Abstract
Background
Eosinophilic esophagitis (EoE) is a clinicopathologic entity of increasing worldwide prevalence. IL-5 is essential for eosinophil trafficking and anti-IL-5 therapy decreases esophageal eosinophilia. EoE is associated with a prominent mast cell infiltration.
Objective
We investigated whether anti-IL-5 (mepolizumab) treatment reduced esophageal mast cell accumulation in pediatric EoE biopsy specimens from a previous randomized anti-IL-5 trial.
Methods
A sub-analysis was completed for children treated with 0.55, 2.5, or 10mg/kg of mepolizumab monthly for 12 weeks followed by no treatment until week 24. Quantitative immunochemistry was used to assess eosinophils, tryptase-positive mast cells, IL-9+ cells, and mast cell-eosinophil couplets prior to and following treatment.
Results
43 patient biopsies had adequate tissue for paired analysis. 40% of subjects responded to anti-IL-5 (defined as <15 eosinophils per hpf following mepolizumab therapy) and 77% of all subjects had decreased numbers of mast cells following anti-IL-5. In responders, epithelial mast cells decreased from 62 to 19 per hpf (p<0.001), were significantly lower than in non-responders following therapy (p<0.05), and correlated with eosinophil numbers (r=0.75, p<0.0001). Mast cells and eosinophils were found in couplets prior to therapy and these were significantly decreased only in responders following anti-IL-5 (p<0.001). Esophageal eosinophils comprised the majority of cells that made the mast cell growth factor IL-9. IL-9+ cells decreased from 102 to 71 per hpf (p<0.001) following anti-IL-5.
Conclusions
Pediatric EoE patients had significantly fewer mast cells, IL-9+ cells, and mast cell-eosinophil couplets in the esophageal epithelium following anti-IL-5 therapy. Since eosinophils were one source of IL-9, they may support esophageal mastocytosis.
Keywords: Eosinophilic esophagitis, pediatric, eosinophils, mast cells, IL-5, IL-9
Introduction
Eosinophilic esophagitis (EoE) is a disease of increasing worldwide prevalence. Diagnostic criteria are an isolated esophageal eosinophilia of ≥ 15 eosinophils per high power field (hpf) on hematoxylin and eosin stain at 400x light microscopy, especially in the context of concurrent acid blockade with proton pump inhibitor therapy (1). Symptoms vary by patient age but vomiting, failure to thrive, abdominal pain, and dysphagia are common (1). Tissue remodeling is likely to be a significant contributing mechanism for strictures, dysmotility, and food impactions (2–5). Successful therapeutic interventions in EoE include elimination diets, topical corticosteroids, and amino acid formulas (6–10).
IL-5 is a pivotal cytokine for eosinophilopoesis and trafficking to target organs (11). Disease modifying effects have been seen using anti-IL-5 treatment in adult patients with eosinophilic asthma, chronic rhinosinusitis, Churg Strauss Syndrome, and the hypereosinophilic syndrome (HES) (12–15). Animal models of aeroallergen induced EoE demonstrate that IL-5 is required for eosinophilopoesis and trafficking into the esophagus (11). Recent human studies have demonstrated that anti-IL-5 (mepolizumab) can reduce eosinophil counts in the esophagus by 54% in adult patients with EoE (16). A randomized trial in children treated with mepolizumab demonstrated that 32% of subjects had residual esophageal eosinophilia of <20 eosinophils per high power field (hpf) and that 8.8% had complete eosinophil resolution following therapy (17). Similarly, a randomized controlled trial of reslizumab showed an up to 67% reduction in esophageal eosinophilia after treatment (18).
Both eosinophils and mast cells play a role in EoE pathogenesis. A specific mast cell gene signature is found in both adult and pediatric EoE (19, 20). In addition, mast cell and eosinophil products such as TGFβ1 can increase esophageal smooth muscle contractility (4). However, a single adult study of EoE did not find any decreases in the numbers of esophageal mast cells following anti-IL-5 therapy at doses of 750 and 1500 mg monthly (16). Although cultured mast cells derived from cord blood progenitors and bone marrow mast cells from subjects with systemic mastocytosis and HES express the IL-5 receptor, IL-5 is considered a relatively eosinophil-specific cytokine (22–24). As such, anti-IL-5 effects on mast cells are likely largely indirect; for example, via a reduction of mast cell growth factors from eosinophils.
