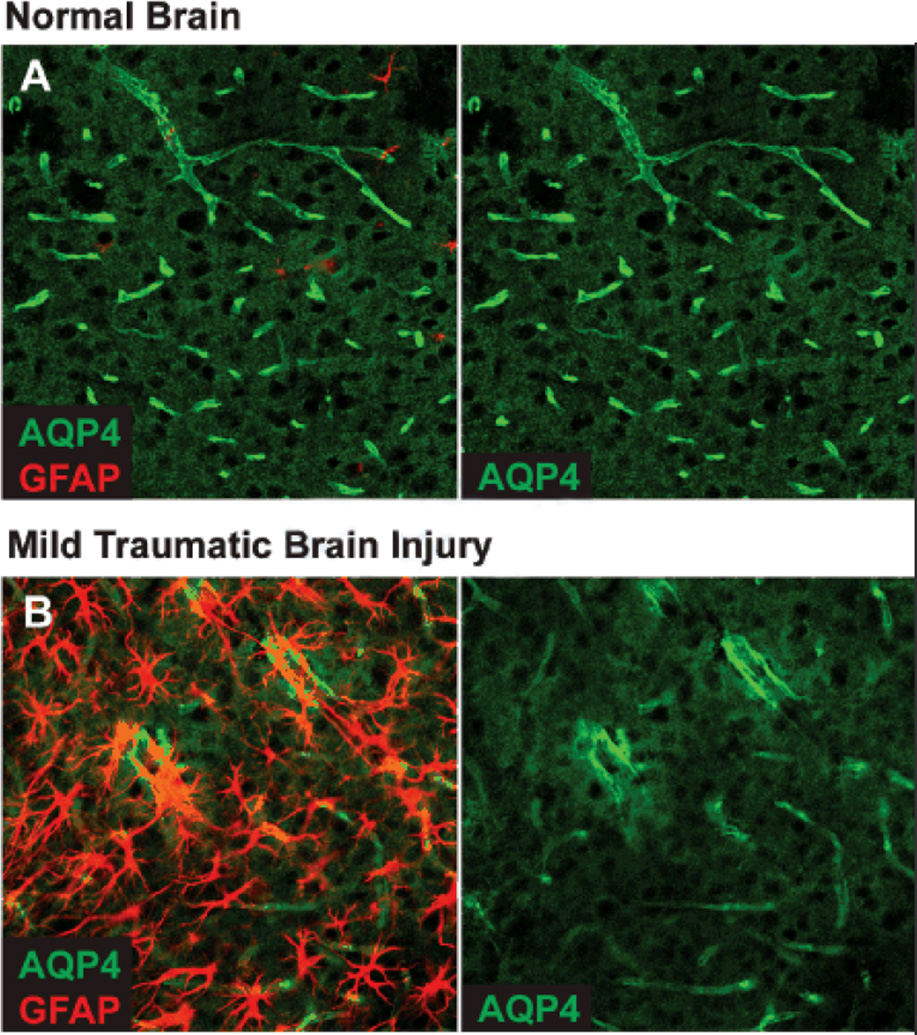
Figure 2. Changes in AQP4 localization after diffuse injury.
(A) Immunofluorescent double-labeling demonstrates that in the healthy young mouse brain, AQP4 expression is highly localized to perivascular astrocytic endfeet surrounding to entirety of the cerebral microvasculature. (B) 7 days after mild traumatic brain injury, widespread reactive astrogliosis (GFAP-immunoreactivity) is observed throughout the ipsilateral cortex. In regions of reactive astrogliosis, AQP4 localization is severely perturbed, exhibiting a loss of polarization to the endfoot process and increased somal labeling. Similar expression patters are observed after diffuse microinfarction (manuscript under review).
Soluble amyloid β is (Aβ) present in the ISF of the healthy young brain and the failure of Aβ clearance is thought to underlie the deposition of Aβ plaques associated with Alzheimer’s disease progression10,11. We next evaluated whether soluble Aβ constitutes one of the solutes cleared from the brain interstitium along the glymphatic pathway. When fluorescently-labeled Aβ was injected into the cortex or striatum, it accumulated around the same paravascular pathways observed with other fluorescent tracers14. We also measured the clearance of radio-labeled Aβ injected directly into the striatum of wild type and Aqp4-null mice. In Aqp4-null mice, radio-labeled Aβ clearance was reduced by ~65% compared to wild type animals, suggesting that AQP4-dependent bulk flow along the glymphatic pathway constitutes a key mechanism of clearance of soluble Aβ from the brain interstitium14.
The effect of diffuse gliotic injury on glymphatic pathway function
Reactive astrogliosis is a cellular response to injury common to many mechanistically distinct forms of brain injury, including ischemic and traumatic brain injury, and characterized by changes in astrocyte morphology and molecular expression patterns17–19. While more severe ischemic and traumatic brain injury is accompanied by glial scar formation, low-intensity injury frequently results in diffuse and long-lasting reactive astrogliosis. This is reflected in two recent studies from our group. In a mouse model of diffuse microinfarction exhibiting only low-level aggregate ischemic burden, widespread reactive gliosis was evident throughout the cortex and striatum for up to a month post-injury (manuscript under review). Similarly, in a mild traumatic brain injury model, widespread cortical and subcortical reactive gliosis was evident for at least one month post-injury in the absence of frank tissue destruction (manuscript under review).