In this study, we utilized biopsy specimens from a previously completed 3-arm anti-IL-5 trial in order to investigate the effects of anti-IL-5 on esophageal mastocytosis in pediatric EoE patients (17). We report that anti-IL-5 significantly reduced the numbers of tryptase positive cells in the esophageal epithelium. We demonstrate that this effect was most pronounced in responders to anti-IL-5 (defined as <15 eosinophils per hpf following therapy) and that mast cell numbers increased at week 24. Mast cells and eosinophils were found in close proximity before therapy but these couplets were significantly decreased in responders at week 12. Double immunofluorescence demonstrated that eosinophils made IL-9 in the esophagus and IL-9 expressing cells were diminished following mepolizumab treatment. As such, eosinophils may be important instigators and/or propagators of esophageal mastocytosis in EoE.
Methods
Human Subjects
Anonymized esophageal biopsies archived during a 3 active-arm, multicenter clinical trial of mepolizumab in pediatric EoE (MEE103219, clinical trials. gov number NCT00358449) Aceves, PI UCSD site) were obtained from GlaxoSmithKline (17). Esophageal biopsies from 43 of the original 57 patients were studied. In this clinical trial, subjects were given monthly doses of anti-IL-5 for 3 months followed by 3 months without therapy. Endoscopy with biopsy was done at weeks 12 and 24. Only paired tissue samples were studied (n=43 for weeks 0 and 12, n=41 of these 43 at week 24). 54 biopsies were obtained from GlaxoSmithKline. 11 were excluded as 8 biopsies did not have adequate esophageal tissue in paired specimens prior to and following therapy, and 3 biopsy specimens no longer met the initial clinical trial diagnostic criteria of ≥20 eosinophils per hpf. The original study and this post-hoc analysis were approved by the Institutional Review Board of the University of California, San Diego.
Immunostaining
Archived, formalin-fixed, paraffin-blocked esophageal biopsy specimens were used for all of the studies. All subjects (n=43) had 0 and 12 week biopsies, 41 of these 43 had 24 week biopsies with adequate tissue for analysis (3–6 evaluable hpf). In total, 127 biopsy specimens were analyzed per stain unless otherwise indicated. IL-9-MBP double immunofluorescence could be completed on a subset of 6 subjects pre- and post-therapy due to limited adequate residual tissue. These subjects had IL-9 positive cells and eosinophils at similar levels to that detected in single stained slides. Residual LP was not present for analysis in this subset of biopises. Biopsies were immunostained using the Vectastain ABC system (Vector Laboratories, Burlingame, Calif) or Alexa immunofluorescence as previously described (2). Esophageal biopsy specimens were incubated with primary antibodies specific for human tryptase (1:150ab2378Abcam, Boston, Mass, for 1 hour at room temperature), IL-9 (1:100sc46654, Santa Cruz Biochemicals, Santa Cruz, California, overnight at 4°C), or major basic protein (1:50BMK13, MA135360, Thermoscientific, overnight at 4°C) and detected using the appropriate species of biotinylated or fluorescence conjugated secondary antibodies. All immunohistochemical slides were counterstained with hematoxylin (Fischer Scientific, Fair Lawn, New Jersey) prior to quantitation and immunofluorescence slides were stained with DAPI.
Histologic Evaluation
The peak number of tryptase-positive mast cells, IL9-positive cells, and eosinophil-mast cell couplets defined as mast cells found within ≤ 3.4 microns (1/2 of the cell length of an average tryptase positive cell) of an eosinophil were counted in 3–6 separate hpf in the epithelium at x400 magnification by an observer blinded to therapy. Results are expressed as the peak mean number of cells per hpf. Image capture and analysis was done using ImagePro (MediaCybernetics, Bethesda MD). Biopsies were scored using our previously reported histology scoring tool (10). Degranulation was defined as absent (0) or present (1) by the presence of granules outside of the eosinophil cytoplasm.
Statistics
All statistical analyses and graphing were carried out using GraphPad Prism (San Diego, CA) software. Comparisons between cell counts of any two groups were made using a Mann Whitney test for unpaired variables. Pre-post analysis was done using a Wilcoxon test for paired variables. Comparisons between 3 groups was done using a one-way ANOVA. Correlations between groups were determined by generating Spearman correlation coefficients. A two tailed p value <0.05 was considered statistically significant.
Results
Anti-IL-5 and esophageal eosinophils, eosinophil degranulation, eosinophil clusters
For this post-hoc analysis, we re-analyzed the numbers of eosinophils and eosinophil (degranulation/clusters) and epithelial characteristics. Although these features were previously reported for the original cohort (17), this re-analysis was critical for accurately analyzing the relationship between the eosinophils and mast cells in the post-hoc study. All of the subjects in this study had paired biopsy specimens available at weeks 0 and 12 and 41 had week 24 biopsies with adequate tissue. Overall, the peak numbers of eosinophils in the esophagus decreased in 86% of subjects from a median of 79 (mean=92) prior to anti-IL-5 therapy to 18 (mean=30) per hpf following therapy (p<0.0001). For the purposes of this study, “response” was defined as <15 esophageal epithelial eosinophils per hpf following 3 infusions of intravenous anti-IL-5 (mepolizumab). Among the anti-IL-5 responder group (40%, n=17), eosinophils decreased from a median of 74 (mean=93, 95% CI 62, 124) to 7 (mean=7, 95% CI 5, 9) per hpf (p<0.0001). Subjects with ≥ 15 eosinophils per hpf following therapy (defined as “non-responders”, n=26) also had reduced esophageal eosinophils from 83 per hpf (mean=91, 95% CI 73, 109) to 31 per hpf (mean=44, 95% CI 32, 56) (p<0.0001) (Figure 1a). The residual numbers of eosinophils at week 12 was significantly lower in the responder than in the non-responder group (p<0.0001) (Fig 1a). Eosinophils were decreased in children in every dosing group at 12 weeks and increased at week 24 (Figures 1a,b). LP eosinophil numbers did not decrease significantly in either responder (pre-therapy mean = 16, 95% CI -5, 38; post-therapy mean = 7, 95% CI -2, 16, n=6, p = 0.5) or non-responder groups (pre-therapy mean = 10, 95% CI 4, 15; post-therapy mean = 9, 95% CI 4, 15, n=21, p=0.5) but due to the small numbers of responders with adequate LP for evaluation this should be interpreted cautiously.
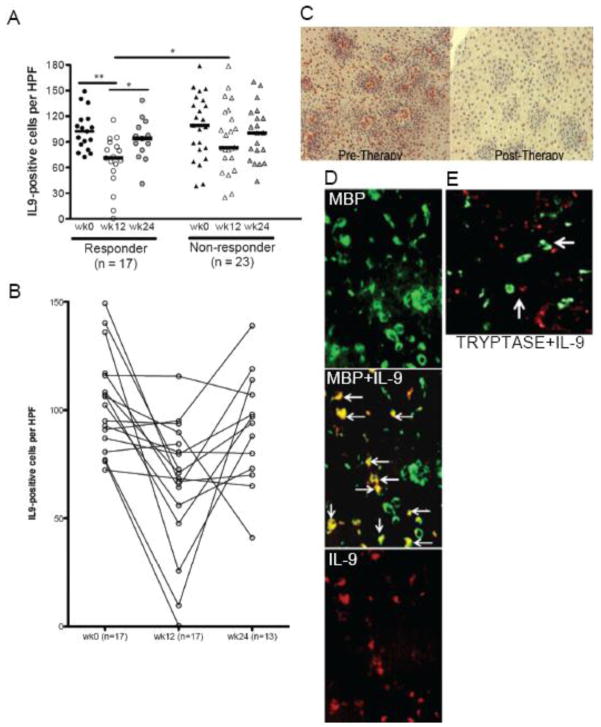
Figure 1. Anti-IL-5 decreases esophageal eosinophils.