Changes in AQP4 expression are often observed in conjunction with reactive astrogliosis. After ischemic or traumatic brain injury20, 21, AQP4 expression is typically elevated. As these studies employ moderate to severe ischemic and traumatic brain injury, much of this may be attributable to altered AQP4 expression within the glial scar. In our own studies of microinfarction (manuscript under review) and mild traumatic brain injury, changes in AQP4 expression within regions of diffuse reactive gliosis are more complex. General AQP4 expression is elevated in gliotic regions 7 days after diffuse microinfarction, but normalizes by 14 days post-injury. The distribution of AQP4 expression, however, remains perturbed for at least 1 month post-injury. Rather than the highly polarized perivascular localization observed in normal brain, AQP4 in reactive astrocytes exhibits a marked reduction in polarity, with lower perivascular APQ4 immunoreactivity and higher somal AQP4 immunoreactivity. Similar patterns of AQP4 dysregulation are also observed in reactive astrocytes following mild traumatic brain injury (Figure 2B).
In light of the critical role that perivascular AQP4 plays in the glymphatic clearance of interstitial solutes, including soluble Aβ14, changes in AQP4 localization following diffuse injury may have critical implications for the pathogenesis of conditions such as vascular dementia and traumatic brain injury. We propose that mis-localization of AQP4 from the perivascular endfeet to the astrocytic soma prevents the efficient directional flux of water into and out of the paravascular spaces that contribute to interstitial solute clearance (Figure 1B). This may cause the widespread failure of waste clearance from the diffusely gliotic brain tissue, resulting in the accumulation of neurotoxic metabolites such as Aβ, in addition to the extracellular and intracellular cytotoxic protein aggregates that are the hallmark of neurodegenerative diseases such as Alzheimer’s disease and chronic traumatic encephalopathy. In this way, reactive gliosis, through its detrimental effects on interstitial waste clearance, may be a key driver of pathology under conditions of diffuse ischemic or traumatic brain injury, and may represent a key target for therapeutic intervention.
Acknowledgments
Sources of Funding: This work was supported by the NIH (JI, MN), American Heart Association (JI), Department of Defense (MN), and the Harold and Leila Y. Mathers Charitable Foundation (MN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
References
- 1.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: Significance for physiology and pathology. Neurochemistry international. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240:F319–F328. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 3.Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain pathology. 1992;2:269–276. doi: 10.1111/j.1750-3639.1992.tb00703.x. [DOI] [PubMed] [Google Scholar]
- 4.Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiological reviews. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Schmid-Schonbein GW. Microlymphatics and lymph flow. Physiol Rev. 1990;70:987–1028. doi: 10.1152/physrev.1990.70.4.987. [DOI] [PubMed] [Google Scholar]
- 6.Sykova E, Nicholson C. Diffusion in brain extracellular space. Physiological reviews. 2008;88:1277–1340. doi: 10.1152/physrev.00027.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Groothuis DR, Vavra MW, Schlageter KE, Kang EW, Itskovich AC, Hertzler S, et al. Efflux of drugs and solutes from brain: The interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J Cereb Blood Flow Metab. 2007;27:43–56. doi: 10.1038/sj.jcbfm.9600315. [DOI] [PubMed] [Google Scholar]
- 8.Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: Is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal fluid research. 2005;2:6. doi: 10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Praetorius J. Water and solute secretion by the choroid plexus. Pflugers Arch. 2007;454:1–18. doi: 10.1007/s00424-006-0170-6. [DOI] [PubMed] [Google Scholar]
- 10.Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Advances in neurology. 1990;52:431–439. [PubMed] [Google Scholar]
- 11.Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a 'paravascular' fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain research. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 12.Ichimura T, Fraser PA, Cserr HF. Distribution of extracellular tracers in perivascular spaces of the rat brain. Brain research. 1991;545:103–113. doi: 10.1016/0006-8993(91)91275-6. [DOI] [PubMed] [Google Scholar]
- 13.Pullen RG, DePasquale M, Cserr HF. Bulk flow of cerebrospinal fluid into brain in response to acute hyperosmolality. The American journal of physiology. 1987;253:F538–F545. doi: 10.1152/ajprenal.1987.253.3.F538. [DOI] [PubMed] [Google Scholar]
- 14.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates csf flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Science translational medicine. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin 4 in rat brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3d reconstruction. Glia. 2010;58:1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 17.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 18.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends in neurosciences. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verkhratsky A, Sofroniew MV, Messing A, deLanerolle NC, Rempe D, Rodriguez JJ, et al. Neurological diseases as primary gliopathies: A reassessment of neurocentrism. ASN Neuro. 2012:4. doi: 10.1042/AN20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal CJ, Lee EY, Gyorgy A, Ecklund JM, Agoston DV, Ling GS. Effect of penetrating brain injury on aquaporin4 expression using a rat model. Journal of neurotrauma. 2007;24:1609–1617. doi: 10.1089/neu.2007.0301. [DOI] [PubMed] [Google Scholar]
- 21.Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM. Activation of p2×7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PloS one. 2012;7:e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]