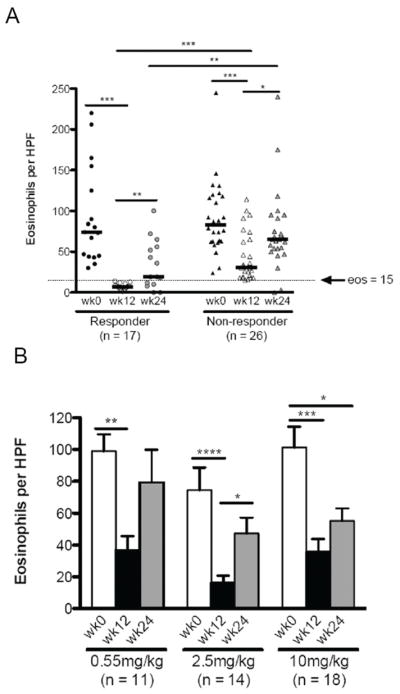
Peak eosinophils prior to and following therapy at weeks 0, 12, 24 by response category (A) and by dose (B). Responders: <15 eosinophils per high power field at week 12. Bars represent median eosinophil counts (A) and mean with standard error (B). A: *p<0.01, **p<0.001, ***p<0.0001. B: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
The eosinophil degranulation score (0=absent, 1=present) decreased from a median of 1 (mean=1) to 0 (mean=0.28) (p<0.0001) only in anti-IL-5 responders. The presence of eosinophil clusters was improved in both responder and non-responder groups (mean score 0.44 and 0.58 prior to therapy, 0 and 0.27 post-therapy in responders and non-responders, respectively). Both eosinophil features were significantly lower post-therapy in responders than in non-responders (p<0.0001 and p<0.05 for degranulation and clusters, respectively).
Anti-IL-5 and esophageal epithelial tryptase positive and lamina propria mast cells
In the overall group, mast cells were diminished in 33/43 (77%) of patients (Figure 2a). Among the responders (n=17), mast cell numbers decreased from a median of 62 per hpf (mean=64, 95% CI 49, 79) to 19 per hpf (mean=25, 95% CI 16, 35) following therapy (p=0.0002) (Figure 2b). By comparison, the numbers of lamina propria mast cells remained static in both responder (median 7 and 10 per hpf prior to and following therapy) and non-responder (7 and 9 per hpf prior to and following post-therapy) groups (data not shown). Mast cells decreased following anti-IL-5 therapy in the responder group and the 2.5mg/kg group (Figures 2a,b).
Figure 2. Anti-IL-5 decreases esophageal mast cells.
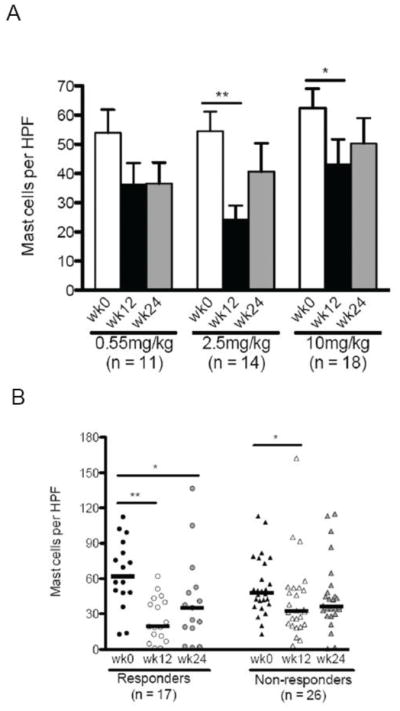
Peak mast cells by dose (A) and response (B) at weeks 0, 12, 24. Responders: <15 eosinophils per high power field (hpf) at week 12. Bars represent mean with standard error (A) and medians (B). A: *p<0.05, **p<0.001. B: *p<0.05, **p<0.001
Anti-IL-5 and mast cell-eosinophil couplets
The presence of mast cell-eosinophil couplets has been demonstrated in allergic rhinitis patients and mast cell-eosinophil direct contact is a likely mechanism by which mutual pro-survival factors are released (23). We utilized double immunofluorescence to assess the presence of and quantitate the numbers of eosinophil-mast cell couplets (Fig 3a). We defined eosinophil-mast cell couplets as a mast cell found within a distance of 3.4 microns from an eosinophil. We analyzed all biopsy specimens with adequate tissue at 0, 12, and 24 weeks of therapy for mast cell-eosinophil couplets. Responders (n=16) had a mean of 6.4 and 1.0 couplets per hpf prior to and following therapy whereas non-responders had a mean of 4 and 2 couplets per hpf prior to following therapy (p<0.001 for responders only) (Fig 3b). At 24 weeks, the non-responders had 3.7 couplets while the responders had 1 couplet per hpf (Fig 3b). The numbers of couplets in the LP were low and did not change significantly in the small numbers of responders or non-responders with adequate LP for analysis (n=12, data not shown). Couplets increased in the epithelium at week 24 in the non-responders only (Fig 3b). Consistent with the concept that mast cells and eosinophils may be in proximity and important for mutual survival/activation, the numbers of eosinophils correlated with the numbers of mast cells in the esophagus of pediatric EoE patients who responded to anti-IL-5 treatment (r=0.75, p<0.0001, supplemental Figure 1). Re-evaluating the correlation between eosinophils and mast cells when not counting eosinophils within an eosinophil cluster demonstrated similar results (r=0.74, p<0.0001). By evaluating mast cell counts at varying eosinophil numbers at week 12, it was clear that there was a close relationship between mast cell and eosinophil numbers (supplemental Figure 1).
Figure 3. Anti-IL-5 decreases eosinophil-mast cell couplets.
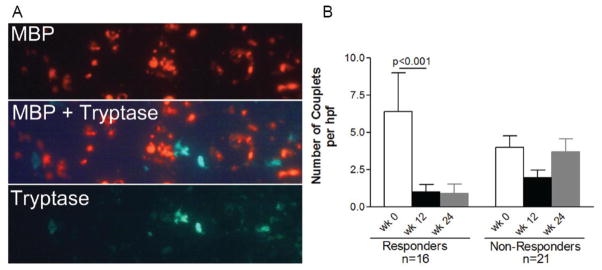
Representative image showing that eosinophils and mast cells are found in close proximity in the esophagus of eosinophilic esophagitis subjects (major basic protein (MBP)=red, tryptase=green) (A). Numbers of eosinophil-mast cell couplets prior to and following anti-IL-5 therapy by response at weeks 0, 12, and 24 (B). Bars represent mean with standard error.
Anti-IL-5 and epithelial IL-9 positive cells
We were interested in determining if there was a potential indirect, eosinophil-dependent mechanism by which mast cells would be decreased in pediatric EoE subjects following anti-IL-5 therapy. IL-9 is a well-described mast cell growth and survival factor which is expressed by eosinophils and other cell types. In the overall group the number of IL-9 positive cells decreased from a median of 107 (baseline) to 80 (week 12) per hpf (p<0.01). The change in IL-9 positive cells was not dose dependent. There was a significant decrease in IL-9 positive cells per hpf in the epithelium of responder patients following anti-IL-5 therapy (prior to therapy median=102, mean=103, 95% CI 91, 115; following therapy median=71, mean=66, 95% CI 50, 82) (p=0.0005) (Figure 4a-c). In addition there was a significant difference between week 12 IL-9 positive cells in responders as compared with non-responders following therapy (p=0.02) (Figure 4a,c). Individually, all but 2 responders had decreased IL-9 positive cell counts per hpf post-therapy (Fig 4b). The numbers of IL-9 positive cells increased at week 24 to 93 per hpf in the responders (Figure 4b).
Figure 4. Anti-IL-5 decreases IL-9 positive cells.
Peak IL-9 positive cells by response at weeks 0, 12, 24 (A) Bars: median number of IL-9+ cells (*p<0.05, **p<0.001). Individual IL-9 counts among responders (B). Representative images of IL-9+ cells prior to and following anti-IL-5 (week 12) in a responder (C) and of MBP (eosinophils, green), IL-9 positive cells (red) and overlapping cells (yellow, arrows) (D). Mast cells (tryptase, green) do not make IL-9 (red) Arrows show mast cell and IL-9 cells in proximity (E).
The numbers of IL-9 positive cells correlated positively with the numbers of mast cells (r=0.44, p<0.01) in responders. Double immunofluorescence demonstrated that eosinophils were one but not the only cellular source of IL-9 in EoE (Figure 4d). The numbers of eosinophils correlated positively but not strongly with IL-9 positive cells (r=0.34, p<0.01). Using the limited subset of biopsies that had adequate residual tissue (n=12 biopsies, n=6 subjects), double immunofluorescence demonstrated that 70±6% of IL-9 positive cells were eosinophils. There was a significant decrease in the numbers of epithelial IL-9 positive eosinophils and a non-significant trend towards reduction of non-eosinophil IL-9 positive cells following anti-IL-5 (supplemental Figure 2). Tryptase positive cells were not a source of IL-9 (Figure 4e). As such, it appears that eosinophils may be the majority but not the only cells that produce IL-9 in EoE.
Relationship to clinical features
We assessed whether mast cell numbers in the esophagus associated with any of the symptom complex for the EoE subjects. Reported symptoms included stomach pain, chest/throat pain, regurgitation, vomiting, sensation of food stuck in the throat, difficulty drinking, pain with drinking, difficulty eating solid food, and pain when eating solid food. These symptoms were graded on a severity scale from 1–6 in the original clinical trial. Subjects who demonstrated decreases in stomach, chest/throat pain, pain with drinking and eating solids, and/or sensation of food stuck in throat all had statistically significant decrease in the numbers of epithelial mast cells and eosinophils. In the subgroup of patients with a >70% decrease in mast cells, the mast cell numbers correlated with the severity of stomach pain (r=0.56, p<0.05), chest/throat pain (r=0.51, p<0.05), and drinking pain (r=0.60, p<0.05). By contrast, there was no correlation between eosinophil numbers and symptom severity. No subjects were symptom-free at week 12. As such, it is possible that in a subset of subjects the degree of mastocytosis correlates with a subset of clinical symptoms.
Discussion
In this study we have demonstrated a number of novel findings in pediatric EoE patients treated with anti-IL-5. Since IL-5 is considered essentially an eosinophil-specific interleukin, anti-IL-5 therapy offers a unique opportunity to assess the effects of eosinophils on disease pathogenesis in human subjects. To this end, we analyzed biopsy specimens obtained during an international, randomized, blinded, multi-center pediatric 3 dose trial of mepolizumab (17) and found, surprisingly, that pediatric EoE patients had decreased numbers of esophageal epithelial tryptase-positive mast cells following anti-IL-5 therapy. This result was unexpected for a number of reasons. Firstly, adult subjects treated with mepolizumab do not have changes in mast cell numbers following therapy. Secondly, mast cells in target tissues have not been shown to be directly dependent on IL-5 suggesting that the effects of anti-IL-5 would occur indirectly via a cell type that is directly affected by anti-IL-5 therapy. We also show for the first time that both eosinophil and non-eosinophil inflammatory cells express IL-9 in the esophagus of pediatric EoE patients and that IL-9 positive cells decrease following anti-IL-5 treatment. Lastly, we demonstrate the presence of eosinophil-mast cell couplets in pediatric EoE biopsies that decrease following anti-IL-5 therapy. Together, these data suggest that esophageal eosinophils in EoE may be an important component in the initiation and/or maintenance of esophageal mastocytosis. The differences between our pediatric observation of decreased mast cells and that reported in adult subjects treated with mepolizumab could be explained by the lower doses used in the children (doses of 750 to 1500 mg/month used in adults which would correspond to 10–20 mg/kg dose in an average adult), a difference in the anti-inflammatory responses in children who have a shorter disease duration than adults, and/or the numbers of subjects analyzed (43 children as opposed to 5 adults on mepolizumab).
Since decreases in eosinophils correlated directly with decreases in mast cell numbers, since esophageal eosinophils are one cell type that produces IL-9, and since eosinophil-mast cell couplets are present only prior to therapy, eosinophils may be important to recruit, activate, and/or enhance the survival of esophageal mast cells. However, the decrease in mast cells and IL-9 positive cells was incomplete following treatment, even among responder patients. Our data demonstrate that both eosinophil and non-eosinophil inflammatory cells are IL-9 producers in EoE. The majority of IL-9 positive cells appear to be eosinophils and as such the decrease in IL-9 positive cells may be due to, in large part, the direct reduction in eosinophil numbers. In contrast, mast cells do not appear to produce IL-9 in these EoE subjects. In addition, it is likely that other mast cell chemoattractants and growth factors contribute to the genesis and maintenance of esophageal mastocytosis in EoE. We were unable to evaluate the expression of other mast cell growth factors such as IL-3 and GM-CSF due to technical difficulties with the antibodies on the archived specimens. However, an analysis of SCF expression demonstrated no consistent changes in staining pattern following therapy (data not shown).
It has been previously noted that mast cells and eosinophils are found in couplets in the nasal tissue of patients with allergic rhinitis (23). In addition, mast cells and eosinophils can have physical interactions allowing cross-stimulation, with eosinophil products activating mast cells and mast cell derived mediators activating eosinophils (23). Our data show that mast cells and eosinophils tend to cluster together in the esophageal epithelium of chronic pediatric EoE. Interestingly, the pattern of a single mast cell surrounded by multiple eosinophils was common and reminiscent of the complex monolayers formed in co-cultures of eosinophils and mast cells (23). Quantitation of these couplets showed that there were fewer of epithelial couplets in responders but not in non-responders at week 12 of anti-IL-5 therapy. In contrast there were no decreases in eosinophil, mast cell, or couplet numbers in the LP. These data suggests that eosinophil products could play a role in prolonging tryptase positive, mucosal mast cell survival in EoE. A sub-analysis using varying mast cell cut-offs revealed that numbers could correlate with symptoms of pain. These data suggest that in some subjects the severity of mast cell inflammation may correlate with a subset of symptoms.
Cultured mast cells derived from cord blood progenitors and bone marrow derived mast cells from subjects with systemic mastocytosis and HES express IL-5 receptor (21–22, 24). In addition, basophils express tryptase and the IL-5 receptor and there are reports of increased activated basophil populations in the circulation of children with EoE (25). As such, anti-IL-5 could also exert potential direct effects on mast cells and basophils. In addition the effect of IL-9 on IL-5 production in mast cells has not been studied in EoE. It has been shown in murine asthma models that IL-9 deficiency significantly decreases mast cell progenitors in the lungs independently of IL-5 (26) and that IL-9 induces IL-5 receptor expression on cultured eosinophils (27). Thus, it is possible that treatment with anti-IL-5, by decreasing IL-9 producing eosinophils, decreases mast cell survival and that this may have subsequent feedback. These subjects merit further study.
In conclusion, our study suggests that eosinophils have an important role in EoE pathogenesis and are not likely to be innocent bystanders or a disease epiphenomenon in EoE. Pathogenic effects of eosinophils in EoE may be multi-pronged and include the promotion of basal cell proliferation as well as mast cell recruitment and survival. In turn, mast cells can activate eosinophils, have damaging effects on the esophageal epithelium via the production of serine proteases, and alter smooth muscle contraction. The potentially complex interplay between mast cells, eosinophils, and IL-9 warrants further investigation in EoE.
Supplementary Material
Supplemental Figure 1. Eosinophils and mast cells numbers have a close relationship. The percent of subjects with varying decreases in esophageal epithelial eosinophils (clear bars) and the numbers of mast cells (filled bars) in the given eosinophil category. The mean peak numbers of eosinophils and mast cells prior to (pre) and post (post) anti-IL-5 are indicated above the respective bar.
Supplemental Figure 2. IL-9 producing eosinophil and non-There is a significant decrease in IL-9 producing eosinophils and a non-signficant decrease in non-eosinophilic cells following anti-IL-5 therapy (n=6 subjects pre- and post-mepolizumab).
Clinical Implications.
12 weeks of anti-IL-5 therapy decreases mast cell numbers in eosinophilic esophagitis which may occur via a mechanism that involves the reduction of IL-9 producing cells including eosinophils.
Acknowledgments
Support: Supported by a research grant from GlaxoSmithKline (S.A.), NIH/NIAID grants AI092135 (SA), AI38425, AI70535, and AI72115 (DB)
Abbreviations
- EoE
Eosinophilic esophagitis
- Hpf
High power field
- HES
Hypereosinophilic Syndrome
- MBP
Major Basic Protein
- TGFβ1
Transforming Growth Factor-beta-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–12. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology. 2008;134:204–14. doi: 10.1053/j.gastro.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol. 2010;126:1198–204. doi: 10.1016/j.jaci.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Kagalwalla AF, Akhtar N, Woodruff SA, et al. Eosinophilic esopahgitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clinical Immunol. 2012 doi: 10.1016/j.jaci.2012.03.005. e published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4:1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Gonsalves N, Yang G-y, Doerfler, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; Food reintroduction identifies causative factors. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.03.001. e published ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 9.Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–91. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Dohil R, Newbury R, Fox L, Bastian J, Aceves S. Oral viscous budesonide is effective in children with eosinophilic esophagitis in a randomized, placebo-controlled trial. Gastroenterology. 2010;139:418–29. doi: 10.1053/j.gastro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol. 2002;168:2464–9. doi: 10.4049/jimmunol.168.5.2464. [DOI] [PubMed] [Google Scholar]
- 12.Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, Hargreave FE, O’Byrne PM. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. Nair P, N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 13.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. Mepolizumab HES Study Group. N Engl J Med. 2008;358(12):1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010;125:1336–43. doi: 10.1016/j.jaci.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 15.Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. Allergy Clin Immunol. 2006;118:1133–41. doi: 10.1016/j.jaci.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 16.Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010;59:21–30. doi: 10.1136/gut.2009.178558. [DOI] [PubMed] [Google Scholar]
- 17.Assa’ad AH, Gupta SK, Collin MH, Thomson M, Heath AT, Smith DA, Perschy TL, et al. Treatment of pediatric eosinophilic esophagitis with mepolizumab: a randomized, double blind, controlled clinical trial. Gastroenterology. 2011;141:1593–604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 18.Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: Results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 201;129:456–463. doi: 10.1016/j.jaci.2011.11.044. [DOI] [PubMed] [Google Scholar]
- 19.Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, et al. Involvement of mast cells in eosinophilic esophagitis. J Allergy Clin Immunol. 2010;126:140–9. doi: 10.1016/j.jaci.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu Blatman KS, Gonsalves N, Hirano I, Bryce PJ. Expression of mast cell-associated genes is upregulated in adult eosinophilic esophagitis and responds to steroid or dietary therapy. J Allergy Clin Immunol. 2011;127:1307–8. doi: 10.1016/j.jaci.2010.12.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochi H, DeJesus NH, Hsieh FH, Austen KF, Boyce JA. IL-4 and -5 prime human mast cells for different profiles of IgE-dependent cytokine production. Proc Natl Academy of Sciences. 2000;97:10509–13. doi: 10.1073/pnas.180318697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahl C, Hoffman HJ, Saito H, Schiotz PO. Human mast cells express receptors for IL-3, IL-5, and GM-CSF; a partial map of receptors on human mast cells cultured in vitro. Allergy. 2004;59:1087–96. doi: 10.1111/j.1398-9995.2004.00606.x. [DOI] [PubMed] [Google Scholar]
- 23.Elishmereni M, Alenius, Bradding P, et al. Physical intereactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro. Allergy. 2011;66:376–85. doi: 10.1111/j.1398-9995.2010.02494.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilson TM, Maric I, Shukla J, et al. IL-5 receptor a levels in patients with marked eosinophilia or mastocytosis. J Allergy Clinical Immunol. 2011;128:1086–92. doi: 10.1016/j.jaci.2011.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siracusa MC, Saenz SA, Hill DA, et al. TSLP promotes interleukin-3-independent basophil hematopoiesis and type 2 inflammation. Nature. 2011;477:229–33. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones TG, Hallgren J, Humbles A, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J of Immunol. 2009;183:5251–60. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gounni AS, Gregory B, Nutku E, et al. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood. 2009;96:2163–71. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Eosinophils and mast cells numbers have a close relationship. The percent of subjects with varying decreases in esophageal epithelial eosinophils (clear bars) and the numbers of mast cells (filled bars) in the given eosinophil category. The mean peak numbers of eosinophils and mast cells prior to (pre) and post (post) anti-IL-5 are indicated above the respective bar.
Supplemental Figure 2. IL-9 producing eosinophil and non-There is a significant decrease in IL-9 producing eosinophils and a non-signficant decrease in non-eosinophilic cells following anti-IL-5 therapy (n=6 subjects pre- and post-mepolizumab).